Abstract
Development of effective antimicrobial agents continues to be a great challenge, particularly due to the increasing resistance of superbugs and frequent hospital breakouts. There is an urgent need for more potent and safer antibiotics with novel scaffolds. As historically many commercial drugs were derived from natural products, discovery of antimicrobial agents from complex natural product structures still holds a great promise. Herein, we report the total synthesis of natural albomycins δ1 (1a), δ2 (1b), and ε (1c), which validates the structures of these peptidylnucleoside compounds and allows for synthetic access to bioactive albomycin analogs. The efficient synthesis of albomycins enables extensive evaluations of these natural products against model bacteria and clinical pathogens. Albomycin δ2 has the potential to be developed into an antibacterial drug to treat Streptococcus pneumoniae and Staphylococcus aureus infections.
Albomycins are promising drug candidates for the treatment of bacterial infections. Here, the authors describe the total syntheses of albomycins δ1, δ2, and ε, and evaluate their antimicrobial activity, identifying albomycin δ2 as a strong agent against S. pneumoniae and S. aureus infections.
Introduction
Sideromycins are a class of antibiotics covalently linked to siderophores1. They are actively transported into bacterial cells via siderophore uptake pathways commonly found in bacterial pathogens, by the so-called “Trojan horse” strategy, resulting in outstanding cell envelope permeability and very low minimum inhibitory concentrations (MICs). These pathogen-specific antibiotics are promising drug candidates for the treatment of various bacterial infections2–4. A few naturally occurring sideromycins have been discovered. Among these, albomycins, originally reported as grisein, were first isolated from soil microorganism Streptomyces griseus in 19475–9. Albomycins exhibited potent inhibitory activities against a number of Gram-negative, as well as Gram-positive bacteria, including multi-drug resistant strains1,10,11. For instance, albomycins exhibited an MIC value of 10 ng/mL against Streptococcus pneumoniae and 5 ng/mL against Escherichia coli, which is almost tenfold more potent than penicillin12. Moreover, no toxicity was observed during in vivo studies of albomycins, and it was well tolerated and safe up to a maximum dose evaluated in mice10. Albomycins have been successfully used to treat human bacterial infections in the Soviet Union10.
The structures of albomycins were fully elucidated by Benz and coworkers in 198213,14, 35 years after their initial isolation. Albomycins δ1 (1a), δ2 (1b), and ε (1c) are all composed of a tri-δ-N-hydroxy-L-ornithine peptide siderophore and a thionucleoside warhead with six consecutive chiral centers, and differ only in the C4 substituent (R) of the pyrimidine nucleobase (Fig. 1). As for 1b, the thionucleoside warhead is a potent seryl-tRNA synthetase inhibitor known as SB-21745215. The highly complex and densely functionalized structures, together with their important therapeutic potential, have made albomycins attractive targets for synthesis. Different synthetic strategies for the tri-δ-N-hydroxy-L-ornithine peptide siderophore have been described by the groups of Benz16,17 and Miller18–20. The synthesis of the thionucleoside moiety of 1a was briefly described by Holzapfel et al. in 199121, though with incomplete data. However, no total synthesis of albomycins has been reported. In one study22, an oxygen analog of 1a was synthesized. Surprisingly, a single replacement of the sulfur with oxygen resulted in the complete loss of antibacterial activity, suggesting a critical role of the sulfur atom in the activity of albomycins. A biosynthetic approach has also been attempted for the generation of albomycin analogs23, but albomycin production by S. griseus is difficult and has yet to be scaled up efficiently24. Herein, we describe the total synthesis of the three natural albomycins (1a–c), which features a Pummerer reaction for nucleobase introduction and an aldol reaction to expand the side chain of thionucleoside. Their biological evaluations demonstrate that albomycin δ2 is a promising lead candidate for treating S. pneumoniae and S. aureus infections and warrants further development.
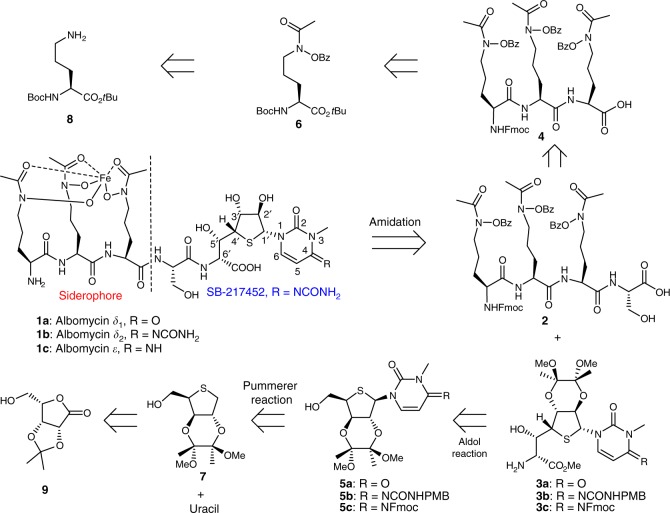
Fig. 1.
Chemical structures of albomycins δ1, δ2, and ε, and their synthetic strategies. The key disconnections involve a Pummerer reaction for nucleobase introduction and an aldol reaction to expand the side chain of thionucleoside. tBu tert-butyl, Bz benzoyl, Fmoc fluorenylmethyloxycarbonyl, PMB p-methoxybenzyl
Results
Synthetic strategy
The retrosynthetic analysis of albomycins (1a–c) in a collective fashion is shown in Fig. 1. The amide bond linking to the thionucleoside core was first disconnected to generate tetrapeptide fragment 2 and thionucleosides 3a–c. Tetrapeptide 2 could be readily accessed via the condensations of tripeptide 4 with L-serine tert-butyl ester. Tripeptide 4 could be derived from amino acid 6, which could arise from the direct oxidation of protected L-ornithine 8. We anticipated that thionucleosides 3a–c could be installed by substrate-directed asymmetric aldol condensation reaction from thionucleosides 5a-c, which could in turn be prepared through a Pummerer reaction from thiosugar 7 and uracil. Thiosugar 7 could be further traced back to the known L-(+)-lyxose derivative 9. Due to the structural feature and labile nature of albomycins, the protecting group strategy had to be delicately selected to accomplish the total synthesis. Because of the presence of reducible hydroxamic acids in the siderophore moiety and an imine group in the thionucleoside moiety of 1b and 1c, protecting groups such as Cbz and Bn which usually need to be deprotected under reducing conditions of H2 and Pd/C in the final stage were avoided. As for the hydroxyl groups in the thionucleoside fragment, orthogonality of protecting groups and their influence on stereoselectivity of uracilation were also key considerations.
Total synthesis of albomycins δ1, δ2, and ε
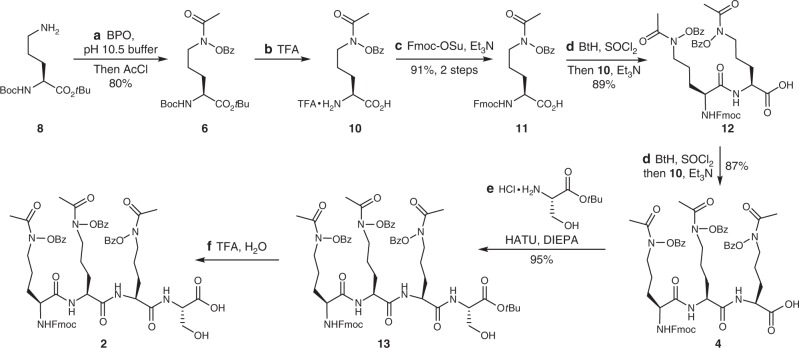
Our synthetic efforts commenced with the preparation of tetrapeptide 2 (Fig. 2). The core component hydroxamic acid 6 of tetrapeptide 2 was first synthesized from N2-Boc-L-ornithine tert-butyl ester 825. Oxidation of the free amine in N2-Boc-L-ornithine tert-butyl ester 8 with benzoyl peroxide25–27, followed by acylation under biphasic conditions afforded the fully protected hydroxamate 6 in 80% yield. Removal of tert-butyl-carbonate (Boc) and tert-butyl (t-Bu) groups with trifluoroacetic acid (TFA) provided the TFA salt 10. The resulting amine group was protected with Fmoc to deliver acid 11 in 91% yield over two steps. Then, we turned to coupling 11 and 10 under active ester-mediated coupling conditions (DCC, NHS)20, which proceeded smoothly to produce dipeptide 12. Unfortunately, application of the same conditions to synthesize tripeptide 4 led to significant epimerization. Failure to optimize the condition after numerous efforts prompted us to explore an alternative strategy. Fortunately, under active amide-mediated coupling conditions developed by Katritzky28–31, tripeptide 4 could be obtained in an iterative fashion from 11 in 77% yield over two steps without any detectable epimerization. Tripeptide 4 was condensed with L-serine tert-butyl ester hydrochloride in the presence of HATU and DIPEA to generate tert-butyl ester 13 in 95% yield. Treatment of 13 with TFA in dichloromethane at 0 °C proceeded smoothly to yield the corresponding tetrapeptide 2.
Fig. 2.
Synthesis of tetrapeptide 2. Reagents and conditions: a BPO (2.0 equiv), pH 10.5 buffer, CH2Cl2, 3 h, then AcCl (1.2 equiv), CH2Cl2, 80%; b TFA, H2O, 12 h; c Fmoc-OSu (1.2 equiv), Et3N (3.0 equiv), DMF, −15 °C, overnight, 91% (2 steps); d BtH (4.0 equiv), SOCl2 (1.0 equiv), THF, 50 min, then 10 (1.1 equiv), Et3N (3.1 equiv), CH3CN/H2O (5:2), −15 °C, 2 h, 12: 89%, 4: 87%; e H-Ser(tBu)-OtBu hydrochloride (1.1 equiv), HATU (1.5 equiv), DIPEA (2.0 equiv), DMF, −15 °C, 2 h, 95%; f TFA, H2O, CH2Cl2, 0 °C, 18 h. BPO benzoyl peroxide, AcCl acetyl chloride, TFA trifluoroacetic acid, Fmoc-OSu N-(9-fluorenylmethoxycarbonyloxy)-succinimide, DMF N,N-dimethyformamide, DCC dicyclohexylcarbodiimide, NHS N-hydroxysuccinimide, BtH 1H-benzotriazole, THF tetrahydrofuran, HATU 1-[bis(dimethylamino) methylene]-1H-1,2,3-triazolo [4,5-b] pyridinium 3-oxid hexafluorophosphate, DIPEA N,N-diisopropylethylamine
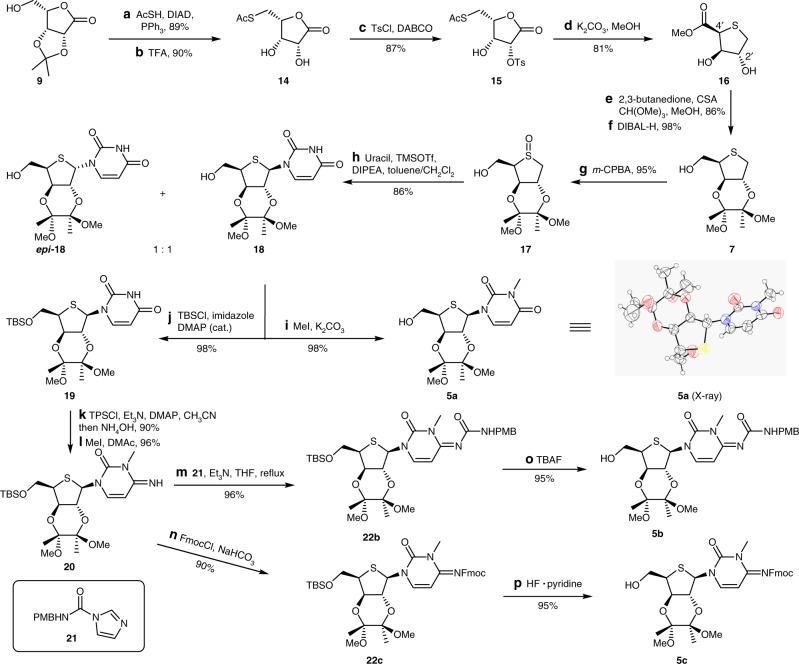
With tetrapeptide 2 in hand, we initiated the synthesis of thionucleosides 5a-c (Fig. 3). At first, lactone 932 was transformed into lactone 14 by a two-step sequence involving Mitsunobu reaction and removal of the isopropylidene protecting group with TFA. Selective mono-tosylation of lactone 14 with tosyl chloride/DABCO generated tosylate 15 in 87% yield. In this case, use of pyridine led to low conversion because of its weaker basicity, whereas Et3N to a bis-tosylation byproduct which further underwent elimination to produce the corresponding α,β-unsaturated tosylate. The transformation of 15 to 16 with K2CO3 in MeOH at room temperature gave the product with the undesired configuration at C4' exclusively. A variety of bases and temperatures were examined for the 5-membered thio-ring closure reaction, and eventually the use of K2CO3 as a base at −30 °C in MeOH smoothly led to 16 in 81% yield without any detectable epimerization. It was surprising that the reaction temperature had such an impact on the stereoselectivity. As mentioned above, the proper choice of protecting group for the trans-1,2-diol is crucial, because the neighboring group effect could influence the stereoselectivity of the following uracilation reaction33. Initially, when the trans-1,2-diol was protected as a diester, the Pummerer reaction indeed provided the desired product as a single diastereomer with moderate yield. However, this protected diester was incompatible with the subsequent selective methyl ester reduction and later stage aldol reaction. Thus, trans-1,2-diol 15 was protected with 2,3-butanedione under acid catalysis, followed by reduction of the methyl ester with DIBAL-H to afford sulfide 7. Treatment of sulfide 7 with m-CPBA provided the corresponding sulfoxide 17 in 95% yield, and subsequent Pummerer reaction34,35 with DIPEA as a base gave rise to 18 and epi-18 in 86% yield as a 1:1 diastereoisomeric mixture. Commonly used Et3N was not suitable for the Pummerer reaction because it could act as a nucleophile, leading to formation of a triethylammonium adduct, and thus reduced yield36. The N-methyl group was introduced by a conventional procedure to afford 5a, which is an advanced intermediate for the synthesis of albomycin δ1. The structure of 5a was verified by X-ray crystallographic analysis (Fig. 3). To achieve the synthesis of albomycin δ2 and ε, a practical access to the imine 20 was required. First, 18 was protected as its TBS ether 19, which was treated with TPSCl in the presence of DMAP and Et3N, followed by NH4OH to produce a cytosine derivative in 90% yield over two steps in one pot, and then methylation of N3 delivered 20 in 96% yield. For the subsequent installation of the N4 carbamoyl group, aminolysis of N4-phenoxycarbonyl37 did not take place due to the steric hindrance of the N3 methyl group. Inspired by the N-PMB carbamoylimidazole urea formation reported by Batey et al.38, we developed a scalable and efficient protocol to prepare 22b. In the presence of Et3N, 20 was allowed to react with 21 under reflux, giving rise to 22b in 96% yield. Additionally, Fmoc was chosen as the protecting group for 20, leading to 22c, which is a key intermediate for the synthesis of albomycin ε.
Fig. 3.
Synthesis of intermediates 5a-c and X-ray crystal-structure diagram of 5a. Reagents and conditions: a AcSH (2.2 equiv), DIAD (2.2 equiv), PPh3 (2.2 equiv), THF, 0 °C, 5 h, 89%; b TFA, CH2Cl2, H2O, 5 h, 90%; c TsCl (1.2 equiv), DABCO (1.1 equiv), CH3CN, 1 h, 87%; d K2CO3 (1.5 equiv), MeOH, −30 °C, 3 h, 81%; e 2,3-butanedione (1.2 equiv), trimethoxymethane (4.0 equiv), CSA (0.1 equiv), MeOH, reflux, 24 h, 86%; f DIBAL-H (2.1 equiv), CH2Cl2, 0 °C, 2 h, 98%; g m-CPBA (1.0 equiv), CH2Cl2, 0 °C, 0.5 h, 95%; h uracil (2.0 equiv), TMSOTf (8.0 equiv), DIPEA (8.0 equiv), toluene, CH2Cl2, 3 h, d.r. 1:1, 86%; i methyl iodide (1.2 equiv), K2CO3 (1.5 equiv), DMF, 2 h, 98%; j TBSCl (2.2 equiv), imidazole (2.2 equiv), DMAP (0.1 equiv), CH3CN, 2 h, 98%; k TPSCl (2.0 equiv), Et3N (2.0 equiv), DMAP (2.0 equiv), CH3CN, 1 h, then NH4OH, 20 h, 90%; l methyl iodide (3.0 equiv), DMAc, 3.5 h, 96%; m 21 (2.0 equiv), Et3N (1.0 equiv), THF, reflux, 96%. n Fmoc-Cl (2.0 equiv), NaHCO3 (4.0 equiv), THF/H2O, 90%; o TBAF (1.3 equiv), THF, 2 h, 95%; p HF·pyridine (5.0 equiv), THF, 36 h, 95%. AcSH thiolacetic acid, DIAD diisopropyl azodiformate, DABCO 1,4-diazabicyclo[2.2.2]octane, CSA camphorsulfonic acid, DIBAL-H diisobutylaluminum hydride, TPSCl 2,4,6-triisopropylbenzenesulfonyl chloride, DMAP 4-dimethylaminopyridine, DMAc N, N-dimethylacetamide, TBAF tetrabutylammonium fluoride
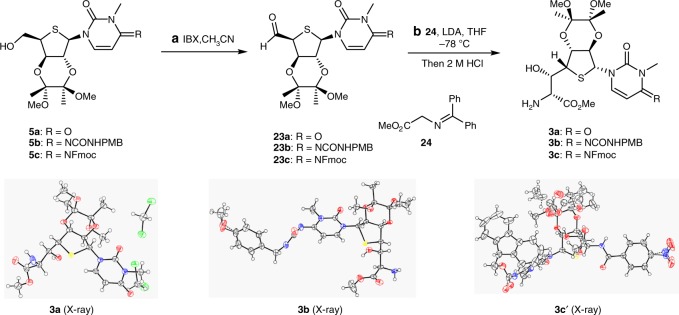
With fragments 5a–c in hand, the next challenge was to install the side chain with the desired stereochemistry (Fig. 4). Oxidation of alcohols 5a–c with IBX gave the corresponding aldehydes 23a–c in excellent yields. Side-chain extension via aldol reaction required a delicate choice of protecting groups for the glycine moiety, which is essential for good stereoselectivity. Inspired by Trost’s work39, we found that the lithium salt of N-(diphenylmethylene) glycine methyl ester could react with aldehydes 23a–c at −78 °C, and subsequent treatment of the condensation products with 2 M aqueous HCl yielded 3a (86%, 2 steps, d.r. 4:1), 3b (83%, 2 steps, d.r. 3:1), and 3c (75%, 2 steps, d.r. 3:1) respectively. The structures of 3a and 3b were unambiguously established by X-ray crystallographic analysis, and the molecular structure of 3c was substantiated by X-ray crystallography of its benzoyl derivative 3c' (Fig. 4).
Fig. 4.
Synthesis of fragments 3a–c and X-ray crystal-structure diagrams of 3a, 3b, and 3c'. Reagents and conditions: a IBX (2.0 equiv), CH3CN, 55 °C, 5 h; b 24 (1.1 equiv), LDA (1.1 equiv), THF, −78 °C, then 2 M HCl, 3a: 86% (d.r. 4:1), 3b: 83% (d.r. 3:1), 3c: 75% (d.r. 3:1), (over 2 steps). IBX 2-iodoxybenzoic acid, LDA lithium diisopropylamide
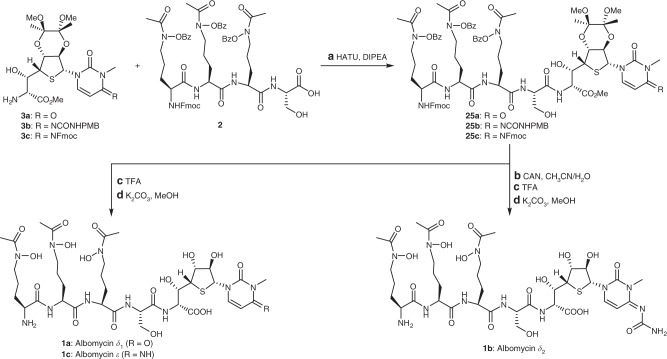
With key fragments 2 and 3a–c in place, their assembly into albomycins (1a–c) was embarked (Fig. 5). Thionucleosides 3a–c were first condensed with tetrapeptide 2 to afford 25a–c in excellent yields. The final deprotection steps from 25a–c to the final albomycins (1a–c) required delicate adjustments of the reaction conditions in order to preserve the sensitive amide bond40 on the cytosine of 1b and 1c. First, oxidative deprotection of the N-PMB group in 25b proceeded smoothly. Addition of CF3CO2H and H2O to the resulting crude product removed the bisacetal moiety within the molecules. The reaction time for these two deprotection steps needed to be carefully controlled, otherwise it would lead to hydrolysis of the imine. After surveying numerous reaction conditions, we found that aqueous K2CO3 (150 mg/mL) could efficiently remove the remaining protecting benzoyl, methyl ester, and Fmoc groups in one pot to afford albomcyin δ2 (1b). These three deprotection steps (25b to 1b) were carried out without purifying the intermediates and the final product albomycin δ2 (1b) was purified with SephadexTM G-15. We subsequently exploited the flexibility of this sequential deprotection. To our pleasure, following the same protocals, both albomycins δ1 (1a) and ε (1c) were obtained in good yields. It’s worth noting that albomcyin ε (1c) was not stable in D2O, and slowly converted to albomycin δ1 (1a) over time. Albomycin δ2 (1b) was much more stable than albomcyin ε (1c), and remained unchanged in D2O at 4 °C for over one month.
Fig. 5.
Total synthesis of albomycins δ1, δ2, and ε. Reagents and conditions: a HATU (1.5 equiv), DIPEA (2.0 equiv), DMF, −15 °C, 2 h, 25a: 93%, 25b: 91%, 25c: 90%; b CAN (10.0 equiv), CH3CN/H2O, 30 min; c TFA, H2O, 1 h; d K2CO3, MeOH, H2O, 1a: 80% (over 2 steps), 1b: 72% (over 3 steps), 1c: 75% (over 2 steps). CAN ceric ammonium nitrate
Biological assessment
As the three naturally occurring albomycins became synthetically accessible, we evaluated their potential as therapeutic agents and determined their MIC values against three Gram-positive and three Gram-negative bacteria species following the protocol from the Clinical and Laboratory Standards Institute (CLSI). Commercial antibiotic, ciprofloxacin, was used as a positive control in the MIC determination experiment. As shown in Table 1, albomycin δ1 (1a) and δ2 (1b) exhibited 8-fold more potency than ciprofloxacin against S. pneumoniae ATCC 49619. 1b inhibited S. aureus USA 300 strain NRS38441, a virulent methicillin resistant MRSA strain, with an MIC of 0.125 µg/mL, and was 16-fold more potent than ciprofloxacin. 1b exhibited an MIC value of 0.5 µg/mL against Bacillus subtilis ATCC 6633, which was less active compared to ciprofloxacin. As for Escherichia coli BJ 518342, 1b showed about 8-fold higher potency than 1a. Particularly impressive is the potency of compound 1a towards the fastidious Neisseria gonorrhoeae ATCC 49226 with a 3.9 ng/mL MIC value while 1b was completely inactive. These results led us to conclude that the C4 substituent of nucleobase in albomycins played an important role rendering their antibacterial activity. Biochemical analysis of albomycin nucleobase analogs will test this hypothesis. All three albomycins displayed no activity towards Gram-negative bacteria Salmonella typhi, and albomycin ε (1c) was inactive to all these strains, which was previously unknown.
Table 1.
MIC values (μg/mL) of albomycins δ1 (1a), δ2 (1b), and ε (1c)
| Entry | Gram-(+) | Gram-(−) | ||||
|---|---|---|---|---|---|---|
| S. pneumoniae ATCC 49619 | S. aureus USA 300 NRS 384 | B. subtilis ATCC 6633 | E. coli BJ 5183 | N. gonorrhoeae ATCC 49226 | Salmonella typhi | |
| Ciprofloxacin | 0.5 | 2 | 0.0625 | 0.0039 | 0.0039 | >512 |
| 1a | 0.0625 | >512 | >512 | 0.25 | 0.0039 | >512 |
| 1b | 0.0625 | 0.125 | 0.5 | 0.0312 | >512 | >512 |
| 1c | >512 | >512 | >512 | >512 | >512 | >512 |
World Health Organization (WHO) published a priority list of antibiotic-resistant pathogenic bacteria for developing new and effective antibiotic treatments. Both S. pneumoniae and S. aureus are on the list due to their increasing multidrug resistance. Ciprofloxacin, vancomycin and penicillin G are on the WHO Model List of Essential Medicines (EML). Penicillin G is among the first medications against bacterial infections and vancomycin has been hailed as the last line of defense. To explore the potential to develop albomycin δ2 into an effective antibiotic, we screened albomycin δ2 against a random collection of 27 clinical S. pneumoniae and S. aureus isolates (three of them are MRSA strains), and compared it with ciprofloxacin, vancomycin, and penicillin G (Table 2) in inhibiting the growth of these clinical pathogens. All these S. pneumoniae and S. aureus strains were freshly isolated from patients in clinic. As shown in Table 2, albomycin δ2 exhibited excellent anti-S. pneumoniae and S. aureus activities better than the other three antibiotics in most cases, while many of these strains displayed severely natural resistance to penicillin G. The MIC values of albomycin δ2 were well below those of ciprofloxacin, vancomycin, and penicillin G, and in a number of cases reaching 1000 times lower. The influence of iron concentration on the antibacterial activity was also studied by conducting assays in iron-rich and iron-deficient media (Table 3). The antibiotic activity of albomycin δ2 against S. pneumoniae strains was significantly increased in iron-deficient media, which most closely mimics the physiological situation in a human host, wherein iron is sequestered in macromolecules such as heme43. Two isolates, strains S15 and S29, which showed the most resistance to albomycin δ2 in iron-rich media, became highly susceptible under iron-depleted conditions. The MIC values of albomycin δ2 against S. aureus and E. coli strains were not influenced by iron concentration. The three control antibiotics did not show any dependence on iron concentration in any of the strains tested. These results suggest that albomycin δ2 is a promising antibiotic candidate for further clinical drug development.
Table 2.
MIC values (µg/mL) of albomycin δ2 (1b) against clinical isolates of S. pneumoniae and S. aureus
| Strains | 1b | Cipro. | Vanco. | Peni. | Strains | 1b | Cipro. | Vanco. | Peni. |
|---|---|---|---|---|---|---|---|---|---|
| S. pneumoniae 30 | 0.0039 | 1 | ≤1 | ≥2 | S. pneumoniae S13 | 2 | 0.5 | ≤1 | ≥2 |
| S. pneumoniae 33 | 0.0039 | 1 | 0.5 | ≤0.0625 | S. pneumoniae S15 | 8 | 0.5 | 0.25 | ≥2 |
| S. pneumoniae 10 | 0.0078 | 0.5 | 0.5 | ≥2 | S. pneumoniae 29 | 16 | >4 | ≤1 | ≥2 |
| S. pneumoniae 28 | 0.0078 | 1 | ≤1 | 1 | S. aureus S13 | 0.0625 | 0.25 | 1 | ≥0.5 |
| S. pneumoniae 31 | 0.0078 | 0.5 | 0.5 | 1 | S. aureus S19 | 0.0625 | 0.25 | 1 | ≥0.5 |
| S. pneumoniae 32 | 0.0078 | 1 | 0.5 | ≤0.0625 | S. aureus S15 | 0.125 | 1 | 1 | ≥0.5 |
| S. pneumoniae 42 | 0.0078 | 1 | 0.5 | ≥2 | S. aureus S17 | 0.125 | 0.25 | 1 | ≥0.5 |
| S. pneumoniae S14 | 0.0078 | 0.5 | 0.25 | 0.5 | S. aureus S21 | 0.125 | 0.5 | 1 | ≥0.5 |
| S. pneumoniae 26 | 0.0156 | 1 | ≤1 | ≥2 | S. aureus S12 (MRSA) | 0.25 | 0.5 | 1 | ≥0.5 |
| S. pneumoniae 43 | 0.0156 | 1 | 0.5 | ≥2 | S. aureus S14 (MRSA) | 0.25 | 0.25 | 1 | ≥0.5 |
| S. pneumoniae 44 | 0.0156 | 1 | 0.5 | ≤0.0625 | S. aureus S16 | 0.25 | >4 | 1 | ≥0.5 |
| S. pneumoniae 09 | 0.0312 | 1 | 0.5 | ≥2 | S. aureus S18 (MRSA) | 0.25 | 0.5 | 2 | ≥0.5 |
| S. pneumoniae 27 | 0.0625 | 0.5 | ≤1 | ≥2 | S. aureus S22 | 0.25 | 0.5 | 1 | ≥0.5 |
| S. pneumoniae 23 | 0.25 | 1 | ≤1 | 0.5 |
These clinical pathogens were freshly isolated from Children’s Hospital of Chongqing Medical University, Chongqing, China
Cipro. Ciprofloxacin, Vanco. Vancomycin, Peni. Penicillin G
Table 3.
MIC values (μg/mL) of albomycin δ2 (1b) against S. pneumoniae, S. aureus and E. coli strains in MHII and MHII-Fe
| Strains | Albomycin δ2 (1b) | Ciprofloxacin | Vancomycin | Penicillin G | ||||
|---|---|---|---|---|---|---|---|---|
| MHII | MHII-Fe | MHII | MHII-Fe | MHII | MHII-Fe | MHII | MHII-Fe | |
| S. pneumoniae ATCC 49619 | 0.0625 | 0.0078 | 0.5 | 0.5 | 0.25 | 0.25 | 0.5 | 0.5 |
| S. pneumoniae S14 | 0.0078 | 0.0039 | 0.5 | 0.5 | 0.25 | 0.25 | 0.5 | 0.5 |
| S. pneumoniae S15 | 8 | 0.125 | 0.5 | 0.5 | 0.25 | 0.25 | ≥2 | ≥2 |
| S. pneumoniae 29 | 16 | 0.5 | >4 | >4 | ≤1 | ≤1 | ≥2 | ≥2 |
| S. aureus ATCC 29213 | 0.5 | 0.5 | 0.5 | 1 | 2 | 2 | ≥0.5 | ≥0.5 |
| S. aureus S17 | 0.125 | 0.125 | 0.25 | 0.25 | 1 | 1 | ≥0.5 | ≥0.5 |
| S. aureus S19 | 0.0625 | 0.0625 | 0.25 | 0.25 | 1 | 1 | ≥0.5 | ≥0.5 |
| E. coli ATCC 25922 | 0.25 | 0.25 | 0.0312 | 0.0312 | – | – | – | – |
| E. coli BJ 5183 | 0.0312 | 0.0312 | 0.0039 | 0.0039 | – | – | – | – |
MHII Mueller–Hinton broth II, MHII-Fe Mueller–Hinton broth II + 100 μM 2,2′-bipyridine
Discussion
In summary, the successful execution of the convergent strategy has led to the total synthesis of albomycins δ1 (1a), δ2 (1b), and ε (1c). Antibacterial assessment of albomycins revealed that C4 substituent on the nucleobase in albomycin plays an essential role in their antibacterial activity. Albomycin δ2 exhibited potent antimicrobial activities against clinical S. pneumoniae and S. aureus isolates including MRSA. Further studies to evaluate albomycin δ2 as a potentially effective and safe antibiotic are ongoing.
Methods
General
All air-sensitive and water-sensitive reactions were carried out under a nitrogen atmosphere with dry solvents under anhydrous conditions, unless otherwise noted. Tetrahydrofuran (THF) was distilled over sodium and benzophenone, dichloromethane (CH2Cl2), N, N-dimethylformamide (DMF), triethylamine (Et3N), and N,N-diisopropylamine (DIPEA) over calcium hydride. All other solvents, as well as starting materials and reagents were obtained from commercial sources and used without further purification. Reactions were monitored by analytical thin-layer chromatography (TLC) on Merck silica gel 60 F254 plates (0.25 mm), visualized by ultraviolet light and/or by staining with phosphomolybdic acid in EtOH. Retention factor (Rf) values were measured using a 5 × 2 cm TLC plate in a developing chamber containing the solvent system described. Yields refer to the isolated yields after silica gel flash column chromatography, unless otherwise stated. 1H NMR spectra were obtained on an Agilent 400MR or 600MR DD2 spectrometer at ambient temperature. Chemical shifts were reported in parts per million (ppm), relative to either a tetramethylsilane (TMS) internal standard or the signals due to the solvent. 13C NMR spectra were obtained with proton decoupling on an Agilent 400MR or 600MR DD2 (100 MHz or 150 MHz) spectrometer and were reported in ppm with residual portium for internal standard. Multiplicity is defined as: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet, br = broad, or combinations of the above. Coupling constants (J) are reported in Hertz. High resolution mass spectra were obtained on a Bruker SolariX 7.0 T spectrometer. Melting point was determined by WRS-2A Digital Melting Point Apparatus. Optical rotations were measured with a Rudolph polarimeter. Crystallographic data were obtained from a single-crystal X-ray diffractometer.
Further experimental data
For NMR spectra of the synthesized compounds, see Supplementary Figs. 1–62. For detailed experimental procedures, see Supplementary Figs. 63–92, and Supplementary Methods. For the comparisons of 1H and 13C NMR spectroscopic data of the natural and synthetic albomycin δ2, see Supplementary Tables 1 and 2. For the crystallographic data of compounds 3a, 3b, 3c', and 5a, see Supplementary Figs. 93–96, and Supplementary Tables 3–6.
Clinical pathogens isolation and identification
The clinical pathogens were isolated according to National Guide to Clinical Laboratory Procedures and characterized by culturing in the specifically appropriate media followed by the VITEK mass spectrometry microbial identification system (bioMerieux, France). Antimicrobial susceptibility was performed for all tested clinical bacterial strains by 96-well microdilution method following Clinical and Laboratory Standards Institute (CLSI) recommendations.
Susceptibility testing
Minimal inhibitory concentration (MIC) assays were adapted according to the recommendations of the Clinical and Laboratory Standard Institute (CLSIM07). For N. gonorrhoeae, MICs were determined by the standard agar dilution method on GC medium base with Isovitalex (Topbio, Shandong, China) at 37 °C with 5% CO2; for S. pneumoniae, MICs were tested using 96-well microdilution plates in Mueller–Hinton broth II with 5% horse-blood (Solarbio, Beijing, China) at 37 °C with 5% CO2; other strains were detected by the standard 96-well microdilution method in Mueller–Hinton broth II (Solarbio, Beijing, China) at 37 °C. Dilutions of the compound were made in quadruplicate in 96-well culture dishes. Strains were taken from an exponentially growing culture and diluted to 5 × 105 CFU/mL. The bacteria were cultured in the presence of tested compounds for about 20 h and bacterial growth was monitored visually following the CLSI guidelines. S. aureus ATCC 29213, S. pneumoniae ATCC 49619, and E.coli ATCC 25922 were used as quality control strains, which were purchased from National Center for Clinical Laboratories.
Electronic supplementary material
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 21572027 and 21372267).
Author contributions
Y.H. and S.C. conceived and designed this project. Y.H. and Z.L. wrote the manuscript and compiled the Supplementary Information. Z.L., X.X., S.Z., and J.G. designed the synthetic route. Z.L., X.X., and S.Z. performed the synthesis and characterization. X.Y. carried out antibacterial assays. Z.L. and S.Z. solved the X-ray structures and prepared the X-ray section of the Supplementary Information. Q.Z. and C.J. isolated and identified the clinical pathogens. All authors discussed the results and commented on the manuscript. Z.L., X.X., and S.Z. contributed equally to this research.
Data availability
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1839504 for 5a, CCDC 1839506 for 3a, CCDC 1839508 for 3b, and CCDC 1839510 for 3c'. These data can be obtained free of charge from The CCDC via (https://www.ccdc.cam.ac.uk/data_request/cif). The authors declare that other data supporting the findings of this study are available within the paper and its supplementary information files and also are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zihua Lin, Xiaobo Xu, Sheng Zhao.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05821-1.
References
- 1.Braun V, Pramanik A, Gwinner T, Köberle M, Bohn E. Sideromycins: tools and antibiotics. Biometals. 2009;22:3–13. doi: 10.1007/s10534-008-9199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wencewicz, T. A. & Miller, M. J. Sideromycins as pathogen-targeted antibiotics. In Topics in Medicinal Chemistry (Springer, Berlin, Heidelberg, 2017).
- 3.Górska A, Sloderbach A, Marszałł MP. Siderophore-drug complexes: potential medicinal applications of the ‘Trojan horse’ strategy. Trends Pharmacol. Sci. 2014;35:442–449. doi: 10.1016/j.tips.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Page MGP. Siderophore conjugates. Ann. NY Acad. Sci. 2013;1277:115–126. doi: 10.1111/nyas.12024. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds DM, Schatz A, Waksman SA. Grisein, a new antibiotic produced by a strain of Streptomyces griseus. Proc. Soc. Exptl. Biol. Med. 1947;64:50–54. doi: 10.3181/00379727-64-15695. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds DM, Waksman SA. Grisein, an antibiotic produced by certain strains of Streptomyces griseus. J. Bacteriol. 1948;55:739–752. doi: 10.1128/jb.55.5.739-752.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braznikova G. Iron-containing antibiotic produced by Actinomyces subtropicus. Nov. Med. 1951;23:3. [Google Scholar]
- 8.Waksman SA. Penalty of isolationism. Science. 1957;125:585–587. doi: 10.1126/science.125.3248.585. [DOI] [PubMed] [Google Scholar]
- 9.Stapley ED, Ormond RE. Similarity of albomycin and grisein. Science. 1957;125:587–589. doi: 10.1126/science.125.3248.587. [DOI] [PubMed] [Google Scholar]
- 10.Gause GF. Recent studies on albomycin, a new antibiotic. Brit. Med. J. 1955;12:1177–1179. doi: 10.1136/bmj.2.4949.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pramanik A, et al. Albomycin is an effective antibiotic, as exemplified with Yersinia enterocolitica and Streptococcus pneumoniae. Int. J. Med. Microbiol. 2007;297:459–469. doi: 10.1016/j.ijmm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Pramanik A, Braun V. Albomycin uptake via a ferric hydroxamate transport system of Streptococcus pneumoniae R6. J. Bacteriol. 2006;188:3878–3886. doi: 10.1128/JB.00205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benz G, et al. Constitution of the deferriform of the albomycins δ1, δ2 and ε. Angew. Chem. Int. Ed. Engl. 1982;21:527–528. doi: 10.1002/anie.198205271. [DOI] [Google Scholar]
- 14.Benz, G. et al. Constitution of the deferriform of the albomycins δ1, δ2 and ε. Angew. Chem. Suppl. 1322−1335 (1982).
- 15.Stefanska AL, Fulston M, Houge-Frydrych CSV, Jones JJ, Warr SR. A potent seryl tRNA synthetase inhibitor SB-217452. J. Antibiot. 2000;53:1346–1353. doi: 10.7164/antibiotics.53.1346. [DOI] [PubMed] [Google Scholar]
- 16.Benz GAlbomycins., III Synthesis of N5-acetyl-N5-hydroxy-L-ornithine from L-glutamic acid. Liebigs. Ann. Chem. 1984;8:1424–1433. doi: 10.1002/jlac.198419840804. [DOI] [Google Scholar]
- 17.Benz G, Schmidt D. Albomycins, IV. Isolation and total synthesis of (N5-acetyl-N5-hydroxy-L-ornithyl)(N5-acetyl-N5-hydroxy-L-ornithyl)-N5-acetyl-N5-hydroxy-L-ornithine. Liebigs. Ann. Chem. 1984;8:1434–1440. doi: 10.1002/jlac.198419840805. [DOI] [Google Scholar]
- 18.Dolence EK, Minnick AA, Miller MJ. N5-acetyl-N5-hydroxy-L-ornithine-derived siderophore-carbacephalosporin β-lactam conjugates: iron transport mediated drug delivery. J. Med. Chem. 1990;33:461–464. doi: 10.1021/jm00164a001. [DOI] [PubMed] [Google Scholar]
- 19.Dolence EK, Lin C, Miller MJ. Synthesis and siderophore activity of albomycin-like peptides derived from N5-acetyl-N5-hydroxy-L-ornithine. J. Med. Chem. 1991;34:956–968. doi: 10.1021/jm00107a013. [DOI] [PubMed] [Google Scholar]
- 20.Lin YM, Miller MJ. Practical synthesis of hydroxamate-derived siderophore components by an indirect oxidation method and syntheses of a DIG-siderophore conjugate and a biotin-siderophore conjugate. J. Org. Chem. 1999;64:7451–7458. doi: 10.1021/jo990769y. [DOI] [Google Scholar]
- 21.Bredenkamp MW, Holzapfel CW, Swanepoel AD. Synthesis of the thionucleoside moiety of albomycin δ1. S. Afrl. Chem. 1991;44:31–33. [Google Scholar]
- 22.Paulsen H, Brieden M, Benz G. Branched and chain-extended sugars, XXXI. Synthesis of the deferri form of the oxygen analog of δ1-albomycin. Liebigs. Ann. Chem. 1987;7:565–575. doi: 10.1002/jlac.198719870702. [DOI] [Google Scholar]
- 23.Zeng Y, et al. Biosynthesis of albomycin δ2 provides a template for assembling siderophore and aminoacyl-tRNA synthetase inhibitor conjugates. Acs. Chem. Biol. 2012;6:1000–1007. doi: 10.1021/cb300173x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni A, et al. A branch point of Streptomyces sulfur amino acid metabolism controls the production of albomycin. Appl. Environ. Microbiol. 2016;82:467–477. doi: 10.1128/AEM.02517-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heemstra JR, Walsh CT, Sattely ES. Enzymatic tailoring of ornithine in the biosynthesis of the Rhizobium cyclic trihydroxamate siderophore vicibactin. J. Am. Chem. Soc. 2009;131:15317–15329. doi: 10.1021/ja9056008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milewska MJ, Chimiak A. An alternative synthesis of N5-acetyl-N5-hydroxy-L-ornithine from L-ornithine. Synthesis. 1990;3:233–234. doi: 10.1055/s-1990-26840. [DOI] [Google Scholar]
- 27.Bergeron RJ, Phanstiel OIV. The total synthesis of nannochelin: a novel cinnamoyl hydroxamate-containing siderophore. J. Org. Chem. 1992;57:7140–7143. doi: 10.1021/jo00052a030. [DOI] [Google Scholar]
- 28.Katritzky AR, Yoshioka M, Narindoshvili T, Chung A, Khashab NM. N-Fmoc-protected(α-dipeptidoyl)benzotriazoles for efficient solid-phase peptide synthesis by segment condensation. Chem. Biol. Drug. Des. 2008;72:182–188. doi: 10.1111/j.1747-0285.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- 29.Katritzky AR, Angrish P, Hür D, Suzuki K. N-(Cbz- and Fmoc-α-aminoacyl)benzotriazoles: stable derivatives enabling peptide coupling of Tyr, Trp, Cys, Met, and Gln with free amino acids in aqueous media with complete retention of chirality. Synthesis. 2005;3:397–402. doi: 10.1055/s-2005-861782. [DOI] [Google Scholar]
- 30.Katritzky AR, Todadze E, Shestopalov AA, Cusido J, Angrish P. Selective peptide chain extension at the C-terminus of aspartic and glutamic acids utilizing N-protected (α-aminoacyl)benzotriazoles. Chem. Biol. Drug. Des. 2006;68:42–47. doi: 10.1111/j.1747-0285.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 31.Katritzky AR, Haase DN, Johnson JV, Chung A. Benzotriazole-assisted solid-phase assembly of Leu-enkephalin, amyloid beta segment 34-42, and other “difficult” peptide sequences. J. Org. Chem. 2009;74:2028–2032. doi: 10.1021/jo8026214. [DOI] [PubMed] [Google Scholar]
- 32.Batra H, et al. A concise, efficient and production-scale synthesis of a protected L-lyxonolactone derivative: an important aldonolactone core. Org. Process Res. Dev. 2006;10:484–486. doi: 10.1021/op050222n. [DOI] [Google Scholar]
- 33.Naka T, Nishizono N, Minakawa N, Matsuda A. Nucleosides and nucleotides. 189. Investigation of the stereoselective coupling of thymine with meso-thiolane-3,4-diol-1-oxide derivatives via the Pummerer reaction. Tetrahedron Lett. 1999;40:6297–6300. doi: 10.1016/S0040-4039(99)01287-3. [DOI] [Google Scholar]
- 34.Naka T, Minakawa N, Abe H, Kaga D, Matsuda A. The stereoselective synthesis of 4′-β-thioribonucleosides via the Pummerer reaction. J. Am. Chem. Soc. 2000;122:7233–7243. doi: 10.1021/ja000541o. [DOI] [Google Scholar]
- 35.Yoshimura Y, et al. A practical synthesis of 4′-thioribonucleosides. Tetrahedron Lett. 2006;47:591–594. doi: 10.1016/j.tetlet.2005.11.049. [DOI] [Google Scholar]
- 36.Kotoulas SS, Koji VV, Bogdanovi GM, Koumbis AE. Synthesis of novel pyrimidine apiothionucleosides and in vitro evaluation of their cytotoxicity. Tetrahedron. 2015;71:3396–3403. doi: 10.1016/j.tet.2015.03.089. [DOI] [Google Scholar]
- 37.Miyata, K. et al. Conformational studies of 4-N-carbamoyldeoxycytidine derivatives and synthesis and hybridization properties of oligodeoxyribonucleotides incorporating these modified bases. Eur. J. Org. Chem. 16, 3626−3637 (2006).
- 38.Duspara PA, Sadequl Islam M, Lough AJ, Batey RA. Synthesis and reactivity of N-alkyl carbamoylimidazoles: development of N-methyl carbamoylimidazole as a methyl isocyanate equivalent. J. Org. Chem. 2012;77:10362–10368. doi: 10.1021/jo302084a. [DOI] [PubMed] [Google Scholar]
- 39.Trost BM, Miege F. Development of ProPhenol ligands for the diastereo- and enantioselective synthesis of β-hydroxy-α-amino esters. J. Am. Chem. Soc. 2014;136:3016–3019. doi: 10.1021/ja4129394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maehr H. Antibiotics and other naturally occurring hydroxamic acids and hydroxamates. Pure Appl. Chem. 1971;28:603–636. doi: 10.1351/pac197128040603. [DOI] [PubMed] [Google Scholar]
- 41.Choe D, et al. Genome-scale analysis of Methicillin-resistant Staphylococcus aureus USA300 reveals a tradeoff between pathogenesis and drug resistance. Sci. Rep. 2018;8:2215. doi: 10.1038/s41598-018-20661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi N, Yoshikura H, Kobayashi I. An Escherichia coli strain, BJ5183, that shows highly efficient conservative (two-progeny) DNA double-strand break repair of restriction breaks. Gene. 2003;303:89–97. doi: 10.1016/S0378-1119(02)01107-1. [DOI] [PubMed] [Google Scholar]
- 43.Ito A, et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016;60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1839504 for 5a, CCDC 1839506 for 3a, CCDC 1839508 for 3b, and CCDC 1839510 for 3c'. These data can be obtained free of charge from The CCDC via (https://www.ccdc.cam.ac.uk/data_request/cif). The authors declare that other data supporting the findings of this study are available within the paper and its supplementary information files and also are available from the corresponding author upon request.