Abstract
Neuroimmune signaling is increasingly identified as a critical component of neuronal processes underlying memory, emotion and cognition. The interactions of microglia and astrocytes with neurons and synapses, and the individual cytokines and immune signaling molecules that mediate these interactions are a current focus of much research. Here, we discuss neuroimmune activation as a mechanism triggering different states that modulate cognitive and affective processes to allow for appropriate behavior during and after illness or injury. We propose that these states lie on a continuum from a naïve homeostatic baseline state in the absence of stimulation, to acute neuroimmune activity and chronic activation. Importantly, consequences of illness or injury including cognitive deficits and mood impairments can persist long after resolution of immune signaling. This suggests that neuroimmune activation also results in an enduring shift in the homeostatic baseline state with long lasting consequences for neural function and behavior. Such different states can be identified in a multidimensional way, using patterns of cytokine and glial activation, behavioral and cognitive changes, and epigenetic signatures. Identifying distinct neuroimmune states and their consequences for neural function will provide a framework for predicting vulnerability to disorders of memory, cognition and emotion both during and long after recovery from illness.
Keywords: neuroimmune, learning and memory, vulnerability, persistent changes, cognition, brain states
Introduction
Behavioral states or brain states are defined as co-ordinated patterns of activity in the brain. Whether that state is a “feeling” (philosophical, Oosterwijk et al., 2012), a systems/circuit activation (Quilichini and Bernard, 2012), or a pattern of kinase activity (Tronson et al., 2012; Mucic et al., 2015), brain states can be thought of as a snapshot of what is happening in the brain in that moment. By adjusting to the precise current conditions of the animal’s environment, changes in state are essential for efficient processing of information relevant to that place and time, and as such, are accompanied by changes in information processing in the hippocampus (Anderson et al., 2014) and cortex (Tsuno and Mori, 2009). In this review, we discuss neuroimmune activity as a continuum of states—including a baseline homeostatic state, acute activation, chronic low-grade activation and a “vulnerable” state that persists long after a major illness or injury—that interact with neural function and cognition.
The Immune System: A Primer
In the periphery, the immune system is divided into two interrelated but separable systems—the innate immune system and the adaptive immune system. The innate immune system enacts the rapid response to infectious agents and injury via specialized receptors that recognize viruses (double stranded RNA), bacteria (e.g., lipopolysaccharides, LPS), or nuclear and cytosolic proteins that are released during cellular damage. The innate immune response is characterized by an expansion and subsequent resolution of cytokine production. The initial response includes cytokines (e.g., interleukins IL-1β, IL-6; tumor necrosis factor α (TNFα), interferon γ (IFN γ)) and chemokines (e.g., CX3CL1, CSF1-3) that recruit additional immune cells, and increase transcription and production of cytokines. This also increases production of regulatory cytokines (e.g., IL-4, IL-10) that, together with intracellular signaling proteins (e.g., SOCS, suppressor of cytokine signaling; PIAS1) suppress cytokine production and immune cell recruitment, thereby resolving the immune response (Wang and Campbell, 2002; Colton, 2009; Schmitz et al., 2011; Becher et al., 2017; Prieto and Cotman, 2017). The adaptive immune system is, in turn, activated by the innate immune response, with a slower response and resulting in the generation of antibodies to the infectious pathogen (Janeway, 2001).
Neuroimmune processes are activated during peripheral illness due to vagal nerve activity and immune signals that cross the blood brain barrier (McCusker and Kelley, 2013), and as a consequence of neural injury or infection. The neuroimmune response in the brain is predominantly considered an innate immune response, however components of the adaptive immune system in the meningeal compartment, including T-cells, are critical for normal neural function (Kipnis et al., 2012), cognitive function, and production of cytokines in the brain during illness or injury (Marin and Kipnis, 2017). Acute activation of neuroimmune processes function to both heal neural injury and attack infection in the brain (Kreutzberg, 1996; Berczi et al., 2009; Kawabori and Yenari, 2015; DiSabato et al., 2016; Sochocka et al., 2017), and as an important adaptive process, triggering sickness behaviors and physiological responses that allow the peripheral immune system to perform at optimal levels (McCusker and Kelley, 2013).
Naïve Homeostatic Baseline State
Although we commonly refer to illness or injury as triggering “activation” of the immune and neuroimmune systems, the naïve homeostatic baseline is not the absence of neuroimmune signaling or activity. Instead, astrocytes and microglia constantly interact with neurons and play active roles in regulation of neural function and synaptic plasticity. Astrocytes provide energy and regulate glutamate during synaptic transmission and plasticity (Suzuki et al., 2011; Gold, 2014; Nortley and Attwell, 2017; Alberini et al., 2018). Microglia provide continuous surveillance, synaptic pruning, and regulation of synaptic plasticity via complement and cytokine interactions (Nimmerjahn et al., 2005; Wu et al., 2015; Lenz and Nelson, 2018; Pósfai et al., 2018). Importantly, neurons produce and have receptors for “immune” proteins including cytokines, chemokines and complement, thereby providing a basis for ongoing communication with glial cells (Freidin et al., 1992; Veerhuis et al., 2011; McCusker and Kelley, 2013; Paolicelli et al., 2014).
Cytokines and chemokines are also produced during this baseline state and during non-inflammatory stimulation such as learning and induction of long-term plasticity (Jankowsky et al., 2000; del Rey et al., 2013). These cytokines play both permissive (e.g, IL-1β; (Goshen et al., 2007); IL-4 (Gadani et al., 2012)) and suppressive (e.g., IL-1β (Avital et al., 2003; Goshen et al., 2007); IL-6 (Balschun et al., 2004)) roles in synaptic plasticity and memory formation during adulthood. Rather than the (neuro)immune system as existing as either “off” or “on,” it is more accurately conceptualized as a continuum of activity from a homeostatic baseline state, through undetectable response to cellular stress, up through low-grade activity, and occasionally a fully active disease or acute inflammatory state (e.g., Chovatiya and Medzhitov, 2014; Marques et al., 2016). In accordance with this view and consistent with the growing evidence for a critical role of immune cells and signaling in normal memory, affective and cognitive processes, the neuroimmune system is not inactive in the absence of inflammatory stimulation, but instead engages in ongoing interactions with synapses, neurons and circuits in the brain (Capuron and Miller, 2011; McCusker and Kelley, 2013; Donzis and Tronson, 2014; Wu et al., 2015; Tronson and Collette, 2017; Dantzer, 2018).
Nevertheless, the levels of cytokines (Erickson and Banks, 2011; Biesmans et al., 2013; Speirs and Tronson, 2018) and cytokine expression (Skelly et al., 2013) in the brain during non-stimulated conditions are very low, often at concentrations that are barely detectable (Erickson and Banks, 2011; Biesmans et al., 2013). During activation by LPS, for example, whereas cytokines in serum can be hundreds or thousands fold higher than baseline, in the brain, these changes are limited to 1.5–10 fold range (Erickson and Banks, 2011; Biesmans et al., 2013; Speirs and Tronson, 2018) with occasional chemokines showing hundreds of fold increases (e.g., CXCL10, CSF3; Speirs and Tronson, 2018). The difficulty in measuring immune signaling in the brain (and in the periphery, Chovatiya and Medzhitov, 2014) in the baseline state therefore makes it difficult to assess the precise roles in these baseline processes.
Acute Neuroimmune Activation State
Acute immune activation, whether by illness, injury, or experimental administration of LPS or other immune trigger, results in the induction of broad networks of cytokines and immune molecules, including complement, IL-1β, IL-6 and TNFα, followed by the regulatory cytokines (IL-10, IL-4) and downstream signaling pathways (Donzis and Tronson, 2014).
That the activation of the neuroimmune system drives a unique brain and behavioral state is clear from the distinct consequences of illness on behavior, cognition, emotion and learning and memory (Dantzer et al., 1998; Yirmiya and Goshen, 2011; Donzis and Tronson, 2014). The roles of cytokines and immune molecules in the modulation of memory has been a particular point of recent interest (Pugh et al., 1998; Marin and Kipnis, 2013; del Rey et al., 2013). Elevations of IL-1β results in disruption of memory and synaptic plasticity (Cunningham et al., 1996; Ross et al., 2003; Goshen et al., 2007; Gonzalez et al., 2013); and IL-6 activation acts as a brake on plasticity (Balschun et al., 2004; Sparkman et al., 2006). In addition to cytokines, other immune signaling pathways including complement signaling are critical for neuroimmune activation (Jacob et al., 2007), physiological effects including fever (Boos et al., 2005) and synaptic plasticity (Boulanger, 2009; Bitzer-Quintero and González-Burgos, 2012; Zhang et al., 2014). For example, the complement proteins are produced by neurons and microglia during infection, triggering microglial infiltration and activation, loss of hippocampal synapses and spatial memory impairments during West Nile Virus infection, effects prevented by knockout of the C3 or blockade of C1qa complement production (Vasek et al., 2016). Immune signaling molecules are therefore a defining feature, and drive outcomes of, the acute neuroimmune activation state.
Morphological and transcriptional changes of microglia and astrocytes are also indicative of neuroimmune activation. Activated microglia show a shift in morphology from a ramified, surveying state to an ameboid state by withdrawing long, thin processes and extending short, thick, phagocytic processes towards the source (Stence et al., 2001). Similarly, astrocytes have several distinct “reactive” transcriptional profiles in response to immune stimulation (Zamanian et al., 2012; Liddelow and Barres, 2017). Activated microglia are a primary source of cytokine and chemokine production during immune events (Colonna and Butovsky, 2017), and thereby alter neuronal-glia communication (Tremblay et al., 2011; Paolicelli et al., 2014). Indeed, inhibition of microglial activity prevents immune challenge-induced impairments in memory, suggesting a requirement for these neuroimmune cells in cognitive deficits during acute neuroimmune activity (Yang et al., 2015; Vasek et al., 2016; Wang et al., 2016; Wadhwa et al., 2017).
The role of a small number of cytokines, notably IL-1β, IL-6 and IL-4, in modulating behavior, cognition and memory processes is well established. Nevertheless, the functional role of specific immune signaling molecules depends on the current immune milieu and the specific immune challenge. For example, IFNγ can enhance immune signaling as observed in models of cerebral malaria and after LPS, or downregulate inflammatory responses in the brain, as in experimental allergic encephalomyelitis (Heremans et al., 1989). Similarly, the role of IL-1 in the brain differs between neurodegenerative and neuroinflammatory states, and depends on the subsets of immune cells and cytokines present (Becher et al., 2017). The precise activation state and transcriptional profile of immune cells also differs depending on the type of immune stimulation, with different immune triggers resulting in different patterns of gene expression and cytokine signaling (Chovatiya and Medzhitov, 2014; Becher et al., 2017; Prieto and Cotman, 2017). This means that in considering the individual roles of cytokines in modulation of neural processes, we also need to bear in mind the broader context of what other cytokines and immune cells are concurrently present.
A special subset of neuroimmune activation is chronic inflammation. As with acute immune activation, chronic inflammation is defined by persistent activation of cytokines and glial cells, and results in changes in behavior and neural function. In models of sepsis, for example, microglial activation persists for weeks or months after surgery, presumably contributing to persistent cognitive deficits (Weberpals et al., 2009; Singer et al., 2016; Olivieri et al., 2018). Similarly, after traumatic brain injury, there is long-term inflammatory activity (Gentleman et al., 2004). In patients of arthritis and periodontal disease, chronic low-grade inflammation is associated with cognitive deficits, depression and increased risk for Alzheimer’s disease (Kamer et al., 2008; Chou et al., 2016; Simos et al., 2016), and animal models suggest a causal link between chronic inflammatory conditions and altered cognitive function and increased depression-like behaviors (Brown et al., 2018; Ding et al., 2018). Long-term increases in cytokine levels in the brain likely result in a notably different cytokine network and therefore a brain states that is distinct from acute neuroimmune activation states.
Collectively, these studies point to neuroimmune activation as being crucial for determining the profile and function of neural activity that mediates changes in behavior, cognition, affect and memory. Furthermore, the importance of specific patterns of immune signaling on cognition and behavior suggests that acute neuroimmune activation is not one, but multiple distinct brain state, depending on the specific immune trigger and, as described below, previous immune experience.
Persistent Changes in Brain State After Immune Challenge
A defining feature of the peripheral immune system is that acute activation results in permanent changes to immune function that persist after resolution of inflammatory signaling. The best-known example of this is the adaptive immune system, which becomes able to produce new antibodies after exposure to a pathogen (Janeway, 2001). Independently, the innate immune system also shows “trained” immunity, in which a transient immune challenge results in increased responsiveness to subsequent immune stimuli (Netea and van der Meer, 2017). Such long-lasting changes may be thought of as a distinct, shifted baseline state that results in functional adaptation for subsequent illnesses. If the innate immune system in the periphery encodes experiences as long-lasting changes in function, it is likely that persistent changes are also encoded in the neuroimmune system.
Permanent changes in neuroimmune function have been observed after prenatal or early life immune challenge in and effect termed “microglia priming.” Here maternal immune challenge during pregnancy or early life immune challenge result in increased responsiveness of microglia to immune challenge during adulthood (Schwarz and Bilbo, 2012; Bilbo, 2013; Haley et al., 2017). More recently, several studies demonstrate similar priming effects after immune challenge in adults, with changes in microglial and astrocytic function and behavior (Fenn et al., 2014; Norden et al., 2015; Muccigrosso et al., 2016; Liddelow and Barres, 2017; Olivieri et al., 2018; Wendeln et al., 2018). Importantly, these effects persist even when the pathogenic process is eliminated long before. By altering interactions between neurons and glial cells and the quantity and specific patterns of cytokines produced (ŠiŠková and Tremblay, 2013; Wendeln et al., 2018), prior illness has a lasting impact on the effect of subsequent acute immune challenge. For example, an LPS challenge results in altered gene expression and increased cytokines in response to stroke at least 6 months later, suggesting persistent changes in neuroimmune function even when animals have shown a full recovery (Wendeln et al., 2018).
Long-lasting functional changes are mediated by epigenetic modifications triggered by immune activation. In the periphery, methylation of histone H3 mediates increased innate immune responses (Kleinnijenhuis et al., 2012). Similarly, in the brain, acetylation and methylation of H3 are associated with altered astrocyte and microglial function after immune challenge (Schaafsma et al., 2015; Haley et al., 2017; Wendeln et al., 2018). Together, these findings demonstrate that a prior immune challenge causes persistent changes in neuroimmune function, or neuroimmune “training,” and results in vulnerability to later immune events.
One question arising from these lasting changes in neuroimmune function is whether there are also changes in the homeostatic baseline state of the brain. If so, transient illness may cause not only the initial acute immune state, but also a new permanently altered brain state. There are several pieces of evidence that support changes in brain function that persist long after the resolution of immune challenge. In addition to altered neuroimmune reactivity, peripheral illness can result in decreased neuronal connectivity, synaptic spines and plasticity (Kondo et al., 2011; Maggio et al., 2013; Huerta et al., 2016). Alternatively, long-lasting increases in permeability of blood-brain barrier may also contribute to persistent changes in baseline homeostatic state of the brain (Saunders et al., 2008; Strbian et al., 2008). Consistent with these data, we have recently observed memory impairments that emerge and persist months after an immune challenge (Tchessalova and Tronson, 2018). Changes in neuronal connectivity, neuroimmune function, and their interactions mediate what and how information is processed, and contribute to persistent changes in cognitive function, mood and memory observed after illness or injury.
Collectively, these data suggest that rather than returning to the original homeostatic baseline state after resolution of an immune challenge, the brain reaches a new homeostatic state with different neuronal connections, a “trained” neuroimmune system, and epigenetic modifications that lead to dysregulated gene expression (Figure 1). A shift in baseline state and neuronal connectivity could result in changes to neural and neuroimmune responses to environmental stimuli, including immune and other sensory information, and thus altered cognitive processes and vulnerability to memory, affective and neurodegenerative disorders.
Figure 1.
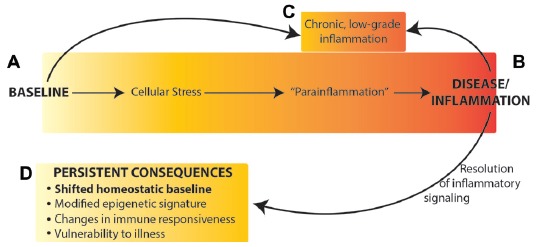
Neuroimmune activation occurs along a continuum from the naïve (homeostatic) baseline (A), to an active inflammatory state (B) or chronic inflammation (C). We propose that resolution of inflammatory signaling does not result in return to the original baseline, but rather results in persistently altered homeostatic baseline (D) mediated by epigenetic changes in the brain. Figure adapted from Chovatiya and Medzhitov (2014).
Discussion and Conclusions
The work reviewed here clearly demonstrates that neuroimmune activation modulates neural and cognitive function both acutely and with effects that persist long after recovery from illness and the resolution of a transient immune response. There is growing evidence for the role of neuroimmune signaling in both normal and pathological memory, cognitive and affective processes. In particular, the role of individual cytokines in the regulation of synaptic plasticity and memory, and the dysregulation during an immune response, has been highlighted by recent research. Importantly, activation of neuroimmune signaling is more complex than single effectors. Instead, the neuroimmune response includes activation of glial cells, cytokines and many other immune signaling molecules in a co-ordinated manner (Schmitz et al., 2011; Becher et al., 2017; Prieto and Cotman, 2017), which in turn causes a distinct set of regulatory cytokines and signaling molecules that mediate resolution of the response (McCusker and Kelley, 2013). This activation and resolution cycle further results in long lasting epigenetic modifications and changes in neuronal connectivity that persist and modulate cognitive function and behavior long after the end of the immune challenge. We therefore propose that neuroimmune activation can be viewed as a continuum of states in the brain: from acute or chronic activation, to resolution, to persistent changes in baseline homeostatic state(s), each with distinctive patterns of glial cell activation, cytokine and immune signaling, epigenetic modifications, and behavioral change (Figure 1).
How to Define Neuroimmune “States”?
An important unresolved question is how to define neuroimmune states? In general, neuroimmune activation is commonly defined by behavioral changes, by the activation of microglia, or by increased level or expression of immune molecules, in particular cytokines and chemokines (Dantzer et al., 1998; McCusker and Kelley, 2013; del Rey et al., 2013; Becher et al., 2017). Behavioral changes, notably sickness behaviors and associated febrile response and weight loss, are commonly used to assess immune (and neuroimmune) activation. Yet sickness responses are not specific to immune trigger—as noted by McCusker and Kelley (2013), “symptoms are commonly expressed by sick animals despite the broad spectrum of possible pathogens.” Therefore, sickness behaviors are not sensitive enough to the precise neuroimmune state of the brain beyond “on” and “off”. Morphological changes of microglia are useful for defining when the neuroimmune system is active but is likely not sufficient for identifying changes in homeostatic baseline or other long lasting, low-grade changes. Alternatively, identifying the precise patterns of cytokines expressed and neuroimmune cells recruited after an immune challenge allows for a detailed definition of different active states. The two disadvantages of this being the sole approach to identifying state, however, are: (1) that levels of cytokines at baseline are very low, thus it is difficult to clearly identify what is happening in the absence of acute neuroimmune activation; and (2) if broad patterns of cytokines define a state it is not sufficient to simply measure a small number of cytokines, and this approach rapidly becomes cumbersome and expensive. Thus, in order to use cytokines and immune signaling to define states, it will be critical to identify a smaller cluster of key proteins distinct for each state. Importantly, behavior, glial activity and cytokine levels are expected to revert to very low baseline (or close to baseline) levels after a transient immune challenge, even if there is a shift in the homeostatic baseline state (Figure 1).
Alternatively, defining an epigenetic landscape or signature has been one approach for defining states (Bonasio et al., 2010; Tronson et al., 2012; Nicol-Benoit et al., 2013). Here, unique epigenetic signatures of histone acetylation, phosphorylation and methylation and DNA methylation during different forms of neuroimmune activation, and that persist after resolution of immune signaling will better define the multiple acute and long-lasting brain states that can be induced by neuroimmune signaling (Figure 2). Indeed, epigenetic modifications and the resulting changes in gene expression profiles likely both define the state and drive the distinct patterns of signaling, behavior and cognitive changes that mediate.
Figure 2.
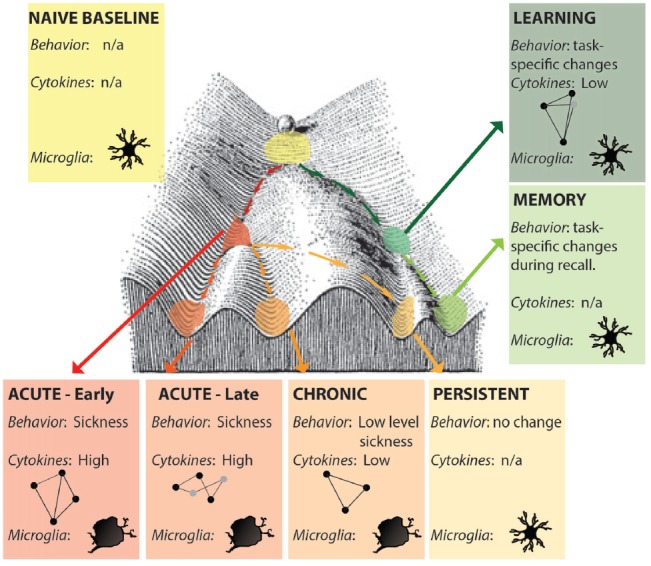
Distinct neuroimmune states. Each state is a unique snapshot of behavior, immune signaling, cellular changes, active cytokine networks and epigenetic signature at that time. Early- and late- acute immune states are distinguished by activation of different cytokine networks (represented by ball-and-stick figures); microglia show activated morphologies only during acute and chronic activation states; persistent changes are indistinguishable from naïve baseline in the absence of additional stimulation. Learning and memory may also be considered distinct neuroimmune (and epigenetic) states. Figure adapted from Waddington (1957).
Implications of “Neuroimmune States” for Cognition and Behavior
There are several advantages to defining immune activation and post-activation as brain states. This view provides a framework to go beyond the idea of neuroimmune system as “on” or “off,” and towards an understanding of multiple active immune states such as those mediating different behavioral outcomes of bacterial or viral infections and provides a way to conceptualize sustained changes that persist long after resolution of acute neuroimmune activity.
There is clear evidence for roles of individual cytokines on cognition, memory and affective processes. Indeed, the activation of specific cytokine networks during learning suggests that, in addition to acute, chronic, and persistent states, neuroimmune signaling during learning and other cognitive tasks may be unique states themselves (Figure 2) that result in distinct epigenetic changes that mediate memory storage (Li et al., 2013). In addition, acute neuroimmune activation critically modulates memory and plasticity, yet depending on circumstances, it can either impair (Pugh et al., 1998; Goshen et al., 2007; Cross-Mellor et al., 2009; Cloutier et al., 2012) or enhance (Goshen et al., 2007; Mori et al., 2014; Delpech et al., 2015) memory and cognitive function. One important factor here is that the impact of immune signaling on cognition and behavior depends on the context of the specific immune milieu at this time (Becher et al., 2017). Indeed, in the periphery, determining the precise immune state can aid in minimizing side effects of immunologic treatments for systemic disease (Morel et al., 2017). By identifying distinct neuroimmune states, we will be better able to predict the effects of illness, immune activation, and specific cytokines on neuronal-glia interactions, plasticity and functional changes in cognition and behavior.
Finally, identifying persistent changes and shifts in the homeostatic baseline state as a consequence of illness or injury will provide a predictive marker for vulnerability or resilience to stress (Fonken et al., 2018), immune challenges (Norden et al., 2015; Muccigrosso et al., 2016), and neurodegeneration (Perry and Holmes, 2014; Hoeijmakers et al., 2016; McManus and Heneka, 2017) later in life. This is a particularly important avenue for research into individual differences and sex differences in susceptibility to immune-related disorders of cognition, memory and emotion (Perry et al., 2016; Snyder et al., 2016; Tronson and Collette, 2017; Choleris et al., 2018; Fisher et al., 2018; Speirs and Tronson, 2018).
Author Contributions
DT and CP wrote the manuscript. NT developed the initial concept, wrote the discussion and edited the manuscript. All three authors contributed to the figures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alberini C. M., Cruz E., Descalzi G., Bessières B., Gao V. (2018). Astrocyte glycogen and lactate: new insights into learning and memory mechanisms. Glia 66, 1244–1262. 10.1002/glia.23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. B., Grossrubatscher I., Frank L. (2014). Dynamic hippocampal circuits support learning- and memory-guided behaviors. Cold Spring Harb. Symp. Quant. Biol. 79, 51–58. 10.1101/sqb.2014.79.024760 [DOI] [PubMed] [Google Scholar]
- Avital A., Goshen I., Kamsler A., Segal M., Iverfeldt K., Richter-Levin G., et al. (2003). Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13, 826–834. 10.1002/hipo.10135 [DOI] [PubMed] [Google Scholar]
- Balschun D., Wetzel W., del Rey A., Pitossi F., Schneider H., Zuschratter W., et al. (2004). Interleukin-6: a cytokine to forget. FASEB J. 18, 1788–1790. 10.1096/fj.04-1625fje [DOI] [PubMed] [Google Scholar]
- Becher B., Spath S., Goverman J. (2017). Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 17, 49–59. 10.1038/nri.2016.123 [DOI] [PubMed] [Google Scholar]
- Berczi I., Quintanar-Stephano A., Kovacs K. (2009). Neuroimmune regulation in immunocompetence, acute illness and healing. in. Ann. N Y Acad. Sci. 1153, 220–239. 10.1111/j.1749-6632.2008.03975.x [DOI] [PubMed] [Google Scholar]
- Biesmans S., Meert T. F., Bouwknecht J. A., Acton P. D., Davoodi N., De Haes P., et al. (2013). Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm. 2013:271359. 10.1155/2013/271359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo S. D. (2013). Frank A. Beach award: programming of neuroendocrine function by early-life experience: a critical role for the immune system. Horm. Behav. 63, 684–691. 10.1016/j.yhbeh.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer-Quintero O. K., González-Burgos I. (2012). Immune system in the brain: a modulatory role on dendritic spine morphophysiology? Neural Plast. 2012:348642. 10.1155/2012/348642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasio R., Tu S., Reinberg D. (2010). Molecular signals of epigenetic states. Science 330, 612–616. 10.1126/science.1191078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos L., Szalai A. J., Barnum S. R. (2005). C3a expressed in the central nervous system protects against LPS-induced shock. Neurosci. Lett. 387, 68–71. 10.1016/j.neulet.2005.07.015 [DOI] [PubMed] [Google Scholar]
- Boulanger L. M. (2009). Immune proteins in brain development and synaptic plasticity. Neuron 64, 93–109. 10.1016/j.neuron.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Brown E., Mc Veigh C. J., Santos L., Gogarty M., Müller H. K., Elfving B., et al. (2018). TNFα-dependent anhedonia and upregulation of hippocampal serotonin transporter activity in a mouse model of collagen-induced arthritis. Neuropharmacology 137, 211–220. 10.1016/j.neuropharm.2018.04.023 [DOI] [PubMed] [Google Scholar]
- Capuron L., Miller A. H. (2011). Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 130, 226–238. 10.1016/j.pharmthera.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E., Galea L. A. M., Sohrabji F., Frick K. M. (2018). Sex differences in the brain: implications for behavioral and biomedical research. Neurosci. Biobehav. Rev. 85, 126–145. 10.1016/j.neubiorev.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R. C., Kane M., Ghimire S., Gautam S., Gui J. (2016). Treatment for rheumatoid arthritis and risk of Alzheimer’s disease: a nested case-control analysis. CNS Drugs 30, 1111–1120. 10.1007/s40263-016-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovatiya R., Medzhitov R. (2014). Stress, inflammation and defense of homeostasis. Mol. Cell 54, 281–288. 10.1016/j.molcel.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier C. J., Kavaliers M., Ossenkopp K.-P. (2012). Lipopolysaccharide inhibits the simultaneous establishment of LiCl-induced anticipatory nausea and intravascularly conditioned taste avoidance in the rat. Behav. Brain Res. 232, 278–286. 10.1016/j.bbr.2012.04.021 [DOI] [PubMed] [Google Scholar]
- Colonna M., Butovsky O. (2017). Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35, 441–468. 10.1146/annurev-immunol-051116-052358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C. A. (2009). Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418. 10.1007/s11481-009-9164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Mellor S. K., Foley K. A., Parker L. A., Ossenkopp K.-P. (2009). Lipopolysaccharide dose dependently impairs rapid toxin (LiCl)-induced gustatory conditioning: a taste reactivity examination of the conditioned taste aversion. Brain Behav. Immun. 23, 204–216. 10.1016/j.bbi.2008.09.006 [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Murray C. A., O’Neill L. A., Lynch M. A., O’Connor J. J. (1996). Interleukin-1β (IL-1β) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci. Lett. 203, 17–20. 10.1016/0304-3940(95)12252-4 [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2018). Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 98, 477–504. 10.1152/physrev.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., Bluthé R. M., Layé S., Bret-Dibat J. L., Parnet P., Kelley K. W. (1998). Cytokines and sickness behavior. Ann. N Y Acad. Sci. 840, 586–590. 10.1111/j.1749-6632.1998.tb09597.x [DOI] [PubMed] [Google Scholar]
- Delpech J.-C., Saucisse N., Parkes S. L., Lacabanne C., Aubert A., Casenave F., et al. (2015). Microglial activation enhances associative taste memory through purinergic modulation of glutamatergic neurotransmission. J. Neurosci. 35, 3022–3033. 10.1523/JNEUROSCI.3028-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A., Balschun D., Wetzel W., Randolf A., Besedovsky H. O. (2013). A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6 and TNFα operates during long-term potentiation and learning. Brain Behav. Immun. 33, 15–23. 10.1016/j.bbi.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Ding Y., Ren J., Yu H., Yu W., Zhou Y. (2018). Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun. Ageing 15:6. 10.1186/s12979-017-0110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSabato D. J., Quan N., Godbout J. P. (2016). Neuroinflammation: the devil is in the details. J. Neurochem. 139, 136–153. 10.1111/jnc.13607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzis E. J., Tronson N. C. (2014). Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 115, 68–77. 10.1016/j.nlm.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M. A., Banks W. A. (2011). Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav. Immun. 25, 1637–1648. 10.1016/j.bbi.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn A. M., Gensel J. C., Huang Y., Popovich P. G., Lifshitz J., Godbout J. P. (2014). Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol. Psychiatry 76, 575–584. 10.1016/j.biopsych.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. W., Bennett D. A., Dong H. (2018). Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiol. Aging [Epub ahead of print]. 10.1016/j.neurobiolaging.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken L. K., Frank M. G., Gaudet A. D., D’Angelo H. M., Daut R. A., Hampson E. C., et al. (2018). Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav. Immun. 70, 257–267. 10.1016/j.bbi.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidin M., Bennett M. V., Kessler J. A. (1992). Cultured sympathetic neurons synthesize and release the cytokine interleukin 1 beta. Proc. Natl. Acad. Sci. U S A 89, 10440–10443. 10.1073/pnas.89.21.10440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S. P., Cronk J. C., Norris G. T., Kipnis J. (2012). IL-4 in the brain: a cytokine to remember. J. Immunol. 189, 4213–4219. 10.4049/jimmunol.1202246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman S. M., Leclercq P. D., Moyes L., Graham D. I., Smith C., Griffin W. S. T., et al. (2004). Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci. Int. 146, 97–104. 10.1016/j.forsciint.2004.06.027 [DOI] [PubMed] [Google Scholar]
- Gold P. E. (2014). Regulation of memory–From the adrenal medulla to liver to astrocytes to neurons. Brain Res. Bull. 105, 25–35. 10.1016/j.brainresbull.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P., Machado I., Vilcaes A., Caruso C., Roth G. A., Schiöth H., et al. (2013). Molecular mechanisms involved in interleukin 1-beta (IL-1β)-induced memory impairment. Modulation by alpha-melanocyte-stimulating hormone (α-MSH). Brain Behav. Immun. 34, 141–150. 10.1016/j.bbi.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Goshen I., Kreisel T., Ounallah-Saad H., Renbaum P., Zalzstein Y., Ben-Hur T., et al. (2007). A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 32, 1106–1115. 10.1016/j.psyneuen.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Haley M. J., Brough D., Quintin J., Allan S. M. (2017). Microglial priming as trained immunity in the brain. Neuroscience [Epub ahead of print]. 10.1016/j.neuroscience.2017.12.039 [DOI] [PubMed] [Google Scholar]
- Heremans H., Dillen C., Dijkmans R., Grau G., Billiau A. (1989). The role of cytokines in various animal models of inflammation. Lymphokine Res. 8, 329–333. [PubMed] [Google Scholar]
- Hoeijmakers L., Heinen Y., van Dam A.-M., Lucassen P. J., Korosi A. (2016). Microglial priming and Alzheimer’s disease: a possible role for (early) immune challenges and epigenetics? Front. Hum. Neurosci. 10:398. 10.3389/fnhum.2016.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta P. T., Robbiati S., Huerta T. S., Sabharwal A., Berlin R. A., Frankfurt M., et al. (2016). Preclinical models of overwhelming sepsis implicate the neural system that encodes contextual fear memory. Mol. Med. [Epub ahead of print]. 10.2119/molmed.2015.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A., Hensley L. K., Safratowich B. D., Quigg R. J., Alexander J. J. (2007). The role of the complement cascade in endotoxin-induced septic encephalopathy. Lab. Investig. 87, 1186–1194. 10.1038/labinvest.3700686 [DOI] [PubMed] [Google Scholar]
- Janeway C. A. (2001). How the immune system works to protect the host from infection: a personal view. Proc. Natl. Acad. Sci. U S A 98, 7461–7468. 10.1073/pnas.131202998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky J. L., Derrick B. E., Patterson P. H. (2000). Cytokine responses to LTP induction in the rat hippocampus: a comparison of in vitro and in vivo techniques. Learn. Mem. 7, 400–412. 10.1101/lm.32600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer A. R., Dasanayake A. P., Craig R. G., Glodzik-Sobanska L., Bry M., de Leon M. J. (2008). Alzheimer’s disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. J. Alzheimers Dis. 13, 437–449. 10.3233/JAD-2008-13408 [DOI] [PubMed] [Google Scholar]
- Kawabori M., Yenari M. A. (2015). The role of the microglia in acute CNS injury. Metab. Brain Dis. 30, 381–392. 10.1007/s11011-014-9531-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J., Gadani S., Derecki N. C. (2012). Pro-cognitive properties of T cells. Nat. Rev. Immunol. 12, 663–669. 10.1038/nri3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L. A. B., Ifrim D. C., Saeed S., et al. (2012). Bacille calmette-guerin induces nod2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U S A 109, 17537–17542. 10.1073/pnas.1202870109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Kohsaka S., Okabe S. (2011). Long-term changes of spine dynamics and microglia after transient peripheral immune response triggered by LPS in vivo. Mol. Brain 4:27. 10.1186/1756-6606-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg G. W. (1996). Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318. 10.1016/0166-2236(96)10049-7 [DOI] [PubMed] [Google Scholar]
- Lenz K. M., Nelson L. H. (2018). Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 9:698. 10.3389/fimmu.2018.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wei W., Ratnu V. S., Bredy T. W. (2013). On the potential role of active DNA demethylation in establishing epigenetic states associated with neural plasticity and memory. Neurobiol. Learn. Mem. 105, 125–132. 10.1016/j.nlm.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Liddelow S. A., Barres B. A. (2017). Reactive astrocytes: production, function and therapeutic potential. Immunity 46, 957–967. 10.1016/j.immuni.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Maggio N., Shavit-Stein E., Dori A., Blatt I., Chapman J. (2013). Prolonged systemic inflammation persistently modifies synaptic plasticity in the hippocampus: modulation by the stress hormones. Front. Mol. Neurosci. 6:46. 10.3389/fnmol.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin I., Kipnis J. (2013). Learning and memory … and the immune system. Learn. Mem. 20, 601–606. 10.1101/lm.028357.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin I. A., Kipnis J. (2017). Central nervous system: (immunological) ivory tower or not? Neuropsychopharmacology 42, 28–35. 10.1038/npp.2016.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques R. E., Marques P. E., Guabiraba R., Teixeira M. M. (2016). Exploring the homeostatic and sensory roles of the immune system. Front. Immunol. 7:125. 10.3389/fimmu.2016.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker R. H., Kelley K. W. (2013). Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 216, 84–98. 10.1242/jeb.073411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus R. M., Heneka M. T. (2017). Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res. Ther. 9:14. 10.1186/s13195-017-0241-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P. A., Lee R. E. C., Faeder J. R. (2017). Demystifying the cytokine network: mathematical models point the way. Cytokine 98, 115–123. 10.1016/j.cyto.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F., Nisticò R., Mandolesi G., Piccinin S., Mango D., Kusayanagi H., et al. (2014). Interleukin-1β promotes long-term potentiation in patients with multiple sclerosis. Neuromolecular Med. 16, 38–51. 10.1007/s12017-013-8249-7 [DOI] [PubMed] [Google Scholar]
- Muccigrosso M. M., Ford J., Benner B., Moussa D., Burnsides C., Fenn A. M., et al. (2016). Cognitive deficits develop 1month after diffuse brain injury and are exaggerated by microglia-associated reactivity to peripheral immune challenge. Brain Behav. Immun. 54, 95–109. 10.1016/j.bbi.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucic G., Sase S., Stork O., Lubec G., Li L. (2015). Networks of protein kinases and phosphatases in the individual phases of contextual fear conditioning in the C57BL/6J mouse. Behav. Brain Res. 280, 45–50. 10.1016/j.bbr.2014.11.024 [DOI] [PubMed] [Google Scholar]
- Netea M. G., van der Meer J. W. M. (2017). Trained immunity: an ancient way of remembering. Cell Host Microbe 21, 297–300. 10.1016/j.chom.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Nicol-Benoit F., Le Goff P., Michel D. (2013). Drawing a Waddington landscape to capture dynamic epigenetics. Biol. Cell 105, 576–584. 10.1111/boc.201300029 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Norden D. M., Muccigrosso M. M., Godbout J. P. (2015). Microglial priming and enhanced reactivity to secondary insult in aging and traumatic CNS injury and neurodegenerative disease. Neuropharmacology 96, 29–41. 10.1016/j.neuropharm.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nortley R., Attwell D. (2017). Control of brain energy supply by astrocytes. Curr. Opin. Neurobiol. 47, 80–85. 10.1016/j.conb.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Olivieri R., Michels M., Pescador B., Ávila P., Abatti M., Cucker L., et al. (2018). The additive effect of aging on sepsis-induced cognitive impairment and neuroinflammation. J. Neuroimmunol. 314, 1–7. 10.1016/j.jneuroim.2017.11.014 [DOI] [PubMed] [Google Scholar]
- Oosterwijk S., Lindquist K. A., Anderson E., Dautoff R., Moriguchi Y., Barrett L. F. (2012). States of mind: emotions, body feelings and thoughts share distributed neural networks. Neuroimage 62, 2110–2128. 10.1016/j.neuroimage.2012.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R. C., Bisht K., Tremblay M.-Ã. (2014). Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 8:129. 10.3389/fncel.2014.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Holmes C. (2014). Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10, 217–224. 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- Perry D. C., Sturm V. E., Peterson M. J., Pieper C. F., Bullock T., Boeve B. F., et al. (2016). Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J. Neurosurg. 124, 511–526. 10.3171/2015.2.jns14503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pósfai B., Cserép C., Orsolits B., Dénes Á. (2018). New insights into microglia-neuron interactions: a neuron’s perspective. Neuroscience [Epub ahead of print]. 10.1016/j.neuroscience.2018.04.046 [DOI] [PubMed] [Google Scholar]
- Prieto G. A., Cotman C. W. (2017). Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine Growth Factor Rev. 34, 27–33. 10.1016/j.cytogfr.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C. R., Kumagawa K., Fleshner M., Watkins L. R., Maier S. F., Rudy J. W. (1998). Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain. Behav. Immun. 12, 212–229. 10.1006/brbi.1998.0524 [DOI] [PubMed] [Google Scholar]
- Quilichini P. P., Bernard C. (2012). Brain state-dependent neuronal computation. Front. Comput. Neurosci. 6:77. 10.3389/fncom.2012.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross F. M., Allan S. M., Rothwell N. J., Verkhratsky A. (2003). A dual role for interleukin-1 in LTP in mouse hippocampal slices. J. Neuroimmunol. 144, 61–67. 10.1016/j.jneuroim.2003.08.030 [DOI] [PubMed] [Google Scholar]
- Saunders N. R., Ek C. J., Habgood M. D., Dziegielewska K. M. (2008). Barriers in the brain: a renaissance? Trends Neurosci. 31, 279–286. 10.1016/j.tins.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Schaafsma W., Zhang X., van Zomeren K. C., Jacobs S., Georgieva P. B., Wolf S. A., et al. (2015). Long-lasting pro-inflammatory suppression of microglia by LPS-preconditioning is mediated by RelB-dependent epigenetic silencing. Brain Behav. Immun. 48, 205–221. 10.1016/j.bbi.2015.03.013 [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Weber A., Roxlau T., Gaestel M., Kracht M. (2011). Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochim. Biophys. Acta 1813, 2165–2175. 10.1016/j.bbamcr.2011.06.019 [DOI] [PubMed] [Google Scholar]
- Schwarz J. M., Bilbo S. D. (2012). Sex, glia and development: Interactions in health and disease. Horm. Behav. 62, 243–253. 10.1016/j.yhbeh.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos P., Ktistaki G., Dimitraki G., Papastefanakis E., Kougkas N., Fanouriakis A., et al. (2016). Cognitive deficits early in the course of rheumatoid arthritis. J. Clin. Exp. Neuropsychol. 38, 820–829. 10.1080/13803395.2016.1167173 [DOI] [PubMed] [Google Scholar]
- Singer B. H., Newstead M. W., Zeng X., Cooke C. L., Thompson R. C., Singer K., et al. (2016). Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One 11:e0149136. 10.1371/journal.pone.0149136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ŠiŠková Z., Tremblay M. È. (2013). Microglia and synapse: interactions in health and neurodegeneration. Neural Plast. 2013:425845. 10.1155/2013/425845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly D. T., Hennessy E., Dansereau M.-A., Cunningham C. (2013). A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, TNF-α and IL-6 challenges in C57BL/6 mice. PLoS One 8:e69123. 10.1371/journal.pone.0069123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H. M., Asthana S., Bain L., Brinton R., Craft S., Dubal D. B., et al. (2016). Sex biology contributions to vulnerability to Alzheimer’s disease: a think tank convened by the women’s Alzheimer’s research initiative. Alzheimers Dement. 12, 1186–1196. 10.1016/j.jalz.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochocka M., Diniz B. S., Leszek J. (2017). Inflammatory response in the CNS: friend or Foe? Mol. Neurobiol. 54, 8071–8089. 10.1007/s12035-016-0297-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman N. L., Buchanan J. B., Heyen J. R. R., Chen J., Beverly J. L., Johnson R. W. (2006). Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell. J. Neurosci. 26, 10709–10716. 10.1523/JNEUROSCI.3376-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs I. C., Tronson N. C. (2018). Sex differences in hippocampal cytokines after systemic immune challenge. bioRxiv:1101/378257 [Preprint]. 10.1101/378257 [DOI] [Google Scholar]
- Stence N., Waite M., Dailey M. E. (2001). Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33, 256–266. [DOI] [PubMed] [Google Scholar]
- Strbian D., Durukan A., Pitkonen M., Marinkovic I., Tatlisumak E., Pedrono E., et al. (2008). The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience 153, 175–181. 10.1016/j.neuroscience.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Stern S. A., Bozdagi O., Huntley G. W., Walker R. H., Magistretti P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. 10.1016/j.cell.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchessalova D., Tronson N. C. (2018). Sex-specific memory deficits after subchronic immune challenge. bioRxiv:1101/379339 [Preprint]. 10.1101/379339 [DOI] [Google Scholar]
- Tremblay M.-È., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. (2011). The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069. 10.1523/JNEUROSCI.4158-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson N. C., Collette K. M. (2017). (Putative) sex differences in neuroimmune modulation of memory. J. Neurosci. Res. 95, 472–486. 10.1002/jnr.23921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson N. C., Corcoran K. A., Jovasevic V., Radulovic J. (2012). Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci. 35, 145–155. 10.1016/j.tins.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno Y., Mori K. (2009). Behavioral state-dependent changes in the information processing mode in the olfactory system. Commun. Integr. Biol. 2, 362–364. 10.4161/cib.2.4.8719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasek M. J., Garber C., Dorsey D., Durrant D. M., Bollman B., Soung A., et al. (2016). A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543. 10.1038/nature18283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R., Nielsen H. M., Tenner A. J. (2011). Complement in the brain. Mol. Immunol. 48, 1592–1603. 10.1016/j.molimm.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington C. H. (1957). The Strategy of the Genes. A Discussion of Some Aspects of Theoretical Biology. London: George Allen & Unwin, Ltd. [Google Scholar]
- Wadhwa M., Prabhakar A., Ray K., Roy K., Kumari P., Jha P. K., et al. (2017). Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J. Neuroinflammation 14:222. 10.1186/s12974-017-0998-z [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang J., Campbell I. L. (2002). Cytokine signaling in the brain: putting a SOCS in it? J. Neurosci. Res. 67, 423–427. 10.1002/jnr.10145 [DOI] [PubMed] [Google Scholar]
- Wang H.-L., Liu H., Xue Z.-G., Liao Q.-W., Fang H. (2016). Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J. Cell. Mol. Med. 20, 1632–1639. 10.1111/jcmm.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weberpals M., Hermes M., Hermann S., Kummer M. P., Terwel D., Semmler A., et al. (2009). NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J. Neurosci. 29, 14177–14184. 10.1523/JNEUROSCI.3238-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeln A.-C., Degenhardt K., Kaurani L., Gertig M., Ulas T., Jain G., et al. (2018). Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338. 10.1038/s41586-018-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Dissing-Olesen L., MacVicar B. A., Stevens B. (2015). Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 36, 605–613. 10.1016/j.it.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang M., Kang X., Jiang C., Zhang H., Wang P., et al. (2015). Thrombin-induced microglial activation impairs hippocampal neurogenesis and spatial memory ability in mice. Behav. Brain Funct. 11:30. 10.1186/s12993-015-0075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R., Goshen I. (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25, 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Zamanian J. L., Xu L., Foo L. C., Nouri N., Zhou L., Giffard R. G., et al. (2012). Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Malik A., Choi H. B., Ko R. W. Y., Dissing-Olesen L., MacVicar B. A. (2014). Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 82, 195–207. 10.1016/j.neuron.2014.01.043 [DOI] [PubMed] [Google Scholar]


