Abstract
Head and Neck Squamous Cell Carcinoma (HNSCC) is the 6th most common cancer globally and commonly presents with locally advanced disease, which has a recurrence rate of around 50% despite aggressive multi-modality treatment involving surgery, radiotherapy and chemotherapy or EGFR inhibition where appropriate. As understanding of the underlying cancer biology and the complex interactions within the tumor microenvironment improves, there is gathering interest in and evidence for the role of immunomodulating agents in the management of HNSCC. Immune checkpoint inhibitors, which aim to hinder the inhibitory interaction between programmed cell death protein 1 (PD-1) and its ligand PD-L1, have demonstrated durable improvements in patient outcomes in advanced / metastatic HNSCC, with both pembrolizumab and nivolumab being granted FDA approval in 2016. There are numerous ongoing clinical trials exploring the role of checkpoint inhibitors both as single agents and in combination, administered with established treatment modalities such as chemotherapy and radiotherapy, as well as alongside other novel immune modulators. These trials are not limited to advanced / metastatic HNSCC, but also to the neo-adjuvant or adjuvant settings. As studies complete and more results become available, the role immunotherapy agents will have within the treatment strategies for HNSCC may change, with increasing biomarker selection resulting in personalized therapy aiming to further improve patient outcomes.
Keywords: immune checkpoint inhibitors, head and neck cancer, head and neck squamous cell carcinoma, cancer immunology, cancer immunotherapy
Head and neck cancer encompasses malignancies that arise in the paranasal sinuses, nasal cavity, oral cavity, pharynx and larynx. In Europe, there are around 139,000 new cases of head and neck cancer per year, 90% of which are squamous cell carcinoma (HNSCC) (1). While both smoking and alcohol consumption have long been established as risk factors for the development of HNSCC, human papillomavirus (HPV) has emerged as a driver for a significant proportion of oropharyngeal squamous cell carcinomas and increasingly is being recognized as its own distinct clinical entity with a more favorable prognosis than non-HPV associated HNSCC (2, 3).
The 2016 FDA approval of the Programmed death-1 (PD-1) monoclonal antibodies pembrolizumab and nivolumab for the treatment of HNSCC heralded the dawn of a new era of treatment for a patient population that historically has a 50% recurrence rate despite aggressive multi-modality treatment involving surgery, radiotherapy, chemotherapy and, where appropriate, EGFR inhibition (4).
Intact immune surveillance is critical to control carcinogenesis and in order to propagate locally and metastasise cancer cells must develop mechanisms that allow them to evade elimination by the host immune system. Immunotherapy works on the premise that the host immune system can be activated to overcome these acquired mechanisms, allowing the recognition of cancer as non-self and eliminate it.
In order for this to happen, T-lymphocytes must be able to infiltrate the tumor and mount appropriate responses (5) and higher numbers of CD3+, CD8+ and FOXP3+ tumor infiltrating lymphocytes (TILs) are associated with a favorable outcome in in several malignancies, including HNSCC (6). In particular, the presence of CD8+ “effector” T-cells and the ratio between CD8+ and FOXP3+ regulatory T-cells (Tregs) correlates with improved prognosis. Tregs are known to actively suppress immune responses (7) and as such their presence within the tumor microenvironment may assist immune evasion in head and neck cancers. However, further understanding is required as although their presence is linked to a decreased survival in several tumor types, a positive correlation has been reported in other cancers such epithelial ovarian cancer, colorectal cancer and lymphoma (8–10).
Some cancers evade T-cell directed immune effects by developing ways of excluding the T-cells from the tumor microenvironment. However, HNSCC has been found to be one of the most immune-infiltrated cancer types (11) suggesting other mechanisms are involved and T-cell homing, infiltration and activity are under a number of influences generated by the tumor. In HNSCC several suppressive mechanisms have been identified that include:
Deficiencies or alterations of tumor human leukocyte antigen (HLA) class I molecules expression (12, 13) along with overexpression of antigens causing T-cell tolerance (14)
Increases in immunosuppressive cytokines such Il-10, (15) IL-6 (16), and TGF-β (17)
Aberrant activation of the transcription factors Signal Transducers and Activators of Transcription 3 (STAT3) (18) and NF-kB, (17) which are notably linked to IL-6 and TGF-β signaling respectively.
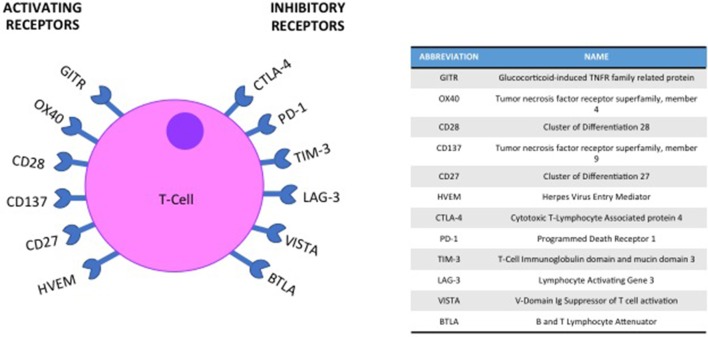
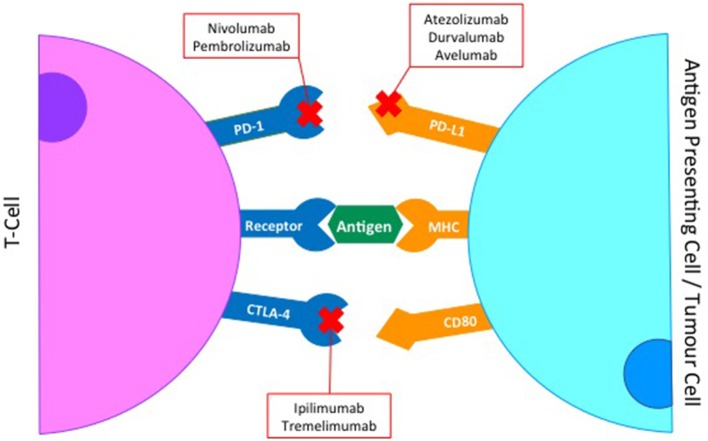
To avoid autoimmunity a series of checkpoints exist on the surface of immune cells, with the activation of a T-cell response being a careful balance of co-inhibitory and co-stimulatory molecules and their ligands (19) (Figure 1). In HNSCC immunomodulatory agents exist that block interaction between co-inhibitory receptors such as Cytotoxic T-Lymphocyte-associated antigen 4 (CTLA-4), Programmed death-1 (PD-1) or Lymphocyte activation gene-3 (LAG-3) and their ligands. Conversely, biologics that mimic ligand activated signaling in co-stimulatory molecules such as Glucocorticoid-induced tumor necrosis factor receptor (GITR) have also been evaluated. Both these approaches aim to achieve the same outcome; an enhanced activation of an immune response to tumor cells (Figure 2).
Figure 1.
Co-inhibitory and co-stimulatory checkpoints are a key element of T-cell immune regulation.
Figure 2.
Monoclonal antibodies to key co-inhibitory immune checkpoints.
PD-1/PD-L1 axis
PD-1 is a member of the CD28 receptor family which is expressed on activated T- and B-cells, monocytes and a subset of thymocytes. The expression of PD-1 on activated T cells enhances the suppressive function of Tregs and this effect is mediated by the interaction with its ligands, PD-L1 and PD-L2, which are expressed on antigen presenting cells, endothelial and epithelial cells as well as on activated lymphocytes. The interaction between PD-1 and PD-L1 negatively regulates immune responses by decreasing cytokine production and inducing T lymphocyte anergy and apoptosis. Upregulation of PD-L1 can occur in tumor cells and allows cancer cells to escape from host immune systems by functionally inactivating T-cell immune surveillance. The inhibition of this interaction can enhance T-cell response and mediate clinical anti-tumor activity (20, 21). Expression of PD-L1 is often high in HNSCC tumors, with positivity being quoted between 46 and 100% across several studies, this wide range likely owing to differences in staining technique, sample preservation and possibly sampling error (22).
Nivolumab is an IgG4 PD-1 monoclonal antibody designed to block co-inhibitory signaling through the PD-1/PD-L1 axis. Following early phase studies demonstrating promising data, a phase III trial, CheckMate 141, compared nivolumab with the physician's choice of second line single agents (docetaxel, methotrexate or cetuximab) in a 2:1 randomisation in patients with platinum resistant recurrent / metastatic HNSCC. It recruited 361 patients and response rates (RR) were low in all arms, but higher in the nivolumab cohort (13.3%) with 6 complete responses (CR) and 26 partial responses (PR). In the chemotherapy arm, RR was 5.8%, including 1 CR and 6 PR. The median overall survival (OS) was 7.5 months with nivolumab, vs. 5.1 months in the control arm, and patients who received nivolumab had a statistically significant 30% lower risk of death (HR 0.70; 95% CI 0.51 to 0.96). There was also an improvement in the estimated progression free survival (PFS) at 6 months; 19.7% with nivolumab and 9.9% for those who received physician's choice. This increased benefit at later time-points gives promise to durable benefit for responding patients, as seen with nivolumab in other tumor types where clinical data are more mature (23). In addition to the higher efficacy seen, treatment with nivolumab was also more tolerable than the standard therapies, with a reduction in grade 3 or 4 adverse events; 13.1 vs. 35.1% and a relative improvement in patient reported outcomes (PROMS) and other Quality of Life parameters (24). Outcomes of patients randomized to nivolumab who were Treated Beyond Progression (TBP) were presented at the European Society of Medical Oncology 2017 Annual Congress (ESMO 2017). Of those patients who had progressed, 62 patients (42%) received at least one further dose of nivolumab and of these 15 (24%) had a subsequent reduction in target lesion size with 3 patients achieving >30% reduction, with no increase in grade 3 or 4 toxicities (25). These findings of unusual patterns of response to immune checkpoint inhibitors have also been described in other patient groups (26, 27) and offers reassurance that continuing therapy beyond progression is safe and may have a role to play in patients maintaining clinical benefit.
Pembrolizumab, another IgG4 PD-1 antibody, has also been examined as a treatment for patients with recurrent or metastatic HNSCC. KEYNOTE-012 was a phase Ib study which included an expansion cohort of 132 HNSCC patients. A response rate of 18% at 9 months was reported (including 4 CR and 20 PR), with a median OS of 8 months and a 6-month PFS of 23%. Treatment was well tolerated with grade 3 or 4 toxicity reported in 9% of patients (28) These data led to the accelerated approval of pembrolizumab by the FDA. Outcomes from the confirmatory phase III trial comparing pembrolizumab with standard therapies (KEYNOTE-040) in patients with platinum-resistant relapsed / metastatic head and neck squamous cell cancers were also presented at ESMO 2017 (29), although the final published manuscript is awaited. This study was similar in design to the CheckMate 141 study, although with a complex hierarchical statistical analysis plan. It demonstrated a median OS of 8.4 months with pembrolizumab compared to 7.1 months in the control arm, with a hazard ratio of 0.81 (95% confidence interval 0.66–0.99), which did not reach statistical significance (p = 0.020) within the multiplicity statistical model. The overall survival data may have been confounded by some patients (12%) within the control arm going on to receive immune checkpoint inhibitors post progression (cross-over effect). The evaluation of other inhibitors of the PD-1/PD-L1 axis in HNSCC, such as durvalumab, atezolizumab and avelumab, are currently ongoing.
GITR
GITR is a member of the Tumor Necrosis Factor superfamily, expressed on the surface of CD25+ CD4+ Tregs. GITR activation by its ligand (GITRL) reduces Treg recruitment and abrogates their suppressive function (30, 31).
AMG228 is a IgG1 antibody that binds to GITR and recently completed phase I clinical trial. Of the 30 patients treated, 10 had HNSCC. Of the 29 evaluable patients, none had an objective response. Treatment-emergent toxicity was experienced in 90% of patients; most common being hypophosphatemia, fatigue, anemia, nausea and pyrexia. There was no evidence of altered T cell activity observed despite complete target coverage in both tumor and peripheral blood (32).
The promise of PD-1 inhibitors has been realized for a small proportion of patients with advanced HNSCC. Further work is ongoing, exploring mechanisms of primary and secondary resistance to these agents within HNSCC, aiming to find better predictive biomarkers and methods to increase likelihood for response.
Combination checkpoint inhibition
Emerging evidence has implicated the upregulation of alternative immune checkpoints in resistance to PD-1/PD-L1 axis interruption (33). The use of combination checkpoint inhibitors (CPI) has been successful in improving response rates and survival in other tumor types, with an increasing number of immune checkpoint inhibitors targeting many co-stimulatory and co-inhibitory interactions transitioning from pre-clinical to clinical evaluation in HNSCC.
CTLA-4 is commonly expressed on the surface of activated T cells, where it binds to B7, preventing interaction with co-stimulatory CD28, leading to negative regulation of T cell proliferation and IL-2 production. Blockade of CTLA-4 correlates with an increase in T-Cell activation and maintenance of high-frequency T-cell receptor clonotypes (34, 35). Dual blockade of PD-L1 and CTLA-4 has been shown to improve response rate and anti-tumor activity when compared to monotherapy alone in metastatic melanoma (36) and the combination is being explored in other solid cancers. In HNSCC, this combination approach is being evaluated in four separate trials (Table 1).
Table 1.
Ongoing trials evaluating combination PD-L1 and CTLA-4 blockade in HNSCC.
| Trial | Phase | Cohort | Drugs | Trial code |
|---|---|---|---|---|
| CheckMate 356 | I/II | EBV positive nasopharyngeal cancers, HPV positive HNSCC | Ipilimumab and Nivolumab | U111-1166-0687 |
| CheckMate 714 | II | Recurrent or metastatic HNSCC | Ipilimumab and Nivolumab vs. Nivolumab and Placebo | NCT02823574 |
| CheckMate 651 | III | First line treatment for HNSCC | Ipilumumab and Nivolumab vs. Cetuximab with platinum and flurouracil | NCT02741570 |
| KESTERAL | III | Recurrent or metastatic HNSCC | Durvalumab and Tremelimumab vs. Durvalumab monotherapy | NCT02551159 |
Durvalumab, IgG1 antibody to PD-L1; EBV, Epstein-Barr Virus; Ipilimumab, IgG1 antibody to CTLA-4; Tremelimumab, IgG2 antibody to CTLA-4.
LAG-3 is expressed on the surface of activated CD4+ and CD8+ T-cells and certain subtypes of natural killer and dendritic cells. It is thought to negatively regulate T cell activation and proliferation and is also expressed by Treg cells and required for their optimal suppressive function (37–41). CA224-020 is a phase I/IIa dose escalation and expansion study, exploring BMS-986016 (an anti-LAG-3 antibody) alone and in combination with Nivolumab in advanced solid tumors, including a HNSCC cohort (NCT01968109). Data from the head and neck cohorts are eagerly awaited.
Checkpoint inhibition in combination with other immune modulators
The immune ecosystem is a complex network of interconnected cells, cytokines and signaling pathways and as such augmenting the antitumor effect of checkpoint inhibitors is not limited to CPI combinations alone. As understanding of this complex biology improves, a number of other agents with the potential to modulate the tumor microenvironment are currently being investigated for the treatment of HNSCC.
SCORES is a phase Ib/II trial of Durvalumab (a PD-L1 inhibitor) in combination with either AZD9150 or AZD5069 in advanced solid tumors including recurrent / metastatic HNSCC (NCT02499328). AZD9150 inhibits STAT3, with pre-clinical evidence of activity in lymphoma and lung cancer (42). Pre-clinical studies have also shown that STAT3 inhibition can increase chemo and radiotherapy sensitization in HNSCC, particularly Nasopharyngeal Cancer (NPC) (43, 44). AZD5069 is a novel selective antagonist of CXC Chemokine receptor 2 (CXCR2), a G protein-coupled receptor for a number of cytokines. It is overexpressed in HNSCC and is implicated in disease proliferation via IL-8 signaling (45, 46). Initial results of patients with HNSCC treated in the AZD9150 and Durvalumab arm were announced at the ESMO Congress 2017. Of 35 patients, 15 had prior PD-L1 treatment and 20 were CPI-naïve. In the CPI- naïve arm, a 25% objective RR was reported (4 confirmed PR and 1 unconfirmed PR) with a 45% DCR at 12 weeks and 30% patients remaining on treatment at 25 weeks. Overall the combination was felt to be tolerable, with G3/4 thrombocytopenia and increase in liver enzymes reported for 3.4% of those dosed, and two treatment related discontinuations (unspecified). These early data are promising and mature results are awaited. In the PD-L1 pretreated arm, 1 complete response and 1 unconfirmed response were reported, with a 20% DCR at 12 weeks (47).
Indoleamine 2,3-dioxygenase 1 (IDO1) is a catabolizing enzyme that induces immune tolerance by suppressing T-cells and has been found to be associated with poor outcome in laryngeal squamous cell carcinoma (48). IDO1 is being heavily investigated as a novel target for immune therapies, with several inhibitors in clinical development. KEYNOTE-037 is a phase I/II study evaluating pembrolizumab given concurrently with epacadostat, an oral inhibitor of IDO1. Patients with recurrent / metastatic HNSCC were eligible for this study if they had received at least one line of platinum based chemotherapy and were CPI-naïve. An interim update of this study was presented at ASCO 2017, when data from 38 patients were presented, 36 of whom were efficacy-evaluable at this early cut off. An ORR of 31% was reported, with a disease control rate of 58%, regardless of the number of previous lines of treatment. The most common treatment related SAEs were fatigue (24%), nausea (11%) and decreased weight (11%). These data suggesting promising anti-tumor activity with good tolerability have led to plans for a phase III study (49).
Checkpoint inhibition in combination with viral therapy
Oncolytic viruses have been found to reduce tumor burden and prime an anti-tumor immunity in a number of preclinical studies (50, 51) and when used in combination with PD-1 inhibition may overcome CPI resistance by broadening neoantigenome-directed T-cell responses (52). Several oncolytic viruses are currently being evaluated in HNSCC. KEYNOTE-137 is an ongoing phase Ib/III randomized study exploring the combination of Talimogene Laherparepvec (T-VEC) with pembrolizumab in recurrent metastatic HNSCC (NCT02626000). T-VEC is a modified, live, attenuated herpes simplex virus type 1 that is designed to promote an antitumor response through selective replication in tumor cells and production granulocyte macrophage colony-stimulating factor (GM-CSF) to stimulate systemic antitumor immunity. It has already been licensed as a single agent for the treatment of unresectable metastatic melanoma after demonstrating an improved durable response rate (DRR) and mOS relative to GM-CSF (53, 54).
Checkpoint inhibition in combination with chemoradiotherapy
In addition to interest in combining immune checkpoint inhibitors to other novel agents, there is also a rationale to combine with both chemotherapy and radiotherapy, which are both known to modulate the tumor microenvironment as well as inducing immunogenic tumor cell death. There is particular interest in exploring CPI in combination with chemo-radiation in locally advanced head and neck cancers. Upregulation of PD-L1 by tumor cells following administration of chemo-radiotherapy (CRT) has been demonstrated in a number of preclinical models (55–57) with an improvement in RR, PFS and median time to death being achieved with the addition of adjuvant durvalumab to CRT in locally advanced, unresectable Non-Small-Cell Lung Cancer as demonstrated in the large phase III PACIFIC study (58).
Higher numbers of CD3+ and CD8+ TILs have been shown to positively correlate with clinical outcome to definitive CRT in HNSCC (59). In addition, in a pilot study of 20 patients, CRT was shown to alter the immune landscape in HNSCC, with an increase in the number of CD8+ T effector cells, CD4+ regulatory cells and T cells expressing PD1, TIM3 and LAG3. It is important to note that in this study, most patients were male (90%) with locally advanced human papillomavirus (HPV) associated disease, 80% of which originated from the oropharynx and thus results may not be representative of all HNSCC entities (60).
A safety study demonstrating the tolerability of pembrolizumab in addition to cisplatin-based CRT for locally advanced HNSCC was presented at ASCO 2017. This 27-patient study delivered a fixed dose of pembrolizumab 4–7 days prior to CRT, 3 weekly for the duration of CRT and a further five doses following completion. Patients predominantly had HPV positive oropharyngeal tumors (74%) and all received their planned RT dose, with 85% achieving target cisplatin dosing and 78% completing the planned doses of pembrolizumab. The addition of checkpoint blockade was not felt to significantly increase the toxicity experienced by patients, however three patients did have treatment discontinued due to immune related adverse events (G2 peripheral motor neuropathy, G3 AST elevation and G1 Lhermitte-like syndrome). The study has now progressed into expansion cohorts of both HPV positive and negative tumors to confirm tolerance and gain preliminary evidence of efficacy (61).
Cetuximab is also used with RT for the radical treatment of locally advanced HNSCC. It is an influencer of natural killer cell response and consequently dendritic cell maturation (62, 63) and is thought to increase the expression of inhibitory checkpoints PD-1, TIM-3 and CTLA-4 on TILs (64, 65). There are a number of current trials attempting to determine the benefit of checkpoint inhibition alongside varying combinations of cetuximab and RT in locally advanced HNSCC, with the combination with avelumab as part of REACH (NCT02999087) being of particular interest due to the propensity of both avelumab and cetuximab to activate the antibody-dependent cellular cytotoxicity (ADCC) pathway (63, 66).
Immune-related adverse events
Treatment with CPIs can have inflammatory side effects which are termed immune related adverse events (irAEs). Although the exact mechanism is unknown, it is likely due to the role that immune checkpoints have in maintaining immune homeostasis and by inhibiting their action, T cells are able to react with self-antigens, with different checkpoint inhibitors having distinct immune toxicity profiles (67). These autoimmune manifestations are more common in patients with pre-existing autoimmune disorders however they may still be safely administered to this population if used with caution and appropriate patient selection (68).
A pooled retrospective analysis of the safety profile of nivolumab in 576 patients with advanced melanoma found the 49% of patients had an irAE; most commonly skin, gastrointestinal, endocrine and hepatic and were classed as grade 3–4 in 4% of patients. The time of onset varied depending on the organ system involved, with skin irAEs manifesting at 5 weeks whereas renal toxicity had had median time of onset of 15 weeks. Approximately 24% required systemic immunosuppressive treatment with the majority of cases resolving (69). In CheckMate 141, the side effect profile of nivolumab in a HNSCC population showed lower rates of both gastrointestinal and hepatic toxicities when compared to the standard of care treatments but did demonstrated an increase in skin toxicity (15.7%), endocrinopathies (7.6%) and pneumonitis (2.1%) (24).
The results of a 114-case series of patients with metastatic HNSCC treated with anti-PD-1 therapy was presented at ASCO 2018, demonstrating that patients who manifested an irAE had improved outcomes compared to those that did not. In total, 59 irAEs were recorded in 49 patients with ORR being higher in irAE positive group (30.6 vs. 12.3% p = 0.02) and an improvement in both PFS (6.9 vs. 2.1 months; p = 0.0004) and mOS (12.5 vs. 6.8 months; p = 0.007) were reported, which remained significant on multivariate analysis (70). A similar observation has been noted in patients with metastatic melanoma and persisted regardless if they required treatment with a systemic immunosuppressant for treatment of their irAE (69).
The development of irAE's can be serious and in some cases fatal, and as such careful consideration must be taken before initiating their use, particularly in the adjuvant or neo-adjuvant setting. As our understanding of the mechanism that drive these systemic manifestations improve, we may develop biomarkers that help identify those who are more likely to develop them which will assist in informed decision making and toxicity monitoring.
Biomarkers
As improvements in the understanding of the interaction between cancer and the host immunity are complimented by increasing numbers of immunomodulatory drugs, there has been a drive to develop potential biomarkers to select patients most likely to benefit from treatment and assist in monitoring response.
Both the nivolumab and pembrolizumab studies outlined above explored the impact of HPV on outcome. In Checkmate-141, OS appeared to be longer with nivolumab regardless of p16 status, however the increase was more pronounced in patients with p16 positive tumors (mOS of 9.1 months with nivolumab vs. 4.4 months with standard therapy) than in p16 negative tumors (mOS 7.5 vs. 5.8 months respectively) (24). KEYNOTE-012 also reported better outcomes in the patients with HPV-positive tumors relative to HPV negative ones (RR 32% vs. 14%, 6 month PFS 37% vs. 20% and 6-month OS 70% vs. 56% (28).
These trials also examined tumor cell PD-L1 expression as a potential biomarker, with data suggesting increased benefit in PD-L1 positive disease. In the overall survival analysis of Checkmate-141, patients with PD-L1 expression >1% treated with nivolumab had a hazard ratio for death of 0.55 (95% CI 0.36–0.83) when compared to standard therapy. Where PD-L1 expression was < 1%, this HR was 0.89 (95% CI 0.54–1.45) (24). Similarly, KEYNOTE-012 reported tumor PD-L1 expression using immunohistochemistry, with positivity being defined as >1%. Patients with PD-L1 expression >1% who received pembrolizumab had improved RR of 22 vs. 4% in those with PD-L1,1%, with median OS 303 vs. 151 days respectively (28). KEYNOTE-040 described an OS HR of 0.54 (95% CI 0.35–0.82) with pembrolizumab in patients with tumors with PD-L1 expression >50% but also evaluated PD-L1 expression on both tumor and associated immune cells (CPS score) describing OS HR of 0.75 for PD-L1 CPS >1% with pembrolizumab compared to control patients.
Of the 61 PD-L1 positive HNSCC in KEYNOTE-012, 43 had RNA expression profiling and survival data were evaluated with multi-gene expression signatures that had previously been derived in melanoma patients. Of these signatures, the 6-gene INF-γ was the top-performing, with significant associations to OR (p = 0.005) and PFS (<0.001). On evaluation of the individual signature genes, INF-γ inducible MHC-II expression was felt to be the biological link. Using an optimal cutoff for INF-γ, positive predictive value for response was 40% with a negative predictive value of 95%; AUC = 0.8 (95%CI 0.61–0.95) within this patients population, which may assist in identifying clinical benefit from anti-PD-1 therapy in patients who are PD-L1 positive (71).
The somatic mutational load (ML) and INF-γ gene expression profile were found to be independently predictive of response to pembrolizumab in the 73 patients within KEYNOTE-012 who had HPV and Epstein-Barr Virus (EBV) negative HNSCC, with ML and INF-γ gene expression profile being significantly associated with OR (p = 0.064 and p = 0.001; AUROC 0.82 and 0.74 respectively). The INF-γ gene expression profile also remained a significant predictor in HPV and EBV positive patients showing promise as a predictor of response regardless of viral status (72).
Combinations of these biomarkers may give additive value to patient selection for CPI therapy. Other potential biomarkers that have shown promise include epigenetic modification of genes associated with homologous recombination, such as RAD51 and XRCC3, which are thought to alter checkpoint expression (73) and the identification of different HNSCC subtypes, each with a distinct tumor microenvironment (74) but how these influence survival or response to immunotherapy has yet to be addressed.
Conclusion
Immunotherapy looks set to revolutionize the treatment of HNSCC, with the approval of nivolumab and pembrolizumab (FDA) already offering new therapeutic options in recurrent / metastatic disease. As our knowledge of the biological processes driving HNSCC improves, along with greater understanding of the important features of the tumor microenvironment, so too does the rationale for combination strategies and the parallel development of predictive biomarkers. These approaches should support an era where a personalized approach to immunotherapy treatment translates into improved outcomes for patients with this disease.
Author contributions
Both M-JD and MF were involved in selecting the content and outline and the writing of this article.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. MF is supported by the UCL/UCLH NIHR Biomedical Research Centre and runs early phase studies in the NIHR UCLH Clinical Research Facility supported by the UCL ECMC.
References
- 1.Gregoire V, Lefebvre JL, Licitra L, Felip E, Group E-E-EGW . Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2010) 21(Suppl 5):v184-6. 10.1093/annonc/mdq185 [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. (2010) 363:24–35. 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. (2006) 24:736–47. 10.1200/JCO.2004.00.3335 [DOI] [PubMed] [Google Scholar]
- 4.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet (2008) 371:1695–709. 10.1016/S0140-6736(08)60728-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanitis E, Dangaj D, Irving M, Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol. (2017) 28(Suppl. 12):xii18–xii32. 10.1093/annonc/mdx238 [DOI] [PubMed] [Google Scholar]
- 6.de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology (2017) 6:e1356148 10.1080/2162402X.2017.1356148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. (2007) 13:108–16. 10.1016/j.molmed.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 8.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE (2009) 4:e6412. 10.1371/journal.pone.0006412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. (2009) 27:186–92. 10.1200/JCO.2008.18.7229 [DOI] [PubMed] [Google Scholar]
- 10.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood (2006) 108:2957–64. 10.1182/blood-2006-04-018218 [DOI] [PubMed] [Google Scholar]
- 11.Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight (2016) 1:e89829. 10.1172/jci.insight.89829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res. (2005) 33:113–33. 10.1385/IR:33:2:113 [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. (2006) 176:3402–9. 10.4049/jimmunol.176.6.3402 [DOI] [PubMed] [Google Scholar]
- 14.Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. (2015) 33:3293–304. 10.1200/JCO.2015.61.1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jebreel A, Mistry D, Loke D, Dunn G, Hough V, Oliver K, et al. Investigation of interleukin 10, 12 and 18 levels in patients with head and neck cancer. J Laryngol Otol. (2007) 121:246–52. 10.1017/S0022215106002428 [DOI] [PubMed] [Google Scholar]
- 16.Choudhary MM, France TJ, Teknos TN, Kumar P. Interleukin-6 role in head and neck squamous cell carcinoma progression. World J Otorhinolaryngol Head Neck Surg. (2016) 2:90–7. 10.1016/j.wjorl.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freudlsperger C, Bian Y, Contag Wise S, Burnett J, Coupar J, Yang X, et al. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene (2013) 32:1549–59. 10.1038/onc.2012.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mali SB. Review of STAT3 (signal transducers and activators of transcription) in head and neck cancer. Oral Oncol. (2015) 51:565–9. 10.1016/j.oraloncology.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 19.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. (2013) 12:130–46. 10.1038/nrd3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. (2009) 229:114–25. 10.1111/j.1600-065X.2009.00767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature (2017) 545:495–9. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. (2014) 50:627–32. 10.1016/j.oraloncology.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. (2014) 32:1020–30. 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddad R, Blumenschein G, Fayette J, Jr, Guigay J, Colevas AD, Licitra L, et al. Treatment beyond progression with nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck in the phase 3 CheckMate 141 Study: a biomarker analysis and updated clinical outcomes. Ann Oncol. (2017) 28:mdx374.001 10.1093/annonc/mdx374.001 [DOI] [Google Scholar]
- 26.Long GV, Weber JS, Larkin J, Atkinson V, Grob JJ, Schadendorf D, et al. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. (2017) 3:1511–9. 10.1001/jamaoncol.2017.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. (2016) 2:1179–86. 10.1001/jamaoncol.2016.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow LQ, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. (2016) 34:3838–45. 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen EE, Harrington KJ, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, et al. Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): Phase 3 KEYNOTE-040 trial. Ann Oncol. (2017) 28:mdx440.040 10.1093/annonc/mdx440.040 [DOI] [Google Scholar]
- 30.Schaer DA, Budhu S, Liu C, Bryson C, Malandro N, Cohen A, et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res. (2013) 1:320–31. 10.1158/2326-6066.CIR-13-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. (2010) 59:1367–77. 10.1007/s00262-010-0866-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran B, Carvajal RD, Marabelle A, Patel SP, LoRusso P, Rasmussen E, et al. Dose escalation results from a first-in-human, phase 1 study of the glucocorticoid-induced TNF receptor-related protein (GITR) agonist AMG 228 in patients (Pts) with advanced solid tumors. J Clin Oncol. (2017) 35:2521 10.1200/JCO.2017.35.15_suppl.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. (2016) 7:10501. 10.1038/ncomms10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. (2014) 371:2189–99. 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blank CU, Enk A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol. (2015) 27:3–10. 10.1093/intimm/dxu076 [DOI] [PubMed] [Google Scholar]
- 36.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:1270–1. 10.1056/NEJMoa1504030 [DOI] [PubMed] [Google Scholar]
- 37.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. (1990) 171:1393–405. 10.1084/jem.171.5.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol. (2005) 174:688–95. 10.4049/jimmunol.174.2.688 [DOI] [PubMed] [Google Scholar]
- 39.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. (2009) 182:1885–91. 10.4049/jimmunol.0800185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, et al. LAG-3 expression defines a subset of CD4+CD25highFoxp3+ regulatory T cells that are expanded at tumor sites. J Immunol. (2010) 184:6545–51. 10.4049/jimmunol.0903879 [DOI] [PubMed] [Google Scholar]
- 41.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity (2004) 21:503–13. 10.1016/j.immuni.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 42.Hong D, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med. (2015) 7:314ra185. 10.1126/scitranslmed.aac5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan Y, Zhou F, Zhang R, Claret FX. Stat3 inhibitor Stattic exhibits potent antitumor activity and induces chemo- and radio-sensitivity in nasopharyngeal carcinoma. PLoS ONE (2013) 8:e54565. 10.1371/journal.pone.0054565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi M, Cui C, Dodge CT, Bhayani MK, Lai SY. Targeting STAT3 inhibits growth and enhances radiosensitivity in head and neck squamous cell carcinoma. Oral Oncol. (2012) 48:1220–6. 10.1016/j.oraloncology.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholls DJ, Wiley K, Dainty I, MacIntosh F, Phillips C, Gaw A, et al. Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J Pharmacol Exp Ther. (2015) 353:340–50. 10.1124/jpet.114.221358 [DOI] [PubMed] [Google Scholar]
- 46.Chan LP, Wang LF, Chiang FY, Lee KW, Kuo PL, Liang CH. IL-8 promotes HNSCC progression on CXCR1/2-meidated NOD1/RIP2 signaling pathway. Oncotarget (2016) 7:61820–31. 10.18632/oncotarget.11445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen E. Phase 1b/2 study (SCORES) assessing safety, tolerability, and preliminary anti-tumour activity of durvalumab plus AZD9150 or AZD5069 in patients with advaned solid malignancies and recurrent/metastatic squamous cell carcinoma of the head and neck (R/M-SCCHN). Ann Oncol. (2017) 28:dx376.001 10.1093/annonc/mdx376.001 [DOI] [Google Scholar]
- 48.Ye J, Liu H, Hu Y, Li P, Zhang G, Li Y. Tumoral indoleamine 2,3-dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch. (2013) 462:73–81. 10.1007/s00428-012-1340-x [DOI] [PubMed] [Google Scholar]
- 49.Hamid O, Bauer T M, Spira A I, Olszanskit A J, Patel S P, Wasser J S. Epacadostat plus pembrolizumab in patients with SCCHN: Preliminary phase I/II results from ECHO-202/KEYNOTE-037. J Clin Oncol. (2017) 35:6010 10.1200/JCO.2017.35.15_suppl.6010 [DOI] [Google Scholar]
- 50.Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. (2008) 14:7358–66. 10.1158/1078-0432.CCR-08-0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woller N, Knocke S, Mundt B, Gurlevik E, Struver N, Kloos A, et al. Virus-induced tumor inflammation facilitates effective DC cancer immunotherapy in a Treg-dependent manner in mice. J Clin Invest. (2011) 121:2570–82. 10.1172/JCI45585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woller N, Gurlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, et al. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol Ther. (2015) 23:1630–40. 10.1038/mt.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greig SL. Talimogene laherparepvec: first global approval. Drugs (2016) 76:147–54. 10.1007/s40265-015-0522-7 [DOI] [PubMed] [Google Scholar]
- 54.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. (2015) 33:2780–8. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 55.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. (2014) 124:687–95. 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. (2014) 74:5458–68. 10.1158/0008-5472.CAN-14-1258 [DOI] [PubMed] [Google Scholar]
- 57.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. (2008) 45:1470–6. 10.1016/j.molimm.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 58.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 59.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. (2014) 110:501–9. 10.1038/bjc.2013.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sridharan V, Margalit DN, Lynch SA, Severgnini M, Zhou J, Chau NG, et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br J Cancer. (2016) 115:252–60. 10.1038/bjc.2016.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powell SF, Gitau MM, Sumey CJ, Reynolds JT, Lohr M, McGraw S, et al. Safety of pembrolizumab with chemoradiation (CRT) in locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). J Clin Oncol. (2017) 35. 10.1200/JCO.2017.35.15_suppl.6011 [DOI] [Google Scholar]
- 62.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. (2013) 19:1858–72. 10.1158/1078-0432.CCR-12-2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pahl JH, Ruslan SE, Buddingh EP, Santos SJ, Szuhai K, Serra M, et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin Cancer Res. (2012) 18:432–41. 10.1158/1078-0432.CCR-11-2277 [DOI] [PubMed] [Google Scholar]
- 64.Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, et al. CTLA-4+ Regulatory T Cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. (2015) 75:2200–10. 10.1158/0008-5472.CAN-14-2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jie HB, Srivastava RM, Argiris A, Bauman JE, Kane LP, Ferris RL. Increased PD-1+ and TIM-3+ TILs during cetuximab therapy inversely correlate with response in head and neck cancer patients. Cancer Immunol Res. (2017) 5:408–16. 10.1158/2326-6066.CIR-16-0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, et al. Antibody-Dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. (2015) 3:1148–157. 10.1158/2326-6066.CIR-15-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 68.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. (2017) 28:368–76. 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
- 69.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. (2017) 35:785–92. 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 70.Corey CFSK, Arun K, Rajesh A, Allison D, Yi-Hung CT, Elaine K, et al. Association of immune-related adverse events (irAEs) with improved response, progression-free survival, and overall survival for patients with metastatic head and neck cancer receiving anti-PD-1 therapy. ASCO (2018). [Google Scholar]
- 71.Tanguy Y, Seiwert BB, Jared W, Joseph PE, Jennifer Y, Erin M, et al. Inflamed-phenotype gene expression signatures to predict benefit from the anti-PD-1 antibody pembrolizumab in PD-L1+ head and neck cancer patients. J Clin Oncol. (2015) 33:6017. 10.1200/jco.2015.33.15_suppl.6017 [DOI] [Google Scholar]
- 72.Robery IH, Tanguy YS, Laura QMC, Shilpa G, Jared W, Iris G. Genomic determinants of response to pembrolizumab in head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. (2017) 35 10.1200/JCO.2017.35.15_suppl.6009 [DOI] [Google Scholar]
- 73.Rieke DT, Ochsenreither S, Klinghammer K, Seiwert TY, Klauschen F, Tinhofer I, et al. Methylation of RAD51B, XRCC3 and other homologous recombination genes is associated with expression of immune checkpoints and an inflammatory signature in squamous cell carcinoma of the head and neck, lung and cervix. Oncotarget (2016) 7:75379–93. 10.18632/oncotarget.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. (2015) 21:870–81. 10.1158/1078-0432.CCR-14-2481 [DOI] [PubMed] [Google Scholar]