Abstract
Cocaine addiction is associated with dysfunction of the prefrontal cortex (PFC), which facilitates relapse and compulsive drug taking. To assess if cocaine’s effects on both neuronal and vascular activity contribute to PFC dysfunction, we used optical coherence tomography (OCT) and multiwavelength laser speckle (MW-LSI) to measure vascularization and hemodynamics, and used GCaMP6f to monitor intracellular Ca2+ levels ([Ca2+]in) as a marker of neuronal activity. Rats were given short (1hr; ShA) or long (6hr; LgA) access cocaine self-administration. As expected, LgA but not ShA rats escalated cocaine intake. In naïve rats, acute cocaine decreased oxygenated hemoglobin, increased deoxygenated hemoglobin, and reduced cerebral blood flow in PFC, likely due to cocaine-induced vasoconstriction. ShA rats showed enhanced hemodynamic response and slower recovery after cocaine, versus naïve. LgA rats showed a blunted hemodynamic response, but an enhanced PFC neuronal [Ca2+]in increase after cocaine challenge associated with drug intake. Both ShA and LgA groups had higher vessel density, indicative of angiogenesis, presumably to compensate for cocaine’s vasoconstricting effects. Cocaine self-administration modified the PFC cerebrovascular responses enhancing it in ShA and attenuating it in LgA animals. In contrast, LgA but not ShA animals showed sensitized neuronal reactivity to acute cocaine in the PFC. The opposite changes in hemodynamics (decreased) and neuronal responses (enhanced) in LgA rats indicate that these constitute distinct effects, and suggest that the neuronal and not the vascular effects are associated with escalation of cocaine intake in addiction whereas its vascular effect in PFC might contribute to cognitive deficits that increase vulnerability to relapse.
Keywords: addiction, hemodynamics, intracellular calcium, prefrontal cortex, self-administration
Introduction
Cocaine is the most commonly abused psychostimulant in the United States. In 2015, approximately 36 million Americans used cocaine and 900 000 suffered from a cocaine use disorder (0.3% of the population; Center for Behavioral Health Statistics and Quality, 2016). The chronic use of cocaine has been linked to damage to the cerebrovascular system (Volkow et al. 1988) and serious adverse cerebral and cardiovascular events, including myocardial infarctions and cerebral hemorrhages and stroke (Qureshi et al. 2001; Toossi et al. 2010). In fact, use of cocaine within the previous 24 hours was associated with a 6.4-fold increase in the risk of an ischemic stroke (Cheng et al. 2016). These effects of cocaine are observed even in young adults, who are otherwise at a low risk for such events. Given the frequently unhealthy life styles of chronic drug users, including the co-morbid use of other drugs, isolating the effects of cocaine can be difficult; for this reason, effective animal models of drug use are essential.
The prefrontal cortex (PFC) is critical for executive function and is implicated in the loss of self-regulation and compulsive pattern of cocaine intake observed in addicted individuals (Volkow et al. 2016). Brain imaging studies in humans have shown that individuals suffering from a cocaine use disorder have reduced activity in the PFC, as measured by decreases in cerebral blood flow (Volkow et al. 1988) and in glucose metabolism (Volkow et al. 1992; Volkow et al. 1993), and by reduced grey matter volumes (Liu et al. 1998; Franklin et al. 2002). PFC hypoactivity during abstinence is associated with cognitive impairments. For example, cocaine abusers have impairment in the Go-Nogo tasks, a measure of response inhibition, failing to inhibit responses on Nogo signals, that are associated with reduced activity in the anterior cingulate cortex (ACC; Kaufman et al. 2003) and PFC (Hester & Garavan 2004). Rodent models, have reported that chronic cocaine exposure changes the morphology (Robinson et al. 2001) and function (Nasif et al. 2005a; Nasif et al. 2005b) of PFC pyramidal neurons. As with humans, chronic cocaine in rats impairs PFC dependent executive function and response inhibition (Stalnaker et al. 2006; Allen & Leri 2014; George et al., 2008). Taken together, these studies highlight the importance of the PFC, and suggest that it is particularly vulnerable to the effects of chronic cocaine.
Here we tested the hypothesis that both neuronal and hemodynamic changes contribute to PFC dysfunction with chronic cocaine exposure. For this purpose, we concomitantly examined hemodynamic and neuronal effects of cocaine in the PFC of rats with a history of cocaine self-administration under short (1hr; ShA) or long (6hr; LgA) access conditions. The escalation model utilized here has both face and construct validity. First, one can show that 7 of the 7 items in the DSM-IV and 7 of the 11 items in the DSM-V, including most of the criteria required for severe use disorder are met in this animal model and include tolerance, withdrawal, substance taken in larger amount than intended, unsuccessful efforts to quit, considerable time spent to obtain the drug, important social, work or recreational activities given up because of use, and continued use despite adverse consequences (George et al., 2014). Clearly, after significant escalation of drug intake, this animal model provides support for the loss of control over drug intake and is particularly useful as an animal model to study the transition to compulsive-like drug seeking behavior (defined here as habitual responding, responding to alleviate a negative emotional state, and responding in the face of punishment (Moore et al., 2017)).To assess hemodynamics in the PFC we used optical coherence tomography (OCT) to measure vascularization, and multiwavelength laser speckle (MW-LSI) to measure dynamic changes in the concentration of oxygenated hemoglobin ([HbO2]), deoxygenated hemoglobin ([HbR]), and cerebral blood flow velocity (CBFv; Ren et al. 2012; Zhang et al. 2016). To assess neuronal activity in PFC we used an AAV1.Syn.GCaMP6f.WPRE.SV40 virus to express GCaMP6f in neurons in the right PFC in order to monitor intracellular Ca2+ ([Ca2+]in), which serves as a marker of neuronal activity (Chen et al. 2013a). We hypothesized that animals who escalated their cocaine consumption would show enhanced neuronal excitability to acute cocaine exposure in PFC, whereas animals who did not increase their cocaine consumption would not. We also hypothesized that regardless of escalation, cocaine would lead to impaired perfusion in PFC.
Materials and Methods
Animals
Subjects were 28 male Sprague Dawley rats (Taconic), approximately 250–300g at the beginning of the experiment. Rats were kept on a 12:12hr light cycle (lights off at 1900hr), with ad libitum access to food and water throughout the experiment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Stony Brook University.
Virus Infusion
To measure changes in PFC neuronal activity (measured with [Ca2+]in), one week prior to commencing testing half of the rats (n=14) were anesthetized with isofluorane, mounted on a stereotaxic frame and their scalps were opened. AAV1.Syn.GCaMP6f.WPRE.SV40 virus (Penn Vector Core) was infused into the right PFC (A/P: +3; M/L: 0.8); two infusions of 0.5µL were made (D/V: −1.4 and −1mm from skull) at a rate of 0.2µL/min, and the injector was left in place for 20min following each infusion to allow for diffusion. At the time of imaging, 5–6 weeks had elapsed since virus infusion to allow for GCaMP6f expression.
Cocaine Self-Administration
Experimental timeline is shown in Fig. 1A. Self-administration procedures were adapted from Wee et al. (2007). In brief, animals underwent a second surgery to implant a chronic indwelling catheter. Rats were anesthetized with isofluorane, and a 14.5cm piece of micro-renthane tubing (MRE037) was passed from the rat’s back to the ventral side. An incision was made in the right jugular vein and the catheter inserted. The dorsal terminal of the catheter was attached to a 22 gauge cannula fixed with dental cement to a surgical mesh that held the cannula secure in the middle of the rat’s back (just posterior to scapula). Rats were flushed daily with heparin (60units/mL)/Cefazolin (150mg/mL) solution to maintain catheter patency and prevent infection. Following 1 week of recovery, animals began self-administration (acquisition) training. Rats were divided into two groups, administering either cocaine (1mg/kg/infusion; 100ul/infusion) or saline (active lever response resulted in 100ul/kg/infusion of saline). All sessions during acquisition were 1hr in duration. Sessions commenced with the illumination of the house light and insertion of levers into the chamber. Pressing the “active” lever resulted in illumination of a cue light and a 4sec infusion. Following the infusion there was a 20sec timeout period during which the cue light remained illuminated and further responses had no consequences. Responses to pressing the “inactive” lever were recorded, but had no consequences. Animals remained in acquisition phase until they reached a criterion of 3 consecutive days with less than 10% variability in infusion number (Ahmed & Koob 1999). Animals were then transitioned into the escalation phase. Saline administering rats remained the same, but rats self-administering cocaine were further divided into two groups that had either a long (6hr), or continued to have a short (1hr) access sessions. All other parameters remained the same. Rats continued under these conditions for 17 days (Fig. 1A). Twenty-four hours after the last cocaine self-administration session or saline session (for Naïve group), all animals were exposed to an acute cocaine challenge (1mg/kg intravenous) and their hemodynamic and neuronal responses measured (Fig. 1B).
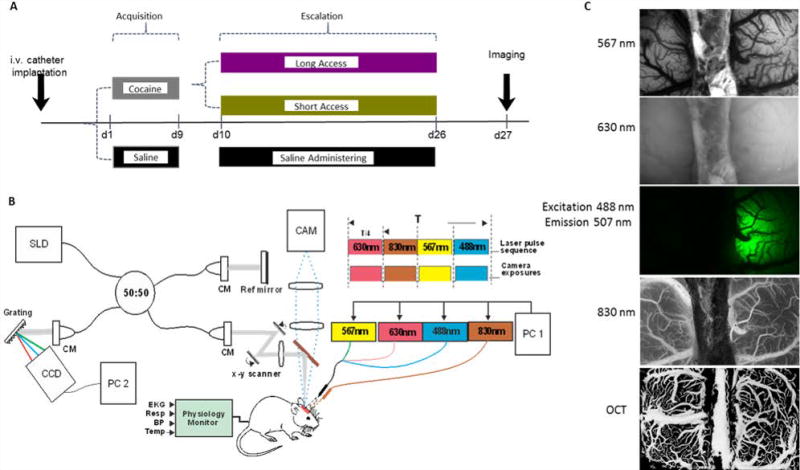
Figure 1.
Panel A displays a diagram of the experimental design. After catheter implantation, rats were divided into cocaine self-administering or saline administering (naïve) groups. The cocaine administering group was further subdivided into ShA and LgA groups for the escalation period. All animals underwent the window implantation and imaging 24hrs after their last session and received a cocaine infusion during imaging. Panel B shows a schematic diagram of the OCT system (left; CM, collimator; BBS, broadband source (λ ¼ 1.3 µm)) and MW-LSI (right) with 4 alternating light sources. Panel C shows representations of images produced from OCT and the 567, 630, 488, and 830 nm wavelengths of the MW-LSI system.
PFC Imaging in vivo
Twenty-four hours following the last self-administration session, rats underwent surgery to implant a cranial window over the PFC, followed by MW-LSI and OCT imaging (Fig. 1C).
Surgical Preparation
For imaging, rats were anesthetized with isofluorane and a 16 gauge IV catheter was inserted into their trachea (Angiocath, BD) and attached to a respirator to control breathing. An incision was made proximal to the left hind limb to expose the left femoral artery and vein and catheters (0.58mm ID, 0.99mm OD, Scientific Commodities Inc.) were inserted into the femoral artery to monitor arterial blood pressure (MAP), and into the femoral vein for drug delivery during imaging. Rats were mounted on a stereotaxic frame (Kopf 900) and a 4×6mm2 portion of skull was removed above the frontal cortex (A/P: +2 to +5; M/L: −3 to +3). The dura was carefully removed to expose the brain surface and covered with 1.25% agarose gel and a cover glass (0.15mm thick; VWR micro coverglass) affixed to the skull using crazy glue (Gorilla Glue). The animal’s mean arterial blood pressure (MAP) (mean = 82.28, SE=1.88), body temperature (37–39°C), and respiration (43 breaths/min) were monitored and recorded (Small Animal Monitoring and Gating system, model 1025L, SA Instruments Inc.), and end-tidal CO2 (pCO2) was monitored (Poet IQ2, Criticare Technologies) throughout the experiment and kept stable at 30–35mmHg.
Image Acquisition
We used an MW-LSI optical imaging system developed in our laboratory using procedures previously described (Chen et al., 2016). In brief, the cranial window was sequentially illuminated by 3 LEDs (λ1= 568nm, λ2= 630nm and λexcitation=488nm; Spectra Light Engine, Lumicor), and a laser diode (λ4= 830nm; DL8142-201-830, ThorLabs), at a rate of 12.5Hz/channel. Images were captured with a zoom fluorescence microscope (AZ100, Nikon) connected to a sCMOS camera (pixel size: 6.5µm; Zyla4.3, Andor) using modified Solis software (version 4.26, Andor).
In parallel, we used OCT (Yuan et al., 2011) to image the morphology of neurovascular networks in PFC. The OCT system utilized a broadband laser (λ=1.3µm, Δλ≥90nm; Inphenix) as illumination source to achieve axial resolution of 8µm. An achromate (f40mm/0.1NA) was applied to focus the illuminating light beam and collect backscatter light from tissue. The lateral resolution was determined to be ~φ12µm. The output light from a spectrometer was captured by a line array detector which provides up to 140 fps A-line acquisition rate. A 2-D raster scanning system enables a field of view (FOV) of 5×4×2mm3.
Image acquisition was 40min in duration and consisted of a 10min baseline period followed by 30min of imaging after an injection of cocaine (1mg/kg iv). We selected the 1 mg/kg dose because it is a dose that is commonly used in self-administration studies, and it is equivalent to doses self-administered by cocaine abusers (Gatley et al., 1999). It also has the advantage of being a dose frequently used in rodent studies (Yuan et al., 2011; Du et al., 2006), which facilitates the comparisons of our results to those of others. For rats that administered saline, this was their first, and only, cocaine exposure and they are thus are labeled as the ‘Naïve’ group.
Image Processing
The hemodynamic changes in the cortical tissue, i.e., the changes in oxygenated hemoglobin (ΔHbO2) and deoxygenated- hemoglobin (ΔHbR) were calculated from λ1 and λ2 images obtained from MW-LSI system according to the equation:
Where , and are the molar extinction coefficients for HbO2 and HbR at the two wavelengths, Rλ1(t) and Rλ2(t) are the measured diffuse reflectance matrices (2-D images) at these wavelengths, and Lλ1(t) and Lλ2(t) are the path lengths of light propagation, which are estimated to be =95.2um and =98.8um (Jacques, 2013). Cerebral blood flow velocity was calculated by reconstructing LSI flow image series by computing the speckle variances in both the spatial and temporal domains (Chen et al. 2016).
Changes in intracellular calcium concentration ([Ca2+]in) were calculated from fluorescence (λexcitation=488 nm, λemission=509 nm). To control for absorption differences due to blood flow changes, values in regions expressing GCaMP were divided by the equivalent left hemisphere region, which had no expression. Results are presented as the percent change from baseline, this controls for differences in GCaMP expression across different animals.
To assess vessel density, the optical coherence angiography (OCA) images were reconstructed using the speckle variation approach (Mariampillai et al. 2010), and 3-D optical doppler tomography (ODT) image reconstructions were performed by the phase subtraction method (Zhao et al. 2000). Frangi filter (Frangi et al. 1998) was utilized for vessel segmentation and OCA image enhancement (see Fig. 6A–F for demonstration). The vessel skeleton was obtained from the segmented vessel mask using the skeletonize algorithm implemented in ImageJ (Arganda-Carreras et al. 2010). To quantify size-dependent vasoconstriction induced by chronic cocaine, the skeleton was divided into three groups: small (<100µm), medium (100–200 µm) and large (>200 µm) vessels. Vessel density of each group was calculated by:
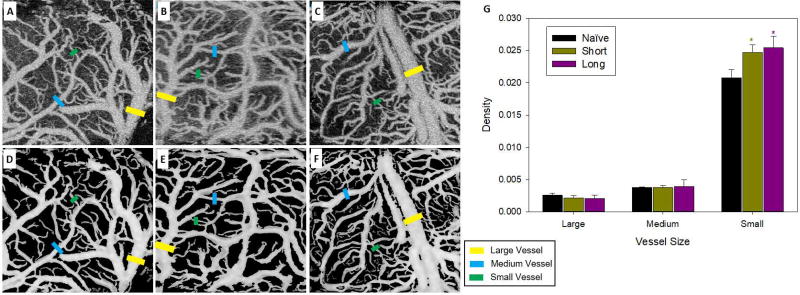
Figure 6.
OCT angiography showed increased density of small vessels following cocaine self-administration. Panels A, B, and C show representative images of OCT angiography for naïve, ShA, and LgA animals respectively. Panels D, E, and F show the same images after correction to enhance contrast and vessel clarity. Yellow, blue, and green bars indicate exemplars of large, medium, and small vessels. Panel G shows the mean density of large, medium, and small vessels for each group. * indicates a significant (p<0.05) difference from the naive group.
Statistical Analysis
Results are reported as mean ± standard error (SE). Data was analyzed using one-way or two-way mixed model analyses of variance (ANOVAs) as appropriate and the Student-Newman-Keuls method was used for post-hoc analysis. All statistical tests were performed using SigmaStat software (Systat Software INC), and all alpha levels were set at 0.05, with only significant effects reported.
Results
Cocaine Self-Administration: Long access (LgA) animals show escalation of drug intake
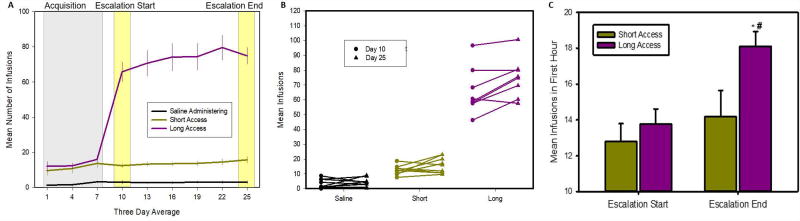
LgA rats showed escalation of drug intake, while ShA rats did not. Fig. 2 shows the mean number of infusions taken (0 or 1mg/kg cocaine) across three day periods. A two-way mixed model ANOVA on drug taking in the first hour of session showed a significant group by day interaction [F(8,100)=2.03; p=0.05]. Post-hoc comparisons showed that both LgA (16.4mg/kg first hour cocaine, p<0.001) and ShA (13.8mg/kg/day, p<0.001) took more infusions than saline administering rats (3.0 infusions/day saline). While there is no significant difference between the first hour responding of LgA and ShA rats overall (p=0.1), the LgA rats do take significantly more cocaine during the first hour at the end of the escalation period (Fig. 2C; LgA= 18.4; ShA=14.6; p=0.029). One-way ANOVA on total 6hr drug intake (Fig. 2A) for the LgA group also found a significant escalation in the number of infusions taken from the start (65.8mg/kg/day) to end (79.6mg/kg/day) of the escalation period [F(4,28)=3.7, p=0.015], while no significant changes were seen in the saline administering (3.1 infusions/day to 3.1 infusions/day; F(4,36)= 0.16, p=0.95) nor the ShA (12.8mg/kg/day to 14.6mg/kg/day; F(4,36)=1.88, p=0.14) rats. These differences are highlighted in Fig. 2B, which shows the change from start to end of the escalation period (day 10 to day 25) for each animal.
Figure 2.
Cocaine self-administration behavior showing escalation of drug in take. Panel A shows the mean number of infusions (0 or 1mg/kg cocaine) animals received during acquisition (grey shaded area) and escalation period. Data points represent 3 day averages (mean ± s.e.); * indicates significant difference from saline administering animals. Panel B shows the number of infusions at the start (circle) and end (triangle) of the escalation period for each animal; these points are highlighted in yellow on panel A. Panel C shows infusions during the first hour at the start and end of the escalation period; * indicates significant difference from the start, # indicates difference from short access. This shows significant escalation of drug intake in the LgA group.
Oxygenated hemoglobin in PFC is temporarily decreased by acute cocaine
Acute cocaine decreased [HbO2] in PFC and this decrease was enhanced in ShA rats compared to ‘naive’ and LgA rats (Fig. 3). Panels A–C (Fig. 3) show representative images of the percent change in [HbO2] concentration over time following a 1mg/kg cocaine injection at time 0 (for naive animals, this was their first exposure to cocaine). A two-way mixed model ANOVA (Fig. 3D) indicated a significant group by time interaction [F(150,1500)=2.61, p<0.001], with post-hoc tests revealing that all groups had a significant reduction in [HbO2] following cocaine infusion. Specifically, naïve rats when injected acutely with cocaine had a maximum [HbO2] decrease (Pa) of −27.75% (SE=2.29), and remained below baseline 1–25min post cocaine (p<0.05) with a mean latency to peak (Pt) of 3.6min (SE=0.33). ShA rats had a greater [HbO2] decrease (Pa=−33.18%, SE=3.16; Pt=3.5, SE=0.25) than naive rats (2.5–6min post injection p<0.05), and the decrease did not fully recover during the imaging period (from 1–30.5min). LgA rats showed an attenuated [HbO2] decrease (Pa=−22.71%, SE=1.98, Pt=2.7, SE=0.5) from 1–13.5min (p<0.05), that was smaller than for naïve rats (4–12.5min post injection p<0.05) or for ShA rats (2–25 min post injection p<0.05). In ShA rats there was a significant correlation between cocaine-induced decreases in [HbO2] (Fig. 3E) and the number of cocaine infusions in the last day of the self-administration period (Fig. 2B) (r=−0.94, p<0.001; Fig. 3F); such that the more infusions the larger the [HbO2] decreases. In LgA rats this correlation was not significant (r=0.14, p=0.76; Fig. 3G).
Figure 3.
Dynamic response of [HbO2] following a 1mg/kg cocaine infusion was enhanced in ShA rats. Panels A (drug naïve), B (ShA), and C (LgA) show ratio images of [HbO2] in representative animals. Green indicates no change from before cocaine injection with greater decreases indicated by deeper shades of blue. Panel D shows the time course of change in the 3 groups following cocaine infusion on at time 0. Significant differences from the naive group (p <0.05) are indicated by * and bars. Panel E shows the mean and individual peak amplitude (Pa) from baseline. Panels F (ShA) and G (LgA) show correlations between the maximal deviation and the number of infusions obtained at the end of self-administration, with the regression equation, and r value displayed for each.
Cerebral blood flow velocity is reduced by cocaine
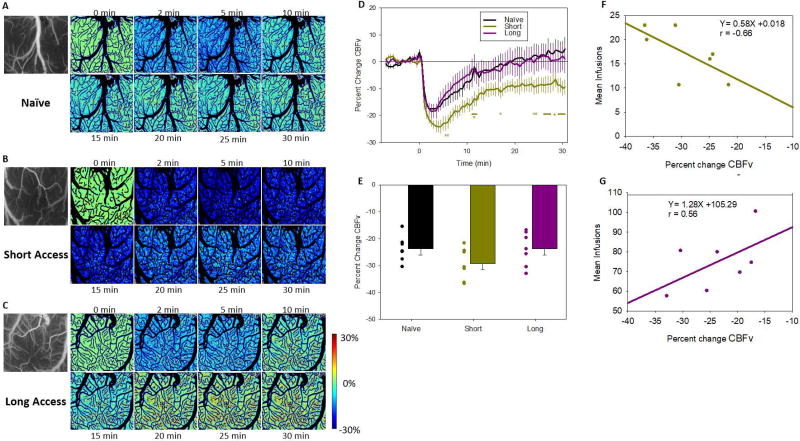
Acute cocaine caused a reduction in cerebral blood flow velocity (CBFv; Fig. 4). Panels A–C show representative images of the percent change in CBFv over time following a 1mg/kg cocaine injection at time 0. A two-way mixed model ANOVA (Fig. 4D) found a significant group by time interaction [F(150,1350)=2.06, p<0.001]. Post-hoc analysis showed that all groups had a significant decrease in CBFv following the cocaine challenge. Naïve rats showed a decrease in CBFv of −20.11% (SE=1.57; Pt=4.75, SE=0.7); that lasted from 1–10.5min post infusion; ShA rats had a decrease of −26.11% (SE=2.05, Pt=3.93, SE=0.44) that lasted from 1–17.5min post infusion; and LgA rats had a decrease of −20.16% (SE=2.23; Pt=3.57, SE=0.75) that lasted only from 1–6.5min post infusion. Furthermore, while there were no group differences between LgA and the naïve groups, differences between ShA and both the naïve and the LgA groups were significant in the later portion of the measurements, as ShA rats showed slower recovery than the other groups (Fig. 4D). Overall, the latency to peak amplitude was slower for CBFv (Pt=4.02, SE=0.37) than for either [HbO2] (Pt=3.3, SE=0.22) or [HbR] (Fig S1; Pt=3.02, SE=0.22) [F(2,38)=7.71, p=0.002], suggesting that the reduction in [HbO2] could not be solely accounted by a reduction in CBFv and might also reflect increases in cortical metabolism. Though the correlations between changes in CBFv and cocaine self-administration did not reach significance for the ShA or LgA rats, the associations were in the opposite direction (ShA: r=−0.66, p=0.11, Fig. 4F; LgA: r=0.56, p=0.19, Fig. 4G) and the difference in the slopes between the ShA and LgA was significant (z=2.39, p=0.008); for the ShA greater CBFv decreases tended to be associated with larger cocaine intake, whereas for the LgA larger CBFv decreases tended to be associated with lower cocaine intake.
Figure 4.
Dynamic changes in CBFv following a 1mg/kg cocaine infusion. Panels A (drug naïve), B (ShA), and C (LgA) show ratio images of CBFv in representative animals. Green indicates no change from before cocaine injection with greater decreases indicated by deeper shades of blue. Panel D shows the time course of change in the 3 groups following cocaine infusion on at time 0. Significant differences from the naive group (p <0.05) are indicated by * and bars. Panel E shows mean and individual Pa from baseline. Panels F (ShA) and G (LgA) show correlations between the maximal deviation and the number of infusions obtained at the end of the self-administration, with the regression equation and r value displayed for each.
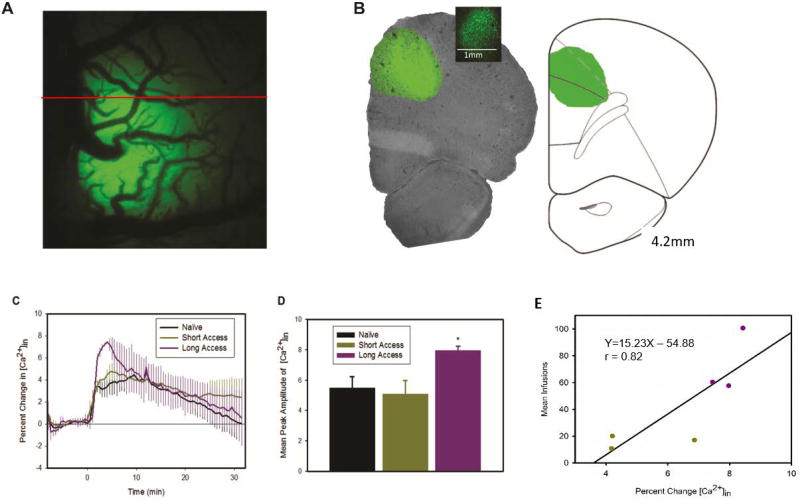
Neuronal calcium concentration is increased by acute cocaine
Panel A of Fig. 5 shows a representative image of GCaMP6f neuronal expression in the right PFC, where the virus was infused. Fluorescence was primarily expressed in the anterior cingulate cortex (ACC), which has been implicated in reinstatement/relapse (Kalivas & McFarland 2003) and the prelimbic cortex (PrL), which has been associated with levels of drug intake (Chen et al. 2013b), as well as, reinstatement (Kalivas & McFarland 2003). A two-way mixed model ANOVA (Fig. 5C) revealed a significant time effect [F(79,632)=17.49, p<0.001] but no group [F(2,8)=0.21, p=0.82] or interaction effects [F(158,632)=0.92, p=0.73]. Post hoc analyses revealed that cocaine induced increases in [Ca2+]in were significant for the three groups from 1.5–22min post injection. Though the group effects were not significant a planned comparison showed that the peak amplitude (Pa) in LgA (7.95%, SE=0.28) was significantly larger than in naive rats (5.50%, SE=0.73; t(6)=−2.47, p=0.048) and ShA rats (5.08%, SE=0.89, p=0.037) but there were no differences between ShA and naïve rats (p=0.74; Fig. 5D). For ShA and LgA rats, a significant correlation was found between the number of cocaine infusions at the end of the escalation period (day 25) and the maximal increase in neuronal [Ca2+]in (r=0.82, p=0.046; Fig. 5E).
Figure 5.
GCaMP6f fluorescence showing enhanced response to 1mg/kg cocaine infusion in LgA rats. Panel A shows a representative surface image of the unilateral expression of GCaMP6f, the red line indicates approx. position of slice. Panel B shows a coronal slice displaying location of expression in the anterior cingulate cortex and the dorsal portion of the prelimbic cortex; the inset is a magnified slice showing cellular expression. Panel C shows the time course of fluorescence change resulting from a cocaine infusion at time 0. Panel D shows the mean peak amplitude of fluorescence for each group. Planned comparisons show that LgA have a significantly larger increase than in naïve rats (p<0.05). Panel E shows correlation between maximal [Ca2+]in and the number of infusions obtained at the end of the self-administration period (day 25), with the regression equation and r value displayed.
Cocaine self-administration increases the number of blood vessels in PFC
OCT angiography revealed significantly higher density of small blood vessels (<100µm) in ShA and LgA than in naïve rats (Fig. 6). A two-way ANOVA showed a significant group by vessel size interaction [F(4,30)=4.25, p<0.01] (Fig. 6G). Post-hoc analysis showed that both the ShA (0.025, SE=0.001, p=0.001) and LgA (0.025, SE=0.001, p=0.001) rats had an increase in small vessels relative to naïve rats (0.021, SE=0.001), consistent with angiogenesis following chronic cocaine exposure. There were no differences in density for middle or larger vessels.
Discussion
Here we demonstrate that chronic cocaine self-administration differentially alters hemodynamic and neuronal [Ca2+]in responses in the PFC of rats. The nature of these alterations depends on the prior history of cocaine self-administration, with limited access (1hr) enhancing the hemodynamic responses (decreases in CBFv and tissue oxygenation) to acute cocaine, while extended access (6hr), which produced escalation of drug intake, blunting the hemodynamic response to acute cocaine but enhancing neuronal reactivity in PFC. Finally, we show that neuronal reactivity but not hemodynamic reactivity to cocaine correlated with doses of cocaine self-administered in both ShA and LgA rats suggesting the hypothesis that cocaine’s neuronal effects and not its vascular actions in PFC are likely to contribute to the escalation of drug intake during bingeing in addiction. On the other hand the reductions in CBFv in PFC during cocaine exposure may be hypothesized to contribute to cognitive deficits that increase vulnerability to relapse in addiction (Garavan et al., 2008; Gorini et al., 2014).
In drug naïve rats, acute cocaine decreased [HbO2], increased [HbR] (supplemental material) and reduced CBFv. These changes are most likely driven by cocaine-induced vasoconstriction (Ren et al. 2012); though changes in cortical metabolism might also contribute. Locally, constriction of cerebral vessels disrupts flow and reduces blood volume, limiting the availability of oxygen to the tissue. In the current study, [HbO2] reductions in PFC occurred more rapidly than CBFv decreases, indicating that they were initially triggered by neuronal activation and concomitant increase in oxygen metabolism reducing [HbO2] in the region. Therefore, these findings indicate that cocaine-induced decreases in [HbO2] reflect both increased local consumption of oxygen and cocaine-induced vasoconstriction resulting in CBFv reductions.
Following self-administration, ShA rats showed enhanced hemodynamic responses to cocaine. Compared to naïve rats, ShA animals had both a greater reduction in HbO2/CBFv and a slower recovery. This is consistent with our previous findings in rats that received noncontingent cocaine administration, which led to a sensitized cerebrovascular response to a cocaine challenge (Zhang et al. 2016). Though the mechanism(s) that contributed to the enhanced hemodynamic response in ShA animals are unclear, these might relate to cocaine’s dopaminergic and noradrenergic effects in DA and adrenergic receptors in cerebral blood vessels. ShA rats also showed a correlation between the number of self-administered cocaine infusions and the reduction in [HbO2] (as well as the increase in [HbR]; see supplemental material) following cocaine intake. Rats which took more cocaine had a greater reduction in blood oxygenation. This is consistent with a sensitization to the hemodynamic responses to cocaine in ShA rats. However, such a correlation was not observed in LgA rats, which were the only ones that escalated cocaine intake, which indicates that hemodynamic effects of cocaine in PFC are not involved in the escalation of cocaine intake.
During self-administration, LgA rats (6hrs of self-administration per day) displayed an escalation in cocaine intake that was not seen in ShA rats, which is considered analogous to the drug escalation observed in human addiction (Ahmed & Koob 1999) and has been argued to reflect a hedonic tolerance as brain reward thresholds increase (decreased reward) in parallel with escalation in cocaine intake (Ahmed and Koob, 1998). When tested 24hrs following their last self-administration session, LgA displayed a blunted hemodynamic response to a cocaine challenge relative to drug naïve and ShA animals, and showed an enhanced neuronal [Ca2+]in response when compared to drug naïve and ShA rats. The results in LgA rats, with cocaine inducing an attenuated hemodynamic response (blunted CBFv and [HbO2] decreases) may also reflect tolerance to the hemodynamic effects of cocaine, and differs from the findings observed after passive cocaine exposures which showed sensitized hemodynamic responses (Zhang et al. 2016). However, it is common for different effects to be observed between self-administered and experimenter-given cocaine (Hemby et al. 1997). Our finding of a blunted hemodynamic response to an acute cocaine injection in LgA animals is consistent with Lu et al. (2012) who also found a reduced BOLD signal in mPFC (PrL) and ACC following cocaine injection in rats with a history of cocaine self-administration (Lu et al. 2012).
Alhough the mechanism(s) underlying the blunted hemodynamic responses to cocaine under LgA conditions are unclear we postulate that they could reflect reduced sensitivity to the effects of cocaine on DA/NE neurotransmission. In the PFC, DA terminals have been found closely associated with capillaries and arterioles and stimulation results in vasoconstriction (Krimer et al., 1998). Imaging studies in human drug users have found that stimulant-induced increase of DA is markedly attenuated in cocaine abusers, whether they are evaluated shortly after cocaine ingestion (Volkow et al. 2014), or after detoxification (Volkow et al. 1997). Similar blunting of dopaminergic response has also been observed following cocaine self-administration in rats (Calipari et al. 2013). Though most studies reporting on blunted DA increases with chronic cocaine exposures have focused on the NAc in rodents (Mateo et al. 2005) and additionally in the striatum in humans (Volkow et al., 2014) it is unclear whether they are restricted only to brain reward regions or generalize to the rest of the brain. If similar changes in DA or NE reactivity were present in the peripheral sympathetic system (Mo et al. 1999; Tella et al. 1992) of LgA rats, this could underlie their attenuated vasoconstriction. Indeed, chronic cocaine has been shown to change vascular sensitivity to NE as evidenced by an attenuated increase in in blood pressure and heart rate following a cocaine infusion (Tella et al. 1999). Such a change in vascular reactivity could also contribute to the observed blunting of hemodynamics in LgA rats. Finally, the attenuated hemodynamic response to cocaine in LgA rats could reflect an already reduced CBFv at baseline, as seen with repeated cocaine exposures (Zhang et al. 2016), which results in a ceiling effect.
In contrast to the attenuated vascular effects the LgA animals showed an enhanced neuronal response to acute cocaine compared to naïve rats. Notably, cocaine-induced [Ca2+]in increases were correlated with the amount of cocaine self-administered, which raises the hypothesis that the enhanced neuronal responses play a role in the escalation of cocaine intake in the LgA rats. While chronic cocaine use is associated in preclinical and clinical studies with hypoactivity of the resting PFC (Chen et al. 2013b, Volkow et al. 1992), enhanced PFC neuronal activity is found in cocaine abusers in response to stimulation by drugs or cues (Wayman et al. 2016; Volkow et al. 2005). In fact, we reported that following cocaine self-administration, pyramidal neurons in the medial PFC show a more depolarized resting membrane potential, and require a significantly lower stimulating current to evoke action potentials than those in drug naïve rats (Wayman et al. 2015; Wayman et al. 2016). This hyper-responsivity could account for the increase in neuronal [Ca2+]in induced by chronic cocaine observed in our current and previous studies (Nasif et al. 2005a; Nasif et al. 2005b; Du et al. 2006). Further, our findings also reveal that in LgA animals there is an uncoupling between the vascular (where cocaine’s effects are blunted) and the neuronal adaptations (where excitability is enhanced) after chronic cocaine exposure. While the blunting of cocaine effects on vasoconstriction may seem beneficial, it could have deleterious implications for the individual. For the brain to function normally it requires a precise coupling between CBFv and neuronal activity to ensure that oxygen is delivered as a function of demand. The uncoupling of blood supply with neural activity could increase the risk for ischemia, contributing to the PFC deficits observed with chronic cocaine exposure.
While it is possible that the increase in Ca2+ fluorescence could be caused by the reduction in blood volume/flow rather than an increase in neural activity, this is unlikely since the reductions in hemodynamics were larger in ShA than in LgA rats, yet the increases in [Ca2+]in were larger in LgA rats. Furthermore, in all cases the effects reflect the interaction of acute cocaine with the history of cocaine self-administration; whereas saline injections did not trigger any significant changes in [Ca2+]in or CBFv (see supplemental).
Despite the enhanced PFC neuronal reactivity to an acute cocaine challenge in LgA compared to drug naïve rats, they showed attenuated [HbO2] and CBFv decreases. These blunted hemodynamic responses could reflect flow compensation as a result of the increases in vessel density (angiogenesis). Though we observed evidence for angiogenesis in ShA and LgA rats, both of whom had a higher density of small vessels (<100µm diameter), we observed enhanced CBFv and [HbO2] decreases following acute cocaine in ShA, which contrasted with the attenuated hemodynamic responses observed in LgA rats. The differences between ShA and LgA animals regarding their hemodynamic reactivity despite similar increases in vessel density could reflect differences in the functionality of the new vessels (Zhang et al. 2016; You et al. 2017). We had previously shown increases in cortical vessel densities in rats with chronic passive (non-contingent) cocaine exposure that were associated with an increase in the levels of vascular endothelial factor, a marker of angiogenesis (Zhang et al. 2016). This indicates that angiogenesis is a response to the pharmacological effects of cocaine, and occurs whether cocaine is self-administered or experimenter given, presumably triggered by vasoconstriction-induced hypoxia (You et al. 2017).
Since the neuronal and hemodynamic responses in the current study were measured while the rats were anesthetized, it is possible that the presence of isoflurane may have influenced the results. In fact, previous studies have found that isoflurane can cause internalization of DAT, enhancing the response to cocaine (Votaw et al., 2003, Byas-Smith et al., 2004). Similarly, Kufahl et al. (2009) found that rats anesthetized with isoflurane had greater fos expression in the frontal cortex following a cocaine challenge than rats anesthetized with α-chloralose. This higher level of fos expression was closer to that observed in awake animals when challenged with cocaine (Kufahl et al., 2009). Isoflurane has also been shown to induce vasodilation (Du et al., 2009) and thus it is possible that it might have attenuated cocaine induced reductions in blood supply. However, since prior cocaine exposure history does not appear to interact with isoflurane effects (Mets et al., 2001; Bernards et al., 1997), if isoflurane enhanced neuronal excitability or attenuated hemodynamic effects of cocaine this would have occurred equally in the 3 groups of animals. So, while the magnitude of the effects might have differed had the imaging been done in awake animals, the differences in neuronal and vascular adaptations between the groups are likely to be consistent with those in awake animals. A second limitation of the current study is that only one injection of cocaine (at a dose used by cocaine abusers) was administered as a challenge dosing. Future studies should image animals while they are repeatedly self-administering cocaine to assess if hemodynamic and neuronal responses to cocaine differ throughout a self-administration session. However, such studies will require technological developments that will enable imaging of awake behaving animals for long time periods (i.e., 6 hours for LgA rats).
The current study demonstrates that cocaine self-administration produced cerebrovascular and neuronal adaptations that were dependent on the history of cocaine exposure. Limited access to cocaine produced sensitization of vascular effects similar to that observed following passive cocaine administration. On the other hand, extended access to cocaine resulted in a blunted response to the hemodynamic effects of cocaine but an enhanced neuronal reactivity in the PFC that was associated with the self-administered doses of cocaine. These results suggest that the escalation of cocaine intake is associated with an enhancement of neuronal reactivity to acute cocaine in PFC and this uncoupling between neuronal and hemodynamic responses is likely to contribute to the PFC dysfunction observed in cocaine abusers.
Supplementary Material
Acknowledgments
The authors would like to acknowledge James Li, and Wei Chen for their assistance in data processing and Kevin Clare for ex vivo work. This work was supported in part by National Institutes of Health (NIH) grants R01DA029718 (CD & YP), R01 NS084817 (X-TH & CD) and R01 DA044552 (X-TH), R21DA042597 (YP & CD), and NIH’s Intramural Program of NIAAA (NDV). The authors would also like to thank the NIDA drug supply program for providing the cocaine used in this study.
Footnotes
Authors contribution:
CD and YP were responsible for the study design. CPA performed self-administration and data analysis. CPA and KP conducted MS-LSI imaging and data analysis; AL performed OCT imaging and data analysis. CPA prepared the manuscript and interpreted the findings. NDV, GFK, XTH, CD, and YP participated in data interpretation, discussion, and critical revisions of the manuscript. All authors reviewed content and approved the manuscript.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Allen CP, Leri F. Perseveration in the presence of punishment: The effects of chronic cocaine exposure and lesions to the prefrontal cortex. Behav Brain Res. 2014;261:185–192. doi: 10.1016/j.bbr.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Arganda-Carreras I, Fernandez-Gonzalez R, Munoz-Barrutia A, Ortiz-De-Solorzano C. 3D Reconstruction of histological sections: application to mammary gland tissue. Microsc Res Tech. 2010;73:1019–1029. doi: 10.1002/jemt.20829. [DOI] [PubMed] [Google Scholar]
- Bernards CM, Kern C, Cullen BF. Chronic cocaine administration does not modify haemodynamic responses to isoflurane anaesthesia in sheep. Can J Anaesth. 1997;44(2):202–207. doi: 10.1007/BF03013010. [DOI] [PubMed] [Google Scholar]
- Byas-Smith MG, Li J, Szlam F, Eaton DC, Votaw JR, Denson DD. Isoflurane induces dopamine transporter trafficking into the cell cytoplasm. Synapse. 2004;53:68–73. doi: 10.1002/syn.20037. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DCS, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 2013;38:2385–2392. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013a;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013b;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Chen W, Park K, Volkow ND, Pan YT, Du CW. Cocaine-induced abnormal cerebral hemodynamic responses to forepaw stimulation assessed by integrated multi-wavelength spectroimaging and laser speckle contrast imaging. IEEE J Sel Top Quantum Electron. 2016;22 doi: 10.1109/JSTQE.2015.2503319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YC, Ryan KA, Qadwai SA, Shah J, Sparks MJ, Wozniak MA, Stern BJ, Phipps MS, Cronin CA, Magder LS, Cole JW, Kittner SJ. Cocaine use and risk of ischemic stroke in young adults. Stroke. 2016;47:918–922. doi: 10.1161/STROKEAHA.115.011417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Tully M, Volkow ND, Schiffer WK, Yu M, Luo ZC, Koretsky AP, Benveniste H. Differential effects of anesthetics on cocaine’s pharmacokinetic and pharmacodynamics effects in brain. Eur J Neurosci. 2009;30(8):1565–1575. doi: 10.1111/j.1460-9568.2009.06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Yu M, Volkow ND, Koretsky AP, Fowler JS, Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26(45):11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangi AF, Niessen WJ, Vincken KL, Viergever MA. Multiscale vessel enhancement filtering. Medical image computing and computer-assisted interventation – MICCAI’98. Lect Notes Comput Sci. 1998;1496:130–137. [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51(2):134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, Logan J. Dopamine transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology. 1999;146:93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology. 2014;231:3911–3927. doi: 10.1007/s00213-014-3623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G. Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci. 2014;8(661) doi: 10.3389/fnhum.2014.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques SL. Optical properties of biological tissues: a review. Physics in Medicine and Biology. 2013:5007–5008. doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23(21):7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Mully EC, III, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nature Neuroscience. 1998;1(4):286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Pentkowski NS, Heintzelman K, Neisewander JL. Cocaine-induced Fos expression is detectable in the frontal cortex and striatum of rats under isoflurane but not α-chloralose anesthesia: implications for FMRI. J Neurosci Meth. 2009;181:241–248. doi: 10.1016/j.jneumeth.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18(4):243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Lu H, Chefer S, Kurup PK, Guillem K, Vaupel DB, Ross TJ, Moore A, Yang Y, Peoples LL, Stein EA. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. Neuroimage. 2012;62:1857–1866. doi: 10.1016/j.neuroimage.2012.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Mariampillai A, Leung MKK, Jarvi M, Standish BA, Lee K, Wilson BC, Vitkin A, Yang VXD. Optimized speckle variance OCT imaging of microvasculature. Opt Lett. 2010;35:1257–1259. doi: 10.1364/OL.35.001257. [DOI] [PubMed] [Google Scholar]
- Mets B, Pantuck CB, Diaz J, Soo E. The effect of chronic cocaine administration on hemodynamic stability and neurohumoral mediators during isoflurane anesthesia and shock in rats. Acta Anaesthesiol. Scand. 2001;45(3):377–384. doi: 10.1034/j.1399-6576.2001.045003377.x. [DOI] [PubMed] [Google Scholar]
- Mo W, Arruda JAL, Dunea G, Singh AK. Cocaine-induced hypertension: role of the peripheral sympathetic system. Pharmacological Research. 1999;40(2):139–145. doi: 10.1006/phrs.1999.0503. [DOI] [PubMed] [Google Scholar]
- Moore CF, Sabino V, Koob GF, Cottone P. Neuroscience of compulsive eating behavior. Front Neurosci. 2017;11:469. doi: 10.3389/fnins.2017.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005a;25:3674–3679. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu XT, White FJ. Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 2005b;312:1305–1313. doi: 10.1124/jpet.104.075184. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MFK, Guterman LR, Hopkins LN. Cocaine use and the likelihood of nonfatal myocardial infarction and stroke - Data from the Third National Health and Nutrition Examination survey. Circulation. 2001;103:502–506. doi: 10.1161/01.cir.103.4.502. [DOI] [PubMed] [Google Scholar]
- Ren H, Du C, Yuan Z, Park K, Volkow ND, Pan Y. Cocaine-induced cortical microischemia in the rodent brain: clinical implications. Mol Psychiatry. 2012;17:1017–1025. doi: 10.1038/mp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39(3):257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Franz TM, Burke KA, Schoenbaum G. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. Eur J Neurosci. 2006;24:2643–2653. doi: 10.1111/j.1460-9568.2006.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella SR, Schindler CW, Goldberg SR. Cardiovascular effects of cocaine in conscious rats – relative significance of central sympathetic-stimulation and peripheral neuronal monoamine uptake and release mechanisms. J Pharmacol Exp Ther. 1992;262(2):602–610. [PubMed] [Google Scholar]
- Tella SR, Schindler CW, Goldberg SR. Cardiovascular responses to cocaine self-administration: acute and chronic tolerance. Eur J Pharmacol. 1999;383:57–68. doi: 10.1016/s0014-2999(99)00582-8. [DOI] [PubMed] [Google Scholar]
- Toossi S, Hess CP, Hills NK, Josephson SA. Neurovascular complications of cocaine use at a tertiary stroke center. J Stroke Cerebrovasc Dis. 2010;19:273–278. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood-flow in chronic cocaine users – a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Logan J, Alexoff DL, Jayne M, Fowler JS, Wong C, Yin P, Du C. Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol Psychiatry. 2014;19(9):1037–1043. doi: 10.1038/mp.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw J, Byas-Smith M, Hua J, Voll R, Martarello L, Levey AI, Bowman D, Goodman M. Interaction of isoflurane with the dopamine transporter. Anesthesiology. 2003;98:404–411. doi: 10.1097/00000542-200302000-00021. [DOI] [PubMed] [Google Scholar]
- Wayman WN, Chen L, Hu XT, Napier TC. HIV-1 transgenic rat prefrontal cortex hyper-excitability is enhanced by cocaine self-Administration. Neuropsychopharmacology. 2016;41:1965–1973. doi: 10.1038/npp.2015.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman WN, Chen LH, Napier TC, Hu XT. Cocaine self-administration enhances excitatory responses of pyramidal neurons in the rat medial prefrontal cortex to human immunodeficiency virus-1 Tat. European Journal of Neuroscience. 2015:1195–1206. doi: 10.1111/ejn.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- You J, Volkow ND, Park K, Zhang Q, Clare K, Du C, Pan Y. Cerebrovascular adaptations to cocaine-induced transient ischemic attacks in the rodent brain. JCI Insight. 2017;2(5):e90809. doi: 10.1172/jci.insight.90809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZJ, Lou ZC, Volkow ND, Pan YT, Du CW. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. Neuroimage. 2011;54(2):1130–1139. doi: 10.1016/j.neuroimage.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, You J, Volkow ND, Choi J, Yin W, Wang W, Pan YT, Du CW. Chronic cocaine disrupts neurovascular networks and cerebral function: optical imaging studies in rodents. J Biomed Opt. 2016;21 doi: 10.1117/1.JBO.21.2.026006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YH, Chen ZP, Saxer C, Xiang SH, de Boer JF, Nelson JS. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt Lett. 2000;25:114–116. doi: 10.1364/ol.25.000114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.