Abstract
Background
Even small delays in the treatment of breast cancer are a frequently expressed concern of patients. Knowledge about this subject is important for clinicians to counsel patients appropriately and realistically, while also optimizing care. Although data and quality measures regarding time to chemotherapy and radiotherapy have been present for some time, data regarding surgical care is more recent and no standard exists. This review was written to discuss our current knowledge about the relationship of treatment times to outcomes.
Methods
The published medical literature addressing delays and optimal times to treatment was reviewed in the context of our current time-dependent standards for chemotherapy and radiotherapy. The surgical literature and the lack of a time-dependent surgical standard were also discussed, suggesting a possible standard.
Results
Risk factors for delay are numerous, and tumor doubling times are both difficult to determine and unhelpful in assessing the impact of longer treatment times on outcomes. Evaluation components also have a time cost, and are inextricable from the patient’s workup. Although the published literature has lack of uniformity, optimal times to each modality are strongly suggested by emerging data, supporting the current quality measures. Times to surgery, chemotherapy and radiotherapy all have a measurable impact on outcomes, including disease-free survival, disease-specific survival, and overall survival.
Conclusions
Delays have less of an impact than often thought, but have a measurable impact on outcomes. Optimal times from diagnosis are <90 days for surgery, <120 days for chemotherapy, and, where chemotherapy is administered, <365 days for radiotherapy.
INTRODUCTION
As the most frequent malignancy in women, breast cancer evokes widespread fear and anxiety.1 Concern about the effect of treatment delay on breast cancer outcomes is one which has been present for over a century, even elaborated by Halsted in his 1907 mastectomy series where he stated that “we no longer need the proof which our figures so unmistakably give that the slightest delay is dangerous….”2 Although fear of breast cancer itself can cause delays,3 patients frequently inquire from their physicians about how soon they should begin treatment, concerned that undue delay will impair their likelihood of survival.
In breast cancer, this perception of longer times equating to poorer outcomes may be magnified by the mantra associated with mammography, that “early detection saves lives,” as the obverse tenet would be that late diagnosis kills patients. This perception is widespread, as illustrated by breast cancer claims where the majority of breast cancer lawsuits are based on alleged delay in diagnosis, rather than therapeutic malpractice.4,5 There is also no medical definition of a standard interval to diagnosis or treatment, although published studies often used specific thresholds6–10 to investigate when times become detrimental. As longer times to treatment probably have a gradual and continuous effect on outcomes, series that evaluate progressive time intervals rather than a specific cutoff may capture the effect on survival more realistically.11–13
In evaluating studies it is important to scrutinize the defined beginning and end points of the interval in question; i.e. does the interval start at first symptom, presentation, imaging, diagnosis or treatment, and does it end with a particular component of evaluation, treatment, recurrence, or death. Scrutiny of this issue has increased, and breast cancer quality measures now exist, specifying appropriate treatment intervals,14 even though it remains currently unproven as to whether these specific measures enhance quality or survival.
BIOLOGY OF DELAY
In theory, cellular division and tumor growth should provide the most accurate method by which to assess the impact of delays on breast cancer outcomes. Tumor doubling time, which is the time required for cells to divide, should help determine the harm caused by a longer interval. Unfortunately, tumor doubling times are not constant, likely complicating reliable prediction. Tumors initially have a parabolic exponential growth rate, but limits in blood supply, physical space, and nutrition, along with a tumor’s chaotic growth pattern, cause them to exhibit Gompertzian kinetics15,16 where their rapid rate of expansion at the outset begins to decline and plateau.
Unfortunately, tumor doubling times vary tremendously within and between studies (Table 1) which may be, in part, due to these nonlinear growth kinetics. These investigations use a variety of methods, including review of breast imaging, metastasis development, historical assessment, and local recurrences.17–27 Such studies estimate tumor doubling times to be between 2 and 7,051 days,20,25 with medians varied from 45–260 days.25,26 These disparate estimates suggest that we are very poor at accurately measuring these intervals, and that doubling time estimations are unhelpful in determining the effect of delays on breast cancer survival. This is supported by the fact that prognostic factors such as age, race, tumor size, grade, and lymph node metastases have also not been consistently found to correlate with tumor doubling time.18,23–26
Table 1.
Published medical literature estimating tumor doubling times.
| Study | Method of Estimation | n | Td (days) Median | Td (days) Range |
|---|---|---|---|---|
| Gershon-Cohen, 1963 Cancer | Mammography | 18 | — | 23–209 |
| Kusama, Cancer 1972 | Metastases | 199 | 105 | 6–548 |
| Charlson, JAMA 1974 | History | 219 | — | — |
| Pearlman, Cancer 1976 | Mastectomy Scar Local Recurrence | 82 | — | 2–140 |
| Shackney, Ann Intern Med 1978 | Mastectomy Scar Local Recurrence | 243 | 25, 129* | 3–500+ |
| Von Fournier, Cancer 1980 | Mammography | 147 | 212** | 44–1,869 |
| Arnerlöv, Cancer 1992 | Mammography | 158 | 180 | 18–270 |
| Spratt, Cancer 1993 | Mammography | 448 | 260 | 10–7,051 |
| Tilanus-Linthorst, Eur J Cancer 2005 | Imaging | 25 | 84 BRCA− | ~15–450 |
| Imaging | 30 | 45 BRCA+ | ||
| Weedon-Fekjær, Br Cancer Res 2008 | Mammography | 364,731 | §1.7 years | — |
| Summary | 18 – 364,731 | 45 – 260 | 2 – 7051 | |
Td = Tumor doubling time
Early stage, late stage cancers
Mean
Time to increase: 1.0 cm to 2.0 cm, not a true doubling time
The total life span of a tumor also cannot be accurately determined, further clouding the relationship of tumor doubling times to delays and outcomes. Cancers begin at inception when the first cell has undergone malignant transformation. The cell doubles approximately 20 to 30 times, to reach 1 mm3 to 1 cm3 when it becomes potentially clinically evident. This time period is referred to as the tumor’s silent interval because it is too small to allow detection.20 The time between potential and actual diagnosis or between diagnosis and treatment are the time intervals that we typically scrutinize and try to minimize.
Although we usually measure survival from diagnosis or treatment until death, the time that a patient is at risk for metastatic disease and death from cancer begins at inception, and continues through the majority of that tumor’s lifespan, which occurs before it is of sufficient size to detect. Thus, delays that occur after reaching a detectable size are thought to represent only a small fraction of the time that a tumor has been in existence, posing risk to the patient. This is, in part, why 8% of women currently present with metastatic disease at diagnosis,28 and likely the reason that most studies find that effects of a longer interval, when significant, are relatively small.
SURGERY
At the time of this writing, there is no time-dependent surgery standard, specifying how soon a patient should undergo operative intervention after diagnosis. This may be because, until recently, there has been little data on waiting times to breast cancer surgery in the United States. In 2012, a SEER-Medicare study found that in 72,586 women having invasive breast cancer who had not received neoadjuvant chemotherapy, mean and median times between presentation and surgery were 46 and 29 days, respectively. The median time had lengthened from 21 days in 1992 to 32 days in 2005,29 consistent with the growing complexity of preoperative breast cancer evaluation which includes a greater use of imaging30 that in itself has a time cost.29
The time to surgery is also related to both necessary and desired components of preoperative evaluation and these are inextricable from it. For instance, preoperative MRI use preoperatively adds 6.4 days to the preoperative interval, while fine needle aspirations add 6 days, and core needle and excisional biopsies add 12.7 and 17.4 days respectively.29 Even the ideal paradigm of a preoperative multidisciplinary evaluation by medical oncology, radiation oncology and surgery adds 12.6 days between diagnosis and surgery, or 6.8 days if these are condensed into one day.31
Treatment choices also have an effect on the time to treatment, and many are scheduling related; lengthier procedures take longer to book into open operative time, while coordination with plastic surgery or nuclear medicine may also delay scheduling. In the United States, the use of radionuclide for sentinel node biopsy adds 2.3 days, while adding reconstruction to mastectomy increases the time to operation by 12.2 days.29
The effect of delays on survival has been controversial. Nodal status as a surrogate for outcome has been investigated, and a modeling study in pregnant patients32 found that delaying treatment from 1 to 3 to 6 months was associated with an increased risk of axillary lymph node metastases, although this was based on two assumed tumor doubling times, and not validated in vivo. Meanwhile, a series of 818 clinically node negative breast cancers diagnosed from 2003–2006,33 found that time to surgery was not associated with lymph node status, and a series reviewing 5,283 women presenting 1988–1999 found that a delay of ≥2 months in diagnosis was not associated with nodal metastases or their breast conservation rate.
Studies that evaluate the effect of timing of first treatment on outcome are shown in Table 2, and utilize varied cutoffs with results that are not uniform. One large study utilizing NCDB data found that outcomes declined only after a threshold of >12 weeks between diagnosis and surgery. Meanwhile a study12 evaluating both Surveillance Epidemiology End Results (SEER)-Medicare and NCDB data found that disease-specific survival declined by a relative 24% per month, while overall survival dropped 9–10% per month in each database, resulting in a 3.1–4.6% absolute decline with delays of 90 days. This study also found that >98% of patients in the United States have surgery within 90 days of diagnosis in both datasets, which suggests that this may be a reasonable candidate for a time-dependent surgical threshold if one were to be defined.
Table 2.
Published medical literature evaluating delays to first treatment and survival outcomes for breast cancer
| Study | n | Measure | Median (d) | Comparison (months) | Death Hazard | Notes |
|---|---|---|---|---|---|---|
| Comber, Ir Med J 1998 | 2,424† | Presentation to Treatment | 17 | <1;1–2;2–3;3–4;4–5;>5 | NS | No difference |
| Sainsbury, Lancet 1999 | 36,222 | Presentation to Treatment | N/A | ≤3 vs >3 | NS | No difference |
| Richards, Lancet 1999 | 44,347 | Symptomatic presentation to Treatment | N/A | < 3 vs > 3 | 1.47 [1.42–1.53] | Meta-analysis; Death hazards only included studies with 5 year OS |
| 25,102 | N/A | < 3 vs 3–6 | 1.24 [1.17–1.30] | |||
| 53,013 | N/A | < 6 vs > 6 | 1.45 [1.40–1.50] | |||
| Brazda, Ann Surg Oncol 2010 | 1,337 | Diagnosis to Treatment | 43‡ | <1.5 v 1.5–3 v >3 | NS | <3 v >3 also NS |
| McLaughlin, J Clin Oncol 2012 | 1,786 | Diagnosis to Treatment | 22 | < 2 vs ≥ 2 | 1.66 [1.00–2.77] | All p’s ~0.05 |
| Shin, Ann Surg Oncol 2012 | 2,045 | Diagnosis to Surgery | 14 | 1 vs > 3 | 1.91 [1.06–3.49] | ≤1 wk hazard 1.20 |
| Bleicher, JAMA Oncol 2016 | 95,544 | Diagnosis to Surgery | N/A | <1;1–2;2–3;3–4;4–6 | 1.09 [1.06–1.13] OS** 1.26 [1.02–1.54] DSS** |
SEER-Medicare **per month delay |
| 115,790 | N/A | <1;1–2;2–3;3–4;4–6 | 1.10 [1.07–1.13] OS** | NCDB **per month delay |
||
| Polverini, Ann Surg Oncol 2016 | 420,792 | Diagnosis to Surgery | N/A | <1;1–2;2–3;3–6.5 | 1.14 [1.09–1.20] OS* | NCDB *>12 vs ≤ 12 weeks, others NS |
OS = overall survival; DSS = disease-specific survival; SEER = Surveillance Epidemiology End Results; NCDB = National Cancer Database; NS = nonsignificant
Mean
Total for 4 cancer types; breast numbers not given. Analysis here otherwise for breast only
CHEMOTHERAPY
We currently have a quality measure that specifies that chemotherapy should be administered within 120 days of diagnosis in women <70 having AJCC T1c, Stage II or III, hormone-receptor negative breast cancer. Although two standard chemotherapy regimens were established in trials that specified administration 2–4 weeks after surgery for cyclophosphamide, methotrexate and 5-fluorouracil (CMF),34 and 2–5 weeks afterwards for doxorubicin and cytoxan (AC),35 the time from diagnosis was not specified. There is unfortunately no published data evaluating whether a chemotherapy standard is better focused on time from diagnosis or surgery.
Current paradigm specifies that chemotherapy be given before radiotherapy and not delayed until afterwards, in part because of data showing that local recurrence is higher when chemotherapy is given before radiotherapy, while metastases increase when radiotherapy is given first.36 Studies most frequently assess times from surgery to chemotherapy in 4 week intervals (Table 3).37–41 Results of these studies vary, with some finding declines in disease free,39,40 disease specific,37 and overall survival,37–41 although an impairment from longer intervals is not always found.36,42,43
Table 3.
Published medical literature evaluating delays to chemotherapy and patterns of care, with survival outcomes for breast cancer.
| Study | n | Measure | Median (d) | Comparison | HR, p | Notes |
|---|---|---|---|---|---|---|
| Recht, New Engl J Med 1996 | 244 | Breast Conservation to Chemotherapy |
* XRT = 36 Chemo=136 |
Chemotherapy before vs after XRT | LR p=0.07 OS p=0.11 |
LR more common when chemo given first, systemic recurrence when XRT given first. |
| Cold, Br J Cancer 2005 | 352 | Surgery to Chemotherapy getting CMF | Not Given | 1–3;4,5,6–13 wks | p=0.1627 | Danish Breast Cancer Cooperative Group trials |
| 6,065 | Surgery to Chemotherapy getting CMF IV | p =0.1913 | ||||
| 1,084 | Surgery to Chemotherapy getting CEF | p =0.6567 | ||||
| Hershman, Br Cancer Res Treat 2006 | 5,003 | Surgery to Chemotherapy | Not Given** | <1;1–≤2;2–≤3;>3 mos | DSS=1.69 OS=1.46 |
<3 mos NS; HR for >3 mos; Women ≥65 y, 1992–1999, stages I–II; SEER-Medicare |
| Lohrisch, J Clin Oncol 2006 | 2,594 | Surgery to Chemotherapy | Not Given§ | ≤4;>4–8;>8–12;>12–24 wks | OS=1.6 p=0.005 |
HR ≤12 v >12; British Columbia Cancer Agency |
| Sanchez, Br Cancer Res Treat 2007 | 2,782 | Surgery to Chemotherapy | Not Given | <3;3–6;6–9;>9 wks | DFS p=0.26 OS p=0.605 |
Females Stage I, II, IIIa. |
| Yu, BMC Cancer 2013 | 34,097 | Surgery to Chemotherapy | Not Given | Per 4 week delay | DFS=1.16 OS=1.15 |
Meta-analysis of surgery to chemotherapy studies |
| Gagliato, J Clin Oncol 2014 | 6,827 | Surgery to Chemotherapy | Not Given | ≤30, 31–60, ≥61 days | ≥61d lowers DRFS, OS |
Stage I–III, 1997–2011 |
| Chavez-MacGregor JAMA Oncol 2016 | 13,869 | Surgery to Chemotherapy, HR+ | 46‡ | <31, 31–60, 61–90, ≥91 days | DSS=NS | All intervals HR was NS |
| 6,276 | Surgery to Chemotherapy, HER2+ | DSS=NS | All intervals HR was NS | |||
| 4,698 | Surgery to Chemotherapy, Triple negative | HR=1.53 | HR same for DSS & OS (>90d only:Others NS) | |||
| Raphael, Br Cancer Res Treat 2016 | 14 studies | Surgery to Chemotherapy getting AC | Not Given | 4 week interval increase | OS RR= 1.04–1.08 | Study-level meta-analysis of observational studies |
| Yu, Oncotarget 2017 | 667 | Surgery to Chemotherapy, Luminal A | Not Given† | ≤4;4–8;>8 wks | HR=NS | HRs are for ≤8 vs >8 weeks to chemotherapy |
| 328 | Surgery to Chemotherapy, Luminal B | HR=1.93 | ||||
| 270 | Surgery to Chemotherapy, Triple Negative | HR=2.55 | ||||
| 143 | Surgery to Chemotherapy, HER2 Neu | HR=2.41 | ||||
| Abdel-Rahman, Breast 2018 | 3,390 | Surgery to Chemotherapy, HR− | Not Given | <>6 wks | p =0.006 | Pts from 3 clinical trials; <>3 wks NS for all. P values given but no hazard ratios. XRT delays NS. |
| Surgery to Chemotherapy, HR+ | p =0.268 | |||||
| Surgery to Chemotherapy, Overall | p =0.534 |
HR = hazard ratio; XRT = radiotherapy; LR – local recurrence; CMF = cyclophosphamide, methotrexate, 5-fluorouracil; CEF = cyclophosphamide, epirubicin, 5-fluorouracil; wks = weeks; mos = months; DSS = disease specific survival; DFS = disease free survival; DRFS = distant recurrence-free survival; OS = overall survival; AC = doxorubicin, cyclophosphamide; RR = relative risk; NS = not significant; HR −= Hormone receptor negative; HR+ = Hormone receptor positive
Times are between last breast surgery until radiotherapy for the radiotherapy-first group, and until chemotherapy in the chemotherapy-first group.
Chemotherapy received by 47% within 1 month, 37% between months 1–2, 6% between months 2–3, and 10% after 3 months.
Chemotherapy received by 38% ≤4 weeks, 49% >4 to 8 weeks, 8.4% >8 to 12 weeks, and 4.3% >12 to 24 weeks.
Chemotherapy received by 89.6% ≤4 months and 10.4% >4 months from surgery.
Chemotherapy received by 21% within 31 days, 50% 31–60 days, 19.2% 61–90 days, and 9.8% ≥91 days after surgery.
Chemotherapy received by 60% within 4 weeks, and 6.5% after 8 weeks from surgery.
Delays in chemotherapy after surgery have also been explored by phenotype. In receptor-positive tumors, two studies have shown no relationship,44,45 with a third46 finding that luminal A tumors are not affected, but luminal B tumors have a hazard ratio of 1.93 for intervals >8 weeks. In a recently presented abstract, among 273,521 receptor-positive patients, each additional month lowered outcome by 11.1%.47 In the sole study evaluating receptor-negative tumors,45 times >6 weeks had a significant impact, while three studies evaluating triple negative tumors have all noted an impact on disease-specific or overall survival.44,46,47 One study of 4,698 patients, found that delay-related declines were worse for triple negative tumors, suggesting neoadjuvant therapy be considered routinely, while another found in 36,505 such patients no difference in the decline between triple negative and other phenotypes.47 Finally, in HER2-positive tumors, one study found no impact on disease-specific survival,44 while two noted that overall survival was affected by times to treatment.46,47
RADIOTHERAPY
Times between surgery and radiotherapy have been increasing in some countries,48,49 where longer intervals exist than in the United States.50 When chemotherapy is administered, the relationship between radiation delays and survival is unclear. For instance, in a series of 482 patients with stage I or II breast cancer,51 an analysis adjusting for chemotherapy administration found that increasing time to radiotherapy was not associated with a local recurrence increase. Such nonsignificant results may have been due to a lack of statistical power, systemic therapy mitigating the effect of delay, or timing issues surrounding chemotherapy administration confounding the analysis.
Much of the published literature evaluates timing when only surgery and radiotherapy are administered, making it easier to conceptually assess the impact of delay by eliminating the confounding of that intervening chemotherapy, although such results may not be applicable to patients receiving systemic therapy. Currently, we only have one time-dependent radiotherapy standard covering patients whether they receive chemotherapy or not. This specifies that radiotherapy be initiated within 365 days of diagnosis in patients <70 having breast conservation.14
A series evaluating the SEER-Medicare dataset found that among 18,050 women >65, diagnosed with Stages 0-II breast cancer,52 having breast conservation and radiotherapy, but no chemotherapy, median time from surgery to radiation was 34 days, with one third starting after 6 weeks. An interval to radiotherapy >6 weeks was associated with an adjusted hazard ratio of 1.19 (95%CI 1.01–1.39, p=0.004) for local recurrence, with a 0.5% increase in the local recurrence risk per day.
Meanwhile, an older single-institution series reviewed 653 node-negative Stage I and II patients not receiving systemic therapy,53 dividing them between those starting radiotherapy <4, 5–8, and 9–12 weeks postoperatively. The last group was the smallest, and while no compromise in outcomes <8 weeks was demonstrable, failure rates in the longest group also “did not suggest a greater risk of…recurrence for this group,”53 although 5-year recurrence rates for the three groups would now be considered high at 24%, 21%, and 15% respectively. In contrast, a series evaluating the SEER-Medicare database54 divided 13,907 Stage I–II women having breast conservation who did not receive chemotherapy after their last surgery and found that radiation ≥12 weeks (3 months) had worse disease-specific and overall survival with hazard ratios of 3.81 (95%CI 2.98–4.87, p<0.0001) and 1.91 (95%CI 1.63–2.23, p<0.0001), respectively, when adjusting for demographics and tumor factors. A more recent study of 568 T1/2, node-negative patients treated with breast conservation therapy without systemic therapy55 found that after 11.2 years of follow up, no differences in disease-free survival were found up to 16 weeks with no definitive conclusion possible >16 weeks because of small patient numbers.
Finally, other series have found that longer times to radiotherapy are of no consequence, up to a point. A study of 1,962 women in British Columbia with T1-3 breast cancer who did not receive chemotherapy56 found that intervals of 0–20 weeks did not impair disease free survival. Another study with overlapping authors using the same dataset, subsequently evaluating 6,428 women57 with T1-2, N0 breast cancers treated with breast conservation but no chemotherapy, found no differences in any outcome up to 20 weeks, although there was a decline thereafter. Meanwhile a study analyzing data from three International Breast Cancer Study Group trials58 also found no effect of up to 20 weeks in 964 patients having breast conservation surgery, radiotherapy, and adjuvant endocrine therapy. Although the literature is varied, outcomes appear to remain unchanged after surgery when times to radiotherapy are at least 8 weeks, and likely up to 20 weeks in the absence of chemotherapy with longer times remaining safe when chemotherapy is given in the interim (Table 4).
Table 4.
Published medical literature evaluating times to radiotherapy in patients having radiotherapy without chemotherapy, and outcomes for breast cancer
| Study | n | Cohort | Inclusion | Delay Groups (weeks unless specified) | Interval without decline (weeks) | Findings |
|---|---|---|---|---|---|---|
| Nixon, Int J Rad Oncol Biol Phys 1994 | 653 | Single institution | Stage I, II | <4; 5–8; 9–12 | < 8 | No difference <8 weeks definitive No difference 9–12 weeks uncertain |
| Froud, Int J Rad Oncol Biol Phys 2000 | 1,962 | British Columbia Cancer Agency Outcomes Unit Database | T1-3, N0 | 0–5; 6–8; 9–12; 13+ | ≤20 | No difference <20 weeks (>20 unknown-sample too small) |
| Hershman, Int J Rad Oncol Biol Phys 2006 | 13,907 | SEER-Medicare, 1991–1999 | Stage I, II | <1; ≥1–<2; ≥2–<3; ≥3 months | <12 | No difference <3 months; DSS HR 3.81; OS HR 1.91 >3 months |
| Vujovic, Int J Rad Oncol Biol Phys 2006 | 568 | Single institution | T1-2, N0 | 0–8; >8–12; >12–16; >16 | <16 | No difference <16 weeks definitive No difference >16 weeks uncertain |
| Olivotto, J Clin Oncol 2009 | 6,428 | British Columbia Cancer Agency Outcomes Unit Database | T1-2, N0-1 | 0–4, >4–8;>8–12;>12–16; >16–20;>20–42 | ≤20 | No difference in LRFS, DRFS, BCSS ≤20 weeks; HRs >20 weeks: LRFS NS; DRFS 1.86; BCSS 2.15 |
| Punglia, BMJ 2010 | 18,050 | SEER-Medicare 1991–2002 | Stage 0-II | Continuous modeling | <6 | Time to XRT >6 weeks: HR 1.19 HR 1.005 for LR per day |
| Karlsson, Int J Rad Oncol Biol Phys 2011 | 964 | Data from 3 IBCSG Trials (Trials VII, VIII, IX) | Any T, N± | ≤48, 49–77; 78–112; ≥113 days | ≤20 | No difference <20 weeks |
IBCSG = International Breast Cancer Study Group; d = days; LRFS = local recurrence-free survival; DRFS = distant recurrence-free survival; BCSS = breast cancer-specific survival; HR = hazard ratio; NS = nonsignificant; LR = local recurrence
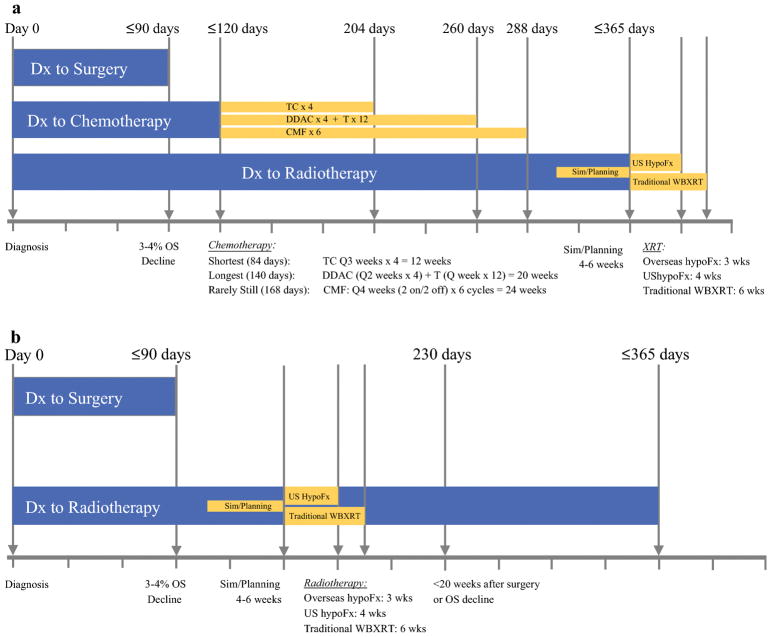
Although the current standard, allowing a full year (365 days) from diagnosis seems lengthy, this allows time for systemic therapy. With 98% of surgeries performed within 90 days, and chemotherapy initiated <120 days according to that time-dependent standard, this allows 84 days and 140 days for our modern-day shortest and longest chemotherapy regimens of TC × 4 and dose dense AC × 4 and T × 12, respectively. If no pauses occur in these regimens, these would complete on day 204 and 260, respectively. For patients even requiring 6 months of CMF, chemotherapy would end on day 288. This would allow only 2–4 weeks to begin simulation and planning, which takes 4–6 weeks, in order to begin radiotherapy by that 365 day threshold (Figure 1a). In a setting where patients do not get chemotherapy, a 365 day interval to radiation is not only unnecessary, but may actually lower survival, even if we consider the 20-week threshold found by the three studies above (Figure 1b).
Figure 1.
Timing of treatments in breast cancer therapy, based upon current quality measures. Current standards specify time to chemotherapy within 120 days of diagnosis, while time to radiotherapy specifies administration within 365 days of diagnosis. With >98% of surgeries in the United States occurring within 90 days, and a drop in overall survival by an absolute 3.1–4.6%, this threshold seems appropriate as it allows one month to begin chemotherapy by the current quality measure. The 365-day quality measure for radiotherapy allows for sufficient time to undergo chemotherapy regimens of varying lengths, while allowing a short time to begin simulation and planning (panel 1a). When chemotherapy is not administered, however, the radiotherapy standard provides an excess of time, even when using 20 weeks postoperative, which is the longest interval found to not confer a survival decline (panel 1b). This suggests that a second standard, measured from time of surgery when chemotherapy is not administered, might optimize care.
Dx = diagnosis; OS = Overall Survival; Sim = simulation; hypoFx = hypofractionation; WBXRT = whole breast radiotherapy; TC = taxotere and cyclophosphamide; DDAC = dose dense doxorubicin and cyclophosphamide; + T = paclitaxel; CMF = cyclophosphamide, methotrexate, and 5-fluorouracil.
CONCLUSION
In short, times to surgery, chemotherapy, and radiotherapy have an impact on outcomes. While there is no time-dependent surgical standard, time from diagnosis to surgery (in the non-neoadjuvant setting) of >90 days, which occurs in <2% of patients in the United States, lowers overall survival by 3.1–4.6%, and would be a reasonable time-dependent standard if one were to be set. Times to chemotherapy as set by the current standard of <120 days from diagnosis would allow for that interval, while limiting any effect of chemotherapy delay. Meanwhile, times to radiotherapy of <365 days in patients receiving chemotherapy, as defined by the current standard, allow appropriate time for systemic therapy, although patients not receiving chemotherapy should likely have radiation far earlier, beginning no more than 20 weeks from surgery where feasible.
SYNOPSIS.
Delays in breast cancer have been a concern for over a century, and current quality measures have begun to reflect the reality that treatment times can affect outcomes. This paper reviews current knowledge about delays and optimizing times to treatment.
References
- 1.Vrinten C, McGregor LM, Heinrich M, et al. What do people fear about cancer? A systematic review and meta-synthesis of cancer fears in the general population. Psychooncology. 2017 Aug;26(8):1070–1079. doi: 10.1002/pon.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halsted WSI. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg. 1907 Jul;46(1):1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jassem J, Ozmen V, Bacanu F, et al. Delays in diagnosis and treatment of breast cancer: a multinational analysis. Eur J Public Health. 2014 Oct;24(5):761–767. doi: 10.1093/eurpub/ckt131. [DOI] [PubMed] [Google Scholar]
- 4.Mitnick JS, Vazquez MF, Plesser KP, Roses DF. Breast cancer malpractice litigation in New York State. Radiology. 1993 Dec;189(3):673–676. doi: 10.1148/radiology.189.3.8234689. [DOI] [PubMed] [Google Scholar]
- 5.Zylstra S, D’Orsi CJ, Ricci BA, et al. Defense of breast cancer malpractice claims. The breast journal. 2001 Mar-Apr;7(2):76–90. doi: 10.1046/j.1524-4741.2001.007002076.x. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on Survival of Longer Intervals Between Confirmed Diagnosis and Treatment Initiation Among Low-Income Women With Breast Cancer. J Clin Oncol. 2012 Dec 20;30(36):4493–4500. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazda A, Estroff J, Euhus D, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010 Oct;17( Suppl 3):291–296. doi: 10.1245/s10434-010-1250-6. [DOI] [PubMed] [Google Scholar]
- 8.Shin HC, Han W, Moon HG, et al. Limited value and utility of breast MRI in patients undergoing breast-conserving cancer surgery. Ann Surg Oncol. 2012 Aug;19(8):2572–2579. doi: 10.1245/s10434-012-2289-3. [DOI] [PubMed] [Google Scholar]
- 9.Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999 Apr 3;353(9159):1132–1135. doi: 10.1016/s0140-6736(99)02374-0. [DOI] [PubMed] [Google Scholar]
- 10.Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999 Feb;79(5–6):858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005 Sep;98(8):238–239. [PubMed] [Google Scholar]
- 12.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol. 2016 Mar 1;2(3):330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polverini AC, Nelson RA, Marcinkowski E, et al. Time to Treatment: Measuring Quality Breast Cancer Care. Ann Surg Oncol. 2016 Oct;23(10):3392–3402. doi: 10.1245/s10434-016-5486-7. [DOI] [PubMed] [Google Scholar]
- 14.American College of Surgeons. National Accreditation Program for Breast Centers. [Accessed March 29, 2018];NAPBC Standards Manual 2018 Edition. 2018 :77. https://accreditation.facs.org/accreditationdocuments/NAPBC/Portal%20Resources/2018NAPBCStandardsManual.pdf.
- 15.Laird AK. Dynamics of Tumor Growth. Br J Cancer. 1964;18(3):490. doi: 10.1038/bjc.1964.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird AK. Dynamics of Tumour Growth - Comparison of Growth Rates and Extrapolation of Growth Curve to One Cell. Br J Cancer. 1965;19(2):278. doi: 10.1038/bjc.1965.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershon-Cohen J, Berger SM, Klickstein HS. Roentgenography of Breast Cancer Moderating Concept of “Biologic Predeterminism”. Cancer. 1963 Aug;16:961–964. doi: 10.1002/1097-0142(196308)16:8<961::aid-cncr2820160802>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Kusama S, Watson FR, Spratt JS, Cunningh C, Donegan WL. Gross Rates of Growth of Human Mammary-Carcinoma. Cancer. 1972;30(2):594. doi: 10.1002/1097-0142(197208)30:2<594::aid-cncr2820300241>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Feinstei Ar. Auxometric Dimension - New Method for Using Rate of Growth in Prognostic Staging of Breast-Cancer. Jama-J Am Med Assoc. 1974;228(2):180–185. doi: 10.1001/jama.228.2.180. [DOI] [PubMed] [Google Scholar]
- 20.Pearlman AW. Breast cancer--influence of growth rate on prognosis and treatment evaluation: a study based on mastectomy scar recurrences. Cancer. 1976 Oct;38(4):1826–1833. doi: 10.1002/1097-0142(197610)38:4<1826::aid-cncr2820380460>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Methylene blue - PubChem Public Chemical Database. [Accessed October 20, 2008];PubChem Public Chemical Database. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6099.
- 22.Shackney SE, Mccormack GW, Cuchural GJ. Growth-Rate Patterns of Solid Tumors and Their Relation to Responsiveness to Therapy. Ann Intern Med. 1978;89(1):107–121. doi: 10.7326/0003-4819-89-1-107. [DOI] [PubMed] [Google Scholar]
- 23.Vonfournier D, Weber E, Hoeffken W, Bauer M, Kubli F, Barth V. Growth-Rate of 147 Mammary Carcinomas. Cancer. 1980;45(8):2198–2207. doi: 10.1002/1097-0142(19800415)45:8<2198::aid-cncr2820450832>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Arnerlov C, Emdin SO, Lundgren B, et al. Breast-Carcinoma Growth-Rate Described by Mammographic Doubling Time and S-Phase Fraction - Correlations to Clinical and Histopathologic Factors in a Screened Population. Cancer. 1992 Oct 1;70(7):1928–1934. doi: 10.1002/1097-0142(19921001)70:7<1928::aid-cncr2820700720>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Spratt JA, Vonfournier D, Spratt JS, Weber EE. Mammographic Assessment of Human Breast-Cancer Growth and Duration. Cancer. 1993 Mar 15;71(6):2020–2026. doi: 10.1002/1097-0142(19930315)71:6<2020::aid-cncr2820710616>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Tilanus-Linthorst MM, Kriege M, Boetes C, et al. Hereditary breast cancer growth rates and its impact on screening policy. Eur J Cancer. 2005 Jul;41(11):1610–1617. doi: 10.1016/j.ejca.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 27.Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10(3):R41. doi: 10.1186/bcr2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Insitute. [Accessed March 29, 2018];Breast Cancer Stage Distribution of SEER Incidence Cases, 2005–2014 By Race/Ethnicity. 2017 https://seer.cancer.gov/explorer/application.php?site=55&data_type=1&graph_type=4&compareBy=race&chk_sex_3=3&chk_sex_2=2&chk_race_1=1&chk_age_range_1=1&advopt_precision=1&showDataFor=sex_2_and_age_range_1.
- 29.Bleicher RJ, Ruth K, Sigurdson ER, et al. Preoperative Delays in the US Medicare Population With Breast Cancer. J Clin Oncol. 2012 Dec 20;30(36):4485–4492. doi: 10.1200/JCO.2012.41.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA. 2010 Apr 28;303(16):1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 31.Churilla TM, Egleston BL, Murphy CT, et al. Patterns of multidisciplinary care in the management of non-metastatic invasive breast cancer in the United States Medicare patient. Breast Cancer Res Treat. 2016 Nov;160(1):153–162. doi: 10.1007/s10549-016-3982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nettleton J, Long J, Kuban D, Wu R, Shaeffer J, ElMahdi A. Breast cancer during pregnancy: Quantifying the risk of treatment delay. Obstet Gynecol. 1996 Mar;87(3):414–418. doi: 10.1016/0029-7844(95)00470-x. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JL, Warneke CL, Mittendorf EA, et al. Delays in primary surgical treatment are not associated with significant tumor size progression in breast cancer patients. Ann Surg. 2011 Jul;254(1):119–124. doi: 10.1097/SLA.0b013e318217e97f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995 Apr 6;332(14):901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990 Sep;8(9):1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 36.Recht A, Come SE, Henderson IC, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996 May 23;334(21):1356–1361. doi: 10.1056/NEJM199605233342102. [DOI] [PubMed] [Google Scholar]
- 37.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006 Oct;99(3):313–321. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 38.Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006 Oct 20;24(30):4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 39.Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM. Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2013 May 16;13:240. doi: 10.1186/1471-2407-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Gagliato DM, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014 Mar 10;32(8):735–744. doi: 10.1200/JCO.2013.49.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016 Nov;160(1):17–28. doi: 10.1007/s10549-016-3960-3. [DOI] [PubMed] [Google Scholar]
- 42.Jara Sanchez C, Ruiz A, Martin M, et al. Influence of timing of initiation of adjuvant chemotherapy over survival in breast cancer: a negative outcome study by the Spanish Breast Cancer Research Group (GEICAM) Breast Cancer Res Treat. 2007 Jan;101(2):215–223. doi: 10.1007/s10549-006-9282-0. [DOI] [PubMed] [Google Scholar]
- 43.Cold S, During M, Ewertz M, Knoop A, Moller S. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG) Br J Cancer. 2005 Sep 19;93(6):627–632. doi: 10.1038/sj.bjc.6602734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol. 2016 Mar;2(3):322–329. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdel-Rahman O. Impact of timeliness of adjuvant chemotherapy and radiotherapy on the outcomes of breast cancer; a pooled analysis of three clinical trials. Breast. 2018 Apr;38:175–180. doi: 10.1016/j.breast.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Yu KD, Fan L, Qiu LX, Ling H, Jiang YZ, Shao ZM. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017 Jul 11;8(28):46549–46556. doi: 10.18632/oncotarget.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mateo AM, Mazor AM, Obeid E, et al. Time to Surgery and the Impact of Delay in Triple Negative Breast Cancers and Other Phenotypes. J Clin Oncol. 2018 May 20;36(Suppl 15) doi: 10.1245/s10434-019-08050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston GM, MacGarvie VL, Elliott D, Dewar RA, MacIntyre MM, Nolan MC. Radiotherapy wait times for patients with a diagnosis of invasive cancer, 1992–2000. Clin Invest Med. 2004 Jun;27(3):142–156. [PubMed] [Google Scholar]
- 49.Jack RH, Davies EA, Robinson D, Sainsbury R, Moller H. Radiotherapy waiting times for women with breast cancer: a population-based cohort study. BMC Cancer. 2007;7:71. doi: 10.1186/1471-2407-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackillop WJ, Zhou Y, Quirt CF. A comparison of delays in the treatment of cancer with radiation in Canada and the United States. Int J Radiat Oncol Biol Phys. 1995 May 15;32(2):531–539. doi: 10.1016/0360-3016(94)00662-5. [DOI] [PubMed] [Google Scholar]
- 51.Benk V, Joseph L, Fortin P, et al. Effect of delay in initiating radiotherapy for patients with early stage breast cancer. Clin Oncol (R Coll Radiol) 2004 Feb;16(1):6–11. doi: 10.1016/j.clon.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Punglia RS, Saito AM, Neville BA, Earle CC, Weeks JC. Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. BMJ. 2010;340:c845. doi: 10.1136/bmj.c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nixon AJ, Recht A, Neuberg D, et al. The relation between the surgery-radiotherapy interval and treatment outcome in patients treated with breast-conserving surgery and radiation therapy without systemic therapy. Int J Radiat Oncol Biol Phys. 1994 Aug 30;30(1):17–21. doi: 10.1016/0360-3016(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 54.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006 Aug 1;65(5):1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 55.Vujovic O, Yu E, Cherian A, Dar AR, Stitt L, Perera F. Eleven-year follow-up results in the delay of breast irradiation after conservative breast surgery in node-negative breast cancer patients. Int J Radiat Oncol Biol Phys. 2006 Mar 1;64(3):760–764. doi: 10.1016/j.ijrobp.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Froud PJ, Mates D, Jackson JS, et al. Effect of time interval between breast-conserving surgery and radiation therapy on ipsilateral breast recurrence. Int J Radiat Oncol Biol Phys. 2000 Jan 15;46(2):363–372. doi: 10.1016/s0360-3016(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 57.Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol. 2009 Jan 1;27(1):16–23. doi: 10.1200/JCO.2008.18.1891. [DOI] [PubMed] [Google Scholar]
- 58.Karlsson P, Cole BF, Colleoni M, et al. Timing of radiotherapy and outcome in patients receiving adjuvant endocrine therapy. Int J Radiat Oncol Biol Phys. 2011 Jun 1;80(2):398–402. doi: 10.1016/j.ijrobp.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]