During their life, T cells are constantly circulating throughout the body. Sphingosine-1-phosphate (S1P), a sphingolipid metabolite, triggers T cell egress from the thymus and secondary lymphoid organs (SLO) into the lymph and the blood. This phenomenon is dependent on the S1P gradient between lymphoid organs (including thymus and SLO) and the lymphatic and blood vessels, which exhibit low, intermediate and high S1P levels, respectively. T cells follow the S1P gradient via the engagement of the G-protein-coupled receptor S1P receptor 1 (S1P1) expressed on T cells.1 S1P production is regulated by various sphingolipid-metabolizing enzymes. The sphingosine kinases 1 and 2 (SKs), encoded by Sphk1 and Sphk2, phosphorylate the sphingosine (Sph) into S1P. Conversely, S1P can be dephosphorylated to Sph by S1P phosphatases 1 and 2, encoded by Sgpp1 and Sgpp2. Alternatively, S1P can be irreversibly degraded by the S1P Lyase (SPL), encoded by Sgpl1, into phosphoethanolamine and hexadecenal. The secretion of S1P was described to involve two transporters, the ATP-binding cassette (ABC) transporter, in a non-specific manner, and the recently identified specific transporter Spinster 2 (SPNS2), encoded by the Spns2 gene.2 As a result, Spns2-deficient mice exhibit decreased concentrations of S1P into the lymph but not blood.3 In a recent study, Mendoza et al. reported that S1P, which is secreted by lymphatic endothelial cells via SPNS2, favors naive T-cell survival by maintaining their mitochondrial content in an S1P1-dependent manner.4 Herein, we discuss the main findings and pathophysiological implications of the latter study as regard to the state of the art.
Maturation of naive T cells occurs in the thymus and rises to ~108 different T-cell receptors (TCR). Newly differentiated single positive CD4 and CD8 thymocytes, which express S1P1 at high levels, migrate from the thymus to the blood stream following the S1P gradient.5 In the blood, high concentrations of S1P induce the internalization of S1P1 allowing for potential relocation of T cells into SLO or tissues. Within SLO, naive T cells patrol until they encounter their specific peptide presented by an antigen-presenting cell (APC). During T cell activation, S1P1 is trapped inside the cells through its interaction with the very early activation antigen marker CD69. This induces T-cell arrest into the lymph node to allow for proliferation and differentiation of antigen specific T cells. S1P1 is then re-expressed by both effector and central memory T cells and exit the lymph node.6 Unactivated naive T cells, which travel through the lymph nodes, do not down-regulate S1P1 at their cell surface and go back directly to the circulation through the sensing of S1P gradient. Of note, transgenic mice with a targeted deletion of Spns2 in endothelial cells exhibited (i) low S1P levels in the lymph and (ii) reduced T-cell content in the blood, spleen and lymph nodes, further arguing for the key role S1P plays in T-cell trafficking.
To evaluate the role of lymphatic endothelial S1P on T-cell homeostasis, Mendoza et al. generated a mouse model (Spns2Δ), exhibiting a conditional deletion of Spns2 in Lymphatic endothelial cells (LEC) by crossing Spns2f/f mice with mice expressing the Cre recombinase under the Lyve 1 promoter (Lyve1-cre). This specific deletion led to a decreased number of naive T cells in SLO, including lymph nodes. Mechanistically, the authors showed that the proportion of apoptotic naive T cells, as evaluated by propidium iodide staining, annexin V labeling and measurement of caspase activity, was doubled in lymph nodes from Spns2Δ mice compared to control mice. To demonstrate that apoptosis contributed, at least in part, to the reduction in naive T-cell content in lymph nodes, WT and Spns2Δ mice were adoptively transferred with naive T cells from BCL2 transgenic mice, which overexpressed the human anti-apoptotic Bcl-2 protein. As a matter of fact, BCL2 overexpression protected naive T cells from apoptosis when infiltrating lymph nodes from Spns2Δ mice. Thus, in the absence of S1P secretion by LEC, naive T cells underwent cell death in lymph nodes, most likely via a mitochondrial apoptotic pathway, a process counteracted by Bcl-2 overexpression. Accordingly, the authors provide evidence that disruption of S1P signaling in naive T cells is associated with a 40% reduction in mitochondrial content, possibly as a consequence of mitophagy (Figure 1).7
Figure 1.
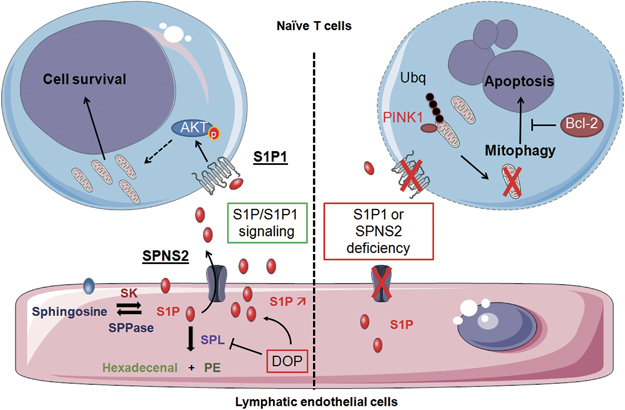
S1P from lymphatic endothelial cells (LEC) is secreted in a SPNS2-dependent manner, promoting naive T-cell survival via S1P1. Sphingosine kinases (SK) 1/2 transform sphingosine into S1P, which is secreted by LEC through the specific transporter SPNS2. S1P can be dephosphorylated by S1P phosphatases 1/2 (SPPases) or irreversibly degraded into phosphoethanolamine (PE) and hexadecenal by S1P lyase (SPL). Inhibition of SPL by DOP (deoxypyridoxine) increases the level of S1P in lymph nodes. S1P binds to S1P1 expressed by naive T cells, which then survive most likely upon activation of the AKT pathway. SPNS2 or S1P1 deficiency impairs this signaling, inducing loss of mitochondria and subsequent apoptosis. Mechanistically, PINK1 accumulates in mitochondria and induces ubiquitinylation of mitochondrial proteins, leading to mitophagy. Overexpression of Bcl-2 counteracts apoptosis induced by the loss of S1P/S1P1 signaling. Bcl-2, B cell lymphoma 2; PINK1, PTEN-induced putative kinase 1; S1P, sphingosine-1-phosphate; S1P1, S1P receptor 1; Ubq, Ubiquitin.
To evaluate the role of S1P in naive T-cell survival, the authors first used a pharmacological approach and injected mice with 4-deoxypyridoxine (DOP), an SPL inhibitor that increases S1P levels. Of note, SPL is the main enzyme involved in the maintenance of low S1P concentration within lymph nodes. Inhibition of SPL was sufficient to reduce the level of apoptotic naive T cells observed in Spns2Δ mice. Next, using a genetic approach, the authors further demonstrated that the S1P pro-survival effect on naive T cells was dependent on the stimulation of S1P1 receptor. Indeed, in thymectomized adult WT mice irradiated and reconstituted with bone marrow from WT or S1pr1-deficient mice, S1pr1 deficiency increased the proportion of apoptotic naive T cells in both LN and spleen. Thus, this study highlights for the first time the critical role of S1P1-dependent S1P signaling in protecting naive T cells from apoptosis. In this context, how LEC produce S1P prior secretion via SPNS2 remains to be determined.
This study is in line with the concept that S1P behaves as an anti-apoptotic sphingolipid metabolite, which impairs the caspase-dependent pro-apoptotic mitochondrial pathway.8 Since S1P antagonizes the pro-apoptotic action of other sphingolipid metabolites such as ceramide and sphingosine, it is tempting to speculate that sphingolipid alterations in naive T cells may contribute, at least in part, to apoptosis induction. S1P can also act intracellularly as a bioactive molecule in cell signaling to prevent apoptosis as demonstrated by S. Spiegel’s group.9 However, S1P signaling is not always associated with cell survival as illustrated by D. Green and colleagues, who demonstrated that intracellular S1P produced by SK2 cooperates with the pro-apoptotic protein Bak to trigger mitochondrial outer-membrane permeabilization, leading to apoptosis.10
Homeostatic proliferation of naive T cells is dependent on two major mechanisms: (i) IL-7 signaling, which is required for naive T-cell development and maintenance and (ii) TCR signaling triggered by low strength interaction with self-peptide associated to MHC. In this context, IL-7 binding to IL-7R seems to be the major pathway for naive T-cell survival through inhibition of the mitochondrial apoptotic pathway. Activated IL-7R leads to the phosphorylation and cytosolic retention of the pro-apoptotic protein Bad through the PI3K/Akt pathway.11 IL-7/IL-7R also induces the expression of the anti-apoptotic protein Bcl-2.12 Moreover, activation of the PI3K/Akt/mTOR pathway in response to IL-7 promotes the trafficking of GLUT-1 to the cell surface and subsequent glucose uptake, which is essential for the maintenance of IL-7R function. S1P1 induces Akt phosphorylation in naive T cells, which can contribute to their survival.13 Moreover, S1P1 can activate additional pathways such as STAT3, which contributes to the expression of the two anti-apoptotic protein Bcl-2 and Bcl-xL. STAT3 can be found in the inner membrane of mitochondria, promoting the integration of the electron transport chain complex I.14 Whether STAT3 contributes to S1P1-dependent naive T-cell survival remains to be investigated. Moreover, one can speculate that S1P1 and IL-7 synergize to promote T-cell survival and expansion.
S1P1-dependent S1P signaling is unlikely restricted to naive T cells. Indeed, effector and central memory T-cell subtypes express S1P1. In addition, central memory T cells, which are mainly located in SLO, use oxidative phosphorylation as a main source of energy production.15 Thus, it is tempting to speculate that the S1P/S1P1 signaling also promotes central memory T-cell survival, by maintaining their mitochondrial pool. Multiple sclerosis patients treated with FTY720 (Fingolimod), a sphingosine analog, develop lymphopenia as a consequence of lymphocyte trafficking alteration. Mechanistically, FTY720 is phosphorylated by sphingosine kinases and the phosphorylated form behaves as a functional antagonist of S1P1, triggering its internalization and desensitization. A growing body of evidence in the literature indicates that FTY720 can trigger autophagy in various cell types, including cancer cells, and displays not only immunosuppressive effects but also anti-cancer properties.16 Whether FTY720 biological activities are dependent, at least in part, on the induction of mitophagy and subsequent cell death of some T-cell subtypes and/or cancer cells remain to be determined.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 2.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza A, Breart B, Ramos-Perez WD, Pitt LA, Gobert M, Sunkara M, et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2:1104–1110. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza A, Fang V, Chen C, Serasinghe M, Verma A, Muller J, et al. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature. 2017;546:158–161. doi: 10.1038/nature22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 6.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 7.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–2836. doi: 10.1182/blood.V98.9.2828. [DOI] [PubMed] [Google Scholar]
- 9.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/S1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 10.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–29166. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 12.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buck MD, O'Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallington-Beddoe CT, Hewson J, Bradstock KF, Bendall LJ. FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy. 2011;7:707–715. doi: 10.4161/auto.7.7.15154. [DOI] [PubMed] [Google Scholar]


