Abstract
Background
Patients with X-linked hyper-IgM syndrome caused by CD40 ligand (CD40L) deficiency often present with episodic, cyclic, or chronic neutropenia, suggesting abnormal neutrophil development in the absence of CD40L-CD40 interaction. However, even when not neutropenic and despite immunoglobulin replacement therapy, CD40L-deficient patients are susceptible to life-threatening infections caused by opportunistic pathogens, suggesting impaired phagocyte function and the need for novel therapeutic approaches.
Objectives
We sought to analyze whether peripheral neutrophils from CD40L-deficient patients display functional defects and to explore the in vitro effects of recombinant human IFN-γ (rhIFN-γ) on neutrophil function.
Methods
We investigated the microbicidal activity, respiratory burst, and transcriptome profile of neutrophils from CD40L-deficient patients. In addition, we evaluated whether the lack of CD40L in mice also affects neutrophil function.
Results
Neutrophils from CD40L-deficient patients exhibited defective respiratory burst and microbicidal activity, which were improved in vitro by rhIFN-γ but not soluble CD40L. Moreover, neutrophils from patients showed reduced CD16 protein expression and a dysregulated transcriptome suggestive of impaired differentiation. Similar to CD40L-deficient patients, CD40L knockout mice were found to have impaired neutrophil responses. In parallel, we demonstrated that soluble CD40L induces the promyelocytic cell line HL-60 to proliferate and mature by regulating the expression of genes of the same Gene Ontology categories (eg, cell differentiation) when compared with those dysregulated in peripheral blood neutrophils from CD40L-deficient patients.
Conclusion
Our data suggest a nonredundant role of CD40L-CD40 interaction in neutrophil development and function that could be improved in vitro by rhIFN-γ, indicating a potential novel therapeutic application for this cytokine.
Keywords: Neutrophils, CD40 ligand, cell development, IFN-γ
Neutrophils are myeloid cells that play a major role in innate immunity through their microbicidal activity, thus representing the first line of defense against invading pathogens.1–5 The neutrophil development program involves a variety of factors that direct myeloid-specific gene expression, which leads to completion of cell differentiation.6,7 CD40 ligand (CD40L)/CD40 interaction, which is mediated locally by membrane/membrane contact and systemically by soluble CD40 ligand (sCD40L) produced by T cells8 and platelets,9 has been implicated in different biological processes and pathologies ranging from cancer to infectious and autoimmune diseases.10–12 Of note, studies of healthy donor cells,13,14 patients with autoimmune diseases and high serum levels of sCD40L,15,16 and mouse models17 demonstrate that the CD40L-CD40 interaction plays an important role during the inflammatory response and in the development of myeloid cells. This latter function is underlined by the fact that bone marrow stromal cells and myeloid progenitors express CD40, suggesting a direct or indirect involvement of CD40L-CD40 interaction in the control of hematopoiesis by influencing the cytokine and growth factor network in the bone marrow.14,18,19
Patients with X-linked hyper-IgM syndrome or CD40L deficiency, which occurs because of loss-of-function mutations in the CD40L gene (CD40LG), can have episodic, cyclic, or chronic neutropenia.20,21 Previous reports indicated a maturation arrest of the myeloid lineage at the promyelocyte-myelocyte stage in the bone marrow of CD40L-deficient patients,21,22 thus suggesting a pivotal role of CD40L-CD40 interaction during neutrophil development. Nonetheless, even when neutrophil counts are normal or CD40L-deficient patients are receiving granulocyte colony-stimulating factor (G-CSF) and immunoglobulin replacement therapy,23,24 these subjects are susceptible to life-threatening infections caused by opportunistic infectious agents, such as fungi and intracellular bacteria. These infections cause high rates of morbidity and mortality,25 pointing to the need for complementary therapeutic options for patients with CD40L deficiency.
Here we aimed to investigate whether peripheral blood neutrophils from CD40L-deficient patients present functional defects. In addition, considering the impaired production of proinflammatory cytokines, such as IFN-γ, by cells from CD40L- and CD40-deficient patients12,26–28 and mice29 and IFN-γ’s priming effect (ie, effects that do not increase cell responses per se but prepare them for markedly enhanced responses on a secondary stimulation) on neutrophils,30–36 we hypothesize that in vitro IFN-γ treatment could improve presumptive impaired immune responses of peripheral neutrophils from patients with CD40L deficiency. Although sCD40L was also shown to possess the ability to prime peripheral blood neutrophils,13 this molecule is not a therapeutic option for CD40L-deficient patients because of severe side effects.37–39 Therefore we explored IFN-γ as a potential new therapy for patients with CD40L deficiency, which could improve disease-related morbidity and mortality rates.25
METHODS
Subjects
We enrolled 6 CD40L-deficient patients (age range, 8–22 years) from 6 unrelated families. The clinical, immunologic, and genetic characteristics of the patients have been described previously20,40 and are summarized in Table E1 in this article’s Online Repository at www.jacionline.org.41,42 The study was initiated in 2009 and completed in 2017, and patients were not neutropenic at the time of blood collection. However, all of them had episodes of intermittent neutropenia during their lives, which improved after G-CSF therapy. At the moment of blood collection, only patient P5 was still receiving G-CSF treatment. For each experiment, a healthy control subject (age range, 23–30 years) was included for comparison.
Informed consent was obtained from the patients or their parents and from healthy control subjects. The blood was collected under institutional guidelines, and approval for the study was obtained from the Ethics Committee of the Institute of Biomedical Sciences, University of São Paulo, according to the Helsinki Convention.
Analysis of human neutrophil function
Neutrophils from healthy control subjects and CD40L-deficient patients were obtained from heparinized blood by means of dextran sedimentation, followed by Ficoll-Hypaque centrifugation, as previously described.43 Viability and purity of fresh isolated neutrophils were consistently greater than 96%, as determined by using Trypan blue exclusion. When indicated, isolated neutrophils were incubated for 2 hours in the presence or absence of 100 U/mL recombinant human IFN-γ (rhIFN-γ; Immukine, Boehringer Ingelheim, Ingelheim am Rhein, Germany) or 500 ng/mL trimeric sCD40L (Life Technologies, Frederick, Md). Neutrophil apoptosis at different time points was analyzed by using flow cytometry after Annexin V–fluorescein isothiocyanate (FITC) staining, as previously described,44,45 in the presence or absence of a carboxy terminal phenoxy group conjugated to the amino acids valine and aspartate (Q-VD-OPh; 10 μmol/L; MP Biomedicals, Illkirch, France), which is a broad-spectrum caspase inhibitor with potent antiapoptotic properties.46
Neutrophil functions were analyzed, as previously described: phagocytic capacity was assessed in whole blood by using flow cytometry with propidium iodide–labeled Paracoccidioides brasiliensis47; respiratory burst (or production of reactive oxygen species [ROS]) was measured by using dihydrorhodamine and luminol-dependent chemiluminescence48,49; neutrophil extracellular trap (NET) release was investigated by fluorescence microscopy and quantified with Sytox Orange50,51; microbicidal activity was determined by counting colony-forming units (CFU)52; neutrophil phenotype (expression of maturation and activation markers) was evaluated by using flow cytometry53–55; and transcriptome profile was studied by using RNA sequencing (RNAseq).45 Details of each method used to investigate neutrophils from patients with CD40L deficiency, as well as studies performed with the promyelocytic leukemia cell line HL-60, are found in the Methods section in this article’s Online Repository at www.jacionline.org.
Neutrophil responses in CD40L-deficient mice
CD40L-deficient (CD40L−/−) mice were generously provided by Dr Richard Flavell (Department of Immunobiology, Yale University School of Medicine, New Haven, Conn) and have been described previously.56 Female C57BL/6 mice were purchased from Harlan Olac (Bicester, United Kingdom). All strains were bred at the animal facility in the Faculty of Medicine and Health Sciences (UAE University, Al Ain, United Arab Emirates), received rodent chow and water ad libitum, and were investigated at 8 to 12 weeks of age. All studies involving animals were carried out in accordance with and after approval of the animal research ethics committee of the Faculty of Medicine and Health Sciences, UAE University. Details of each method used to investigate neutrophil responses in CD40L-deficient mice can be found in the Methods section in this article’s Online Repository.
Statistical analysis
Statistical significance was assessed by using the nonparametric Mann-Whitney test. Data were expressed as medians ± SDs with 25th and 75th percentiles or means and SDs. Statistical analyses were performed with GraphPad Prism 5.01 software (GraphPad Software, San Diego, Calif), and differences with a P value of .05 or less were considered significant.
RESULTS
ROS production by neutrophils from CD40L-deficient patients is reduced, with possible implications for their killing activity
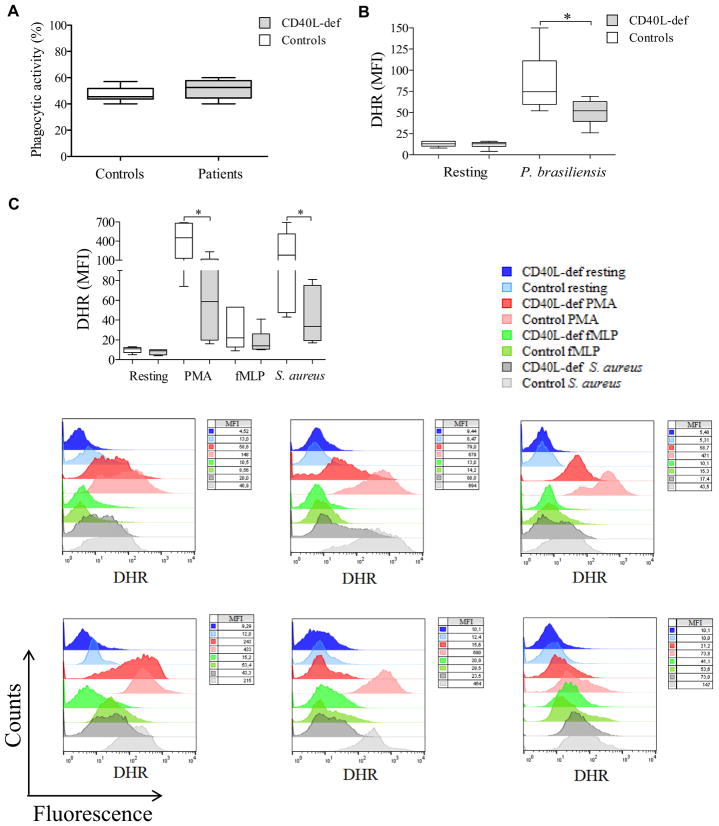
In spite of normal phagocytic capacity (Fig 1, A), neutrophils from patients with CD40L deficiency displayed a reduced respiratory burst in response to P brasiliensis (Pb18, a highly virulent isolate52) when compared with healthy control subjects (Fig 1, B). The defect exhibited by neutrophils from CD40L-deficient patients was not restricted to this pathogen because they also showed a lower respiratory burst response on activation with phorbol 12-myristate 13-acetate (PMA), N-formyl-methionyl-leucyl-phenylalanine (fMLP; but not statistically significant), and Staphylococcus aureus when compared with healthy control subjects (Fig 1, C). Of note, neutrophils from CD40L-deficient patients sometimes displayed a more vigorous respiratory burst after fMLP stimulation than those of healthy control subjects, although this was statistically not significant. This fact can be explained by previously reported observations, suggesting that blood neutrophils respond variably to stimuli, such as fMLP, because of the existence of at least 2 distinct subsets of peripheral blood neutrophils that are differentially primed in vivo and exhibit a diverse pattern of responses at sites of infection.57–60
FIG 1.
Defective respiratory burst in the seting of CD40L deficiency and its relation to neutrophil microbicidal activity. A, Neutrophils from CD40L-deficient patients (n = 6) exhibit normal phagocytic capacity compared with those from healthy control subjects. B and C, The neutrophil respiratory burst from CD40L-deficient patients was assessed by using dihydrorhodamine (DHR) analysis in response to P brasiliensis (Fig 1, B), as well as PMA, fMLP, or S aureus (Fig 1, C), from 6 independent experiments comparing each CD40L-deficient patient with different healthy control subjects. Results were expressed as MFI (n = 6; *P ≤ .05, Mann-Whitney test). D, Respiratory bursts from neutrophils from healthy control subjects treated with various concentrations of catalase were analyzed by using luminol-enhanced chemiluminescence; values were expressed as relative light units (RLU). E, After challenging neutrophils with P brasiliensis (ratio of 2 neutrophils/1 fungus), microbicidal activity was assessed based on CFU values from recovering internalized fungi. CFU values (percentage of controls) were determined in relation to CFU numbers of untreated neutrophils from healthy control subjects. Both the respiratory burst and microbicidal activity of neutrophils from healthy control subjects were assessed in the presence of different doses of catalase (100, 300, and 1000 U/mL). F, Correlation between respiratory burst (data set from Fig 1, D) and microbicidal activity (data set from Fig 1, E) reduction was assessed by using Pearson correlation analysis (n = 3). G, Quantification of NETs release by isolated neutrophils from CD40L-deficient patients in comparison with healthy control subjects. After incubation for 4 hours in the presence of PMA, NET release (n = 5) was analyzed by using Sytox Orange, and results were expressed in relative fluorescence units (RFU), as previously described.62 Patient 4 died before NET analysis was available. Significant differences are denoted by asterisks at a P value of .05 or less (Mann-Whitney test).
Notably, a partially affected respiratory burst, which is an essential mechanism that confers host resistance against opportunistic pathogens,61 possibly affects the killing activity of neutrophils because dose-dependent inhibition of ROS production induced by catalase (a hydrogen peroxide scavenger) abolished the capacity of neutrophils from healthy control subjects to kill P brasiliensis (Fig 1, D and E). A strong correlation between the respiratory burst and inhibition of microbicidal activity was observed (Fig 1, F).
Moreover, NET release, which is a mechanism often associated with ROS production,50,51 was impaired in human CD40L deficiency when neutrophils from patients were stimulated with PMA in comparison with those from healthy subjects (Fig 1, G, and see Fig E1 in this article’s Online Repository at www.jacionline.org).62 However, neither neutrophils from healthy control subjects nor those from CD40L-deficient patients released NETs in response to P brasiliensis (see Fig E1). The reasons for the differences between our finding and previous reports describing that P brasiliensis is able to induce NETs63,64 remain to be investigated. Of note is a previous report demonstrating that variations in the pattern of NET release occur when induced by distinct P brasiliensis strains65; in addition, differences in antigenicity have been associated with distinct isolates of P brasiliensis.66–68 Therefore these observations could explain the apparent opposite results.
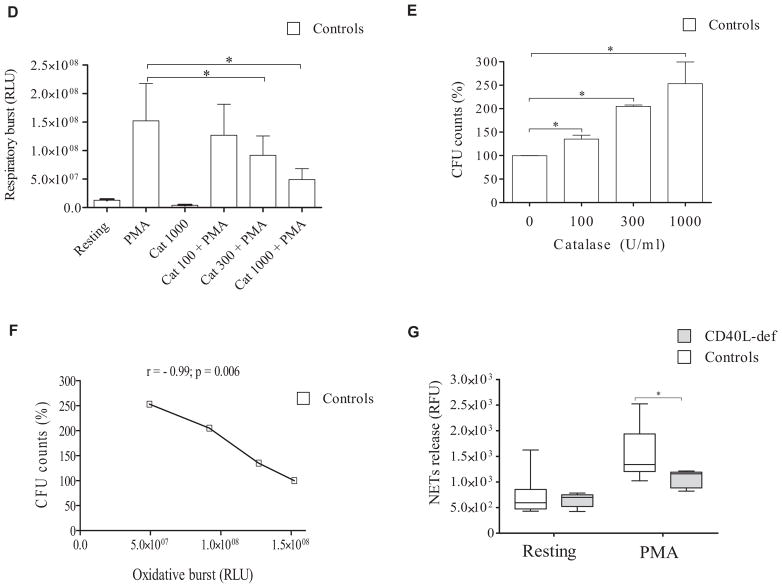
rhIFN-γ improves defective respiratory burst and microbicidal activity of neutrophils from CD40L-deficient patients
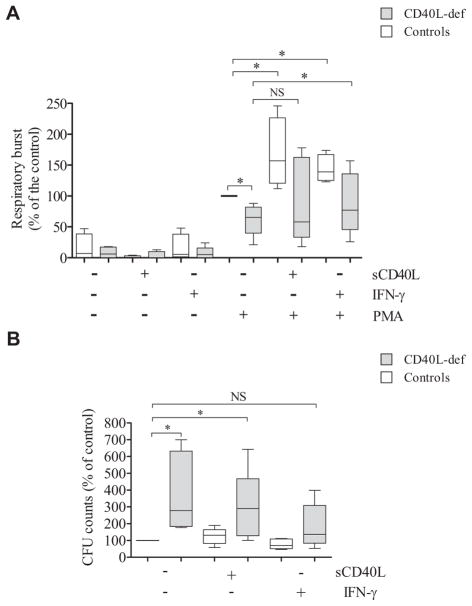
We observed that rhIFN-γ alone was unable to significantly change the respiratory burst capacity of neutrophils from patients and healthy control subjects. However, rhIFN-γ enabled neutrophils from both CD40L-deficient patients and healthy control subjects to increase the PMA-induced respiratory burst (Fig 2, A). Although still showing a reduced respiratory burst compared with rhIFN-γ–primed neutrophils from healthy subjects, rhIFN-γ priming significantly increased the PMA-induced respiratory burst of neutrophils from CD40L-deficient patients when compared with their unprimed neutrophils (Fig 2, A). We also incubated neutrophils in the presence of sCD40L, although it is not a therapeutic option for CD40L-deficient patients because the CD40L-CD40 interaction needs an endogenous tight regulation in vivo to avoid the development of severe side effects.37–39 Although it increased the neutrophil responses of both CD40L-deficient patients and healthy control subjects, sCD40L priming did not significantly increase the PMA-induced respiratory burst of neutrophils from patients when compared with their unprimed neutrophils.
FIG 2.
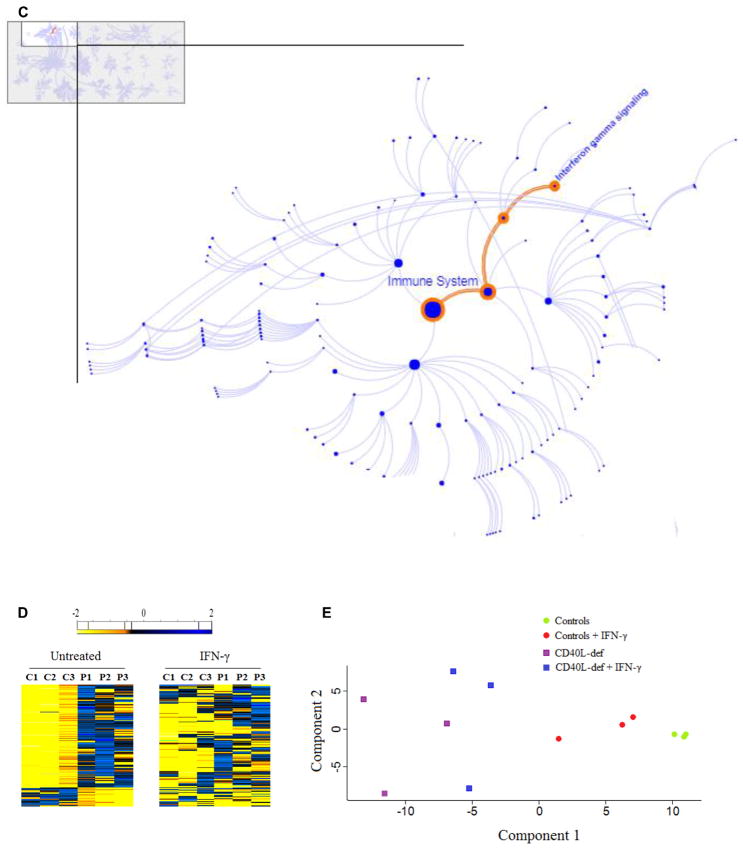
rhIFN-γ improves defects of neutrophil microbicidal activity and respiratory burst, as well as differences in the transcriptome observed in patients with CD40L deficiency. A, Neutrophil respiratory burst was analyzed by using a luminol-enhanced chemiluminescence assay, and values are expressed as relative light units (RLU). The respiratory burst of PMA-stimulated neutrophils from healthy control subjets was considered 100% (n = 6; P ≤ .05, Mann-Whitney test). B, After challenging neutrophils with P brasiliensis (ratio of 2 neutrophils/1 fungus), microbicidal activity was assessed by determining CFU values from recovering internalized fungi. CFU values (percentage of control values) were determined in relation to CFU values of untreated neutrophils from healthy control subjects. Before the analysis, neutrophils remained without (−) or with (+) sCD40L (500 ng/ mL) or rhIFN-γ (100 U/mL) for 2 hours. Untreated neutrophils from healthy control subjects were considered to have a microbicidal activity of 100%. *P ≤ .05. NS, Not significant. C, Functional network analysis of DEGs in neutrophils from CD40L-deficient patients was performed by using the Reactome Knowledgebase (http://reactome.org/), which indicated that IFN-γ signaling is significantly (P = 1.11 × 10−16; false discovery rate = 8.44 × 10−15) impaired in the absence of CD40L-CD40 interaction. The figure shown is a zoomed image of the genome-wide hierarchical visualization of Reactome pathways in a space-filling graphic obtained from the analysis of DEGs in human CD40L deficiency. D, Heat maps of dysregulated genes of neutrophils from CD40L-deficient patients and effect of rhIFN-γ. mRNA from neutrophils was sequenced by using RNAseq. Values in transcripts per million are represented in the heat maps on a Log2 scale in which yellow shows low expression and blue shows high expression. Results of lack of treatment (left panel) and in vitro treatment with rhIFN-γ (100 U/mL) for 2 hours (right panel) are shown in the heat map. DEGs in patients’ neutrophils are compared with those from healthy control subjects; the addition of rhIFN-γ to neutrophil cultures improved the pattern of gene expression by CD40L-deficient neutrophils, reducing the number of DEGs from 164 to 21 genes. E, Principle component analysis of all DEGs observed in neutrophils from 3 CD40L-deficient patients was performed with Perseus (MaxQuant, v1.11; Martinsried, Germany), showing that rhIFN-γ regulates the transcriptome of neutrophils from healthy control subjects and patients, thus reducing differences in the pattern of gene expression of both groups.
Of note, neutrophils from CD40L-deficient patients showed reduced microbicidal activity when challenged with P brasiliensis (Fig 2, B). Similar to the effects on the respiratory burst, both rhIFN-γ and sCD40L increased the microbicidal activity of neutrophils from patients and healthy control subjects. However, only rhIFN-γ–primed neutrophils from CD40L-deficient patients showed microbicidal activity comparable with that from untreated neutrophils from healthy control subjects (Fig 2, B).
Dysregulated transcriptome of neutrophils from CD40L-deficient patients is improved by in vitro treatment with rhIFN-γ
To gain insight into mechanisms by which IFN-γ alters immune responses of neutrophils derived from CD40L-deficient patients, we used a high-throughput approach. The transcriptome of patient-derived neutrophils was evaluated in the absence and presence of rhIFN-γ by using RNAseq.69–71 Because RNAseq analysis demands a large volume of blood to obtain sufficient neutrophil-derived mRNA,45 we had to consider ethical rules and limited the amount of blood that can be safely collected from the patients. Therefore we were only able to compare rhIFN-γ–treated (for 2 hours) but not sCD40L-treated neutrophils with resting neutrophils. RNAseq analysis of untreated neutrophils from CD40L-deficient patients demonstrated a functional network of 164 differentially expressed genes (DEGs; 24 downregulated and 140 upregulated) when compared with those from healthy control subjects (see Fig E2 in this article’s Online Repository at www.jacionline.org).72 Among others, the subsets of DEGs in neutrophils from CD40L-deficient patients include genes directly linked to several immune response aspects, such as innate immune responses (GO:0045087), the oxidation-reduction process (GO:0055114), and genes regulating cell/myeloid differentiation (GO:0030154/GO:0030099; Table I).73–76
TABLE I.
Biological processes dysregulated in patients with CD40L deficiency
| GO term | GO category | Neutrophils | HL-60 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Untreated vs sCD40L treated | DMSO treated vs DMSO + sCD40L treated | ||||||
|
|
|
|
|||||
| Upregulated | Downregulated | Upregulated | Downregulated | Upregulated | Downregulated | ||
| GO:0002376 | Immune system process | AGPAT5, AKT1S1, BNIP3, BST1, CLEC6A, CMTM7, HERC5, HERC6, HLA-C, IRAK1, IRF5, IRG1, LRRK1, PNP, PPIA, PSMA3, PSMC3, SLAMF7, ST6GAL1, TAB1, USP18, XAF1 | BTN3A2, CSF1R, HLA-A | C1QBP, CFL1, PPP3CA, PRKCSH, SYNCRIP | PSME3, RAB5B | CALCOCO2, CIAPIN1, CYLD, MSH2 | CALM3, HCK, IFNGR2, MCM3AP, PTMS |
|
| |||||||
| GO:0045087 | Innate immune response | CLEC6A, HERC5, HLA- C, IRAK1, IRF5, IRG1, PSMA3, PSMC3, SLAMF7, TAB1, USP18, XAF1 | AKT1S1, CSF1R, HLA-A | C1QBP, CFL1, PPP3CA, PRKCSH, SYNCRIP | PSME3 | CALCOCO2, CYLD | CALM3, HCK, IFNGR2, UNC93B1 |
|
| |||||||
| GO:0030154 | Cell differentiation | AGPAT5, ANGPTL6, ARID5B, BNIP3, CAPN2, CMTM7, CPNE1, EVL, FOXK1, HERC1, HERC6, IL6ST, ITGA7, LRRK1, PDLIM5, RBM38, STRBP, TNIK | CSF1R | ARHGDIA, CASC5, CEP57, FOXN2, MSI2, OPA1, PLK4, PPP3CA, SEC23IP, SYNCRIP, TSNAX | CD63, FLNA, GLMN, HSPA8, LEF1 | ACADVL, ELP3, MSH2, RAN, USP33 | ANXA4, CDK16, EIF4G1, HCK, SDCCAG8 |
|
| |||||||
| GO:0030099 | Myeloid differentiation | LRRK1 | CSF1R | LEF1 | |||
|
| |||||||
| GO:0048002 | Antigen processing and presentation of peptide antigen | HLA-C, PSMA3, PSMC3 | HLA-A | PSME3, SEC61A1 | UNC93B1 | ||
|
| |||||||
| GO:0080134 | Regulation of response to stress | ACTL6A, CLEC6A, ERRFI1, F3, HERC5, IL6ST, IRAK1, IRG1, NPC1, NUP62, PFKP, PSMA3, PSMC3, TAB1, TNIK, USP18 | AKT1S1, HLA-A, TNFRSF1A | C1QBP, DNAJC7, OPA1, RAE1, TMEM39B, TNFRSF1A | DDX5, HSPA8, NUP214, PSME3, SCFD1, UBL4A | ABR, CYLD, MAP4K4 | CALM3, HCK, IFNGR2, UNC93B1 |
|
| |||||||
| GO:0007259 | JAK-STAT cascade | ELP2, IL6ST, TNFRSF1A | CSF1R | TNFRSF1A | |||
|
| |||||||
| GO:0055114 | Oxidation-reduction process | ABCD1, GSTT1, HBA2, HK3, HSD17B4, IL6ST, KMO, PFKP, PKM, SCP2 | FAM54B, RRM1 | ACADVL | CALM3 | ||
|
| |||||||
| GO:0006897 | Endocytosis | ADORA2A, FCHO1, HBA2, LMBR1L, NPC1 | CFL1, RABEP1 | SCFD1 | USP33 | HCK | |
|
| |||||||
| GO:0030334 | Regulation of cell migration | EVL, F3, RTN4, STAP1 | CSF1R | ARHGDIA, C1QBP | GNB2L1, LEF1 | ABR, ELP3 | |
|
| |||||||
| GO:0007264 | Small GTPase- mediated signal transduction | ARHGEF3, EVL, LRRK1, RAB28, RHEB | GNGT2 | ARHGDIA, CASC5, CFL1, ZWINT | FLNA, RAB5B | ABR, ARL1, RAN | CALM3 |
|
| |||||||
| GO:0016192 | Vesicle-mediated transport | ADORA2A, CPNE1, DYSF, FCHO1, HBA2, LMBR1L, NPC1, PPIA, SYS1, TSNARE1 | GGA3, RABEP1, COPZ1, CFL1 | ARCN1, CD63, RAB5B, SEC16A, TUBA4A | USP33 | CALM3, CDK16, HCK | |
|
| |||||||
| GO:0007599 | Hemostasis | ADORA2A, DGKA, F3, PPIA | C1QBP, CFL1 | CD63, FLNA, TUBA4A | CALM3 | ||
|
| |||||||
| GO:0010628 | Positive regulation of gene expression | ARID5B, CARD14, CIRBP, FOXK1, GPBP1L1, IRAK1, IRF5, MAML2, NUP62, PSMC3, PTBP1, RPS27L, STAP1, STK36, TAB1, YBX1 | BRD7, FOSB, NRF1, PBX2, TNFRSF1A | C1QBP, PPP3CA, TBP, TFDP1, TNFRSF1A | DDX5, HMGN1, HSPA8, LEF1 | RAN | CDK4, NAMPT |
|
| |||||||
| GO:0045892 | Negative regulation of transcription, DNA-templated | ARID5B, FOXK1, IRAK1, IRG1, TAF1C, YBX1, ZFP161 | BRD7, DEDD, FOSB, NFKBID | AEBP2, C1QBP, HDGF, PA2G4, TBP | DDX5, FLNA, HSPA8, NFXL1, SMARCE1 | CYLD | ANXA4 |
|
| |||||||
| GO:0010608 | Posttranscriptional regulation of gene expression | CIRBP, RBM38, RPS27L,YBX1 | C1QBP, PA2G4, SYNCRIP | EIF3C, GNB2L1 | RAN | CDK4, EIF3K, EIF4G1 | |
|
| |||||||
| GO:0006417 | Regulation of translation | CIRBP, RPS27L | C1QBP, PA2G4, SYNCRIP | EIF3C, GNB2L1 | CDK4, EIF3K, EIF4G1 | ||
|
| |||||||
| GO:0031401 | Positive regulation of protein modification process | CARD14, IL6ST, IRAK1, LRRK1, PPP2R4, PSMA3, PSMC3, STAP1, TAB1, TNIK | BRD7, CSF1R, TNFRSF1A | TNFRSF1A | FNTA, GNB2L1, PSME3 | CALM3 | |
|
| |||||||
| GO:0045859 | Regulation of protein kinase activity | AKT1S1, CARD14, DUSP18, ERRFI1, HERC5, IRAK1, NUP62, STAP1, TAB1, TNIK | CSF1R | GNB2L1 | ELP3 | CALM3, CDK4 | |
Gene Ontology analysis of DEGs in patients with CD40L deficiency was performed by using STRING73 and DAVID74,75 to categorize and group the set of genes upregulated and downregulated in neutrophils from CD40L-deficient patients and HL-60 cells based on a known functional association, as defined by the Gene Ontology Consortium.76 GO, Gene Ontology; JAK-STAT, Janus kinase–signal transducer and activator of transcription.
Subpathway analysis of DEGs involved in the innate immune responses using the Reactome Knowledge base77,78 revealed that the IFN-γ signaling pathway is severely impaired in the absence of CD40L (Fig 2, C), suggesting an explanation for why the function of peripheral neutrophils from CD40L-deficient patients improved when exposed to rhIFN-γ. Furthermore, in agreement with previous reports indicating that IFN-γ priming triggers de novo mRNA synthesis,33,79 the addition of rhIFN-γ to neutrophil cultures improved broadly the pattern of gene expression in neutrophils from CD40L-deficient patients, reducing the number of DEGs to 21 genes (Fig 2, D and E). Taken together, our data suggest that rhIFN-γ improves the dysregulation of a network of molecular interactions in peripheral neutrophils from CD40L-deficient patients.
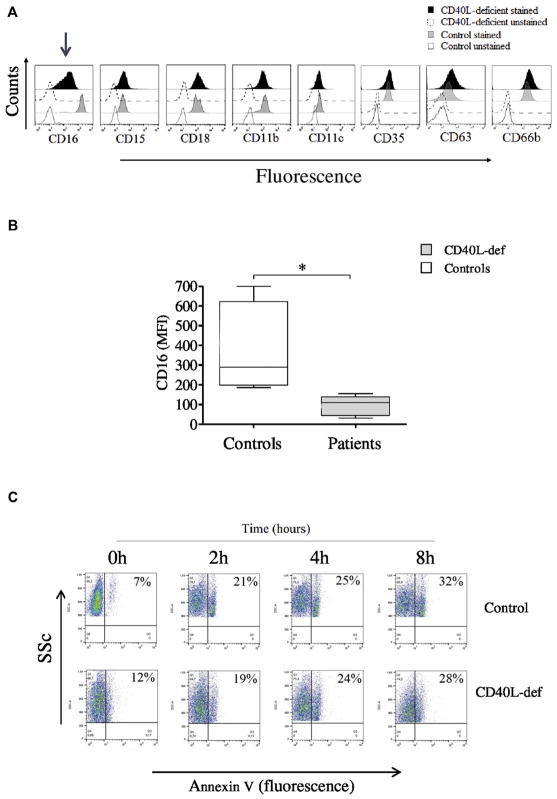
Neutrophils from CD40L-deficient patients exhibit an immature phenotype
Functional defects of neutrophils from CD40L-deficient patients are associated with DEGs involved in cell/myeloid differentiation, thus indicating the presence of immature neutrophils in the blood circulation. In accordance with this, despite normal expression of molecules associated with the myeloid cell lineage (CD15, CD18, CD11b and CD11c, CD35, CD63, and CD66b), neutrophils from CD40L-deficient patients demonstrated reduced expression of CD16 (Fig 3, A and B), a typical marker of neutrophil maturation.80 Of note, neither rhIFN-γ nor sCD40L was able to increase CD16 expression by neutrophils from CD40L-deficient patients (data not shown), indicating a nonredundant role of CD40L-CD40 interaction for the early stages of myeloid cell differentiation. On the other hand, neutrophils from CD40L-deficient patients displayed normal kinetics of the expression of phosphatidylserine on the outer leaflets of their cell membrane in a caspase-dependent manner (Fig 3, C and D, and see Fig E3 in this article’s Online Repository at www.jacionline.org),44–46 as well as normal expression of the activation marker CD62 ligand (CD62L) at resting state, when compared with those from healthy control subjects (Fig 3, E). These findings indicate that the reduced CD16 expression on patients’ neutrophils did not reflect the influence of neutrophils undergoing apoptosis or activation.81–83 However, despite the normal expression of several Toll-like receptors (TLRs; TLR1, TLR2, TLR4, and TLR6; Fig 3, F), the shedding of CD62L from neutrophils from CD40L-deficient patients was slightly decreased in response to PMA and LPS but statistically significant only when activated by the TLR1/TLR2 agonist Pam3CSK in comparison with healthy control subjects (Fig 3, E). Neither rhIFN-γ nor sCD40L was able to improve the shedding of CD62L molecules by neutrophils from CD40L-deficient patients (data not shown), thus suggesting that the absence of CD40L results in multiple neutrophil functional defects.
FIG 3.
Reduced CD16 expression suggests abnormal development of neutrophils in the setting of CD40L deficiency. A, Histograms obtained by using flow cytometric analysis showing expression of different molecules on neutrophils from CD40L-deficient patients and healthy control subjects. Selectively reduced CD16 expression is identified with an arrow. B, Graphics displaying reduced CD16 expression by neutrophils from CD40L-deficient patients compared with those from healthy control subjects. C and D, Dot plots displaying kinetic measurement of phosphatidylserine on the outer leaflet of the cell membrane by using an Annexin V–FITC single staining assay, as previously described,44,45 suggesting no alterations in patients’ neutrophil apoptosis over a short (0 to 8 hours; Fig 3, C) or after a longer (18 hours) period in the presence of IFN-γ or sCD40L when compared with healthy control subjects (Fig 3, D). Neutrophils were analyzed in the presence or absence of the broad-spectrum caspase inhibitor Q-VD-OPh. Graphic data are shown in Fig E3. SSc, Side scatter. E, Graphics showing levels of CD62L expression at rest and after activating neutrophils with PMA and TLR agonist (Pam3CSK4-TLR1/2 agonist; LPS-TLR4 agonist). F, Expression of TLRs on neutrophils from patients and heathy control subjects. Cells were analyzed by using flow cytometry. A significant difference is denoted by an asterisk (n = 5; P < .05, Mann-Whitney test).
At the latest stage of the study, patients P1 to P3 underwent hematopoietic stem cell transplantation, patient P4 died in a cachectic state after recurrent diarrhea caused by the concomitant occurrence of refractory Cryptosporidium parvum and Mycobacterial tuberculosis infection, and patient P6 had severe and refractory leishmaniosis and has been hospitalized for more than 2 months. Therefore experiments to evaluate the simultaneous expression of CD62L and CD11b on neutrophils, which are both adhesion molecules well known to be downregulated and up-regulated, respectively, by means of cell activation84,85 could only be performed with neutrophils from patient P5. Although CD62L shedding in response to PMAwas robust by neutrophils from both the control subject and patient P5, CD62L shedding in response to fMLP was less profound (see Fig E4, A, in this article’s Online Repository at www.jacionline.org). CD11b, on the other hand, was downregulated by exposure to PMA but upregulated by fMLP (see Fig E4, B). These results do not support a clear correlation between CD62L shedding and CD11b upregulation. A possible explanation for this abnormal pattern of CD62L shedding and CD11b upregulation could be provided by the neutrophil isolation method we used because washing steps and erythrocyte lysis are known to upregulate the expression of CD11b by neutrophils.84
Impaired neutrophil immune responses in CD40L−/− mice resemble defects shown by neutrophils of CD40L-deficient patients
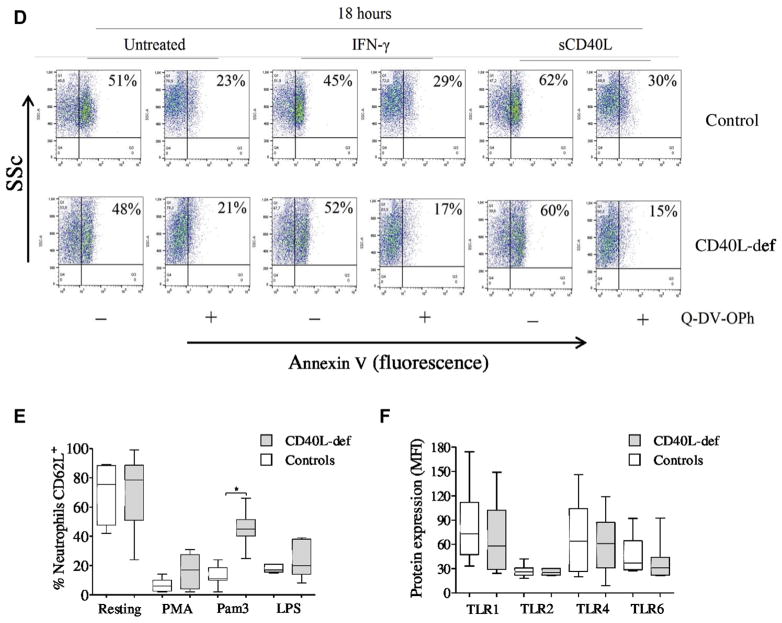
To exclude the possibility that differences shown by neutrophils from CD40L-deficient patients are age related or secondary to infections and/or therapies to which the patients are being subjected, we evaluated whether CD40L−/− mice also present neutrophil defects in response to infection with attenuated Salmonella enterica serovar Typhimurium (BRD509). CD40L−/− mice showed a significantly reduced number of neutrophils in the peritoneal cavity in response to intraperitoneal injection of thioglycollate or BRD509 in comparison with wild-type mice (Fig 4, A). In parallel, CD40L−/− mice were unable to control bacterial proliferation in the peritoneal cavity (Fig 4, B), and their neutrophils displayed reduced respiratory burst (Fig 4, C) while maintaining normal phagocytic capacity (Fig 4, D).
FIG 4.
CD40L-deficient mice show defective neutrophil responses in vivo. A, Recruitment of neutrophils to the peritoneal cavity from wild-type and CD40L (CD154)–deficient (CD154−/−) mice (3 per group) was assessed 0, 24, 48, and 72 hours after inoculation with thioglycollate (TG) or challenge with BRD509, an attenuated aroA−/aroD− mutant strain of Salmonella enterica serovar Typhimurium. B–D, All experimental procedures, including control of bacterial proliferation (Fig 4, B), neutrophil respiratory burst (Fig 4, C), and phagocytic activity (Fig 4, D), are described in detail in the Methods section in this article’s Online Repository. Neutrophil phagocytic activity and respiratory burst capacity were analyzed 24 hours after challenging mice with BRD509. *P ≤ .05.
sCD40L accelerates differentiation of the human promyelocytic cell line HL-60
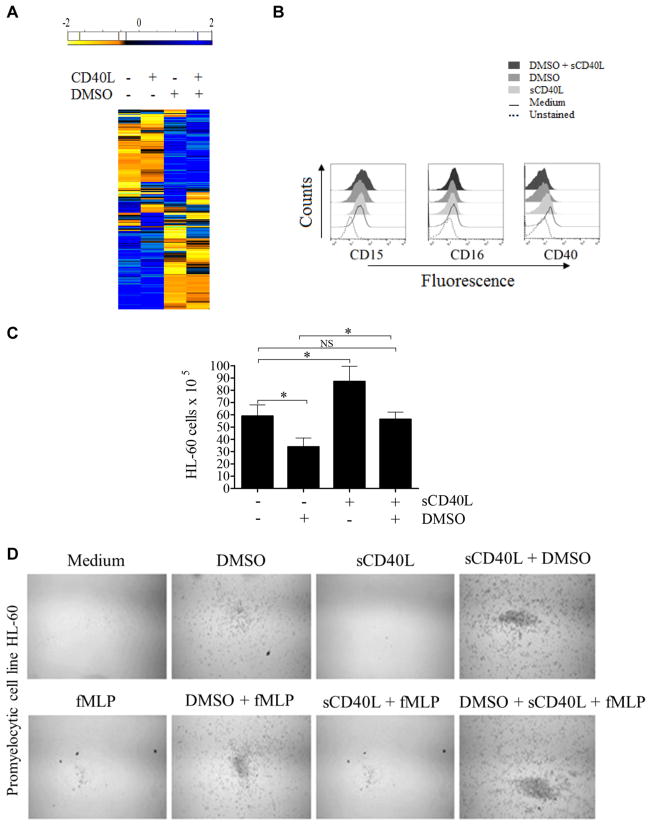
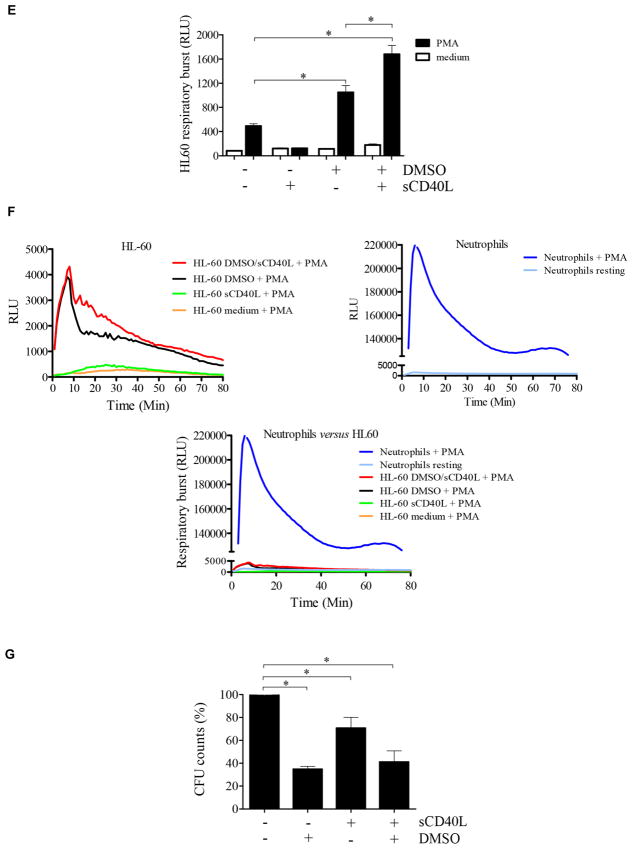
Our findings suggesting defects in maturation and function of neutrophils in the absence of CD40L-CD40 signaling are in agreement with previous reports demonstrating that CD40L-CD40 interaction modulates the development of myeloid cells by regulating the milieu of cytokines in the bone marrow microenvironment.14,18,19 Therefore we hypothesized that the defects we observed could, at least in part, be explained by defective CD40L-CD40 signaling required for maturation of myeloid cells. To investigate this possibility, because bone marrow–derived cells from CD40L-deficient patients could not be obtained for investigation, we assessed the effects of CD40L-CD40 interaction on maturation of the promyelocytic leukemia cell line HL-60, an often used in vitro model to study the development of myeloid cells.86–88 Cell viability in the presence of dimethyl sulfoxide (DMSO), a chemical agent traditionally used to induce granulocyte differentiation,86 and/or sCD40L was not different than the viability of untreated HL-60 cells during 6 days of culture (see Fig E5 in this article’s Online Repository at www.jacionline.org). RNAseq analysis of HL-60 cells demonstrated that sCD40L induced upregulation of 62 genes and downregulation of 19 genes compared with untreated HL-60 cells. Likewise, sCD40L induced upregulation of 19 genes and downregulation of 26 genes in HL-60 cells treated with DMSO plus sCD40L when compared with those cultured in the presence of DMSO alone (Fig 5, A, and Table I). The functional association network of HL-60 genes affected by sCD40L is demonstrated in Fig E6 in this article’s Online Repository at www.jacionline.org.72 Considering the malignant features of HL-60 cells, it is not surprising that the sCD40L-induced genes were not the same DEGs displayed by neutrophils from CD40L-deficient patients. Nevertheless, the HL-60 genes controlled by sCD40L belong to the same Gene Ontology categories (eg, innate immune response, GO:0045087; oxidation-reduction process, GO:0055114; and genes regulating cell/myeloid cell differentiation, GO:0030154/ GO:0030099) when compared with DEGs of peripheral blood neutrophils from CD40L-deficient patients (Fig 5, A, and Table I).
FIG 5.
sCD40L regulates promyelocytic cell development. A, Heat map showing that sCD40L induces transcriptome alterations in the promyelocytic HL-60 cells. HL-60 cells were incubated for 48 hours in the absence and presence of 500 ng/mL sCD40L, 1% DMSO, or both before RNAseq analysis. In the heat map the results obtained in transcripts per million (TPM) are represented in a Log2 scale, where yellow shows low expression and blue shows high expression. Gene Ontology analysis of genes upregulated or downregulated by sCD40L compared with untreated cells or DMSO-treated versus DMSO plus sCD40L–treated cell are listed in Table I. A pool of 3 independent cultures of HL-60 cells in which every experimental condition (resting, DMSO, sCD40L, and DMSO plus sCD40L) has been performed in triplicates. B, Histograms obtained by using flow cytometric analysis showing expression of CD15, CD16, and CD40 on HL-60 cells. C, Equal numbers of promyelocytic HL-60 cells were distributed in 6-well plates and cultured in the presence or absence of 1% DMSO, 500 ng/mL sCD40L, or both. After 6 days, cells were harvested and counted, and cell viability was assessed (see Fig E4). D, HL-60 migration was assessed by using a transwell migration assay. Microscopic images display cells that migrated. Data shown are representative of 3 independent experiments performed in triplicates. E, Respiratory burst of HL-60 cells was induced by PMA (90 mmol/L) and analyzed with a luminol-enhanced chemiluminescence assay. Values are expressed as relative light units (RLU). F, Comparative graphics showing the kinetics of respiratory burst of HL-60 cells (upper left panel), neutrophils (upper right panel), and both overlapping (lower panel). G, HL-60 cells were challenged with P brasiliensis (ratio of 2 neutrophils/1 fungus), and their microbicidal activity was assessed by counting CFU values from recovering internalized fungi. CFU values (in percentages) were determined in relation to CFU numbers of untreated HL-60 cells. *P ≤ .05. NS, Not significant.
Except for a slight reduction in CD40 expression, when HL-60 cells were cultured in the presence of DMSO, sCD40L, or both, we did not observe phenotypic changes (eg, alterations in the expression of CD15 and CD16) by HL-60 cells in the presence of sCD40L (Fig 5, B). This suggests that the direct effects of sCD40L on regulation of CD16 (and consequently cell maturation) occur at early stages of hematopoietic cell differentiation or, alternatively, that additional factors modulate CD16 expression. On the other hand, although DMSO inhibited HL-60 cell proliferation, sCD40L accelerated HL-60 proliferation significantly (Fig 5, C). Moreover, as previously demonstrated,87,89 DMSO-induced differentiation enabled HL-60 cells to migrate toward an fMLP gradient. Addition of sCD40L to the culture alone did not influence the migratory capacity of HL-60 cells. However, sCD40L stimulation in conjunction with DMSO increased both spontaneous (without chemoattractant) and fMLP-induced migration of HL-60 cells (Fig 5, D).
Of note, sCD40L in the presence of DMSO significantly increased the PMA-stimulated respiratory burst of HL-60 cells (Fig 5, E) when compared with cell cultures only in the presence of DMSO. However, PMA-stimulated HL-60 cells cultured in both conditions, DMSO alone or DMSO plus sCD40, showed a very low response in terms of respiratory burst, which was comparable with the response of resting peripheral blood neutrophils from healthy control subjects (Fig 5, F). This fact is in agreement with previous reports showing that although HL-60 cells acquire certain characteristics of mature neutrophils, the magnitude of their respiratory burst response is significantly lower compared with that of primary blood-derived neutrophils.90,91 This might be the reason for not observing even a slight effect of sCD40L on microbicidal activity when comparing DMSO-differentiated with sCD40L plus DMSO–differentiated HL-60 cells (Fig 5, G). However, although sCD40L alone did not increase the respiratory burst of HL-60 cells, it was able to enhance their killing capacity in comparison with untreated cells, suggesting that ROS-independent microbicidal mechanisms are also affected by sCD40L during cell development, a possibility requiring future investigation. As previously reported,92 another peculiarity observed was the fact that 100 U/mL IFN-γ added to the culture of malignant hematologic HL-60 cells induced rapid cell death with only 10% or less of cells remaining alive after 3 days of culture (data not shown).
DISCUSSION
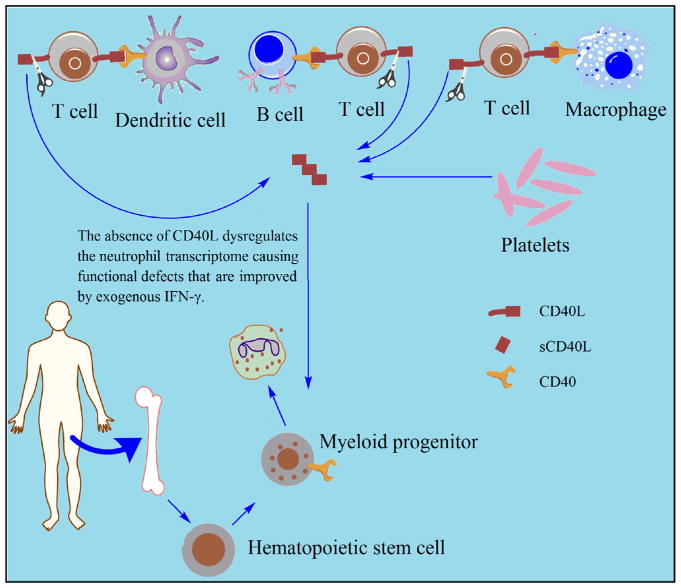
The present work suggests that in addition to the often reported episodic, cyclic, or chronic neutropenia,20,21 CD40L-deficient patients also have functional defects of peripheral blood neutrophils caused by abnormalities in myeloid development (Fig 6). This is not due to the age difference between our CD40L-deficient patients (8–22 years of age when studied) and control subjects (age range, 23–30 years) because CD40L-deficient mice challenged with the attenuated Salmonella strain BRD509 showed similar neutrophil defects. Furthermore, as we routinely observe in our diagnostic laboratory, infants suspected wrongly to have chronic granulomatous disease (CGD), when tested for phagocyte defects (as we assessed in this study), had normal phagocyte responses that were comparable with those of healthy young adult control subjects.40 In addition, after the first year of life, infants have consistently normal neutrophil counts and function (eg, chemotaxis; migration; phagocytosis; respiratory burst; expression of maturation markers, such as CD16; and microbicidal activity) when compared with adults.93–95
FIG 6.
Blood circulating neutrophils of CD40L-deficient patients presented defects in development and function that are improved by the presence of exogenous IFN-γ.
Of note, different to the constant B-cell defects identified in patients with X-linked hyper-IgM syndrome, we hypothesize that the defects we report here might follow the intermittent pattern of neutrophil counts characterized by episodic, cyclic, or chronic neutropenia frequently associated with this immune defect.96 If that is the case, future studies clarifying the periodicity and rhythm of these neutrophil functional defects, as well as which factors determine their occurrence and at what age they become more frequent, will be relevant to improve the treatment of CD40L deficiency.
Respiratory burst defects are associated with markedly reduced ROS production by phagocytes caused by intrinsic defects of the nicotinamide adenine dinucleotide phosphate oxidase components. These defects commonly cause CGD,97 a severe innate immune deficiency rendering affected patients susceptible to recurrent life-threatening infections.97–99 Notably, patients with CGD and female carriers of X-linked CGD with only slightly affected respiratory burst can also experience recurrent and severe infections caused by opportunistic intracellular bacteria and fungi.98,100 In agreement, our findings suggest that even partially impaired respiratory burst shown by neutrophils and previously by macrophages40 from CD40L-deficient patients contributes to their increased susceptibility to infection. Furthermore, correlating the dose-dependent inhibition of the respiratory burst by catalase with reduced killing of P brasiliensis killing by neutrophils from healthy subjects is in line with the existence of an ROS-dependent mechanism of microbial killing.101
The high morbidity and mortality rates of insufficiently treated CD40L-deficient patients20,21,23,25 emphasize the need for novel therapeutic options. Although sCD40L priming improved the response of neutrophils from CD40L-deficient patients,13 the improvement in neutrophil responses was far less than that shown by rhIFN-γ, which is known to have a priming effect on the respiratory burst and fungicidal activity of peripheral blood neutrophils.32,33 Similarly, in vivo treatment with recombinant sCD40L is insufficient to completely correct the effect of CD40L deficiency on adaptive immune responses.102 In line with previous reports demonstrating a role of CD40L-CD40 stimulation during myelopoiesis,14,18,103,104 our finding of significant effects of sCD40L on the maturation of HL-60 cells suggests that sCD40L not only affects mature immune cells but that the CD40L-CD40 interaction is also required for the early development and maturation of hematopoietic cells in primary lymphoid organs. In accordance with this, levels of neither sCD40L nor rhIFN-γ increased CD16 (a glycosylphosphatidylinositol [GPI]–anchored protein serving as maturation marker for neutrophils) expression on the surfaces of neutrophils from CD40L-deficient patients and addition of sCD40L did not enhance the expression of CD16 by HL-60 cells, suggesting that CD40L-CD40 signaling is most important during the earliest stages of hematopoiesis.
A recent study of bone marrow from CD40L-deficient mice suggested that CD40L controls hematopoietic stem and progenitor cell biology by inducing adhesion-mediated quiescence,105 which is an actively maintained state in which signaling pathways are involved in maintaining a poised state that allows rapid activation.106 Other reports have described the effects of CD40L-CD40 interaction on hematopoietic progenitor cell proliferation and differentiation.107,108 Multidimensional flow cytometric studies of normal human bone marrow aspirates demonstrate that expression of FcγRIIIB (also known as CD16) correlates with maturation stages of neutrophils. CD16 expressed on promyelocytic cells is upregulated during the different stages of neutrophil development, reaching its highest expression at late maturation stages (metamyelocytes and mature neutrophils).53,109 Mechanistically, downregulation of CD16 expression by neutrophils from CD40L-deficient patients is likely not due to impaired synthesis of the GPI anchor moiety because their neutrophils expressed CD66b (another GPI-anchored protein)110 normally when compared with healthy control subjects. Of note, CD40L stimulation was shown to enhance the proliferation and differentiation of CD34+ progenitor cells toward the myeloid lineage by regulating the network of several pleiotropic hematopoietic growth factors that are present in the bone marrow, such as production of Fms-like tyrosine kinase 3 ligand, G-CSF, and GM-CSF by bone marrow stromal cells.14,18,111,112 Therefore multiple cytokine/growth factor pathways remain to be investigated to better understand the neutrophil functional and maturation defects in CD40L deficiency reported here.
Historically, peripheral blood neutrophils have been thought not to be able to significantly change their transcriptome profile. However, this concept has been revised when a significant change in the gene expression profile of peripheral neutrophils was observed in response to priming cytokines.33,45 In agreement, we found that rhIFN-γ shifted the transcriptome of peripheral blood neutrophils from CD40L-deficient patients toward that of neutrophils from healthy control subjects, thus improving the responses of neutrophils deprived of CD40L-CD40 interaction during development.14,18,103,104 These observations are in line with the essential need of proper control of gene expression for normal myeloid cell development and function.86,113,114
In concurrence with the network view of the immune system orchestrated by a system-wide intercommunication between a variety of cells and soluble molecules,115–118 the study of CD40L-deficient patients demonstrates that CD40L-CD40 interaction, which was initially thought to be involved predominantly in regulation of B-cell maturation and immunoglobulin class-switch recombination, plays a broader role and is essential for the development and maturation of the adaptive and innate immune system. An important question raised by the data presented here relates to the previously reported defects in the function of T cells,26 dendritic cells,27,28 and macrophages40 in the absence of CD40L-CD40 signaling. Are these abnormalities caused by the absence of CD40L-CD40 interaction in the peripheral immune system, or does the absence of this interaction affect hematopoietic cell development in central lymphoid organs?
Taken together, the data presented here provide new insights into the role played by CD40L/CD40 interaction in innate immunity. We have extended our previously reported findings obtained from studying macrophages40 to neutrophils, suggesting that the defective function of phagocytes in the setting of CD40L deficiency can be improved by rhIFN-γ. Such adjuvant therapy could be considered a potential option for CD40L-deficient patients in cases of life-threatening refractory tract infections or even as a prophylactic therapy administered in a similar way as to patients with CGD.
METHODS
Analysis of neutrophils from CD40L-deficient patients
Phagocytosis
Neutrophil phagocytic capacity was analyzed in whole blood by using flow cytometry with propidium iodide–labeled P brasiliensis, as previously described.E1 After 1 hour of incubation at 37°C, red blood cells were lysed with a red blood cell lysis solution (Qiagen, Santa Clarita, Calif). Neutrophils were stained with anti-CD15, and phagocytosis was analyzed by using flow cytometry.
Analysis of neutrophil respiratory burst
Neutrophil respiratory burst was evaluated, as previously described.E2 Cells were stimulated with PMA (300 ng/mL; Sigma-Aldrich, St Louis, Mo), fMLP (50 nmol/L; Sigma-Aldrich), or heat-killed S aureus for 1 hour at 37°C followed by dihydrorhodamine 123 (Sigma-Aldrich) staining. Neutrophils were stained with anti-CD15 and analyzed by using flow cytometry. Results were expressed as mean fluorescence intensity (MFI). Neutrophil respiratory burst was also measured based on luminol-dependent chemiluminescence, as previously described.E3 In brief, 2 × 105 neutrophils in the absence or presence of PMA (300 ng/mL) and luminol (1 mmol/L; Sigma-Aldrich) were monitored by means of chemiluminescence for 2 hours with a microplate luminometer reader (EG&G Berthold LB96V, Bad Wildbad, Germany). The results were expressed as relative light units during the observation period and shown as a percentage of control cells in relation to the PMA-induced respiratory burst of neutrophils from healthy control subjects. When indicated, experiments were performed in the presence or absence of different catalase concentrations (100, 300, and 1000 U/mL; Sigma-Aldrich) and P brasiliensis (Pb18; a highly virulent isolate).E4
NET release by neutrophils
NET release was analyzed, as previously described.E5,E6 In brief, after PMA (100 ng/mL) or P brasiliensis (1 neutrophil/2 fungi) stimulation for 4 hours, neutrophils from control subjects were seeded on glass coverslips, fixed with PBS plus 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 3% BSA. Samples were incubated with primary antibody to the histone complex (H2A-H2B; kindly provided by Professor Dr Arturo Zychlinsky from the Department of Cellular Microbiology, Max Planck Institute for Infection Biology, Berlin, Germany) and anti-neutrophil elastase mAbs, followed by exposure to a secondary antibody and nuclear staining with Hoechst dye. Cells were analyzed by using fluorescence microscopy. In addition, NET formation was quantified by using Sytox Orange, and results were expressed in relative fluorescence units, as previously described.E7
Microbicidal activity
Neutrophil microbicidal activity was analyzed by means of challenge with the fungus P brasiliensis and determined by determining CFU values, as previously described, with few modifications.E4 Briefly, neutrophils were suspended in 200 μL of RPMI 1640 in 96-well round-bottom cell-culture microplates and challenged with P brasiliensis at a ratio of 2:1 (neutrophils/fungus). After a 2-hour incubation at 37°C, plates were centrifuged and washed, supernatants containing noningested microorganisms were discarded, and plates were washed. Neutrophils were lysed with chilled (4°C) ultrapure water. P brasiliensis CFU values were determined 5 days after the lysates were cultured at 35°C on brain-heart infusion agar (Difco, Detroit, Mich) in plates containing 4% (vol/vol) normal horse serum (Instituto Butantan, São Paulo, Brazil) and 5% P brasiliensis (Pb192) culture filtrate. The CFU index was determined in relation to CFU values of unchallenged neutrophils from healthy control subjects.
Neutrophil phenotyping
Expression of neutrophil maturation markers (CD15, CD16, CD18, CD11b, CD11c, CD35, CD63, and CD66b; all from BD Biosciences, San Jose, Calif) was analyzed in whole blood after red blood cell lysis by using RBC Lysis Solution (Qiagen), according to the manufacturer’s instructions. After staining with fluorophore-conjugated mAbs (Becton Dickinson, Mountain View, Calif), cells were analyzed by using flow cytometry on a BD FACSCanto II Cytometer, and neutrophils were gated according to size (forward scatter) and granularity (side scatter). The MFI for expression of each molecule was obtained by using FlowJo software (TreeStar, Ashland, Ore).
RNAseq and data processing
Neutrophil transcriptome profiles from 3 CD40L-deficient patients and 3 healthy control subjects were analyzed, as previously described.E8 Total RNAwas obtained by using TRIzol (Invitrogen, Carlsbad, Calif), according to the manufacturer’s instructions. RNA integrity and concentration were assessed by using the Agilent 2100 Bio-analyser RNA Nano chip (Agilent, Santa Clara, Calif) and orthogonally validated by means of visualization of the integrity of the 28S and 18S bands on an agarose gel. cDNA libraries were obtained by using the Illumina CBot station and HiScanSQ with the Illumina TruSeq RNA Sample Preparation Kit (Illumina, San Diego, Calif), according to each manufacturer’s instructions. Sequencing was carried out with the Illumina HiSeq 2000 paired-end 100-bp (PE 100) system. Bioinformatics analysis of data obtained was performed, as previously described.E9 The protocol of the transcriptome analysis and data obtained were submitted to ArrayExpress and are publicly available at http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-5316.
Assessment of CD62L shedding
We evaluated the expression and shedding of membrane-bound L-selectin (CD62L) using flow cytometry, as previously described.E10 In brief, after neutrophil TLRs bind their ligands, signaling pathways are activated, triggering CD62L shedding. Briefly, 100 μL of whole blood was incubated for 1 hour without stimulation or with PMA (400 ng/mL), synthetic triacylated lipopeptide Pam3CSK4 (1 μg/mL, a TLR1/2 agonist; InvivoGen, Carlsbad, Calif), or LPS (100 ng/mL, a TLR4 agonist; Sigma-Aldrich) at 37°C in a 5% CO2 atmosphere. After erythrocyte lysis, cells were washed, stained with anti-human CD62L antibody (BD Biosciences), and analyzed by using flow cytometry. When indicated, CD62L shedding was analyzed simultaneously with CD11b expression. MFIs of CD62L and CD11b expression from stimulated cells were compared with the MFIs of unstimulated cells.
Analysis of HL-60 cell responses
HL-60 cells, a useful model for myeloid differentiation, were cultured for 6 days, as previously described,E11 in the presence or absence of 500 ng/mL sCD40L, 1.0% DMSO, or both. After 6 days of incubation, cells were harvested and counted, and cell viability was determined by using Trypan blue exclusion and the LIVE/DEAD Viability/Cytotoxicity Kit (Thermo Fisher Scientific, Waltham, Mass), according to the manufacturer’s instructions.
To assess the HL-60 maturation state, we analyzed the respiratory burst, fungicidal activity, and transcriptome profile, as described above, for neutrophils from CD40L-deficient patients. Their migration was analyzed by using the transwell migration assay (24-well plate; Corning, Corning, NY), as previously described.E12
Neutrophil responses in CD40L-deficient mice
Bacterial strains and growth conditions
The characteristics of the bacterial strains used here have been published elsewhere.E13–E15 BRD509 is an attenuated aroA−/aroD− mutant strain of Salmonella enterica serovar Typhimurium, which is approximately 10,000-fold less virulent than the parental SL1344 strain from which it was derived.E16 Aliquots of frozen bacteria were routinely plated on Salmonella shigella agar in the presence of ampicillin and grown overnight at 37°C. Log-phase bacterial suspensions were prepared in pyrogen-free saline and injected intraperitoneally at a volume of 0.5 mL. Bacterial doses were confirmed based on CFU plate counts.
Enumeration of bacteria in the peritoneal cavity
Quantification of bacterial loads in the peritoneal cavity was carried out, as previously described.E17 Mice (3 per group) were killed at 24 to 72 hours after inoculation. Peritoneal lavage was performed with cold Ca2+- and Mg2+-free saline, and aliquots were centrifuged at 2500g for 5 minutes and resuspended in sterile water. Aliquots (in a volume of 50–100 μL) or appropriate dilutions thereof were plated on Salmonella shigella agar plates, and viable CFU values were determined after an overnight incubation.
Flow cytometric analysis of peritoneal exudate cells
Peritoneal exudate cells (PECs) were analyzed, as previously described.E18 Briefly, PECs were suspended to a concentration of 5 × 106/mL, and 100-mL aliquots were dispensed into wells of a round-bottom 96-well plate. Cells were preincubated with anti-CD16/CD32–specific mAb (clone 2.4G2; BD Biosciences) for 30 minutes on ice to block FcγIII/II receptor sites. Analysis of PECs was carried out by means of 6-color flow cytometric analysis, according to a standard procedure,E19 after staining cells with the following panel of conjugated mAbs: CD3-FITC, CD19–phycoerythrin (PE), CD8–allophycocyanin (APC), CD4–PE-Cy7, and CD11b–APC-Cy7 (all from BioLegend, San Diego, Calif). Analysis of myeloid cell subpopulations was carried out with a second panel of mAbs: CD11b–APC-Cy7, F4/80–PE-Cy7, Ly6C-PE, Ly6G-APC, and anti–MHC class II–FITC (BioLegend). The viability dye 7AAD (BioLegend) was used with each panel of mAbs to exclude nonviable cells from the analysis. Data were collected on 30,000 cells by using a FACS-Canto II (BD Biosciences) and analyzed with FACSDiva software (BD Biosciences).
Measurement of neutrophil phagocytic activity
Twenty-four hours after challenging mice with BRD509, the phagocytic potential of PECs was assessed by using a flow cytometry–based phagocytosis assay kit (catalog no. 500290) from Cayman Chemical (Cayman Pharma, Neratovice, Czech Republic), according to the manufacturer’s instructions. Twenty-four hours after challenging mice with BRD509, PECs were incubated with rabbit IgG-FITC–labeled latex beads for 3 hours at 37°C, followed by surface staining with a panel of mAbs, including CD11b–APC-Cy7, F4/80–PE-Cy7, Ly6C-PE, and Ly6G-APC (BioLegend). Cells were then further processed and analyzed by using flow cytometry, as described above.
Measurement of intracellular ROS
ROS production was measured by using flow cytometry with 2′,7′–dichlorofluorescein diacetate (Sigma-Aldrich) as a fluorescent probe, according to an established protocol.E20 Briefly, 24 hours after challenging mice with BRD509, PECs were stained with 5 μmol/L 2′,7′–dichlorofluorescein diacetate for 30 minutes at 37°C in the dark. Cells were then washed, resuspended, and surface stained with mAbs (CD11b–APC-Cy7, F4/80–PE-Cy7, Ly6C-PE, and Ly6G-APC), followed by analysis on FACSCanto II, as described above.
Supplementary Material
Clinical implications.
Neutrophils from CD40L-deficient patients display defective development and function that are not corrected by therapies currently available. The improved function of CD40L-deficient neutrophils achieved by rhIFN-γ suggests a new potential therapeutic application for this cytokine.
Acknowledgments
We thank all the patients and their families for their participation in this study. We thank Dr Antje Muller from the Department of Rheumatology, University of Lübeck, and Dr Jing Sun from the University of Cincinnati College of Medicine, Cincinnati, Ohio, for critical reading of the manuscript.
Supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 2012/50515-4 to O.C.-M. and grant 2012/51745-3 and 2016/22158-3 to A.C.-N.), the Jeffrey Modell Foundation (to A.C.-N.), and PENSI Institute and Ministério da Saúde do Brasil (PRONAS/PDC 2015 and 25000.077928/2015-06 to A.C.-N.). The animal studies were supported by a UAEU Program for Advanced Research grant (no. 31M193) from the Office of Research and Sponsored Projects, UAE University (to B.K.a.-R.).
Abbreviations used
- APC
Allophycocyanin
- CD40L
CD40 ligand
- CD62L
CD62 ligand
- CFU
Colony-forming units
- CGD
Chronic granulomatous disease
- DEG
Differentially expressed gene
- DMSO
Dimethyl sulfoxide
- FITC
Fluorescein isothiocyanate
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- G-CSF
Granulocyte colony-stimulating factor
- GPI
Glycosylphosphatidylinositol
- MFI
Mean fluorescence intensity
- NET
Neutrophil extracellular trap
- PE
Phycoerythrin
- PEC
Peritoneal exudate cell
- PMA
Phorbol 12-myristate 13-acetate
- Q-VD-OPh
Carboxy terminal phenoxy group conjugated to the amino acids valine and aspartate
- rhIFN-γ
Recombinant human IFN-γ RNAseq, RNA sequencing
- ROS
Reactive oxygen species
- sCD40L
Soluble CD40 ligand
- TLR
Toll-like receptor
Footnotes
Disclosure of potential conflict of interest: T. R. Torgerson has consultant arrangements with Shire Pharmaceuticals, CSL Behring, UCB Pharmaceuticals, and ADMA Biologics and has received grants from the National Institutes of Health. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Uhl B, Vadlau Y, Zuchtriegel G, Nekolla K, Sharaf K, Gaertner F, et al. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood. 2016;128:2327–37. doi: 10.1182/blood-2016-05-718999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 3.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol Mech Dis. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–39. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 5.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–11. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 6.Lian Z, Kluger Y, Greenbaum DS, Tuck D, Gerstein M, Berliner N, et al. Genomic and proteomic analysis of the myeloid differentiation program: global analysis of gene expression during induced differentiation in the MPRO cell line. Blood. 2002;100:3209–20. doi: 10.1182/blood-2002-03-0850. [DOI] [PubMed] [Google Scholar]
- 7.Newburger PE, Subrahmanyam YV, Weissman SM. Global analysis of neutrophil gene expression. Curr Opin Hematol. 2000;7:16–20. doi: 10.1097/00062752-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Mazzei GJ, Edgerton MD, Losberger C, Lecoanet-Henchoz S, Graber P, Durandy A, et al. Recombinant soluble trimeric CD40 ligand is biologically active. J Biol Chem. 1995;270:7025–8. doi: 10.1074/jbc.270.13.7025. [DOI] [PubMed] [Google Scholar]
- 9.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 10.Kato K, Santana-Sahagún E, Rassenti LZ, Weisman MH, Tamura N, Kobayashi S, et al. The soluble CD40 ligand sCD154 in systemic lupus erythematosus. J Clin Invest. 1999;104:947–55. doi: 10.1172/JCI7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AHR, Ugel S, et al. T cell cancer therapy requires CD40-CD40L activation of tumor necrosis factor and inducible nitric-oxide-synthase-producing dendritic cells. Cancer Cell. 2016;30:651. doi: 10.1016/j.ccell.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Subauste CS, Wessendarp M, Sorensen RU, Leiva LE. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999;162:6690–700. [PubMed] [Google Scholar]
- 13.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solanilla A, Dechanet J, El Andaloussi A, Dupouy M, Godard F, Chabrol J, et al. CD40-ligand stimulates myelopoiesis by regulating flt3-ligand and thrombopoietin production in bone marrow stromal cells. Blood. 2000;95:3758–64. [PubMed] [Google Scholar]
- 15.Goules A, Tzioufas AG, Manousakis MN, Kirou KA, Crow MK, Routsias JG. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J Autoimmun. 2006;26:165–71. doi: 10.1016/j.jaut.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Perazzio SF, Soeiro-Pereira PV, dos Santos VC, de Brito MV, Salu B, Oliva MLV, et al. Soluble CD40L is associated with increased oxidative burst and neutrophil extracellular trap release in Behçet’s disease. Arthritis Res Ther. 2017;19:235. doi: 10.1186/s13075-017-1443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Z, Jin R, Yu S, Rivet JJ, Smyth SS, Nanda A, et al. CD40 is essential in the upregulation of TRAF proteins and NF-KappaB-dependent proinflammatory gene expression after arterial injury. PLoS One. 2011;6:e23239. doi: 10.1371/journal.pone.0023239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mavroudi I, Papadaki V, Pyrovolaki K, Katonis P, Eliopoulos AG, Papadaki HA. The CD40/CD40 ligand interactions exert pleiotropic effects on bone marrow granulopoiesis. J Leukoc Biol. 2011;89:771–83. doi: 10.1189/jlb.0610330. [DOI] [PubMed] [Google Scholar]
- 19.Saeland S, Duvert V, Caux C, Pandrau D, Favre C, Vallé A, et al. Distribution of surface-membrane molecules on bone marrow and cord blood CD34+ hematopoietic cells. Exp Hematol. 1992;20:24–33. [PubMed] [Google Scholar]
- 20.Cabral-Marques O, Klaver S, Schimke LF, Ascendino ÉH, Khan TA, Pereira PVS, et al. First report of the hyper-IgM syndrome registry of the latin american society for immunodeficiencies: novel mutations, unique infections, and outcomes. J Clin Immunol. 2014;34:146–56. doi: 10.1007/s10875-013-9980-4. [DOI] [PubMed] [Google Scholar]
- 21.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, et al. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 22.Jasinska A, Kalwak K, Trelinska J, Borowiec M, Piatosa B, Zeman K, et al. Successful haploidentical PBSCT with subsequent T-cell addbacks in a boy with hyperIgM syndrome presenting as severe congenital neutropenia. Pediatr Transplant. 2013;17:37–40. doi: 10.1111/j.1399-3046.2012.01786.x. [DOI] [PubMed] [Google Scholar]
- 23.Cabral-Marques O, Schimke L-F, Pereira PVS, Falcai A, de Oliveira JB, Hackett MJ, et al. Expanding the clinical and genetic spectrum of human CD40L deficiency: the occurrence of paracoccidioidomycosis and other unusual infections in Brazilian patients. J Clin Immunol. 2012;32:212–20. doi: 10.1007/s10875-011-9623-6. [DOI] [PubMed] [Google Scholar]
- 24.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, et al. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003;82:373–84. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 25.de la Morena MT, Leonard D, Torgerson TR, Cabral-Marques O, Slatter M, Aghamohammadi A, et al. Long term outcomes of 176 patients with X-linked hyper IgM syndrome treated with or without hematopoietic cell transplantation. J Allergy Clin Immunol. 2016;136:1282–92. doi: 10.1016/j.jaci.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain A, Atkinson TP, Lipsky PE, Slater JE, Nelson DL, Strober W. Defects of T-cell effector function and post-thymic maturation in X- linked hyper-IgM syndrome. J Clin Invest. 1999;103:1151–8. doi: 10.1172/JCI5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontana S, Moratto D, Mangal S, De Francesco M, Vermi W, Ferrari S, et al. Functional defects of dendritic cells in patients with CD40 deficiency. Blood. 2003;102:4099–106. doi: 10.1182/blood-2003-04-1244. [DOI] [PubMed] [Google Scholar]
- 28.Cabral-Marques O, Arslanian C, Ramos RN, Morato M, Schimke L, Soeiro Pereira PV, et al. Dendritic cells from X-linked hyper-IgM patients present impaired responses to Candida albicans and Paracoccidioides brasiliensis. J Allergy Clin Immunol. 2012;129:778–86. doi: 10.1016/j.jaci.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Al-Ojali SM, Moore CBT, Fernandez-Cabezudo MJ, Al-Ramadi BK. Enhancement of the anti-Salmonella immune response in CD154-deficient mice by an attenuated, IFN-γ-expressing, strain of Salmonella enterica serovar Typhimurium. Microb Pathog. 2012;52:326–35. doi: 10.1016/j.micpath.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Miralda I, Uriarte SM, McLeish KR. Multiple phenotypic changes define neutrophil priming. Front Cell Infect Microbiol. 2017;7:217. doi: 10.3389/fcimb.2017.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Y, Matsushima H, Ohtola JA, Geng S, Lu R, Takashima A. Neutrophil priming occurs in a sequential manner and can be visualized in living animals by monitoring IL-1β promoter activation. J Immunol. 2015;194:1211–24. doi: 10.4049/jimmunol.1402018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tennenberg SD, Fey DE, Lieser MJ. Oxidative priming of neutrophils by interferon-gamma. J Leukoc Biol. 1993;53:301–8. doi: 10.1002/jlb.53.3.301. [DOI] [PubMed] [Google Scholar]
- 33.Ellis TN, Beaman BL. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology. 2004;112:2–12. doi: 10.1111/j.1365-2567.2004.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aas V, Larsen K, Iversen JG. IFN-γ induces calcium transients and increases the capacitative calcium entry in human neutrophils. J Interfon Cytokine Res. 1998;18:197–205. doi: 10.1089/jir.1998.18.197. [DOI] [PubMed] [Google Scholar]
- 35.Gougerot-Podicalo MA, Elbim C, Chollet-Martin S. Modulation of the oxidative burst of human neutrophils by pro- and anti-inflammatory cytokines. Pathol Biol (Paris) 1996;44:36–41. [PubMed] [Google Scholar]
- 36.Jabłońska E, Kiluk M, Markiewicz W, Jabłoński J. Priming effects of GM-CSF, IFN-gamma and TNF-alpha on human neutrophil inflammatory cytokine production. Melanoma Res. 2002;12:123–8. doi: 10.1097/00008390-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Sacco MG, Ungari M, Catò EM, Villa A, Strina D, Notarangelo LD, et al. Lymphoid abnormalities in CD40 ligand transgenic mice suggest the need for tight regulation in gene therapy approaches to hyper immunoglobulin M (IgM) syndrome. Cancer Gene Ther. 2000;7:1299–306. doi: 10.1038/sj.cgt.7700232. [DOI] [PubMed] [Google Scholar]
- 38.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–9. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 39.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Müller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80:1008–14. [PubMed] [Google Scholar]
- 40.Cabral-Marques O, Ramos RN, Schimke LF, Khan TA, Amaral EP, Barbosa Bomfim CC, et al. Human CD40L deficiency dysregulates the macrophage transcriptome causing functional defects that are improved by exogenous IFN-γ. J Allergy Clin Immunol. 2016;139:900–12. doi: 10.1016/j.jaci.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 42.Taschner PEM, den Dunnen JT. Describing structural changes by extending HGVS sequence variation nomenclature. Hum Mutat. 2011;32:507–11. doi: 10.1002/humu.21427. [DOI] [PubMed] [Google Scholar]
- 43.Kotz KT, Xiao W, Miller-Graziano C, Qian W, Russom A, Warner EA, et al. Clinical microfluidics for neutrophil genomics and proteomics. Nat Med. 2010;16:1042–7. doi: 10.1038/nm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amarante-Mendes GP, Finucane DM, Martin SJ, Cotter TG, Salvesen GS, Green DR. Anti-apoptotic oncogenes prevent caspase-dependent and independent commitment for cell death. Cell Death Differ. 1998;5:298–306. doi: 10.1038/sj.cdd.4400354. [DOI] [PubMed] [Google Scholar]
- 45.Wright HL, Thomas HB, Moots RJ, Edwards SW. RNA-Seq reveals activation of both common and cytokine-specific pathways following neutrophil priming. PLoS One. 2013;8:e58598. doi: 10.1371/journal.pone.0058598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–52. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 47.Feriotti C, Loures FV, Frank de Araújo E, da Costa TA, Calich VLG. Mannosyl- recognizing receptors induce an M1-like phenotype in macrophages of susceptible mice but an M2-like phenotype in mice resistant to a fungal infection. PLoS One. 2013;8:e54845. doi: 10.1371/journal.pone.0054845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soeiro-Pereira PV, Falcai A, Kubo CA, Oliveira EB, Jr, Marques OC, Antunes E, et al. BAY 41-2272, a soluble guanylate cyclase agonist, activates human mononuclear phagocytes. Br J Pharmacol. 2012;166:1617–30. doi: 10.1111/j.1476-5381.2011.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–21. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 51.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–9. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pina A, Saldiva PHN, Restrepo LEC, Calich VLG. Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J Leukoc Biol. 2006;79:1202–13. doi: 10.1189/jlb.0106052. [DOI] [PubMed] [Google Scholar]
- 53.Terstappen LW, Safford M, Loken MR. Flow cytometric analysis of human bone marrow. III. Neutrophil maturation. Leukemia. 1990;4:657–63. [PubMed] [Google Scholar]
- 54.Moulding DA, Hart CA, Edwards SW. Regulation of neutrophil FcgammaRIIIb (CD16) surface expression following delayed apoptosis in response to GM-CSF and sodium butyrate. J Leukoc Biol. 1999;65:875–82. doi: 10.1002/jlb.65.6.875. [DOI] [PubMed] [Google Scholar]
- 55.von Bernuth H, Ku C-L, Rodriguez-Gallego C, Zhang S, Garty B-Z, Maródi L, et al. A fast procedure for the detection of defects in Toll-like receptor signaling. Pediatrics. 2006;118:2498–503. doi: 10.1542/peds.2006-1845. [DOI] [PubMed] [Google Scholar]
- 56.Al-Ramadi BK, Fernandez-Cabezudo MJ, Ullah A, El-Hasasna H, Flavell RA. CD154 is essential for protective immunity in experimental salmonella infection: evidence for a dual role in innate and adaptive immune responses. J Immunol. 2006;176:496–506. doi: 10.4049/jimmunol.176.1.496. [DOI] [PubMed] [Google Scholar]
- 57.Elbim C, Chollet-Martin S, Bailly S, Hakim J, Gougerot-Pocidalo MA. Priming of polymorphonuclear neutrophils by tumor necrosis factor alpha in whole blood: identification of two polymorphonuclear neutrophil subpopulations in response to formyl-peptides. Blood. 1993;82:633–40. [PubMed] [Google Scholar]
- 58.Daniels RH, Elmore MA, Hill ME, Shimizu Y, Lackie JM, Finnen MJ. Priming of the oxidative burst in human neutrophils by physiological agonists or cytochalasin B results from the recruitment of previously non-responsive cells. Immunology. 1994;82:465–72. [PMC free article] [PubMed] [Google Scholar]
- 59.Eggleton P, Fisher D, Crawford N. Heterogeneity in the circulating neutrophil pool: studies on subpopulations separated by continuous flow electrophoresis. J Leukoc Biol. 1992;51:617–25. doi: 10.1002/jlb.51.6.617. [DOI] [PubMed] [Google Scholar]
- 60.Eggleton P, Wang L, Penhallow J, Crawford N, Brown KA. Differences in oxidative response of subpopulations of neutrophils from healthy subjects and patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:916–23. doi: 10.1136/ard.54.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meloni-Bruneri LH, Campa A, Abdalla DS, Calich VL, Lenzi HL, Burger E. Neutrophil oxidative metabolism and killing of P. brasiliensis after air pouch infection of susceptible and resistant mice. J Leukoc Biol. 1996;59:526–33. doi: 10.1002/jlb.59.4.526. [DOI] [PubMed] [Google Scholar]
- 62.Reinhardt TA, Sacco RE, Nonnecke BJ, Lippolis JD. Bovine milk proteome: quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J Proteomics. 2013;82:141–54. doi: 10.1016/j.jprot.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Bachiega TF, Dias-Melicio LA, Fernandes RK, de Almeida Balderramas H, Rodrigues DR, Ximenes VF, et al. Participation of dectin-1 receptor on NETs release against Paracoccidioides brasiliensis: role on extracellular killing. Immunobiology. 2016;221:228–35. doi: 10.1016/j.imbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Cano LE, López JA, Hernandez O, González Á, Mejía SP. Human neutrophils produce extracellular traps against Paracoccidioides brasiliensis. Microbiology. 2015;161:1008–17. doi: 10.1099/mic.0.000059. [DOI] [PubMed] [Google Scholar]
- 65.Della Coletta AM, Bachiega TF, de Quaglia e Silva JC, de Soares ÂMVC, De Faveri J, Marques SA, et al. Neutrophil extracellular traps identification in tegumentary lesions of patients with paracoccidioidomycosis and different patterns of NETs generation in vitro. PLoS Negl Trop Dis. 2015;9:e0004037. doi: 10.1371/journal.pntd.0004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biagioni LM, Sadatsune T, Franco MF, Mattos MC. A comparative study of the immunoantigenicity of eight Paracoccidioides brasiliensis isolates. Rev Inst Med Trop Sao Paulo. 1986;28:281–6. doi: 10.1590/s0036-46651986000500001. [DOI] [PubMed] [Google Scholar]
- 67.Hahn RC, Macedo AM, Fontes CJF, Batista RD, Santos NL, Hamdan JS. Randomly amplified polymorphic DNA as a valuable tool for epidemiological studies of Paracoccidioides brasiliensis. J Clin Microbiol. 2003;41:2849–54. doi: 10.1128/JCM.41.7.2849-2854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Macoris S, Sugizaki M, Peraçoli M, Bosco S, Hebeler-Barbosa F, Simões L, et al. Virulence attenuation and phenotypic variation of Paracoccidioides brasiliensis isolates obtained from armadillos and patients. Mem Inst Oswaldo Cruz. 2006;101:331–4. doi: 10.1590/s0074-02762006000300019. [DOI] [PubMed] [Google Scholar]
- 69.Moyerbrailean GA, Davis GO, Harvey CT, Watza D, Wen X, Pique-Regi R, et al. A high-throughput RNA-seq approach to profile transcriptional responses. Sci Rep. 2015;5:14976. doi: 10.1038/srep14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–20. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:447–52. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang DW, Lempicki RA, Sherman BT. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 76.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. The Gene Ontology Consortium. Gene ontology: tool for the unification of biology. Nat Genet. 2011;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Croft D, O’Kelly G, Wu G, Haw R, Gillespie M, Matthews L, et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39:D691–7. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, et al. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2016;44:D481–7. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Worku M, Paape MJ, Marquardt WW. Modulation of Fc receptors for IgG on bovine polymorphonuclear neutrophils by interferon-gamma through de novo RNA transcription and protein synthesis. Am J Vet Res. 1994;55:234–8. [PubMed] [Google Scholar]
- 80.Elghetany MT, Ge Y, Patel J, Martinez J, Uhrova H. Flow cytometric study of neutrophilic granulopoiesis in normal bone marrow using an expanded panel of antibodies: correlation with morphologic assessments. J Clin Lab Anal. 2004;18:36–41. doi: 10.1002/jcla.20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van Den Bosch VJMM. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155:559–66. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berg M, James SP. Human neutrophils release the Leu-8 lymph node homing receptor during cell activation. Blood. 1990;76:2381–8. [PubMed] [Google Scholar]
- 83.Jones J, Morgan BP. Apoptosis is associated with reduced expression of complement regulatory molecules, adhesion molecules and other receptors on polymorphonuclear leucocytes: functional relevance and role in inflammation. Immunology. 1995;86:651–60. [PMC free article] [PubMed] [Google Scholar]
- 84.Macey MG, McCarthy DA, Vordermeier S, Newland AC, Brown KA. Effects of cell purification methods on CD11b and L-selectin expression as well as the adherence and activation of leucocytes. J Immunol Methods. 1995;181:211–9. doi: 10.1016/0022-1759(95)00003-s. [DOI] [PubMed] [Google Scholar]
- 85.Orr Y, Taylor JM, Cartland S, Bannon PG, Geczy C, Kritharides L. Conformational activation of CD11b without shedding of L-selectin on circulating human neutrophils. J Leukoc Biol. 2007;82:1115–25. doi: 10.1189/jlb.0906545. [DOI] [PubMed] [Google Scholar]
- 86.Newburger PE, Chovaniec ME, Greenberger JS, Cohen HJ. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979;82:315–22. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Millius A, Weiner OD. Manipulation of neutrophil-like HL-60 cells for the study of directed cell migration. Methods Mol Biol. 2010;591:147–58. doi: 10.1007/978-1-60761-404-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fleit HB, Wright SD, Durie CJ, Valinsky JE, Unkeless JC. Ontogeny of Fc receptors and complement receptor (CR3) during human myeloid differentiation. J Clin Invest. 1984;73:516–25. doi: 10.1172/JCI111238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hauert AB, Martinelli S, Marone C, Niggli V. Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis. Int J Biochem Cell Biol. 2002;34:838–54. doi: 10.1016/s1357-2725(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 90.Yaseen R, Blodkamp S, Lüthje P, Reuner F, Völlger L, Naim HY, et al. Antimicrobial activity of HL-60 cells compared to primary blood-derived neutrophils against Staphylococcus aureus. J Negat Results Biomed. 2017;16:2. doi: 10.1186/s12952-017-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pullen GR, Hosking CS. Differentiated HL60 promyelocytic leukaemia cells have a deficient myeloperoxidase/halide killing system. Clin Exp Immunol. 1985;62:304–9. [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Y, Weyman CM, Liu H, Almasan A, Zhou A. IFN-γ induces apoptosis in HL-60 cells through decreased Bcl-2 and increased Bak expression. J Interferon Cytokine Res. 2008;28:65–72. doi: 10.1089/jir.2007.0025. [DOI] [PubMed] [Google Scholar]
- 93.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. 2010;29:315–48. doi: 10.3109/08830181003792803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110:18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 95.Carr R, Davies JM. Abnormal FcRIII expression by neutrophils from very pre-term neonates. Blood. 1990;76:607–11. [PubMed] [Google Scholar]
- 96.Leven EA, Maffucci P, Ochs HD, Scholl PR, Buckley RH, Fuleihan RL, et al. Hyper IgM syndrome: a report from the USIDNET Registry. J Clin Immunol. 2016;36:490–501. doi: 10.1007/s10875-016-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–10. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borregaard N, Cross AR, Herlin T, Jones OT, Segal AW, Valerius NH. A variant form of X-linked chronic granulomatous disease with normal nitroblue tetrazolium slide test and cytochrome b. Eur J Clin Invest. 1983;13:243–8. doi: 10.1111/j.1365-2362.1983.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 99.Woodman RC, Newburger PE, Anklesaria P, Erickson RW, Rae J, Cohen MS, et al. A new X-linked variant of chronic granulomatous disease characterized by the existence of a normal clone of respiratory burst-competent phagocytic cells. Blood. 1995;85:231–41. [PubMed] [Google Scholar]
- 100.Battersby AC, Cale CM, Goldblatt D, Gennery AR. Clinical manifestations of disease in X-linked carriers of chronic granulomatous disease. J Clin Immunol. 2013;33:1276–84. doi: 10.1007/s10875-013-9939-5. [DOI] [PubMed] [Google Scholar]
- 101.Perry FE, Elson CJ, Greenham LW, Catterall JR. Interference with the oxidative response of neutrophils by Streptococcus pneumoniae. Thorax. 1993;48:364–9. doi: 10.1136/thx.48.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jain A, Kovacs JA, Nelson DL, Migueles SA, Pittaluga S, Fanslow W, et al. Partial immune reconstitution of X-linked hyper IgM syndrome with recombinant CD40 ligand. Blood. 2011;118:3811–7. doi: 10.1182/blood-2011-04-351254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ezekowitz RA, Sieff CA, Dinauer MC, Nathan D, Orkin SH, Newburger PE. Restoration of phagocyte function by interferon-gamma in X-linked chronic granulomatous disease occurs at the level of a progenitor cell. Blood. 1990;76:2443–8. [PubMed] [Google Scholar]
- 104.Conti F, Aragão Filho WC, Prando C, Deswarte C, Hubeau M, Newburger PE, et al. Phagocyte nicotinamide adenine dinucleotide phosphate oxidase activity in patients with inherited IFN-γR1 or IFN-γR2 deficiency. J Allergy Clin Immunol. 2015;135:1393–5. doi: 10.1016/j.jaci.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seijkens T, Beckers L, Christ A, Moura R, Tjwa M, Lutgens E. Abstract 158: CD40L regulates hematopoietic stem and progenitor cell biology by inducing adhesion-mediated quiescence. Arterioscler Thromb Vasc Biol. 2012;32:158. [Google Scholar]
- 106.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–40. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flores-Romo L, Björck P, Duvert V, van Kooten C, Saeland S, Banchereau J. CD40 ligation on human cord blood CD34+ hematopoietic progenitors induces their proliferation and differentiation into functional dendritic cells. J Exp Med. 1997;185:341–9. doi: 10.1084/jem.185.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Canque B, Camus S, Yagello M, Gluckman JC. IL-4 and CD40 ligation affect differently the differentiation, maturation, and function of human CD34+ cell-derived CD1a+CD14- and CD1a- CD14+ dendritic cell precursors in vitro. J Leukoc Biol. 1998;64:235–44. doi: 10.1002/jlb.64.2.235. [DOI] [PubMed] [Google Scholar]
- 109.van Lochem EG, van der Velden VHJ, Wind HK, te Marvelde JG, Westerdaal NAC, van Dongen JJM. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts. Cytometry. 2004;60:1–13. doi: 10.1002/cyto.b.20008. [DOI] [PubMed] [Google Scholar]
- 110.Hernández-Campo PM, Almeida J, Matarraz S, de Santiago M, Sánchez ML, Orfao A. Quantitative analysis of the expression of glycosylphosphatidylinositol-anchored proteins during the maturation of different hematopoietic cell compartments of normal bone marrow. Cytometry B Clin Cytom. 2007;72:34–42. doi: 10.1002/cyto.b.20143. [DOI] [PubMed] [Google Scholar]
- 111.Vadhan-Raj S, Buescher S, LeMaistre A, Keating M, Walters R, Ventura C, et al. Stimulation of hematopoiesis in patients with bone marrow failure and in patients with malignancy by recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1988;72:134–41. [PubMed] [Google Scholar]
- 112.Anderlini P, Przepiorka D, Champlin R, Körbling M. Biologic and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood. 1996;88:2819–25. [PubMed] [Google Scholar]
- 113.De Kleer I, Willems F, Lambrecht B, Goriely S. Ontogeny of myeloid cells. Front Immunol. 2014;5:423. doi: 10.3389/fimmu.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skalnik DG. Transcriptional mechanisms regulating myeloid-specific genes. Gene. 2002;284:1–21. doi: 10.1016/s0378-1119(02)00387-6. [DOI] [PubMed] [Google Scholar]
- 115.Jerne NK. The immune system. Sci Am. 1973;229:52–60. doi: 10.1038/scientificamerican0773-52. [DOI] [PubMed] [Google Scholar]
- 116.Bergthaler A, Menche J. The immune system as a social network. Nat Immunol. 2017;18:481–2. doi: 10.1038/ni.3727. [DOI] [PubMed] [Google Scholar]
- 117.Menche J, Sharma A, Kitsak M, Ghiassian SD, Vidal M, Loscalzo J, et al. Disease networks. Uncovering disease-disease relationships through the incomplete interactome. Science. 2015;347:1257601. doi: 10.1126/science.1257601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rieckmann JC, Geiger R, Hornburg D, Wolf T, Kveler K, Jarrossay D, et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat Immunol. 2017;18:583–93. doi: 10.1038/ni.3693. [DOI] [PubMed] [Google Scholar]
REFERENCES
- E1.Feriotti C, Loures FV, Frank de Araújo E, da Costa TA, Calich VLG. Mannosyl- recognizing receptors induce an M1-like phenotype in macrophages of susceptible mice but an M2-like phenotype in mice resistant to a fungal infection. PLoS One. 2013;8:e54845. doi: 10.1371/journal.pone.0054845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–21. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Soeiro-Pereira PV, Falcai A, Kubo CA, Oliveira EB, Jr, Marques OC, Antunes E, et al. BAY 41-2272, a soluble guanylate cyclase agonist, activates human mononuclear phagocytes. Br J Pharmacol. 2012;166:1617–30. doi: 10.1111/j.1476-5381.2011.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Pina A, Saldiva PHN, Restrepo LEC, Calich VLG. Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J Leukoc Biol. 2006;79:1202–13. doi: 10.1189/jlb.0106052. [DOI] [PubMed] [Google Scholar]
- E5.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- E6.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–9. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Reinhardt TA, Sacco RE, Nonnecke BJ, Lippolis JD. Bovine milk proteome: quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J Proteomics. 2013;82:141–54. doi: 10.1016/j.jprot.2013.02.013. [DOI] [PubMed] [Google Scholar]
- E8.Wright HL, Thomas HB, Moots RJ, Edwards SW. RNA-seq reveals activation of both common and cytokine-specific pathways following neutrophil priming. PLoS One. 2013;8:e58598. doi: 10.1371/journal.pone.0058598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Cabral-Marques O, Ramos RN, Schimke LF, Khan TA, Amaral EP, Barbosa Bomfim CC, et al. Human CD40L deficiency dysregulates the macrophage transcriptome causing functional defects that are improved by exogenous IFN-γ. J Allergy Clin Immunol. 2016;139:900–12. doi: 10.1016/j.jaci.2016.07.018. [DOI] [PubMed] [Google Scholar]
- E10.Ellis TN, Beaman BL. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology. 2004;112:2–12. doi: 10.1111/j.1365-2567.2004.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Newburger PE, Chovaniec ME, Greenberger JS, Cohen HJ. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979;82:315–22. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Carrigan SO, Weppler AL, Issekutz AC, Stadnyk AW. Neutrophil differentiated HL-60 cells model Mac-1 (CD11b/CD18)-independent neutrophil transepithelial migration. Immunology. 2005;115:108–17. doi: 10.1111/j.1365-2567.2005.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Al-Ojali SM, Moore CBT, Fernandez-Cabezudo MJ, Al-Ramadi BK. Enhancement of the anti-Salmonella immune response in CD154-deficient mice by an attenuated, IFN-γ-expressing, strain of Salmonella enterica serovar. Typhimurium Microb Pathog. 2012;52:326–35. doi: 10.1016/j.micpath.2012.03.002. [DOI] [PubMed] [Google Scholar]
- E14.Fernandez-Cabezudo MJ, Mechkarska M, Azimullah S, Al-Ramadi BK. Modulation of macrophage proinflammatory functions by cytokine-expressing Salmonella vectors. Clin Immunol. 2009;130:51–60. doi: 10.1016/j.clim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- E15.al-Ramadi BK, Adeghate E, Mustafa N, Ponery AS, Fernandez-Cabezudo MJ. Cytokine expression by attenuated intracellular bacteria regulates the immune response to infection: the Salmonella model. Mol Immunol. 2002;38:931–40. doi: 10.1016/s0161-5890(02)00020-2. [DOI] [PubMed] [Google Scholar]
- E16.al-Ramadi BK, Fernandez-Cabezudo MJ, Ullah A, El-Hasasna H, Flavell RA. CD154 is essential for protective immunity in experimental salmonella infection: evidence for a dual role in innate and adaptive immune responses. J Immunol. 2006;176:496–506. doi: 10.4049/jimmunol.176.1.496. [DOI] [PubMed] [Google Scholar]
- E17.al-Ramadi BK, Mustafa N, AbouHaidar M, Fernandez-Cabezudo MJ. Induction of innate immunity by IL-2-expressing Salmonella confers protection against lethal infection. Mol Immunol. 2003;39:763–70. doi: 10.1016/s0161-5890(03)00005-1. [DOI] [PubMed] [Google Scholar]
- E18.Issac JM, Sarawathiamma D, Al-Ketbi MI, Azimullah S, Al-Ojali SM, Mohamed YA, et al. Differential outcome of infection with attenuated Salmonella in MyD88-deficient mice is dependent on the route of administration. Immunobiology. 2013;218:52–63. doi: 10.1016/j.imbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- E19.Ramadi KB, Mohamed YA, Al-Sbiei A, Almarzooqi S, Bashir G, Al Dhanhani A, et al. Acute systemic exposure to silver-based nanoparticles induces hepatotoxicity and NLRP3-dependent inflammation. Nanotoxicology. 2016;10:1061–74. doi: 10.3109/17435390.2016.1163743. [DOI] [PubMed] [Google Scholar]
- E20.Aryappalli P, Al-Qubaisi SS, Attoub S, George JA, Arafat K, Ramadi KB, et al. The IL-6/STAT3 signaling pathway is an early target of manuka honey-induced suppression of human breast cancer cells. Front Oncol. 2017;7:167. doi: 10.3389/fonc.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.