Abstract
Prosthetic joint infection is usually caused by staphylococci. Among the coagulase-negative staphylococci, Staphylococcus lugdunensis is important because it behaves as a pathogen similar to S aureus. It also develops biofilms, and the biofilm phenotype can appear as small-colony variants. Although genetically indistinguishable, they differ in size and antibiotic susceptibility from the parent strain and are responsible for chronic persistent infection and failure of antibiotic treatment. They can also lead to misinterpretation of results. The patient reported here underwent total knee replacement and 2 years later presented with prosthetic joint infection. Tissue samples and prosthesis taken at revision grew S lugdunensis, the majority of which were small-colony variants. Recommendations are made for their detection and identification.
Keywords: Infection, Joint, Prosthetic, Staphylococcus, lugdunensis
Introduction
The incidence of prosthetic joint infection (PJI) in the UK for both hips and knees is approximately 0.6% [1]. Both the high morbidity and mortality and the economic impact make accurate diagnosis and adequate treatment a priority. Staphylococci are responsible for the majority of PJI cases. Staphylococcus lugdunensis is a coagulase-negative staphylococcus (CoNS) that causes a variety of serious infections including PJI [2].
Small-colony variants (SCVs) are members of the bacterial biofilm phenotype characterized by slow growth in small colonies approximately one-tenth the size of the parent strain. SCVs emerge as a result of genetic mutations or metabolic variations due to stress arising from nutrient limitation or exposure to sublethal concentrations of antibiotics [3]. The presence of SCVs has implications for microbiological diagnosis, clinical presentation, and subsequent management [4].
In this report, SCV of S lugdunensis was recovered from an infected knee replacement.
Case history
O.A. is a 50-year-old female with a long history of knee problems and was admitted with pain around her right knee where arthroplasty was performed and a draining sinus. Her previous history included an arthroscopic lateral release in 2003, patellar chondroplasty in 2005, a Fulkerson's osteotomy in 2007, removal of tibial screws in 2007, and a right total knee replacement (TKR) in 2014. After the knee replacement, she required 2 manipulations under anesthesia, the most recent in May 2016. In September 2016, she presented to the emergency department with an increase in pain and a draining sinus that had progressed from an area of induration a few days earlier. Her C-reactive protein was 30 mg/L and erythrocyte sedimentation rate was 42 mm/h.
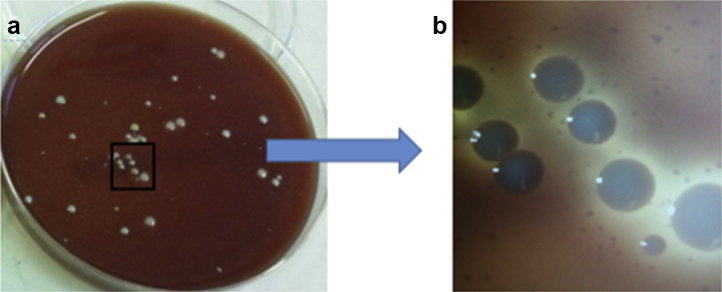
On October 11, 2016, she underwent a first-stage revision of the TKR. Multiple tissue samples and the explanted prosthesis (femoral, tibial, and patellar implants) were sent for microbiological culture and sensitivity. In the laboratory, tissues were homogenized and cultured, and the all the removed prosthesis was sonicated, and the sonicates were cultured. After 48 hours of incubation, tissue samples grew 30-40 cfu/mL of S lugdunensis, and the sonicates grew 350 cfu/mL of full-size parent colonies scattered on a background of a much larger number of SCVs which were not clearly evident until 72 hours of incubation (Fig. 1). Both strains had similar antibiotic susceptibility profiles. If only the larger parent colonies were considered, this could have been seen as probable CoNS contamination. Matrix-assisted laser desorption ionization–time of flight mass spectroscopy showed that both parent and SCVs were indistinguishable and were S lugdunensis.
Figure 1.
(a) Culture of the sonicate from the removed prosthesis on blood agar after 72 hours of incubation, showing large colonies surrounded by high numbers of very small colonies. The marked square is magnified 10 times in (b) to show the small colonies more clearly.
At the revision surgery, while the femoral and patellar components were well fixed, the tibial component was grossly loose. Following debridement, an articulating spacer was created using a standard press-fit condylar Sigma TKR prosthesis (DePuy Synthes, Warsaw, IN). The tibial component had a small cemented stem to provide additional diaphyseal support, and the knee was cemented in place using Palacos R+G (Heraeus Medical, Wehrheim, Germany) with 2 g of vancomycin added. The sinus was excised and wound primarily closed.
After the surgery, she was initially treated with 2 g of ceftriaxone and 600 mg of rifampicin per day, but the ceftriaxone was changed to 800 mg of teicoplanin per day after 5 weeks due to a rash associated with ceftriaxone. After a 6-week course of intravenous antibiotics, a further 6 weeks of oral flucloxacillin 4 g and rifampicin 600 mg per day were prescribed.
The patient has done well after her revision and finds that the articulating spacer provides her with adequate function. She has therefore not yet proceeded to the second stage and she has been followed up for a year at the time of preparation of this report (12 months of follow-up). Her C-reactive protein and erythrocyte sedimentation rate have returned to normal, and the wound has completely healed.
Discussion
S lugdunensis is known to colonize the inguinal region and perineum [5]. It was first described in 1988 [6], and since then, it has been identified as a pathogen causing a wide variety of infections throughout the human body [7].
S lugdunensis can produce clumping factor (bound coagulase) and hence can be misidentified as S aureus if the slide coagulase test is used rather than the tube test [8]. This misidentification has been reported in literature and can be compounded by yellow pigment and DNAse production by some strains [9]. The appearance of SCV can also give an impression of mixed culture, with possible interpretation of contamination. The phenotypic changes in S lugdunensis SCV can also give rise to misidentification as S hominis or other CoNS in certain commercial identification systems [10], [11]. In such situations, matrix-assisted laser desorption ionization–time of flight mass spectroscopy is able to provide more confident identification [9], [12], [13]. S lugdunensis resembles S aureus in pathogenicity [2]. In addition, S lugdunensis isolates are usually β-lactamase deficient and susceptible to penicillins [14]. Hence, recognition of SCV and species identification of S lugdunensis is crucial for proper treatment. Although being clonally similar, their biochemical characterization and antibiotic susceptibilities can vary. Therefore, antibiotic susceptibility testing should be done for each phenotype separately [15]. SCVs of S aureus hemB mutants have been shown to exhibit more adhesiveness to surfaces than the parent strain [16]. Furthermore, development of SCV has been shown to be induced by the slow pattern of antibiotic release from gentamicin beads in patients with osteomyelitis [17].
S lugdunensis appears to have a preference to infect knees more than hips. Among the S lugdunensis PJI episodes (summarized in Table 1), we could identify the site of infection in 84, among which 58 (69%), 24 (28.6%), and 2 (2.4%) episodes were in knees, hips, and other sites, respectively. This observation was previously made by the authors of the largest 2 series of S lugdunensis PJI [2], [29]. Presentation of PJI due to S lugdunensis can vary widely between acute symptoms such as fever and local site inflammation to unexplained dull aching pain at the site of surgery. Delay between time of surgery and presentation can be as short as 3 weeks [29] or as long as 10 years [24].
Table 1.
Summary of S lugdunensis PJI cases reported in the literature.
| Reference | No. | Age (range) | Gender | Comorbidities | Duration | Site |
|---|---|---|---|---|---|---|
| Sampathkumar et al. [8] | 2 | 72 | M | MG, cancer pancreas, asthma | 4 y | TKR |
| 74 | M | Cancer prostate | 6 wk | TKR | ||
| Weightman et al. [18] | 1 | 72 | M | 10 mo | TKR | |
| Sanzeni and Ringberg [19] | 1 | 54 | M | 2 y | THR | |
| Losada et al. [20] | 1 | 69 | M | Rheumatoid arthritis treated with steroids and cyclosporin | TKR | |
| Frank et al. [21] | 6 | |||||
| Lecuire et al. [22] | 7 | (34-86) | From 6 wk up to 9 y and 8 mo | 4 TKR, 3 THR | ||
| Trampuz et al [23] | 3 | |||||
| Shah et al. [2] | 28 | (35-88) | 14 M, 14 F | 3 DM, 5 on steroids, 9 urogenital abnormalities | 25 TKR, 3 THR | |
| Harris et al. [12] | 8 | |||||
| Merino et al. [24] | 1 | 51 | M | Multiple myeloma | 10 y | THR |
| Szabados et al. [9] | 1 | 47 | M | DM, HBV | 2.5 y | THR |
| Tsaras et al. [25] | 3 | |||||
| Tande et al. [15] | 5 | |||||
| Campoccia et al. [26] | 4 | 2 TKR, 2 THR | ||||
| Peel et al. [27] | 7 | |||||
| Marmor et al. [28] | 9 | 6 TKR, 3 THR | ||||
| Lourtet-Hascoet et al. [29] | 28 | (58-78) | 13 M, 15 F | 4 CVD, 2 cancer, 1 DM, 1 rheumatoid disease | 3-56 wk | 16 TKR, 10 THR, 1 foot, 1 shoulder |
| Argemi et al. [30] | 1 | 70 | F | 2 y | TKR |
CVD, cardiovascular disease; DM, diabetes mellitus; F, female; HBV, hepatitis B; M, male; MG, myasthenia gravis; No., number of patients; THR, total hip replacement; TKR, total knee replacement.
The method of treatment could be determined in 69 episodes reported in the literature. Two-stage revision was used in 30 episodes (43.5%), debridement, antibiotics, and implant retention (DAIR) in 24 episodes (34.8%), and 1-stage revision in 9 episodes (13%). However, comparison of the success rate of different strategies was not possible because of the lack of universal definition of a successful treatment and the scarcity of information regarding the success of treatment of each strategy. In the absence of clear recommendations for optimal surgical management of S lugdunensis PJI and bearing in mind its similar virulence, it should be treated as S aureus rather than CoNS [29].
In a report of 38 PJI patients infected with staphylococcal SCVs, 3 patients were infected by S lugdunensis SCV [15]. More recently, a case of normal variant S lugdunensis PJI who was initially treated by DAIR, developed a persistent infection 1 year later, and SCVs were isolated [30]. This highlights the importance of not underestimating the pathogenicity of S lugdunensis. Unlike our case, emergence of SCV in the aforementioned case was most likely due to prior antibiotic use after the DAIR procedure (ofloxacin and rifampicin for 3 months). In our case, SCV had similar susceptibilities to antibiotics as the parent strain which could be explained by it not being induced by antibiotics.
In our case, quantitative bacterial growth from the sonicate was approximately 10-fold higher than the tissue homogenate. Sonication of retrieved implants has shown higher diagnostic sensitivity than periprosthetic tissue samples in both conventional and molecular diagnostic methods of PJI [23], [31]. Therefore, we recommend the use of sonication to enhance the detection and identification of the infecting bacteria and their SCVs in particular. In addition, prolonged incubation of aerobic culture to 72 hours and meticulous inspection of the agar plates are necessary to avoid missing SCVs.
Summary
SCVs are hard to detect and identify by microbiologists in comparison to the parent strain. They can also lead to persistent, latent, or recurrent infections. SCVs are more likely to be associated with prolonged antimicrobial use and more chronic symptoms. Surgeons and microbiologists should be alert to the possibility of misidentified organisms or existence of SCVs when unexplained treatment failure happens.
Acknowledgments
The authors thank the patient for consenting for this case report.
Funding: This work was supported by Newton-Mosharafa Fund through the mission sector of the Egyptian Ministry of Higher Education. M.A. is a Clinical Fellow and PhD student whose PhD is supported by the funder.
Availability of data and materials: For the purpose of confidentiality, the original operative reports, laboratory results, and outpatient clinic records are stored in accordance with the UK Data Protection Act 1988. The patient has consented for her data to be published anonymously.
Consent for publication: Written informed consent was obtained from the patient for publication of this case report.
Ethics approval and consent to participate: Informed consent was given by the patient and is available for review by the Editor-in-Chief of the journal. Samples were collected under the regulations and ethical approval of Nottingham Health Science Biobank.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2018.06.003.
Appendix A. Supplementary data
References
- 1.Public Health England . Public Health England; London: 2016. Surveillance of surgical site infections in NHS hospitals in England, 2015/16. [Google Scholar]
- 2.Shah N.B., Osmon D.R., Fadel H. Laboratory and clinical characteristics of Staphylococcus lugdunensis prosthetic joint infections. J Clin Microbiol. 2010;48(5):1600. doi: 10.1128/JCM.01769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balwit J.M., van Langevelde P., Vann J.M., Proctor R.A. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170(4):1033. doi: 10.1093/infdis/170.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Proctor R.A., von Eiff C., Kahl B.C. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4(4):295. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 5.Vandenesch F., Etienne J., Reverdy M.E., Eykyn S.J. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin Infect Dis. 1993;17(5):871. doi: 10.1093/clinids/17.5.871. [DOI] [PubMed] [Google Scholar]
- 6.Freney J., Brun Y., Bes M. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., Two Species from Human Clinical Specimens. Int J Syst Bacteriol. 1988;38(2):168. [Google Scholar]
- 7.Frank K.L., Del Pozo J.L., Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampathkumar P., Osmon D.R., Cockerill F.R., 3rd Prosthetic joint infection due to Staphylococcus lugdunensis. Mayo Clin Proc. 2000;75(5):511. doi: 10.4065/75.5.511. [DOI] [PubMed] [Google Scholar]
- 9.Szabados F., Anders A., Kaase M. Late periprosthetic joint infection due to Staphylococcus lugdunensis identified by matrix-assisted laser desorption/ionisation time of flight mass spectrometry: a case report and review of the literature. Case Rep Med. 2011;2011:608919. doi: 10.1155/2011/608919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seifert H., Oltmanns D., Becker K., Wisplinghoff H., von Eiff C. Staphylococcus lugdunensis pacemaker-related infection. Emerg Infect Dis. 2005;11(8):1283. doi: 10.3201/eid1108.041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Ami R., Navon-Venezia S., Schwartz D., Carmeli Y. Infection of a ventriculoatrial shunt with phenotypically variable Staphylococcus epidermidis masquerading as polymicrobial bacteremia due to various coagulase-negative Staphylococci and Kocuria varians. J Clin Microbiol. 2003;41(6):2444. doi: 10.1128/JCM.41.6.2444-2447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris L.G., El-Bouri K., Johnston S. Rapid identification of staphylococci from prosthetic joint infections using MALDI-TOF mass-spectrometry. Int J Artif Organs. 2010;33(9):568. doi: 10.1177/039139881003300902. [DOI] [PubMed] [Google Scholar]
- 13.Dupont C., Sivadon-Tardy V., Bille E. Identification of clinical coagulase-negative staphylococci, isolated in microbiology laboratories, by matrix-assisted laser desorption/ionization-time of flight mass spectrometry and two automated systems. Clin Microbiol Infect. 2010;16(7):998. doi: 10.1111/j.1469-0691.2009.03036.x. [DOI] [PubMed] [Google Scholar]
- 14.Tan T.Y., Ng S.Y., He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393. doi: 10.1128/JCM.00740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tande A.J., Osmon D.R., Greenwood-Quaintance K.E., Mabry T.M., Hanssen A.D., Patel R. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. MBio. 2014;5(5):e01910. doi: 10.1128/mBio.01910-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaudaux P., Francois P., Bisognano C. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect Immun. 2002;70(10):5428. doi: 10.1128/IAI.70.10.5428-5437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Eiff C., Lindner N., Proctor R.A., Winkelmann W., Peters G. Development of gentamicin-resistant Small Colony Variants of S. aureus after implantation of gentamicin chains in osteomyelitis as a possible cause of recurrenceZ Orthop Ihre Grenzgeb. 1998;136(3):268. [PubMed] [Google Scholar]
- 18.Weightman N.C., Allerton K.E., France J. Bone and prosthetic joint infection with Staphylococcus lugdunensis. J Infect. 2000;40(1):98. doi: 10.1053/jinf.1999.0563. [DOI] [PubMed] [Google Scholar]
- 19.Sanzeni L., Ringberg H. Fistulating periprosthetic Staphylococcus lugdunensis hip infection cured by intra-articular teicoplanin injections–a case report. Acta Orthop Scand. 2003;74(5):624. doi: 10.1080/00016470310018072. [DOI] [PubMed] [Google Scholar]
- 20.Losada I., Rita M., Freire M., Graña G. Relapsing Staphylococcus lugdunensis septic arthritis associated with a knee prosthesisEnferm Infecc Microbiol Clin. 2003;21(4):214. doi: 10.1016/s0213-005x(03)72921-8. [DOI] [PubMed] [Google Scholar]
- 21.Frank K.L., Hanssen A.D., Patel R. icaA is not a useful diagnostic marker for prosthetic joint infection. J Clin Microbiol. 2004;42(10):4846. doi: 10.1128/JCM.42.10.4846-4849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecuire F., Gontier D., Carrere J., Basso M., Benareau I., Rubini J. Joint prosthesis infection with Staphyococcus lugdunensis: 7 casesRev Chir Orthop Reparatrice Appar Mot. 2007;93(1):88. doi: 10.1016/s0035-1040(07)90209-3. [DOI] [PubMed] [Google Scholar]
- 23.Trampuz A., Piper K.E., Jacobson M.J. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 24.Merino P., Arribi A., Gestoso I., Picazo J., Gimeno L., Del Potro E. Linezolid treatment of a prosthetic joint infection with Staphylococcus lugdunensis in a patient with multiple myeloma. Int J Antimicrob Agents. 2010;35(2):203. doi: 10.1016/j.ijantimicag.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Tsaras G., Osmon D.R., Mabry T. Incidence, secular trends, and outcomes of prosthetic joint infection: a population-based study, olmsted county, Minnesota, 1969-2007. Infect Control Hosp Epidemiol. 2012;33(12):1207. doi: 10.1086/668421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campoccia D., Testoni F., Ravaioli S. Orthopedic implant-infections. Incompetence of Staphylococcus epidermidis, Staphylococcus lugdunensis and Enterococcus faecalis to invade osteoblasts. J Biomed Mater Res A. 2016;104(3):788. doi: 10.1002/jbm.a.35564. [DOI] [PubMed] [Google Scholar]
- 27.Peel T.N., Cole N.C., Dylla B.L., Patel R. Matrix-assisted laser desorption ionization time of flight mass spectrometry and diagnostic testing for prosthetic joint infection in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 2015;81(3):163. doi: 10.1016/j.diagmicrobio.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Marmor S., Bauer T., Desplaces N. Multiplex antibody detection for noninvasive genus-level diagnosis of prosthetic joint infection. J Clin Microbiol. 2016;54(4):1065. doi: 10.1128/JCM.02885-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lourtet-Hascoet J., Bicart-See A., Félicé M.P., Giordano G., Bonnet E. Staphylococcus lugdunensis, a serious pathogen in periprosthetic joint infections: comparison to Staphylococcus aureus and Staphylococcus epidermidis. Int J Infect Dis. 2016;51:56. doi: 10.1016/j.ijid.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Argemi X., Dahyot S., Lebeurre J., Hansmann Y., Ronde Oustau C., Prévost G. Staphylococcus lugdunensis small colony variant conversion resulting in chronic prosthetic joint infection. Med Mal Infect. 2017;47(7):498. doi: 10.1016/j.medmal.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Rak M., KavčIč M., Trebše R., CőR A. Detection of bacteria with molecular methods in prosthetic joint infection: sonication fluid better than periprosthetic tissue. Acta Orthop. 2016;87(4):339. doi: 10.3109/17453674.2016.1165558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.