Summary
Background:
Chronic kidney disease (CKD) is associated with venous thromboembolism (VTE) risk via unknown mechanisms. Whether factors associated with reduced VTE in the general population might also be associated with reduced risk in CKD is unknown.
Objectives:
To determine whether thrombosis biomarkers attenuate VTE risk and if factors associated with reduced VTE are similarly effective in CKD.
Methods:
Baseline biomarkers were measured in a case-cohort (294 VTE cases, 939 non-cases) from the REGARDS study, a nationwide prospective cohort of 30,239 persons age ≥45 with 4.3 years follow-up. The hazard ratio (HR) of VTE per 10 ml/min/1.73m2 lower eGFR, and the percent attenuation of this HR by each biomarker were calculated. Associations of protective factors (physical activity, lower BMI, aspirin, warfarin and statin use) with VTE were estimated in those with and without CKD.
Results:
The HR (95% CI) for VTE with lower eGFR was 1.13 (1.02, 1.25), and VTE risk was attenuated 23% (5, 100%) by D-dimer, 100% (50, 100%) by FVIII and 15% (2, 84%) by CRP. Normal BMI was associated with lower VTE risk in those without CKD (HR 0.47 (0.32, 0.70)) but not with CKD (HR 1.07 (0.51, 2.22)). Statin use, physical activity and warfarin use were associated with lower VTE risk in both groups.
Conclusions:
Procoagulant and inflammatory biomarkers mediated the association of eGFR with VTE. Higher physical activity, statin and warfarin use mitigated risk of VTE in those with and without CKD, but normal BMI did not mitigate risk in CKD.
Keywords: biomarkers, inflammation, kidney, procoagulation, thrombosis
Introduction
Kidney disease has recently emerged as a risk factor for venous thromboembolism (VTE),[1-4] however little is known about mechanisms or clinical implications of this relationship. Low estimated glomerular filtration rate (eGFR) is associated with increased levels of biomarkers of inflammation and procoagulation, including higher levels of C-reactive protein (CRP), D-dimer and factor VIII (FVIII).[5-7]. Since these alterations are also associated with increased VTE risk,[8-19] they might account for the relationship of kidney disease with VTE. That is, CKD induced inflammation and procoagulation may explain part of the relationship between eGFR and VTE and thus could be considered as mediators. If this is the case, then interventions that have an impact on the biology represented by these biomarkers could lower VTE risk associated with CKD.
Epidemiology studies and clinical trials suggest a normal BMI, higher physical activity,[20-22] statins,[23],[24],[25] aspirin and anticoagulants[26],[27, 28] reduce the risk of VTE. However, it is not known if these factors that are protective in the general population are similarly protective in patients with CKD. Knowledge of whether a particular factor is equally, or more, effective in CKD could also shed light on potential mechanisms of VTE in CKD.
In this analysis, we evaluated whether higher levels of D-dimer, Factor VIII (FVIII) and C-reactive protein (CRP), would attenuate the association of eGFR and VTE. We also evaluated whether established risk factors and protective medication use exert similar associations with VTE risk in individuals with versus without CKD.
Methods
Study population and design:
We studied participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort, a prospective cohort study of self-reported blacks and whites in the United States, which has been previously described.[29] In brief, REGARDS enrolled 30,239 black and white women and men age 45 and older between 2003-2007. Exclusion criteria included medical conditions preventing long-term participation, active cancer or active treatment for cancer, residence in or awaiting placement in a nursing home, or inability to communicate in English. For this analysis, we excluded participants missing baseline measures of kidney function (serum creatinine and cystatin C), those with prior self-reported VTE or receiving dialysis at baseline.
Participants were identified from a commercially available list of U.S. residents and recruited through a mailing followed by telephone contact. The cooperation rate was 49% of eligible participants. After completing a computer assisted telephone interview, participants underwent an in-home visit, where informed consent was obtained, anthropomorphic data, medication inventory and urine samples were collected and fasting phlebotomy and an electrocardiogram were performed. Two blood pressure measurements were averaged for analysis, and blood samples were collected after a 10 to 12 hour fast. Blood was centrifuged locally and shipped on ice packs to the University of Vermont for reprocessing and analysis or storage.[30] The study was approved by all institutional review boards at participating universities.
Event ascertainment and definitions:
The primary outcome was incident VTE, consisting of deep vein thrombosis (DVT), pulmonary embolism (PE) or both occurring together. Events were ascertained and validated as previously described.[31] In brief, VTE events were identified by four methods: review of reasons for hospitalization from telephone calls every 6 months up to February 2010; a telephone interview administered from February 2010-February 2011 to identify any self-report of VTE from baseline to time of the call; review of all deaths using periodic searches of the National Death Index, exit interviews or records of last hospital stay; review of medical records for other events such as stroke. For potential cases, in-patient and outpatient medical records were retrieved and then reviewed and adjudicated by two physicians. Major disagreements were settled by a blinded third physician.
Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation[32] based on creatinine and cystatin C.[33] Baseline covariates of interest included age, sex, race (self-reported black or white), region of residence (southeast or non-southeast), body mass index (BMI) in kg/m2, diabetes, hyperlipidemia, cardiovascular disease (CVD), physical activity frequency (self-report of 0, 1-3 or 4 or more times per week), self-report of regular aspirin use, and prevalent statin or warfarin use determined by pill bottle review. Southeast was defined as residing in Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina or Tennessee. Diabetes was defined as fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL, or taking insulin or glucose lowering medications. Hyperlipidemia was defined as cholesterol >240 mg/dL, low density lipoprotein >160 mg/dL or taking medications for high cholesterol. CVD was defined as self-report of pre-baseline myocardial infarction, coronary artery surgery, angioplasty, stenting, silent MI on electrocardiogram or stroke. CKD was defined as eGFR less than 60 ml/min/1.73m2 by convention In REGARDS, albuminuria was not associated with VTE[1] so this was not considered. Normal BMI was defined as 18.5-<25 kg/m2.
Case-Cohort Study Design:
We used previously measured biomarker data for D-dimer and FVIII from a cohort random sample (CRS), selected as previously described.[34] The CRS was randomly selected within strata of race, sex and age to ensure sufficient representation of high-risk groups. To these data, we added measurement of these biomarkers in 294 VTE cases. For analyses conducted in this case-cohort sample we excluded participants missing lab data (Table S1).
Laboratory:
In the full cohort, serum creatinine was measured using an isotope-dilution mass spectrometry-traceable method using the Vitros 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Rochester, NY), with a coefficient of variation (CV) of 1.1%. Serum cystatin C was measured with high sensitivity particle-enhanced immunonephelometry (N Latex Cystatin C on the BNII, Dade Behring, Deerfield, IL), with an intra-assay CV of 2.0-2.8% and an inter-assay CV of 2.3-3.1%. CRP was measured by particle enhanced immunonephelometry using the BNII nephelometer (N High Sensitivity CRP, Dade Behring, Deerfield, IL) with inter-assay CV of 2.1-5.7%.[35]
For this VTE case-cohort study, D-dimer and FVIII were measured in 2015 in VTE cases and harmonized with measurements made previously in the CRS in 2012. The CRS D-dimer was run in plasma using an immunoturbidometric assay on the STA-R analyzer (Diagnostica Stago, Asnières sur Seine, France; inter-assay CV 3.2–27.1%) and VTE cases were run on the STA-R Evolution analyzer (Diagnostica Stago, Asnières sur Seine, France; inter-assay CV 1.5-21.7%). FVIII was measured using an ELISA with a CV of 4-7% (Enzyme Research Laboratories, South Bend, IN), with units of % of normal.
Given that analytes in cases and the cohort sample were measured at two different times, and for D-dimer with two different analyzers, we evaluated for analytical drift in assay results using three approaches (Table S2). We detected a laboratory shift in D-dimer values after the introduction of a new analyzer in 2014, which was verified in a set of experiments. There was no analytic drift observed for FVIII however the correlation between analytes in 2012 and 2015 was weaker, which we attributed to higher analytical CVs. To harmonize results, we adjusted the D-dimer values for VTE cases down by 0.05 µg/mL.
Statistical analysis:
In this report, we examine both the potential of mediation for selected biomarker and effect modification by CKD status.
For the mediation analysis, we compared baseline characteristics among participants with VTE and those in the cohort sample using chi-squared tests and t-tests as appropriate. D-dimer and CRP were highly skewed and thus natural log transformation was used for the analyses. We present back-transformed (geometric) means (95% CI) of log transformed variables in the results. We used linear regression to study the cross-sectional association of eGFR and biomarker levels (D-dimer, FVIII and CRP). We adjusted for demographics (age, sex, race and region of residence), and covariates that are known to be related to these biomarkers: BMI, hypertension, hyperlipidemia, diabetes, history of cardiovascular disease and smoking. Cox proportional hazards models were used to determine the hazard ratio (HR) for VTE per SD increment of each biomarker. Those without an event or who died of non-VTE related causes were censored at that time or at the time of last follow-up, whichever occurred first. We accounted for population weighting of the CRS using the Barlow method.[36] A base model adjusted for age, sex, race, region and BMI. We calculated the adjusted HR of VTE per 10-unit lower eGFR, then added each biomarker separately to the base model. To estimate the magnitude of the potential mediation of the association of CKD with VTE by each biomarker we calculated the percent attenuation of the HR: Percent attenuation = 100% × (HR without biomarker − HR with biomarker) /(HR without biomarker − 1). The 95% CI of the percent attenuation of the HR for eGFR and VTE was calculated using bootstrapping with replacement with 1000 replicate samples.[37] If the percent mediation was greater than 100% we reset that value to 100%. To verify that our results from the case-cohort study were representative of the full REGARDS study, we performed a sensitivity analysis by repeating the same analysis with CRP, which was measured in the entire cohort, and presented these results for qualitative comparison. We have previously published on the competing risk for death in the association of CKD and VTE.[1]
To study effect modification by CKD status, in the full cohort we obtained risk-estimates for established VTE risk factors and protect medication use from stratified Cox models with CKD as the stratification variable. We formally tested for an interaction between each protective factor and CKD using interaction terms, interpreted as significant if p interaction <0.10. Models were adjusted for age, sex, race, region and BMI.
Violations of the proportional hazards assumption were tested visually and with Schoenfeld tests. For the primary predictor (eGFR), non-proportionality was further tested with an interaction term, eGFR × time, and HR were estimated at various time points. For FVIII, Schoenfeld testing demonstrated significant violation of the proportional hazards assumption but this was not confirmed with observed vs predicted plots or smoothed estimates of the log HR. All statistical analyses were performed using Stata software version 14 (StataCorp LP, College Station, TX) and SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The case-cohort sample included 1,467 participants, with 386 being VTE cases. Among these, after excluding those with missing measures of kidney function (n= 88), study biomarkers (n=9), receiving dialysis (n=9), or who reported pre-baseline VTE (n= 143), there were 1,233 participants in the case-cohort sample (294 VTE cases) (Table S1). There were 627 participants lost to follow-up in the larger cohort of 30,239 participants.
The VTE cases were more likely to be male and white compared to non-cases. Comorbid conditions were similar between cases and non-cases other than BMI which was higher in cases. The eGFR was lower in VTE cases than the cohort sample, and markers of inflammation and procoagulation were higher (Table 1).
Table 1:
Baseline characteristics of REGARDS case-cohort participants
| Characteristic | No VTE N=939 | VTE N=294 |
|---|---|---|
| Age (years) | 68 ± 12 | 69 ± 8 |
| Female | 49 | 41 |
| Black | 49 | 38 |
| Southeast | 52 | 53 |
| Hypertension | 60 | 64 |
| Diabetes | 20 | 20 |
| Hyperlipidemia | 41 | 41 |
| Cardiovascular disease | 19 | 23 |
| BMI (kg/m2) | 28.7 ± 5.8 | 30.2 ± 5.7 |
| Physical activity | ||
| - 0 × week | 35 | 41 |
| - 1-3 × week | 36 | 35 |
| - 4+ × week | 29 | 25 |
| Smoking | ||
| - Current | 15% | 12% |
| - Never | 48% | 42% |
| - Past | 37% | 46% |
| eGFR ml/min/1.73m2 | 87 ± 23 | 80 ± 21 |
| D-dimer (median, IQR) µg/ml | 0.5 (0.3-0.9) | 0.7 (0.4 - 1.2) |
| FVIII % | 124 ± 45 | 165 ± 60 |
| CRP (median, IQR) mg/L | 2.1 (0.9- 4.9) | 2.7 (3.1 - 6.1) |
Abbreviations: BMI body mass index. eGFR estimated glomerular filtration rate. FVIII Factor VIII. CRP C-reactive protein. Continuous variables are presented as mean ± standard deviation (SD) unless noted. Frequency are presented as percentages unless noted.
Lower eGFR was associated with higher levels of biomarkers of inflammation and procoagulation. In the cohort sample, adjusting for demographics and cardiovascular risk factors, each 10 ml/min/1.73m2 lower eGFR was associated with a higher level of natural log (ln) D-dimer 0.05 µg/mL (95% CI 0.01 0.08), Factor VIII 6.6% (95% CI 4.5, 8.4%) and ln-CRP 0.08 mg/L (95% CI 0.04, 0.15) (Table 2). The adjusted HRs of VTE were 1.69 (95% CI 1.49, 2.02) per SD higher ln-D-dimer, 2.23 (95% CI 1.98, 2.62) per SD higher FVIII and 1.29 (1.09, 1.52) per SD higher ln-CRP (Table 3).
Table 2:
Association of eGFR and biomarkers of inflammation and procoagulation in the cohort sample
| Difference in biomarker (95% CI) per 10 ml/min/1.73m2 lower eGFR | ||
|---|---|---|
| Unadjusted | Adjusted | |
| D-dimer (µg/ml) | 0.12 (0.09, 0.15) | 0.05 (0.01, 0.08) |
| FVIII (%) | 5.8 (4.5, 7.1) | 6.4 (4.5, 8.4) |
| CRP (mg/L) | 0.07 (0.03, 0.11) | 0.09 (0.04, 0.15) |
Abbrevations: CI, confidence interval; eGFR, estimated glomerular filtration rate; FVIII, Factor VIII; CRP, C-reactive protein. Linear regression models were adjusted for age, sex, race, region, BMI, hypertension, diabetes, hyperlipidemia, cardiovascular disease, smoking. D-dimer and CRP were highly skewed and were log transformed. Back-transformed (geometric) means are presented in this table.
Table 3:
Association of biomarkers and risk of VTE
| HR (95% CI) per SD increment of each biomarker | ||
|---|---|---|
| Unadjusted | Adjusted | |
| D-dimer | 1.77 (1.51, 2.07) | 1.69 (1.41, 2.02) |
| FVIII | 2.14 (1.80, 2.53) | 2.23 (1.89, 2.62) |
| CRP | 1.34 (1.16, 1.56) | 1.29 (1.09, 1.52) |
Abbreviations: HR, hazard ratio; CI, confidence interval; SD, standard deviation; FVIII, Factor VIII; CRP, C-reactive protein. Cox models were adjusted for age, sex, race, region, race*region and BMI. D-dimer and CRP were log transformed.
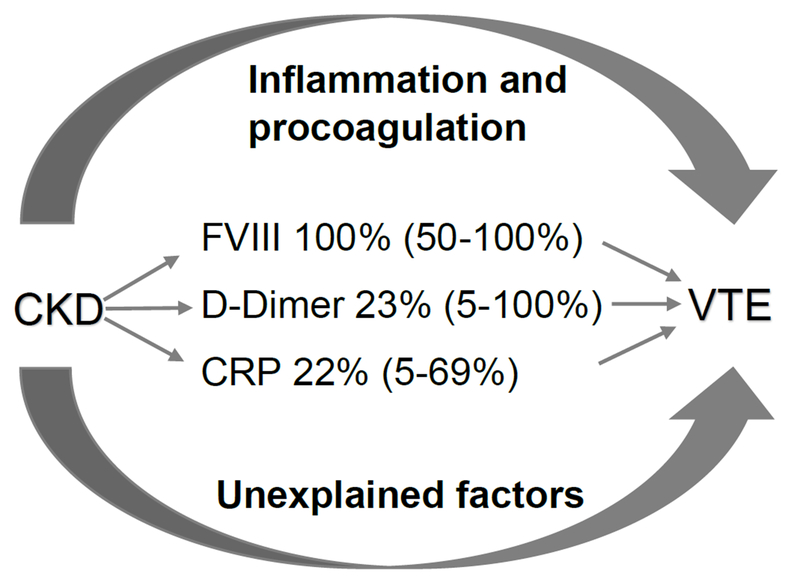
Mean follow-up time was 4.3 years. The eGFR was inversely associated with VTE risk. For each 10 ml/min/1.73m2 lower eGFR, the HR (95% CI) for VTE was 1.13 (1.02-1.25), after adjusting for demographics and VTE risk factors. After adjusting for ln-D-dimer, Factor VIII and ln-CRP individually, the HRs (95% CI) for VTE were attenuated 1.10 (0.99, 1.21), 0.98 (0.87, 1.11) and 1.11 (1.00, 1.22) respectively (Table 4). Including all three biomarkers the HR was 0.99 (0.88-1.11). The percent attenuation of the HRs (95% CI) were 23% (5,100%) for ln-D-dimer, >100% (50, 100%) for Factor VIII, 15% (2, 84%) for ln-CRP and >100 % (44, 100%) for all three biomarkers (Figure 1).
Table 4:
Association of eGFR and incident VTE with adjustment for biomarkers of procoagulation and inflammation
| Model | HR (95% CI) of VTE per 10-unit lower eGFR | HR (95% CI) of VTE by quartile of eGFR (ml/min/1.73m2) | |||
|---|---|---|---|---|---|
| Q1 (7-≤71) | Q2 (>71-≤87) | Q3 (>87-≤103) | Q4 (>103–107) | ||
| Case-Cohort Study | |||||
| Cases (n) | 66 | 74 | 60 | 47 | |
| Cohort sample (n) | 233 | 225 | 239 | 252 | |
| Base Model* | 1.13 (1.02, 1.25) | 1.39 (0.77, 2.49) | 1.19 (0.71, 2.01) | 1.06 (0.65, 1.74) | 1.0 |
| Base Model + D-dimer | 1.10 (0.99, 1.21) | 1.12 (0.62, 2.05) | 1.11 (0.65, 1.91) | 1.06 (0.64, 1.76) | 1.0 |
| Base Model + FVIII | 0.98 (0.87, 1.11) | 0.69 (0.35, 1.37) | 0.60 (0.32, 1.11) | 0.86 (0.50, 1.48) | 1.0 |
| Base Model + CRP | 1.11 (1.00, 1.22) | 1.24 (0.68, 2.24) | 1.15 (0.69, 1.94) | 1.03 (0.63, 1.69) | 1.0 |
| Full Cohort | |||||
| Base Model | 1.09 (1.02, 1.16) | 1.46 (0.91, 2.36) | 1.13 (0.72, 1.80) | 0.97 (0.61, 1.54) | 1.0 |
| Base Model + CRP | 1.07 (1.00, 1.15) | 1.36 (0.84, 2.19) | 1.09 (0.69, 1.72) | 0.96 (0.60, 1.52) | 1.0 |
Abbreviations: HR, hazard ratio; CI, confidence interval; Q, quartile; FVIII, Factor VIII; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate. eGFR as a predictor is presented as a continuous variable (HR per 10 ml/min/1.73m2 decrement) and as quartiles with reference group as the highest quartile of eGFR. Cox models were adjusted for age, sex, race, region, race*region and BMI (Base Model*) and biomarkers individually. D-dimer and CRP were log transformed.
Figure 1:
Potential mediators of the association of eGFR and VTE: role of biomarkers of inflammation and procoagulation
The proportional hazards assumption may have been violated for eGFR and FVIII. Stratification of FVIII at median FVIII values demonstrated proportional hazards for VTE. There was a statistically significant interaction between eGFR and time, thus we modeled the HR for VTE at 1, 3 and 5 years follow up. The association of eGFR and VTE decreased over time but attenuation by FVIII was similar across follow up time periods. (Table S3).
In a sensitivity analysis utilizing the full cohort, for each 10 ml/min/1.73m2 decrease in eGFR the age, sex, race and BMI-adjusted HR (95% CI) of VTE was 1.09 (1.02, 1.16). After the addition of ln-CRP the HR (95% CI) was 1.07 (1.00, 1.15), for a percent attenuation (95% CI) of 22% (5-69%).
The association of mitigating factors for risk of VTE were assessed in the full cohort, and stratified by CKD status, with results shown in Table 5. Statin use was associated with a reduced risk of VTE; the HR was 0.68 (95% CI 0.50, 0.93) in the full cohort with a similar HR in the non-CKD group, and a weaker relationship in the CKD group, although this difference was not statistically significant (p interaction of statin use and CKD 0.61). In the full cohort and in those without CKD, normal BMI was associated with a ~50% lower risk of VTE, but there was no association in those with CKD (p interaction BMI and CKD = 0.07). We observed inverse associations of warfarin use and physical activity with VTE which were similar in those with and without CKD, although the 95% confidence intervals for these HRs included 1.0. Aspirin use was not associated with risk of VTE in the full cohort, or in those with or without CKD.
Table 5:
The association of lifestyle factors and medications with the risk of VTE, stratified by CKD status
| HR (95% CI) of VTE | P interaction of Protective Factor × CKD Status | ||||
|---|---|---|---|---|---|
| Overall (n = 25,936) | CKD (n = 2,473) | No CKD (n = 23,463) | |||
| Regular Aspirin Use | N cases | 124 | 22 | 102 | |
| N non-cases | 11,059 | 1,341 | 9,718 | ||
| 1.08 (0.81, 1.43) | 0.96 (0.51, 1.79) | 1.07 (0.80, 1.41) | 0.64 | ||
| Statin Use | N cases | 70 | 16 | 54 | |
| N non-cases | 8,005 | 1,078 | 6,927 | ||
| 0.68 (0.50, 0.93) | 0.86 (0.46, 1.64) | 0.67 (0.49, 0.93) | 0.61 | ||
| Warfarin Use | N cases | 4 | 2 | 2 | |
| N non-cases | 704 | 177 | 527 | ||
| 0.30 (0.07, 1.21) | 0.70 (0.17, 2.94) | 0.29 (0.07, 1.19) | 0.41 | ||
| Physical activity 1-3 ×/week vs none | N cases | 83 | 12 | 71 | 0.98 |
| N non-cases | 9,214 | 700 | 8,514 | ||
| 0.82 (0.59, 1.14) | 0.88 (0.43, 1.81) | 0.82 (0.59, 1.14) | |||
| Physical activity 4+/week vs none | N cases | 66 | 8 | 58 | |
| N non-cases | 7,622 | 539 | 7,083 | ||
| 0.76 (0.53, 1.07) | 0.73 (0.32, 1.67) | 0.76 (0.53, 1.08) | |||
| BMI<25 vs ≥25 | N cases | 41 | 10 | 31 | |
| N non-cases | 6,410 | 545 | 5,865 | ||
| 0.48 (0.32, 0.70) | 1.07 (0.51, 2.22) | 0.47 (0.32, 0.70) | 0.07 | ||
CKD is defined as eGFR < 60 ml/min/1.73m2. Models were adjusted for age, sex, race, region, race*region, BMI.
Discussion
In this prospective study, the association of eGFR with VTE was attenuated by D-dimer, CRP and in particular Factor VIII. Overall, normal BMI was strongly protective against VTE, but this relationship was present only for those without CKD, and was absent in those with CKD. Statin use was associated with reduced risk of VTE overall, and this association was weaker in those with compared to without CKD, although this difference was not statistically significant. Warfarin use and physical activity were similarly associated with reduced risk of VTE in both those with and without CKD. Baseline aspirin was not associated with lower risk of VTE in either those with or without CKD.
Our findings suggest that CKD-induced activation of inflammation and procoagulation are in the causal pathway between eGFR and VTE. We confirmed prior literature that the studied biomarkers D-dimer[5, 7, 38] FVIII[4, 5, 7, 39] and CRP[40, 41] are more adverse with worsening kidney function (confirming our rationale to choose them for this study). We also confirmed that D-dimer,[8] FVIII[10, 18] and CRP[14] were strongly associated with VTE. We are aware of only one prior study of mediators of the relationship between eGFR and VTE; The Multiple Environmental and Genetic Association (MEGA) case-control study included 2,473 patients with recent VTE and 2,936 matched controls in the Netherlands between 1999-2004 and accounted for genetic mutations and confounders such as recent surgery or immobilization.[4] Similar to findings here, adjustment for FVIII or von Willebrand factor (vWF) measured after the VTE fully explained the association of eGFR and VTE, supporting the conclusion of both studies that FVIII and/or vWF may mediate the association of kidney function with VTE. In that study, there was no mediation by anticoagulant factors, including protein C, protein S or antithrombin. One limitation of the MEGA study is that blood samples were collected three months after the VTE, thus biomarkers might have been elevated due to the recent thrombosis, and eGFR might have changed after the VTE as well. VTE may also have caused greater increases in FVIII and vWF in those with versus without CKD, possibly explaining the observed mediation. In our study, kidney function and biomarkers were measured at baseline, prior to the VTE, and participants with previous VTE were excluded from the analyses. Our findings confirmed the MEGA findings, reducing the likelihood that the discussed factors were important limitations in that study, and strengthening a conclusion that FVIII and/or vWF are mediators of this association. Unlike MEGA, we evaluated inflammation, as measured by CRP, and coagulation activation, measured by D-dimer, as potential mediators of the association of eGFR and VTE. The risk of VTE was attenuated by both biomarkers, but to a lesser extent than by FVIII. An alternative explanation for both studies findings is that these biomarkers are confounders but not mediators of the association of CKD with VTE. We are not aware of other studies addressing these two biomarkers.
Given our finding that FVIII played a larger role in the association of eGFR and VTE than CRP or D-dimer, further discussion is warranted, specifically on the potential role of FVIII as mediator in the association of eGFR with VTE. FVIII is a glycoprotein procofactor that is essential to coagulation. It is produced in liver sinusoidal cells and endothelial cells and circulates bound to vWF in an inactivated form. When blood vessel injury occurs, it separates from vWF and acts a cofactor in the conversion of Factor IX to Factor IXa. When not bound to vWF, FVIII is rapidly catabolized in circulation via LDL-receptor-related protein (LRP), a hepatic clearance receptor that is reduced in kidney disease.[42-44] Our model that included all three biomarkers had similar point estimates to that of the model with just FVIII. One possibility for this is that inflammation as measured by CRP and procoagulation as measured by D-dimer exert their effects upstream, by promoting FVIII activity or by impairing FVIII degradation. Despite the potential joint mechanisms by which inflammation and procoagulation may be increasing the risk of VTE in CKD, Factor VIII appears to play a larger role. FVIII has an established role in the etiology other cardiovascular diseases[45, 46] and in progression of CKD as well[47-49]
As CKD has only recently emerged as a risk factor for VTE, there is little data on primary prevention of VTE in CKD. Here, we studied the potential for lifestyle factors and medications to reduce the risk of VTE in those with and without CKD by determining if there was an interaction between CKD and a protective factor. If we found a factor was associated with reduced risk of VTE in CKD it might elucidate mechanisms of VTE in CKD and point to interventions that could be useful to lower the risk. Our findings raise hypotheses that warfarin use, statin use and higher physical activity might mitigate VTE risk in those with CKD, although power limited our interpretations in some cases leading to confidence intervals that included 1.0. Considering warfarin use, we excluded those who had prior VTE, so participants presumably had a different indication for warfarin, e.g. atrial fibrillation or a mechanical heart valve. The wide confidence intervals (particularly in the CKD group) may be due to low numbers of events, imprecision of the predictor (i.e. baseline use not reflecting use at time of the VTE), under-dosing in CKD (due to concern for bleeding, alterations in non-renal clearance), or difficulties in achieving stable international normalized ratios in CKD. Whether warfarin would be practical in primary prevention of VTE in those with CKD would require a randomized controlled trial to determine, and must take into account the bleeding risk, which might offset any protective effect.[50, 51] Our finding related to statin use confirms prior work[24, 52] and suggests further study of statins (which do not cause bleeding) in VTE prevention in CKD patients. However, there is potential for prevalent user bias since in REGARDS we identified prevalent medication use. If prevalent statin users were healthier than non-users this could account for the lower risk of VTE associated with statins. In REGARDS, prevalent statin use may not reflect healthier participants since participants with kidney disease were more likely to use statins than participants without kidney disease. Our finding of no association of regular aspirin use with VTE risk agree with prior research.[53] An association of increased physical activity with lower VTE risk has been reported previously[20, 21] further study of its role in VTE prevention seems worthy. In contrast, our findings suggest that attaining a normal BMI might lower VTE risk in those without CKD, but not in those with CKD. One hypothesis to explain this is that the inflammatory or pro-coagulant pathways of CKD outweigh any impact that normal BMI might have in reducing VTE risk in CKD. Alternatively, lower BMI in CKD may be due to unmeasured confounding illness, which would mask a protective effect of lower BMI for VTE.
There are several strengths to this study. This was a prospective study where kidney function and biomarker levels were measured prior to onset of VTE, and this might better elucidate mechanistic pathways of CKD-related VTE risk. The study was a contemporary cohort that reflects current use of statins and trends in obesity and diabetes relevant to the U.S. population. We had excellent representation of white and black participants, unlike many studies of VTE epidemiology. In addition, we evaluated the role of lifestyle factors in risk of VTE stratified by CKD status, to attempt to develop hypotheses for how CKD patients could reduce their risk of VTE.
There are limitations of this study to consider. The biomarkers were measured at baseline which may have led to misclassification if biomarker level changed prior to the VTE. The biomarkers in cases were measured later than the cohort random sample, and in some cases with different analyzers, but we took several steps to harmonize the data. Also, we were unable to determine whether low eGFR preceded or was the result of inflammation and procoagulation. It is possible that inflammation and procoagulation cause both CKD and VTE through independent pathways. That is, misclassification of the mediator, unknown confounding between mediator and outcome and interaction between mediator and exposure are potential limitations. We did not have information on cause of CKD, thus were unable to determine if the mediation by inflammatory and procoagulant markers varied by CKD etiology. We demonstrated that the association of eGFR and VTE decreased with time, which may have led to underestimation of the effect size and the mediation by biomarkers. Due the blood sample processing methods in REGARDS we were unable to evaluate the role of platelet function, proteins released by platelets or vWF,[30] all of which may be other factors linking CKD with VTE. Warfarin is known to reduce the risk of VTE but the association was weaker than might be expected in our study. Reasons for this could be insufficient power to detect this association given only 3% of the cohort reported warfarin use or failure of baseline use to reliably indicate continuous use up to the time of VTE. Direct oral anticoagulants could not be evaluated here due to the time when the study was performed. Finally, although we adjusted for established VTE risk factors, there may be other factors not considered that would lead to residual confounding.
Conclusion
In this study, markers of inflammation and procoagulation that are associated with both lower eGFR and VTE may mediate the association of eGFR and VTE. Normal BMI was protective against VTE but not in participants with CKD. Higher physical activity, statin and warfarin use mitigated risk of VTE in those with and without CKD, offering possible targets for intervention studies.
Supplementary Material
Essentials.
Chronic kidney disease (CKD) is associated with procoagulant and inflammatory biomarkers.
We studied the association of CKD and venous thromboembolism (VTE) in a case-cohort study.
Factor VIII, D-dimer and C-reactive protein appeared to explain the association of CKD and VTE.
Statin use was protective against VTE in those with and without CKD.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
This study was supported by a cooperative agreement U01 NS041588 (G. Howard, PI) from the National Institute of Neurological Disorders and Stroke (NINDS) and additional funding from the American Recovery and Reinvestment Act grant RC1HL099460 (N. Zakai, PI) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NHLBI or the National Institutes of Health. Representatives of the funding agency were not directly involved in the collection, management, analysis or interpretation of the data. An unrestricted educational grant from AMGEN Corporation funded cystatin C measurements. The funders had no role in the study design, collection, analysis, interpretation; writing the report nor decision to submit the report for publication.
Footnotes
Addendum
K. L. Cheung, M. Cushman, and N. A. Zakai were responsible for the research idea and study design. S. E. Judd, N. A. Zakai, and M. Cushman were responsible for data acquisition. K. L. Cheung, N. A. Zakai, P. W. Callas, M. Cushman, M. Kurella Tamura, C. A. Peralta, B. K. Mahmoodi, and G. Howard were responsible for data analysis and interpretation. K. L. Cheung and P. W. Callas were responsible for statistical analysis. N. A. Zakai and M. Cushman provided mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. K. L. Cheung takes responsibility that this study has been reported honesty, accurately and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Disclosure of Conflict of Interests
C. A. Peralta reports personal fees from Cricket Health, Inc, outside the submitted work. The other authors state that they have no conflict of interest.
References
- 1.Cheung KL, Zakai NA, Folsom AR, Kurella Tamura M, Peralta CA, Judd SE, Callas PW, Cushman M. Measures of Kidney Disease and the Risk of Venous Thromboembolism in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;70:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoodi BK, Gansevoort RT, Naess IA, Lutsey PL, Braekkan SK, Veeger NJ, Brodin EE, Meijer K, Sang Y, Matsushita K, Hallan SI, Hammerstrom J, Cannegieter SC, Astor BC, Coresh J, Folsom AR, Hansen JB, Cushman M. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation. 2012;126:1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folsom AR, Lutsey PL, Astor BC, Wattanakit K, Heckbert SR, Cushman M. Chronic kidney disease and venous thromboembolism: a prospective study. Nephrol Dial Transplant. 2010;25:3296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ocak G, Vossen CY, Lijfering WM, Verduijn M, Dekker FW, Rosendaal FR, Cannegieter SC. Role of hemostatic factors on the risk of venous thrombosis in people with impaired kidney function. Circulation. 2014;129:683–91. [DOI] [PubMed] [Google Scholar]
- 5.Dubin R, Cushman M, Folsom AR, Fried LF, Palmas W, Peralta CA, Wassel C, Shlipak MG. Kidney function and multiple hemostatic markers: cross sectional associations in the multi-ethnic study of atherosclerosis. BMC Nephrol. 2011;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller C, Katz R, Cushman M, Fried LF, Shlipak M. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Nephrol. 2008;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. [DOI] [PubMed] [Google Scholar]
- 8.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, Heckbert SR. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–8. [DOI] [PubMed] [Google Scholar]
- 9.Douketis J, Tosetto A, Marcucci M, Baglin T, Cushman M, Eichinger S, Palareti G, Poli D, Tait RC, Iorio A. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Annals of internal medicine. 2010;153:523–31. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Cushman M, Heckbert SR, Rosamond WD, Aleksic N. Prospective study of fibrinolytic markers and venous thromboembolism. Journal of clinical epidemiology. 2003;56:598–603. [DOI] [PubMed] [Google Scholar]
- 11.Marcucci M, Smith CT, Douketis JD, Tosetto A, Baglin T, Cushman M, Eichinger S, Palareti G, Poli D, Tait RC, Iorio A. Patient-level compared with study-level meta-analyses demonstrate consistency of D-dimer as predictor of venous thromboembolic recurrences. Journal of clinical epidemiology. 2013;66:415–25. [DOI] [PubMed] [Google Scholar]
- 12.Shrivastava S, Ridker PM, Glynn RJ, Goldhaber SZ, Moll S, Bounameaux H, Bauer KA, Kessler CM, Cushman M. D-dimer, factor VIII coagulant activity, low-intensity warfarin and the risk of recurrent venous thromboembolism. Journal of thrombosis and haemostasis : JTH. 2006;4:1208–14. [DOI] [PubMed] [Google Scholar]
- 13.Tosetto A, Iorio A, Marcucci M, Baglin T, Cushman M, Eichinger S, Palareti G, Poli D, Tait RC, Douketis J. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). Journal of thrombosis and haemostasis : JTH. 2012;10:1019–25. [DOI] [PubMed] [Google Scholar]
- 14.Folsom AR, Lutsey PL, Astor BC, Cushman M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thrombosis and haemostasis. 2009;102:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). The American journal of medicine. 2002;113:636–42. [DOI] [PubMed] [Google Scholar]
- 16.Olson NC, Cushman M, Lutsey PL, McClure LA, Judd S, Tracy RP, Folsom AR, Zakai NA. Inflammation markers and incident venous thromboembolism: the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Journal of thrombosis and haemostasis : JTH. 2014;12:1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luxembourg B, Schmitt J, Humpich M, Glowatzki M, Dressler D, Seifried E, Lindhoff-Last E. Cardiovascular risk factors in idiopathic compared to risk-associated venous thromboembolism: A focus on fibrinogen, factor VIII, and high-sensitivity C-reactive protein (hs-CRP). Thrombosis and haemostasis. 2009;102:668–75. [DOI] [PubMed] [Google Scholar]
- 18.Kraaijenhagen RA, in’t Anker PS, Koopman MM, Reitsma PH, Prins MH, van den Ende A, Buller HR. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thrombosis and haemostasis. 2000;83:5–9. [PubMed] [Google Scholar]
- 19.Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–5. [DOI] [PubMed] [Google Scholar]
- 20.Olson NC, Cushman M, Judd SE, McClure LA, Lakoski SG, Folsom AR, Safford MM, Zakai NA. American Heart Association’s Life’s Simple 7 and risk of venous thromboembolism: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2015;4:e001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabrhel C, Varraso R, Goldhaber SZ, Rimm E, Camargo CA, Jr. Physical inactivity and idiopathic pulmonary embolism in women: prospective study. Bmj. 2011;343:d3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, Heinze G, Kyrle PA. Overweight, obesity, and the risk of recurrent venous thromboembolism. Archives of internal medicine. 2008;168:1678–83. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Zhang P, Tian JH, Yang K. Statins for primary prevention of venous thromboembolism. Cochrane Database Syst Rev. 2014:CD008203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lassila R, Jula A, Pitkaniemi J, Haukka J. The association of statin use with reduced incidence of venous thromboembolism: a population-based cohort study. BMJ open. 2014;4:e005862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrington DM, Vittinghoff E, Lin F, Fong J, Harris F, Hunninghake D, Bittner V, Schrott HG, Blumenthal RS, Levy R, Group HS. Statin therapy, cardiovascular events, and total mortality in the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation. 2002;105:2962–7. [DOI] [PubMed] [Google Scholar]
- 26.Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA : the journal of the American Medical Association. 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 27.Adams NB, Lutsey PL, Folsom AR, Herrington DH, Sibley CT, Zakai NA, Ades S, Burke GL, Cushman M. Statin therapy and levels of hemostatic factors in a healthy population: the Multi-Ethnic Study of Atherosclerosis. Journal of thrombosis and haemostasis : JTH. 2013;11:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahebkar A, Serban C, Mikhailidis DP, Undas A, Lip GY, Muntner P, Bittner V, Ray KK, Watts GF, Hovingh GK, Rysz J, Kastelein JJ, Banach M, Lipid, Blood Pressure Meta-analysis Collaboration G. Association between statin use and plasma D-dimer levels. A systematic review and meta-analysis of randomised controlled trials. Thrombosis and haemostasis. 2015;114:546–57. [DOI] [PubMed] [Google Scholar]
- 29.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 30.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clinical biochemistry. 2014;47:243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakai NA, McClure LA, Judd SE, Safford MM, Folsom AR, Lutsey PL, Cushman M. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129:1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. New England Journal of Medicine. 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, Ahmed A, Thacker EL, Zakai NA. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke; a journal of cerebral circulation. 2014;45:1646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki T, Voeks J, Zakai NA, Jenny NS, Brown TM, Safford MM, LeWinter M, Howard G, Cushman M. Metabolic syndrome, C-reactive protein, and mortality in U.S. Blacks and Whites: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetes Care. 2014;37:2284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. Journal of clinical epidemiology. 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 37.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the Mediation, Confounding and Suppression Effect. Prevention science : the official journal of the Society for Prevention Research. 2000;1:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert-Ebadi H, Bertoletti L, Combescure C, Le Gal G, Bounameaux H, Righini M. Effects of impaired renal function on levels and performance of D-dimer in patients with suspected pulmonary embolism. Thrombosis and haemostasis. 2014;112:614–20. [DOI] [PubMed] [Google Scholar]
- 39.Dekkers IA, Mutsert Rd, Vries APJd, Rosendaal FR, Cannegieter SC, Jukema JW, Cessie Sl, Rabelink TJ, Lamb HJ, Lijfering WM. Determinants of impaired renal and vascular function are associated with elevated levels of procoagulant factors in the general population. Journal of Thrombosis and Haemostasis. 2018;16:519–28. [DOI] [PubMed] [Google Scholar]
- 40.Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;43:244–53. [DOI] [PubMed] [Google Scholar]
- 41.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney international. 2004;65:1009–16. [DOI] [PubMed] [Google Scholar]
- 42.Saenko EL, Yakhyaev AV, Mikhailenko I, Strickland DK, Sarafanov AG. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. The Journal of biological chemistry. 1999;274:37685–92. [DOI] [PubMed] [Google Scholar]
- 43.Milani L, Merkel C, Canel F, Gasparotto ML, Gatta A. Relationship between plasma levels of factor VIII related antigen and reticulo-endothelial function in chronic uremia. Clinical nephrology. 1983;20:235–8. [PubMed] [Google Scholar]
- 44.Kim C, Vaziri ND. Down-regulation of hepatic LDL receptor-related protein (LRP) in chronic renal failure. Kidney international. 2005;67:1028–32. [DOI] [PubMed] [Google Scholar]
- 45.Tracy RP, Bovill EG, Yanez D, Psaty BM, Fried LP, Heiss G, Lee M, Polak JF, Savage PJ. Fibrinogen and factor VIII, but not factor VII, are associated with measures of subclinical cardiovascular disease in the elderly. Results from The Cardiovascular Health Study. Arteriosclerosis, thrombosis, and vascular biology. 1995;15:1269–79. [DOI] [PubMed] [Google Scholar]
- 46.Meade TW, Cooper JA, Stirling Y, Howarth DJ, Ruddock V, Miller GJ. Factor VIII, ABO blood group and the incidence of ischaemic heart disease. British journal of haematology. 1994;88:601–7. [DOI] [PubMed] [Google Scholar]
- 47.Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, Fornage M, Parekh RS, Coresh J, Kao WH. Hemostatic Factors, APOL1 Risk Variants, and the Risk of ESRD in the Atherosclerosis Risk in Communities Study. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bash LD, Erlinger TP, Coresh J, Marsh-Manzi J, Folsom AR, Astor BC. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiramoto JS, Katz R, Peralta CA, Ix JH, Fried L, Cushman M, Siscovick D, Palmas W, Sarnak M, Shlipak MG. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limdi NA, Nolin TD, Booth SL, Centi A, Marques MB, Crowley MR, Allon M, Beasley TM. Influence of kidney function on risk of supratherapeutic international normalized ratio-related hemorrhage in warfarin users: a prospective cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;65:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Limdi NA, Beasley TM, Baird MF, Goldstein JA, McGwin G, Arnett DK, Acton RT, Allon M. Kidney function influences warfarin responsiveness and hemorrhagic complications. Journal of the American Society of Nephrology : JASN. 2009;20:912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunutsor SK, Seidu S, Khunti K. Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis. The Lancet Haematology. 2017;4:e83–e93. [DOI] [PubMed] [Google Scholar]
- 53.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Archives of internal medicine. 2002;162:1182–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.