Abstract
Seed priming is a widely used technique in crops to obtain uniform germination and high-quality seedlings. In this study, we found a long-term effect of seed priming with gibberellic acid-3 (GA3) on plant growth and production in Leymus chinensis. Seeds were germinated on agar plates containing 0–200 μM GA3, and the germinated seedlings were transplanted to clay planting pots and grown for about one year. The clonal tillers grown from the mother plants were transplanted to field conditions in the second year. Results showed that GA3 treatment significantly increased seed germination rate by 14–27%. GA3 treatment also promoted subsequent plant growth and biomass production, as shown by a significant increase in plant height, tiller number, and fresh and dry weight in both pot (2016) and field (2017) conditions. It is particularly noteworthy that the growth-promoting effect of a single seed treatment with GA3 lasted for at least two years. In particular, GA3 treatment at 50 μM increased aboveground fresh and dry weight by 168.2% and 108.9% in pot-grown conditions, and 64.5% and 126.2% in field-grown conditions, respectively. These results imply a transgenerational transmission mechanism for the GA-priming effect on clonal offspring growth and biomass production in L. chinensis.
Introduction
Seed quality is the basis of adequate plant establishment and is associated with the productive success of crops1. Therefore, a variety of strategies are employed in improving seed germination, seedling growth, and productivity. Seed priming, a low-cost and low-risk tool, is considered to be the most effective of these methods1–4. It comprises a pre-sowing treatment of soaking seeds in a specified solution, allowing some metabolic activities to proceed before germination5,6, and can increase germination percentage, shorten germination time, and improve seedling establishment7,8. Based on the priming agents, seed priming can generally be classified into four groups: hydropriming, osmopriming, halopriming, and hormone priming6.
In hormone priming, plant growth regulators such as gibberellic acids (GAs)5, abscisic acid (ABA)9,10, or salicylic acid (SA)1,11,12 have been widely used to increase synchronized seed germination, seedling growth, and also the yield of a variety of crop species, such as rice5,10,13, corn14,15, safflower16, wheat12,17,18, beet1, and sunflower19. However, most studies have focused on the life stages of seed germination and seedling growth, and little attention has been given to priming effects over a long-time span. Moreover, there have been scarce reports of studies on hormone priming in grass species, especially perennial grass species20,21.
GAs play important roles in many essential plant growth and development processes, including seed germination, stem elongation, leaf expansion, flower and fruit development, and floral transition22. They are often used to overcome seed dormancy, and can significantly improve seed germination in many species, mainly through the activation of embryo growth, mobilization of reserves, and weakening of the endosperm layer3,15. It has also been reported that seed priming with GAs improves germination and the growth parameters of shoot length, root length, and seedling weight in Capparis spinosa7, Trigonella foenum-graecum11, Hibiscus sabdariffa L.9, Trifolium repens L.20, Beta vulgaris1, Zea mays L.14, and Medicago sativa21. In addition, seed priming with GAs increased yield in sunflower12 by 123.5% (seeds per head) and wheat19 by 7.0% (grain yield).
The most important function of plants in grassland ecosystems is their contribution to natural grassland ecosystem productivity, which partially relies on the plant biomass of individual plants, especially perennial species23–25. Leymus chinensis, a perennial, rhizomatous grass is one of the most widely distributed types of steppe vegetation in temperate eastern Eurasia26,27. It is one of the species most preferred for consumption by large herbivores because of its high palatability in terms of forage value, and high crude protein28. In recent years, due to human interference, especially overgrazing, L. chinensis grassland has become severely degraded, to unprecedented levels26,27, and one of the most serious problems facing the development of animal husbandry is now the reduction of plant height and productivity in L. chinensis. Therefore, improving the primary productivity of L. chinensis in both natural and artificial grassland has become an urgent necessity.
In this study, the objective was to elucidate the priming effects of gibberellic acid-3 (GA3) on seed germination and subsequent growth and grass production in the perennial grass, L. chinensis.
Results
Effect of GA3 treatment on seed germination
GA3 treatment at all concentration levels significantly enhanced seed germination rates compared with the control treatment, with the highest recorded germination rate being 50 μM (Fig. 1). The effect of GA3 increased with increasing concentration up to 50 μM, while further higher concentrations (100 and 200 μM) slightly compromised the promoting effect when compared to concentrations of 10 and 50 μM. GLM analysis showed that GA3 concentrations had significant promoting effects (df = 5, χ2 = 19.383, p < 0.01), and the Tukey’s test showed that a concentration of 50 μM was the most effective in promoting seed germination, when compared to the control (p < 0.001).
Figure 1.
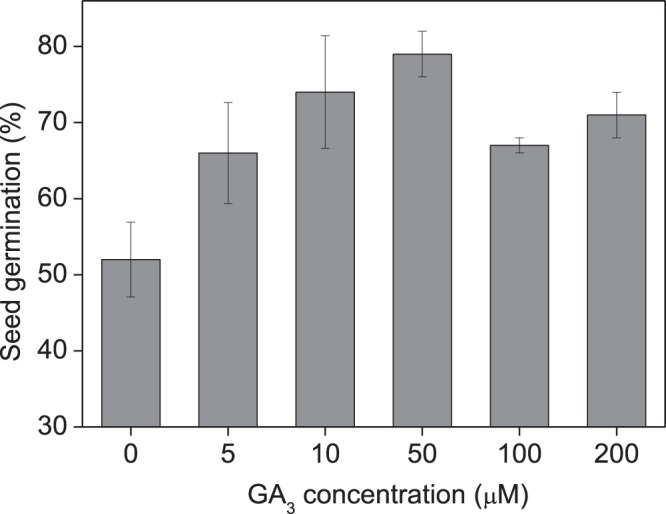
Effect of the application of gibberellic acid-3 (GA3) on seed germination in Leymus chinensis. Seeds were germinated in agar plates containing 0 (control), 5, 10, 50, 100, and 200 μM of GA3, for 28 d under an alternating cycle of 12/12 h of light (fluorescent and incandescent white light of 54 μmol m−2 s−1) at 28/16 °C. Values are mean ± s.e.
Effect of seed priming with GA3 treatment on growth in pot experiments (2016)
Seed treatment with GA3 at all concentration levels promoted subsequent plant height (df = 5, F = 5.704, p < 0.001) and tiller number (df = 5, F = 8.360, p < 0.001) during the whole growth period (Fig. 2). Plant height was highest at 50 μM, and tiller number was greatest at 5 μM and 10 μM GA3, respectively (Fig. 2).
Figure 2.
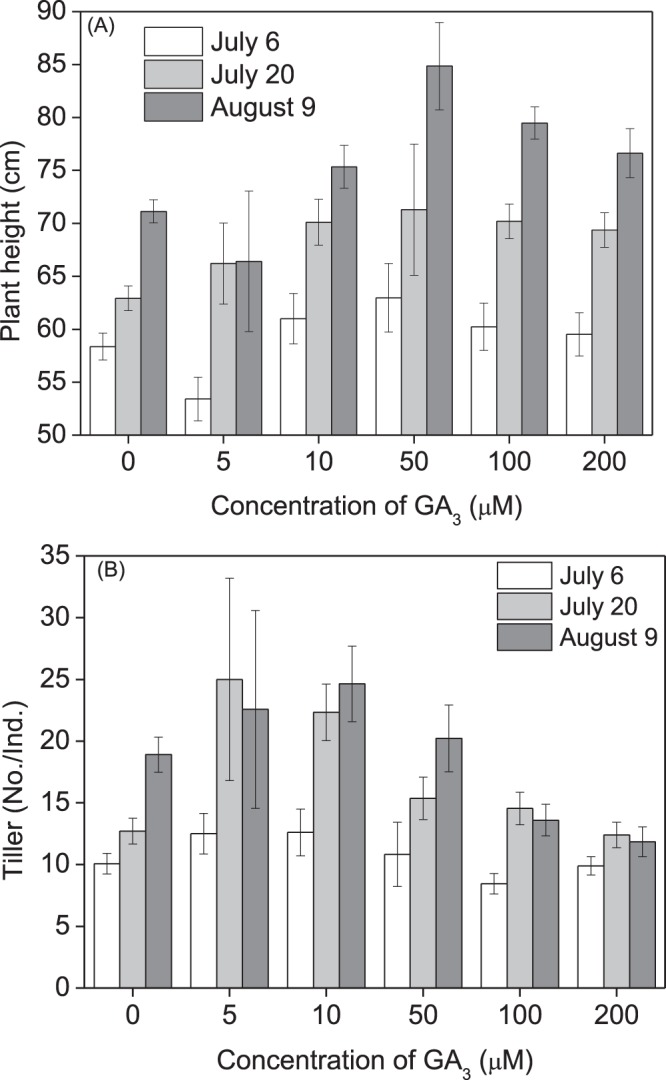
Effect of seed priming with gibberellic acid-3 (GA3) on plant growth in Leymus chinensis grown in pots (2016). Leymus chinensis seeds were germinated in the presence of GA3 at different concentrations, as indicated (0–200 μM), and the seedlings were transplanted to and grown in clay pots. (A) Plant height, (B) tillers per plant. Columns in white, light grey, and grey represent the measurements made on July 6, July 20, and August 9, 2017, respectively. Data are means ± s.e.
Grass production was also markedly enhanced by seed treatment with GA3 (Fig. 3). Both the fresh (df = 5, F = 4.570, p = 0.017) and dry weight (df = 5, F = 4.428, p = 0.019) of shoots were significantly affected by GA3 concentrations, with a GA3 treatment of 50 μM showing the highest promoting effect. No significant effect on grass production was observed when GA3 concentrations was ≥100 μM (Fig. 3).
Figure 3.
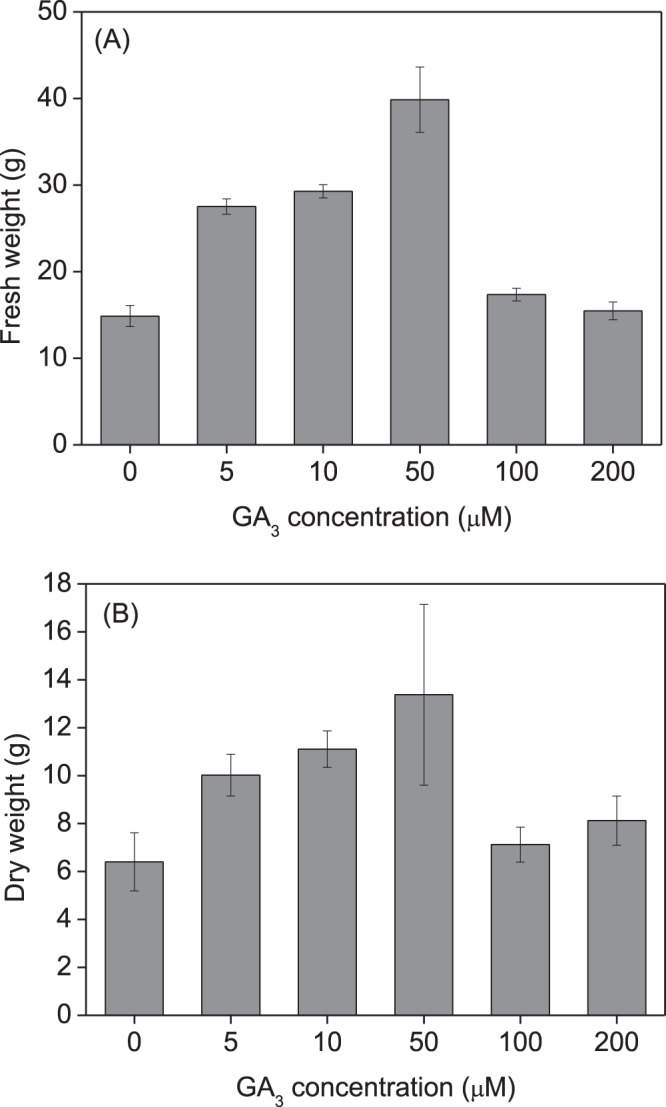
Effect of seed priming with gibberellic acid-3 (GA3) on fresh and dry weight of Leymus chinensis grown in pots (2016). Leymus chinensis seeds were germinated in the presence of GA3 at different concentrations, as indicated (0–200 μM), and the seedlings were transplanted to and grown in clay pots. (A) Fresh weight, (B) dry weight per plant. Data are means ± s.e.
Transgenerational effects of seed priming with GA3 treatment on clonal offspring growth in field conditions (2017)
Seed treatment with GA3 at all concentration levels promoted clonal offspring plant growth during the whole growth period, as shown by the increased plant height and tiller number per plant (Fig. 4). Values of plant height and tiller number were highest at 50 μM GA3 (Fig. 4A). Plant height (df = 5, F = 19.458, p < 0.001) of L. chinensis was significantly affected by GA3 treatment. Significant promotion of tiller number by GA3 concentration (df = 5, F = 11.083, p < 0.001) was observed, especially at a concentration of 50 μM GA3 (Fig. 4B).
Figure 4.
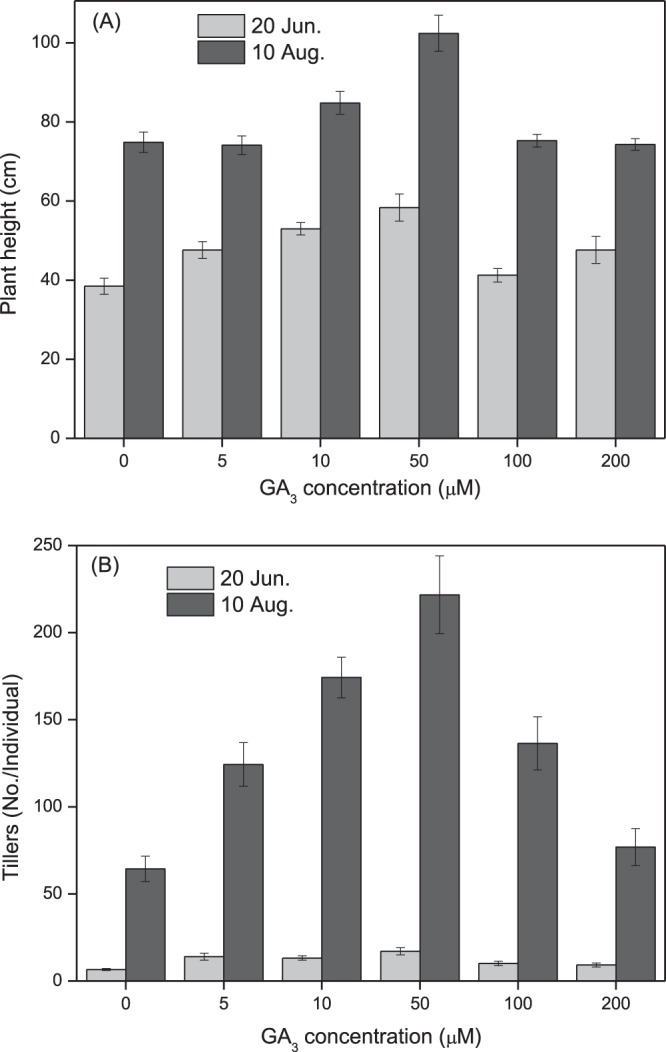
Effect of seed priming with gibberellic acid-3 (GA3) on plant growth of Leymus chinensis in the field experiment (2017). Leymus chinensis seeds were germinated in the presence of GA3 at different concentrations, as indicated (0–200 μM), and the seedlings were transplanted to and grown in clay pots in 2016. The tillers grown from the mother plants were separated individually and transplanted to the field in 2017. (A) Plant height, (B) tillers per plant. The columns in light grey and grey represent the measurements made on July 20 and August 10, 2017, respectively. Data are means ± s.e.
Grass production was also markedly enhanced by seed treatment with GA3 (Figs 5, 6). Both fresh (df = 5, F = 5.279, p = 0.021) and dry (df = 5, F = 8.552, p < 0.001) shoot weights were significantly affected by GA3 concentrations, with the 50 μM GA3 treatment having the highest promoting effect.
Figure 5.
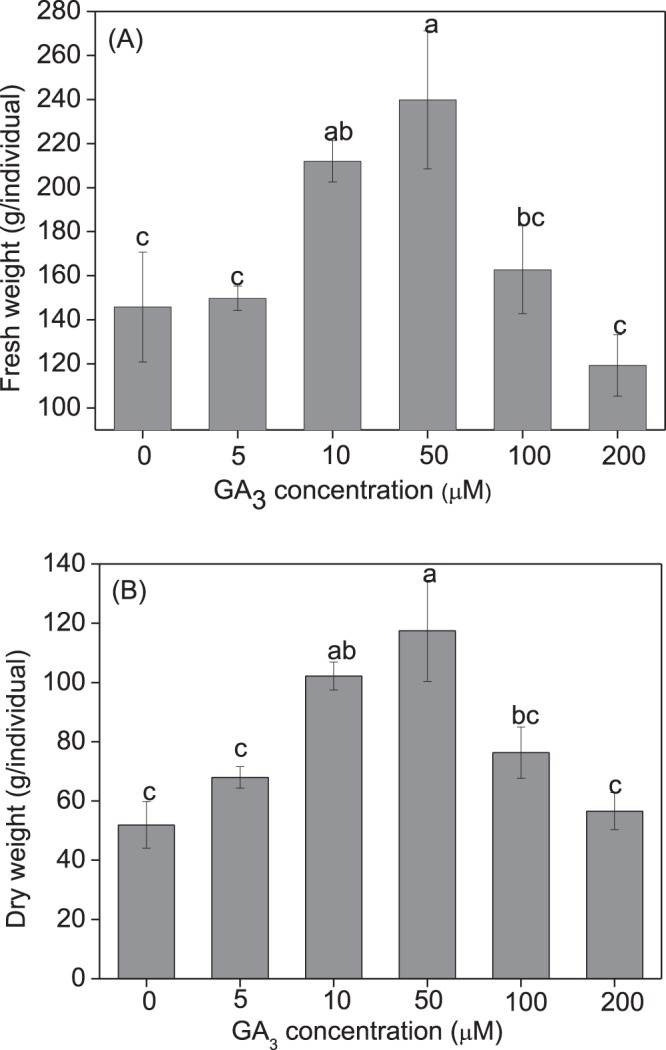
Effect of seed priming with gibberellic acid-3 (GA3) on fresh and dry weight of Leymus chinensis in the field experiment (2017). Leymus chinensis seeds were germinated in the presence of GA3 at different concentrations, as indicated (0–200 μM). The seedlings were transplanted to and grown in clay pots in 2016. The tillers grown from the mother plants were separated individually and transplanted to the field in 2017. (A) Plant height, (B) tillers per plant. The columns in light grey and grey represent the measurements made on July 20 and August 10, 2017, respectively. Data are means ± s.e.
Figure 6.
Image of the effect of priming with gibberellic acid-3 (GA3) on plant growth in transgenerational Leymus chinensis offspring in field conditions (2017).
Discussion
Native perennial species in natural grassland plays a very important role in the broad-scale restoration of degraded ecosystems, where grass reseeding technology has great potential for restoring ecosystem functionality29,30. Leymus chinensis previously dominated native perennial grass species on the eastern Eurasian Steppe and is considered to be the most attractive grass in the restoration of artificially established grasslands. In this study, seed priming with GA3 significantly enhanced seed germination and subsequent plant growth (Figs 2, 4), and grass production (Figs 3, 5) in L. chinensis. In particular, GA3 priming at a concentration of 50 μM enhanced germination rate by 27.0%, and grass production in fresh and dry matter by 168.2% and 108.9% in pot (Fig. 3), and 64.5% and 126.2% in field (Fig. 5) conditions, respectively. It is noteworthy that the significant improvement in grass production for at least two years was obtained by just a single GA3 seed treatment (priming). These results strongly demonstrated that seed priming with GA3 is a simple but effective method for enhancing grass production in L. chinensis, especially in artificial grasslands where seeding is necessary.
The poor seed germination of L. chinensis has been considered an obstacle to the establishment of artificial grasslands28. Several strategies for improving seed germination have been suggested, for example, cold stratification, removal of glumes31, and exogenous hormone treatments28. Seed priming with GA3 has been demonstrated to be a useful tool for activating metabolic germination processes and facilitating increments in physiological processes during seed germination1,4,7, especially for grass seeds exhibiting physiological dormancy (PD)3, e.g., Leymus arenarius32, Setaria viridis33, Tripsacum dactyloides34, and some Triodia species (Poaceae)35. In L. chinensis, we previously proved a positive relationship (p > 0.05) between seed germination and endogenous hormone content during seed development36. In this study, exogenous GA3 treatment at a range of 5–200 μM enhanced germination rate, with the highest effect recorded at 50 μM (Fig. 1). High GA3 concentrations of ≥100 μM showed a less beneficial effect on seed germination compared to concentrations of 10–50 μM. These results are somewhat inconsistent with previous reports that GA3 concentrations as high as 300 μM37 or 2.89 mM38 showed higher promoting effects than other concentrations in L. chinensis seed germination. This discrepancy may be ascribed to different degrees of dormancy in the seed material used in the experiments.
GA-priming has been demonstrated to promote seedling growth in various crop plants4,6; Seed priming using GA3 at appropriate concentrations leads to high germination rates and better seedling growth; however, the beneficial concentration differs among plant species. GA3 treatment showed the highest promoting effect on seed germination and seedling growth in Capparis spinosa at 360.9 μM7, Trigonella foenum-graecum at 180.4 μM11, and 721.8–1443.5 μM for Parthenium argentatum Gray39. The yield attributes of Helianthus annuus L.19 and Triticum aestivum L.12 were also increased by seed treatment with 10–100 μM GA3 for 8 h. In previous studies on L. chinensis, GA spraying at various growth stages remarkably promoted plant growth and grass production40–42. In this study, we showed that seed priming with GA3 significantly promoted plant growth (Figs 2, 4) and enhanced grass production (Figs 3, 5) in L. chinensis, in both pot and field experiments. Similar to the effect on seed germination (Fig. 1), seed treatment with GA3 at 50 μM yielded the highest promoting effect on plant growth (Figs 2–6), and GA3 levels above 50 μM were less beneficial to plant growth than in the range of 5–50 μM (Figs 2–6). This is in accordance with observations that phytohormones only function within a threshold range of concentration levels. However, the most promoting effects of GA3 concentrations on production of the first, second, and following generations in L. chinensis needs further study.
The most significant and unexpected finding in this study was that the beneficial effect of seed priming with GA3 was passed on to clonal offspring for at least two years in L. chinensis (Figs 2–6). The fact that the priming effect was also observed in the next generation plants (Figs 4–6) implies a transgenerational transmission mechanism for GA-priming effects in this species. Transgenerational effects have been observed in many species in passing on to offspring maternal stress responses including responses to drought43, salinity44, and light45,46. Hartmann et al.45 report that far-red irradiated seeds of Chenopodium album and Stellaria media showed a significantly reduced emergence for two years, demonstrating the influence of the maternal far-red-absorbing seed phytochrome Bfr over time45. Very recently, Ren et al.47 have reported that long-term overgrazing-induced memory decreased the photosynthesis of clonal offspring in L. chinensis by decreasing leaf chlorophyll content and Rubisco enzyme activity, and downregulating a series of key genes that regulate photosynthetic efficiency, stomata opening, and chloroplast development47. It would be very interesting to observe further through how many generations GA priming effects can succeed. The molecular and physiological mechanisms underlying the transgenerational effects of GA-priming remain to be explored, although DNA methylation changes induced by environmental cues have been implicated in many studies of transgenerational effects43,48.
Conclusion
In this study, we showed that seed priming with GA3 can significantly enhance seed germination rate and subsequent plant growth and grass production in a perennial grass species, L. chinensis. The GA priming effect was transgenerational, with the clonal offspring also showing enhanced plant growth and grass production. Our findings provide a new practical method for improving perennial grass productivity, especially in artificial grasslands, in which seeding is necessary. On the other hand, some questions remain unresolved, such as how long or through how many generations can the GA-priming effect be preserved, and what molecular and physiological mechanisms underpin the transgenerational transmission of GA-priming effects to clonal offspring.
Materials and Methods
A schematic of the overall experimental design is shown in Fig. 7.
Figure 7.
Schematic of the overall experimental design.
Plant seeds
Matured seeds of L. chinensis were collected from the Da’an Sodic Land Experiment Station (45°35′58″–45°36′28″N, 123°50′27″–123°51′31″E), in the western part of the Songnen Plain, northeast China, in late July, 2015. The collected seeds were air-dried at room temperature, placed in a paper bag, and stored at 4 °C until November 2015, just before the germination experiments were undertaken.
GA3 treatment and seed germination (2015)
A 10 mM GA3 stock solution was prepared by adding 0.0346 g GA3 to a 10 ml volumetric flask and dissolved by a drop of 95% ethylalcohol, after which distilled water was added to make a total solution of 10 ml. Seeds of L. chinensis were surface-sterilized in 0.1% HgCl2 for 10 min and then washed with distilled water several times before being used in the experiments. Approximately 25 seeds were sown in a Petri dish (diameter 9 cm) containing 0.7% (w/v) water agar supplemented with 0 (control, ethylalcohol at a concentration comparable to that in GA solutions), or 5, 10, 50, 100, 200 μM of GA3, with four replicates for each treatment. The Petri dishes were incubated under an alternating cycle of 12/12 h light (fluorescent and incandescent white light of 54 μmol·m−2 s−1) at 28/16 °C. Seed germination was measured every second day until no new germination occurred within three days.
Plant growth in pot experiments (2016)
After determining the germination rate, seedlings were transplanted to clay pots (diameter 30 cm, and height 30 cm) each containing 10 kg of soil, in early January 2016. The clay loam soil was collected from a field based at the Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China. Four seedlings were transplanted into each pot, with four replicates per treatment. Tap water was added to the pots to keep the soil moist. Plant height and tillers per plant were recorded during growth, and grass production (fresh and dry weight of shoots) was measured in late July. The aboveground L. chinensis plant material in each pot were cut separately and the fresh weight recorded immediately. Then, the plants were put in paper bags and dried in an oven at 105 °C for 2 h and 80 °C for another 48 h, and the dry weight of each treatment was recorded.
Plant growth in field experiments (2017)
The L. chinensis tillers were separated individually from the mother plants grown in pots, and transplanted to a field with one individual clonal offspring in each hill at the Institute of Geography and Agroecology on April 24, 2017; the field was the same as that from which soil was collected for the pot experiment, and therefore the soil was of the same type. The row and line spacings were 60 cm and 50 cm, respectively. The plot was irrigated once, immediately after the transplantation. Height and tiller number of plants in each hill were recorded on June 20 and August 10, 2017, respectively, with 30 hills for each GA3 concentration. Grass production (fresh and dry weight of shoots) was measured in late August, 2017 with 10 replicates for each GA3 concentration treatments.
Statistical analysis
Generalized linear models (GLMs) with a binomial error structure and logit link function were used to compare proportional data relating to the final germination of L. chinensis in the GA3 treatments. The differences in the tiller number and plant height among GA3 treatments were analyzed by repeated measures ANOVA using a linear mixed effect model in the lme4 package in R. We treated sampling time as a random effect and allowing concentration to enter the model as a fixed effect. If necessary, data were log transformed to meet assumptions of normality and homogeneity of variance. In addition, data of fresh and dry weights among different treatments were compared separately by one-way analysis of variance (ANOVA). Before the analyses of ANOVA, the normality (shapiro-wilk test) and homoscedasticity (Levene’s test) was conducted. A Tukey’s test was used for multiple comparisons when the among treatments was significant. All of the analyses were carried out using the R statistical platform49.
Acknowledgements
This study was supported from the National Basic Research Program of China (2015CB150802), the National Natural Science Foundation of China (41771058, 41371260, 41001027), and the National Key Research & Development Program of China (2016YFC0501200) and the National Key Basic survey of resources (2015FY110500).
Author Contributions
H.-Y.M., C.-J.J., Z.-W.L. designed the experiment, and H.-Y.M., D.-D.Z., Q.-R.N., J.-P.W., Y.L., M.-M.W., X.-L.L. conducted the experiments and collected the data. H.-Y.M. and Q.-R.N. analyzed the data. H.-Y.M. and C.-J.J. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chang-Jie Jiang, Email: cjjiang@affrc.go.jp.
Zheng-Wei Liang, Email: liangzw@iga.ac.cn.
References
- 1.Dotto L, Silva VN. Beet seed priming with growth regulators. Semina: Ciencias Agrarias. 2017;38:1785–1798. [Google Scholar]
- 2.Maiti R, Pramanik K. Vegetable seed priming: a low cost, simple and powerful techniques for farmers’ livelihood. Int. J. Bio-Resour. Stress Manag. 2013;4:475–481. [Google Scholar]
- 3.Baskin, C. C. & Baskin, J. M. Seeds: ecology, biogeography, and evolution of dormancy and germination (2nd edition). Academic Press, San Diego (2014).
- 4.Paparella S, et al. Seed priming: state of the art and new perspectives. Plant Cell Rep. 2015;34:1281–1293. doi: 10.1007/s00299-015-1784-y. [DOI] [PubMed] [Google Scholar]
- 5.Chunthaburee S, Sanitchon J, Pattanagul W, Theerakulpisut P. Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Not. Bot. Horti. Agrobo. 2014;42:405–413. [Google Scholar]
- 6.Ibrahim EA. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016;192:38–46. doi: 10.1016/j.jplph.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Heydariyan M, Basirani N, Sharifi-Rad M, Khmmari I, Poor SR. Effect of seed priming on germination and seedling growth of the caper (Capparis spinosa) under drought stress. Intl. J. Adv. Biol. Biom. Res. 2014;2:2381–2389. [Google Scholar]
- 8.Abiri R, et al. Quantitative assessment of indica rice germination to hydropriming, hormonal priming and polyethylene glycol priming. Chil. J. Agr. Res. 2016;76:392–400. doi: 10.4067/S0718-58392016000400001. [DOI] [Google Scholar]
- 9.Ali HM, et al. Effects of gibberellic acid on growth and photosynthetic pigments of Hibiscus sabdariffa L. under salt stress. Afr. J. Biotechnol. 2012;11:800–804. [Google Scholar]
- 10.Wei LX, et al. Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crops Res. 2017;203:86–93. doi: 10.1016/j.fcr.2016.12.024. [DOI] [Google Scholar]
- 11.Farahmandfar E, Shirvan MB, Sooran SA, Hoseinzadeh D. Effect of seed priming on morphological and physiological parameters of fenugreek seedlings under salt stress. Intl. J. Agri. Crop Sci. 2013;5:811–815. [Google Scholar]
- 12.Ulfat A, Majid SA, Hameed A. Hormonal seed priming improves wheat (Triticum aestivum L.) field performance under drought and non-stress conditions. Pak. J. Bot. 2017;49:1239–1253. [Google Scholar]
- 13.Khaliq A, et al. Seed priming with selenium: consequences for emergence, seedling growth, and biochemical attributes of rice. Biol. Trace Elem. Res. 2015;166:236–244. doi: 10.1007/s12011-015-0260-4. [DOI] [PubMed] [Google Scholar]
- 14.Ghodrat V, Rousta MJ. Effect of Priming with Gibberellic Acid (GA3) on Germination and growth of corn (Zea mays L.) under saline conditions. Intl. J. Agri. Crop Sci. 2012;4:882–885. [Google Scholar]
- 15.Pallaoro DS, et al. Priming corn seeds with plant growth regulator. J. Seed Sci. 2016;38:227–232. doi: 10.1590/2317-1545v38n3163847. [DOI] [Google Scholar]
- 16.Aymen EM, Kaouther Z, Fredj MB, Cherif H. Seed priming for better growth and yield of safflower (Carthamus tinctorius) under saline condition. J. Stress Physiol. Biochem. 2012;8:135–143. [Google Scholar]
- 17.Afzal I, Basra SMA, Iqbal A. The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress Physiol. Biochem. 2005;1:6–14. [Google Scholar]
- 18.Iqbal M, Ashraf M. Gibberellic acid mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2013;86:76–85. doi: 10.1016/j.envexpbot.2010.06.002. [DOI] [Google Scholar]
- 19.Jafri N, Mazid M, Mohammad F. Responses of seed priming with gibberellic acid on yield and oil quality of sunflower (Helianthus annus L.). Indian J. Agric. Res. 2015;49:235. [Google Scholar]
- 20.Galhaut L, et al. Seed priming of Trifolium repens L. improved germination and early seedling growth on heavy metal-contaminated soil. Water Air Soil Poll. 2014;225:1905. doi: 10.1007/s11270-014-1905-1. [DOI] [Google Scholar]
- 21.Younesi O, Moradi A. Effect of priming of seeds of Medicago sativa “Bami” with gibberellic acid on germination, seedlings growth and antioxidant enzymes activity under salinity stress. J. Hortic. Res. 2014;22:167–174. doi: 10.2478/johr-2014-0034. [DOI] [Google Scholar]
- 22.Khan AS, Chaudhry NY. GA3 improves flower yield in some cucurbits treated with lead and mercury. Afr. J. Biotechnol. 2006;5:149–153. [Google Scholar]
- 23.Díaz S, Cabido M. Plant functional types and ecosystem function in relation to global change. J. Veg. Sci. 1997;8:463–474. doi: 10.1111/j.1654-1103.1997.tb00842.x. [DOI] [Google Scholar]
- 24.Li XL, et al. Pathways of Leymus chinensis individual aboveground biomass decline in natural semiarid grassland induced by overgrazing: a study at the plant functional trait scale. PLOS ONE. 2015;10:e0124443. doi: 10.1371/journal.pone.0124443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latzel V, González APR, Rosenthal J. Epigenetic memory as a basis for intelligent behavior in clonal plants. Front. Plant Sci. 2016;7:1–7. doi: 10.3389/fpls.2016.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma HY, Yang HY, Liang ZW, Ooi MKJ. Effects of 10-year management regimes on the soil seed bank in saline-alkaline grassland. PLOS ONE. 2015;10:e0122319. doi: 10.1371/journal.pone.0122319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, et al. Long-term effects of mowing on plasticity and allometry of Leymus chinensis in a temperate semi-arid grassland, China. Arid Land. 2016;8:899–909. doi: 10.1007/s40333-016-0005-z. [DOI] [Google Scholar]
- 28.Ma HY, et al. Lemmas and endosperms significantly inhibited germination of Leymus chinensis (Trin.) Tzvel. (Poaceae) J. Arid Environ. 2008;72:573–578. doi: 10.1016/j.jaridenv.2007.06.013. [DOI] [Google Scholar]
- 29.Wu GL, Liu ZH, Zhang L. Effects of artificial grassland establishment on soil nutrients and carbon properties in a black-soil-type degraded grassland. Plant Soil. 2010;333:469–479. doi: 10.1007/s11104-010-0363-9. [DOI] [Google Scholar]
- 30.Mganga KZ, Musimba NKR, Nyariki DM. Competition indices of three perennial grasses used to rehabilitate degraded semi-arid rangelands in Kenya. Rangeland J. 2015;37:489–495. doi: 10.1071/RJ15023. [DOI] [Google Scholar]
- 31.Ma HY, Liang ZW, Liu M, Wang MM, Wang SH. Mechanism of the glumes in inhibiting seed germination of Leymus chinensis (Trin.) Tzvel. (Poaceae) Seed Sci. Technol. 2010;38:655–664. doi: 10.15258/sst.2010.38.3.13. [DOI] [Google Scholar]
- 32.Greipsson S. Effects of stratification and GA3 on seed germination of a sand stabilising grass Leymus arenarius used in reclamation. Seed Sci. Technol. 2001;29:1–10. [Google Scholar]
- 33.Sebastian J, Wong MK, Tang E, Dinneny JR. Methods to promote germination of dormant setaria viridis seeds. PLOS ONE. 2014;9:e95109. doi: 10.1371/journal.pone.0095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogis C, Gibson LR, Knapp AD, Horton R. Enhancing germination of eastern gamagrass seed with stratification and gibberellic acid. Crop Sci. 2004;44:549–552. doi: 10.2135/cropsci2004.5490. [DOI] [Google Scholar]
- 35.Erickson TE, Shackelford N, Dixon KW, Turner SR, Merritt DJ. Overcoming physiological dormancy in seeds of Triodia (Poaceae) to improve restoration in the arid zone. Restor. Ecol. 2016;24:S64–S76. doi: 10.1111/rec.12357. [DOI] [Google Scholar]
- 36.Ma HY, Liang ZW, Wu HT, Huang LH, Wang ZC. Role of endogenous hormones, glumes, endosperm and temperature on germination of Leymus chinensis (Poaceae) seeds during development. J. Plant Ecol. 2010;3:269–277. doi: 10.1093/jpe/rtp035. [DOI] [Google Scholar]
- 37.He XQ, et al. Seed dormancy and dormancy-breaking methods in Leymus chinensis (Trin.) Tzvel. (Poaceae) Grass Forage Sci. 2016;71:641–648. doi: 10.1111/gfs.12220. [DOI] [Google Scholar]
- 38.Zhang WD, Bi JJ, Ning TY, Liu XL, He MR. Effects of temperature, light and other treatments on seed germination of Leymus chinensis. Can. J. Plant Sci. 2006;86:143–148. doi: 10.4141/P04-125. [DOI] [Google Scholar]
- 39.Dissanayake P, George DL, Gupta ML. Effect of light, gibberellic acid and abscisic acid on germination of guayule (Parthenium argentatum Gray) seed. Ind. Crop Prod. 2010;32:111–117. doi: 10.1016/j.indcrop.2010.03.012. [DOI] [Google Scholar]
- 40.Cui S, Mu CS. Effects of exogenous Gibberellic acid (GA3) on photosynthesis and transpiration of Leymus chinensis at the tillering and jointing stage. Acta Prataculturae Sinica. 2005;14:97–101. [Google Scholar]
- 41.Li GL, Liu XP, Du GM. Effects of exogenous gibberellic acid and cytokinin on the production performance of Leymus chinensis at returning green stage. Chinese Journal of Grassland. 2009;31:113–117. [Google Scholar]
- 42.Cai Y, Shao L, Li X, Liu G, Chen S. Gibberellin stimulates regrowth after defoliation of sheepgrass (Leymus chinensis) by regulating expression of fructan-related genes. J. Plant Res. 2016;129:1–10. doi: 10.1007/s10265-016-0832-1. [DOI] [PubMed] [Google Scholar]
- 43.González APR, et al. Stress-induced memory alters growth of clonal offspring of white clover (Trifolium repens) Am. J. Bot. 2016;103:1567–1574. doi: 10.3732/ajb.1500526. [DOI] [PubMed] [Google Scholar]
- 44.Yang F, et al. Transgenerational plasticity provides ecological diversity for a seed heteromorphic species in response to environmental heterogeneity. Perspect. Plant Ecol. 2015;17:201–208. doi: 10.1016/j.ppees.2015.03.003. [DOI] [Google Scholar]
- 45.Hartmann KM, Grundy AC, Market R. Phytochrome-mediated long-term memory of seeds. Protoplasma. 2005;227:47–52. doi: 10.1007/s00709-005-0130-6. [DOI] [PubMed] [Google Scholar]
- 46.Galloway LF, Etterson JR. Transgenerational plasticity is adaptive in the wild. Science. 2007;318:1134–1136. doi: 10.1126/science.1148766. [DOI] [PubMed] [Google Scholar]
- 47.Ren W, et al. Long-term overgrazing-induced memory decreases photosynthesis of clonal offspring in a perennial grassland plant. Front. Plant Sci. 2017;8:419. doi: 10.3389/fpls.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herman JJ, Sultan SE. DNA methylation mediates genetic variation for adaptive transgenerational plasticity. Proc. R. Soc. B. 2016;283:20160988. doi: 10.1098/rspb.2016.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Core Development Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http:// www.R-project.org/.




