Abstract
In nature, enzymatic pathways generate Caryl−C(O) bonds in a site-selective fashion. Synthetically, Caryl−C(O) bonds are synthesised in organometallic reactions using prefunctionalized substrate materials. Electrophilic routes are largely limited to electron-rich systems, non-polar medium, and multiple product formations with a limited scope of general application. Herein we disclose a directed para-selective ketonisation technique of arenes, overriding electronic bias and structural congestion, in the presence of a polar protic solvent. The concept of hard–soft interaction along with in situ activation techniques is utilised to suppress the competitive routes. Mechanistic pathways are investigated both experimentally and computationally to establish the hypothesis. Synthetic utility of the protocol is highlighted in formal synthesis of drugs, drug cores, and bioactive molecules.
Electrophilic acylation of arenes is largely limited to electron rich systems, non-polar medium and often displays moderate selectivity. Here, the authors show a directed para-selective ketonisation of arenes, overriding electronic bias and structural congestion, and apply it to the synthesis of bioactive compounds.
Introduction
Carbon−Carbon bonds constitute the major backbone of organic molecules. Prudent construction of such linkages facilitates structural manipulation and complex total synthesis.1 Synthetic methodologies, thriving to transform robust C−H bonds into diverse functional motifs, can potentially shift the retrosynthetic paradigm. Recent exercise on C−H bond functionalization prompted us to sketch a generalised route of site-selective C−C bond formation at a distal para position of an arene, attenuating structural and electronic constraints. Although statistically such transformations are highly probable, inert nature and minimal reactivity distinction impose significant synthetic challenges to target a particular C−H bond. In nature, para-toluene monooxygenase functionalise para C−H bonds of toluene distinctively.2,3 Although synthetic reproduction of such transformation can be attained for biased substrates, it falters in recapitulating the enzymatic efficiency with unbiased and deactivated substrates.
In biosynthetic pathways, ketoacyl-synthase and benzophenone synthase transfer R−C(O) groups to form C−C(O) connectivity.4–6 Synthetically carbonyl cores can be accessed using organometallic reagents and cross-coupling at the expense of sensitive reagents and prefunctionalized reactants.7–13 On the contrary, electrophilic substitutions (Friedel–Crafts acylation) are more atom economical yet biased to electron-rich systems and sensitive to substituents. Electronic effect of the substituents often leads to the inseparable mixtures of isomers whereas it mostly fails with electron-deficient systems. Broadly, the need of non-nucleophilic solvent medium further confines the scope.14–16 In the present work, we intend to circumvent such limitations both in terms of selectivity as well as reactivity of the reagents. Over the last few decades directed C−H activation has offered a promising strategy for superior regioselectivity.17–39 However, directed carbonyl insertion is mostly explored for ortho C−H bonds.40–44 Expanding the idea to distal para positions, spans larger separation and thus tunnels through bulky and strained intermediates, vulnerable to subtle manifold modification.45–49
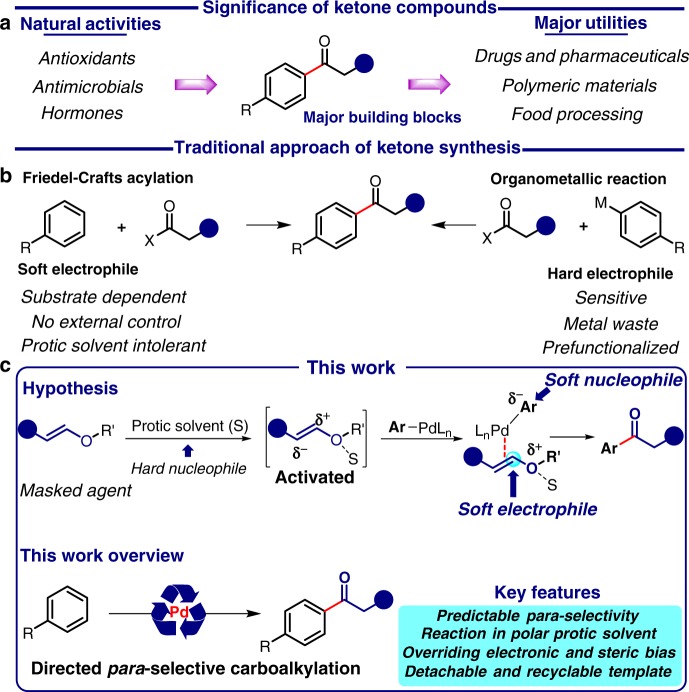
Comprehending the significance of carbonyl scaffold50 and synthetic challenges, herein we disclose directed para-selective ketonisation of arenes by overriding the electronic bias, in the presence of a polar solvent (Fig. 1).
Fig. 1.
Overview of the work. a Diverse functions of ketones. b Classic synthetic routes for ketone synthesis and its drawbacks. c Mechanistic hypothesis for generalised approach and key outline of the work
Results
Design of template and optimisation
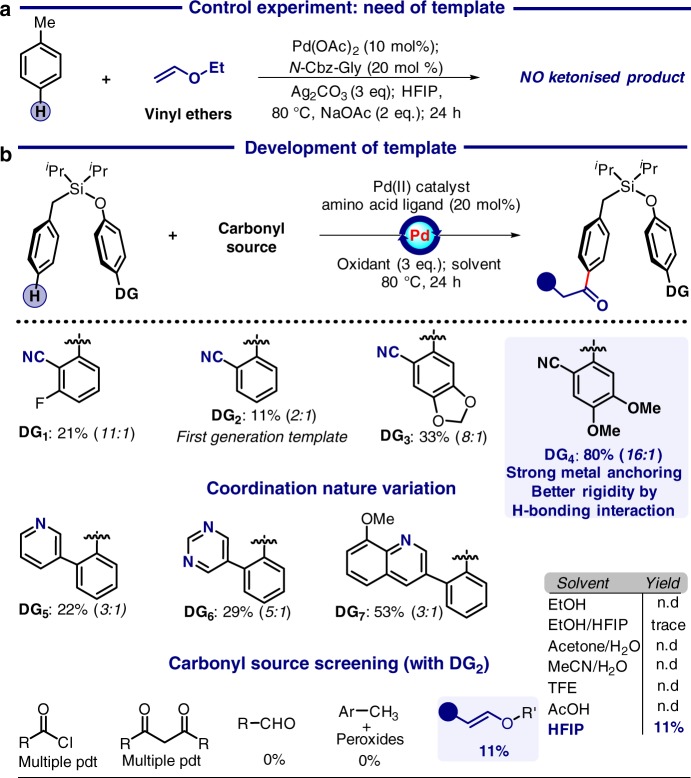
Initially acylation reaction was chosen as the prototype transformation with a toluene model substrate, appended with the first-generation biphenyl nitrile directing template (Fig. 2). A series of different acylating agents were screened. Interestingly, the usage of protic solvent under elevated temperature consumed electrophilic acid chlorides and anhydrides. Therefore, selection of a compatible acylating agent was imperative. In view of competitive nucleophilicity of the solvent and metallacycle, we envisioned to identify a soft masked acylating agent to facilitate interaction with soft metallacycles (Fig. 2). In this regard, first breakthrough was obtained with ethyl vinyl ether in the presence of catalytic Pd(OAc)2 and hexafluoroisopropanol (HFIP) solvent with an overall yield of 11% and 2:1 para-selectivity. Notably, vinyl ethers are electron rich and less reactive and thus less pronounced as cross-coupling partner.51–66 Additionally, the possibility of linear and branched isomer formation along with an additional hydrolysis step to release the carbonyl unit is noteworthy.67 However, in stark contrast, vinyl ether worked satisfactorily under the current condition in a one-pot process. To seek better selectivity and yield different directing groups were tested. Replacement of the linear nitrile group by heterocyclic metal coordinating motifs such as pyridine (DG5), pyrimidine (DG6), and methoxy quinoline systems (DG7) improved yield yet compromised the selectivity. Apparently, methoxy quinoline (DG7), due to its increased bulk, destabilises the necessary orientation by pushing the toluene nucleus away exposing the meta-C–H bond for reaction. In stark contrast, alteration of the electronic environment of the nitrile-based directing group (DG1, DG3, and DG4) offered significant improvement both in yield and selectivity. A yield of 52% with 11:1 para-selectivity was obtained with a second-generation hydrogen-bonded para-directing template (DG4). In particular, the presence of two methoxy groups triggered facile metal-CN binding offering better yield, whereas template-solvent H-bonding interaction generated optimum rigidity to ensure superior selectivity.46 Under optimised condition Pd(OAc)2 along with N-Cbz-Gly gave 80% yield and 16:1 para-selectivity in the presence of NaOAc and Ag2CO3. Control experiment with a simple toluene substrate under the optimised reaction condition gave a mixture of products with no signature of desired para-acylated product formation.68 Such a phenomenon clearly indicates the significant role of the directing template in selective para functionalization.
Fig. 2.
Development of para-ketonisation reaction. a Significance of the directing group. b Screening of the reaction parameters
Scope of the methodology
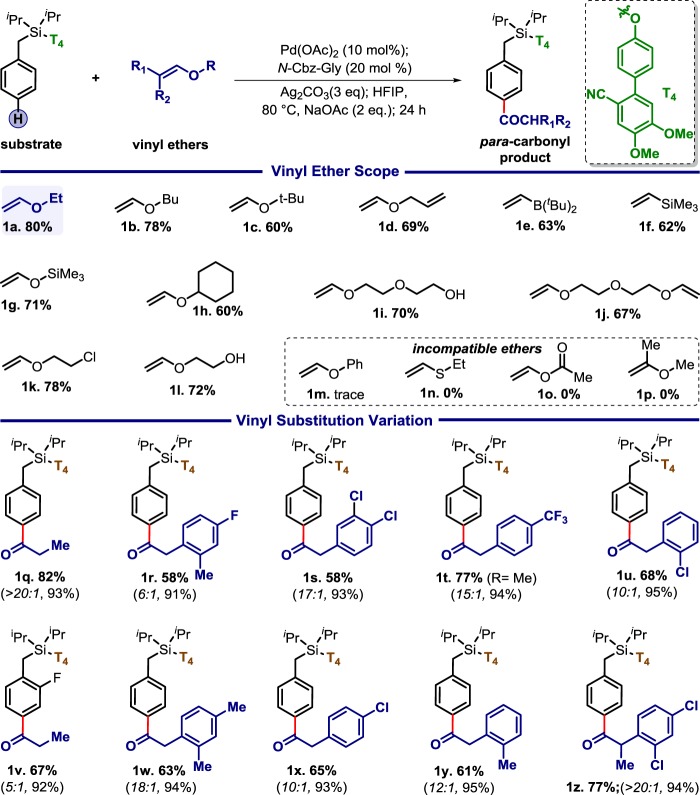
Once optimised, the scope of vinyl ether was tested (Table 1). Both cyclic and acyclic alkenyl ethers offered good yield (1a–1c, 1g, 1h, and 1k). No competitive product formation was observed for allyl vinyl ether, divinyl polyether, and free −OH group (1d, 1i, 1j, and 1l). Interestingly, vinyl silane and vinyl borane were found to be compatible (1e and 1f). A number of substituents both aliphatic and aromatic moieties around the vinyl group were tested successfully. Both electron-rich and -deficient arene rings were tolerated under the standard condition (1u–1r and 1w–1z). Di-substitution vinyl ether led to the formation of corresponding α−di (1z) substituted ketonised product.
Table 1.
The scope of vinyl ether
para: others selectivity for entries 1a–1p is 16:1; yields in parenthesis are based on the recovered starting material.
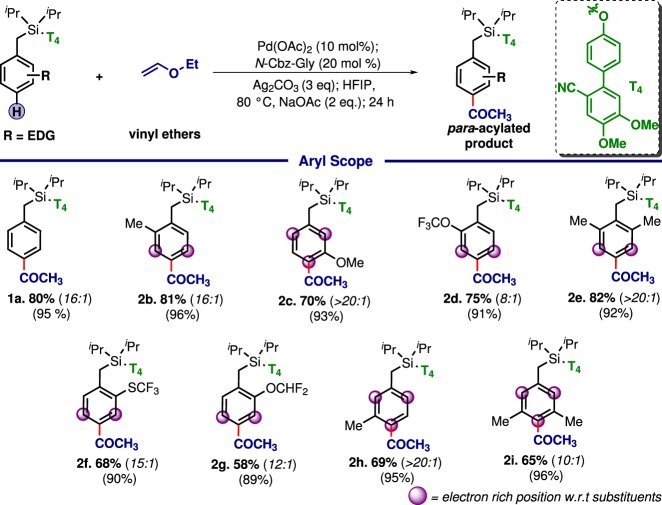
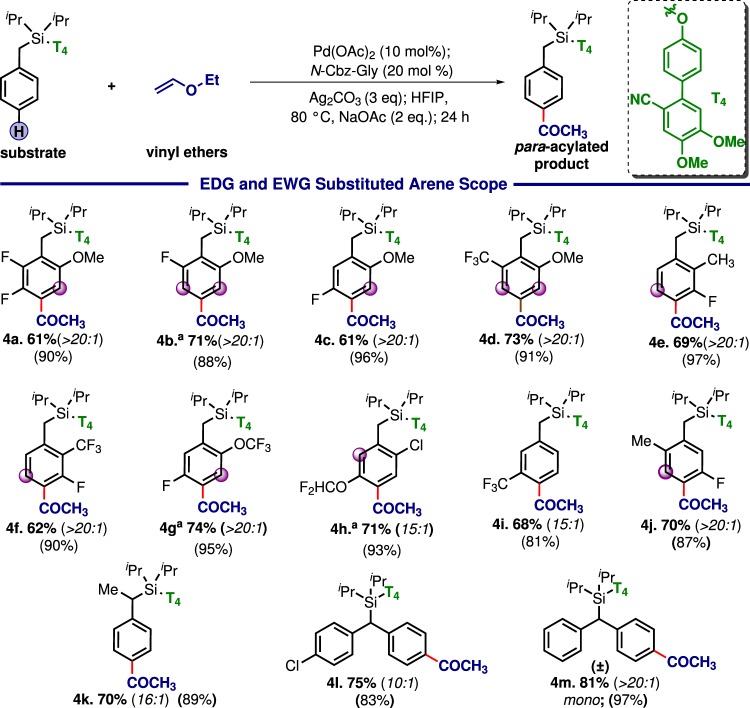
Following the diversification on alkoxy and vinyl substituents, the scope of arenes was explored (Tables 2–4). With electron-rich systems (Tables 2; 2a–2i) a predictable para-selectivity was obtained by virtue of the directing group. Despite the possibility of random electrophilic functionalization, para-ketonised product was obtained in synthetically useful yield and selectivity. However, ortho-trifluoromethoxy toluene (2d) offered a moderate selectivity as compared to methyl (2b) substituent. Although the reason of such an anomaly is unclear, it is worth mentioning that −OCF3 can influence the outcome of a transformation not just by electronic effect but also distorting the planer alignment with the benzene ring which can have a significant impact on the transformation, relied on the appropriate spatial orientation.69,70 Nevertheless, the current methodology complemented the electrophilic route with excellent para-selectivity.
Table 2.
The scope of electron-rich arenes
Table 4.
Scope of arenes
aN-Ac-Gly used as ligand
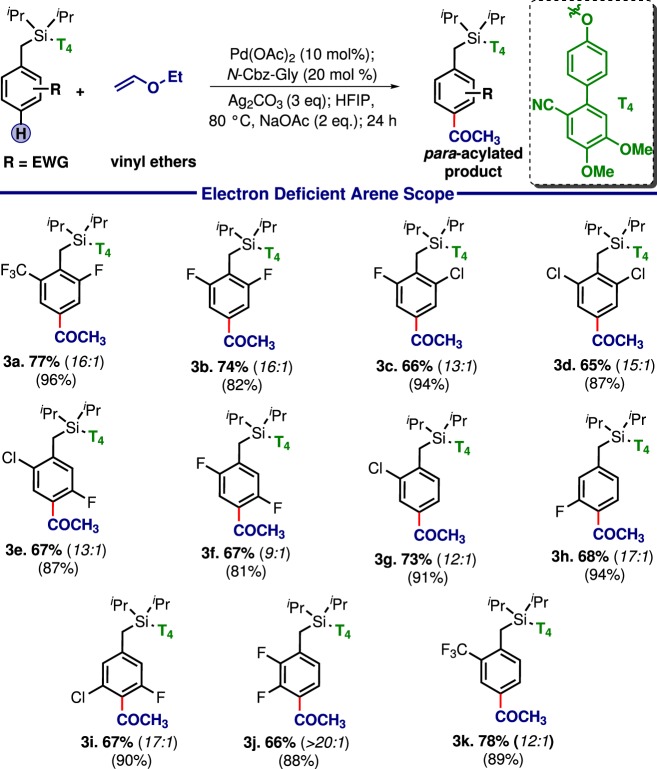
Although electron-deficient systems are not susceptible towards electrophilic substitution reaction, para-ketonisation generated the desired product with excellent yield and selectivity (Table 3: 3a–3k). Poly-halo compounds, specially poly-fluoro, which is having significant medicinal values can be functionalized using the current protocol. Notably, comparable results for both the electron-rich and electron-deficient arenes re-establish the prominent influence of the directing template over other paraphernalia.
Table 3.
Scope with electron-deficient arenes
Arenes with both electron-rich and electron-deficient substituents were also found to be compatible (Table 4). T4 template played the key role to dictate the selectivity. Substrates with benzylic substitution (4k–4m) underwent mono para-acylation successfully (4m).
Experimental mechanistic evidences
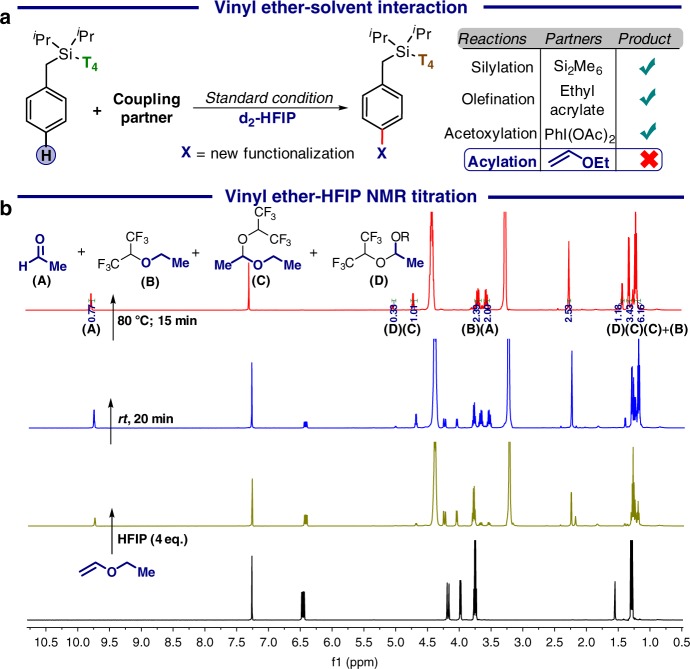
Following the scope of the reaction, a series of control experiments were conducted to gain better insight of the mechanism (Fig. 3). As the vinyl moiety of ether rearranges to the carbonyl group, we were intrigued to understand the stepwise pathway. NMR titration showed a slow hydrolysis of vinyl ether in the presence of HFIP, generating multiple products including aldehyde which was accelerated upon heating. The control experiment revealed that the hydrolysed product is ineffective as the acylation agent. Upon replacement HFIP by d2-HFIP or isopropanol, less acidic variant of HFIP, neither decomposition nor the desired product formation was observed. Seemingly, the protonation of the ether by HFIP is responsible for the generation of the reactive intermediate of ketonisation.
Fig. 3.
Influence of HFIP. a Control experiment for vinyl ether–HFIP interaction. b NMR study of vinyl ether–HFIP interaction
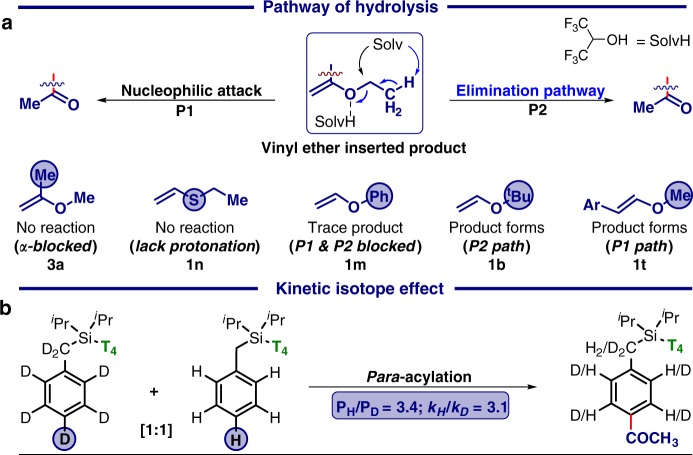
During the scope of the reaction thiovinyl ether, unlike vinyl ether, failed completely (Fig. 4a) to deliver the desired carbonyl product which further strengthens the hypothesis of protonation by HFIP. Once insertion into the palladacycle, vinyl ether can undergo either nucleophilic pathway (P1) or elimination pathway (P2) of hydrolysis to generate the target molecule. The possibility of both hydrolytic pathways can be rationalised from the reactivity of different alkoxy substituted vinyl ethers (Fig. 4a). Product distribution and kinetic isotope effect study of the deuterated substrates (kH/kD = 3.1; PH/PD = 3.4) revealed C−H activation as the rate-limiting step (Fig. 4b).69
Fig. 4.
Understanding the mechanistic features. a Plausible pathways of hydrolysis. b Determination of kinetic isotope effect (KIE)
DFT calculations and mechanistic cycle
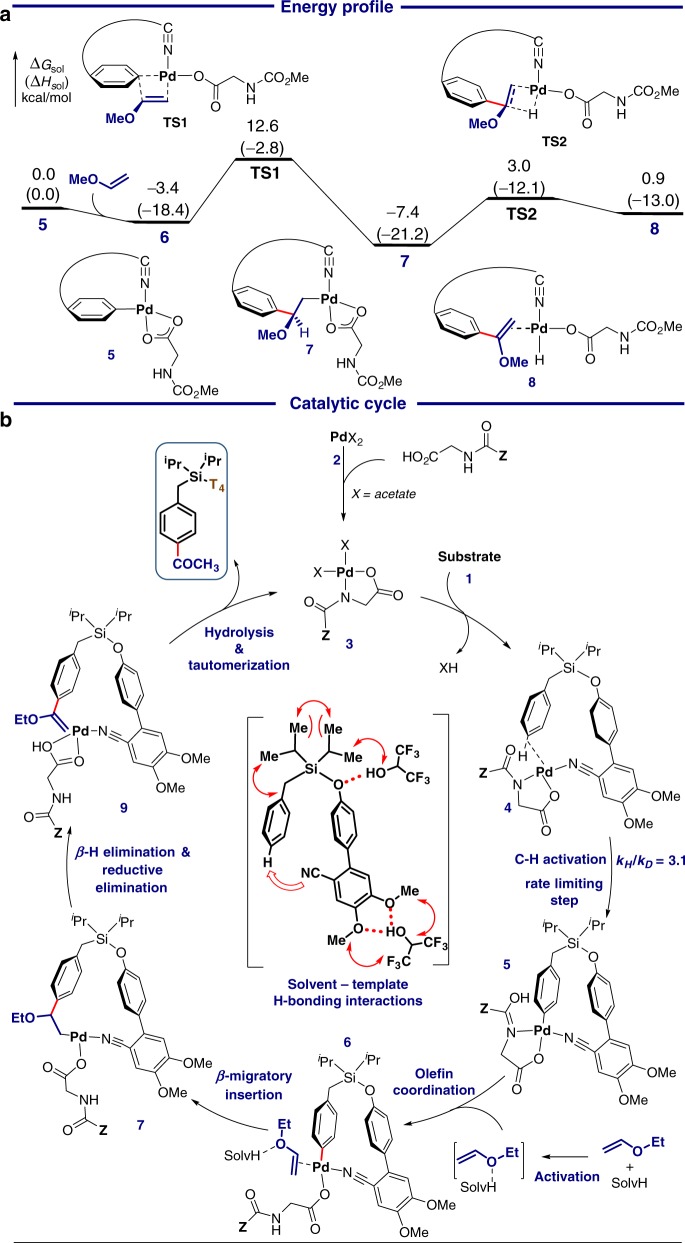
Based on these mechanistic experiments, a plausible catalytic cycle for para-ketonisation was proposed (Fig. 5b). The pathway was evaluated by density functional theory calculations (Fig. 5a). Initial steps of the para-selective ketonisation was found to resemble para-selective C–H silylation of 1.46 Compared to para-C–H palladation, meta-C–H palladation is disfavoured due to greater ring strain and distortion of the 15-membered palladacycle in the transition state.46,68 The para-C–H metalation occurs via the CMD mechanism directed by the Si-based T4-directing group to form palladacycle 5. Subsequent olefin migratory insertion (TS1) requires a relatively low activation free energy of 16.0 kcal/mol. The β-elimination of the benzylic hydrogen (TS2) is facile, requiring only 10.4 kcal/mol, to form the Pd hydride species 8, which upon reductive elimination yields the alkenyl ether product 9. Finally, hydrolysis of alkenyl ether 9 leads to the desired product formation via pathway P1 or P2. It is noteworthy that apart from generating the activated vinyl ether, HFIP solvent molecule forms H-bonding with the methoxy group of the template (T4) which favours the bent geometry of C–H metalation transition state, thus metal binding and improved para selectivity.46
Fig. 5.
Stepwise mechanism of para-ketonisation. a Energy profile of the para-C−H acylation with vinyl methyl ether. Energies are with respect to the palladacycle 5. See SI for the complete energy profile. Method: M06/SDD-6-311 + G(d,p)/ SMD(HFIP)//B3LYP/SDD-6-31G(d). b Plausible catalytic cycle
Template recovery and applications
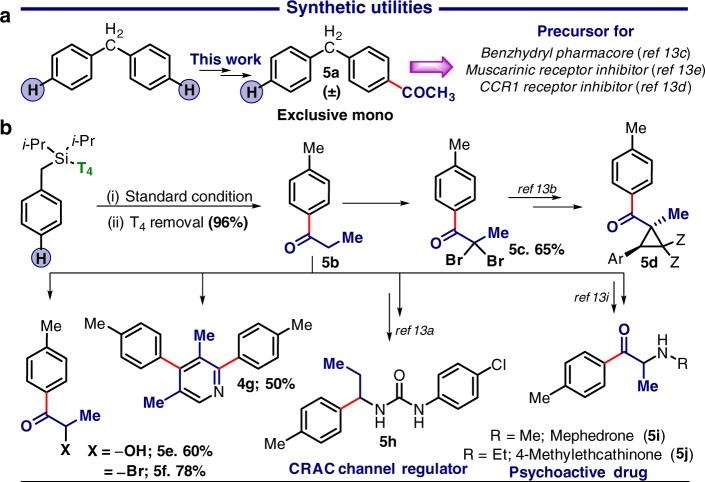
During para-ketonisation, the presence of the template (T4) was essential for improved selectivity and yield, yet its removal is required for further synthetic applications (Fig. 6). Almost a quantitative amount of directing was recovered from the 1q (96%) along with the formation of (p-tolyl)-1-propanone (5b) which was further used for α- functionalization and cyclization. Recovery of T4 from 4m led to the formation of mono ketonised benzhydryl cores (5a).68
Fig. 6.
Synthetic utilities of para-ketonisation. a Selective mono ketonisation of benzhydryl core. b Applicative highlights of the methodology
Discussion
Therefore, we have developed a reusable template-assisted para-selective ketonisation of toluene derivatives with vinyl ethers in the presence of polar protic HFIP. The protocol allows a broad spectrum of vinyl ether and arenes. Also, it can withstand electron deficiency and steric congestion, which is likely to diminish reactivity significantly. The sequence of activation, insertion, and hydrolysis was experimentally investigated and was further supported by computational studies.
Methods
Procedure of para-ketonisation
In an oven-dried screw-capped reaction tube was charged with a magnetic stir-bar, benzylsilyl ether substrate (viscous benzylsilyl ether was weighed first), Pd(OAc)2 (10 mol%), ligand (N-CBZ-Gly or N-Ac-Gly; 20 mol%), Ag2CO3 (3 eq.) and NaOAc (2 eq.). About 1.2 mL (for 0.1 mmol scale) of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) was added followed by vinyl ether (3 eq.). The reaction tube was capped and stirred (900 rpm) on a preheated oil-bath at 80 °C for 24/36 h. Upon completion the mixture was cooled and diluted with EtOAc and filtered through a celite pad. The filtrate was evaporated under reduced pressure and the crude mixture was purified by column chromatography using silica (100–200 mesh size) and petroleum ether/ethyl acetate as the eluent. The selectivity was monitored using 1H-NMR signal in the presence of 1,3,5-trimethoxybenzene as an internal standard. The regioselectivity was determined from 1H-NMR signals of aromatic region and benzylic position.
In the substrate scope table, selectivity was obtained from 1H-NMR.
Electronic supplementary material
Acknowledgements
This activity is supported by research grant from SERB (EMR/2015/000164), India. Financial support received from Lombardy Region and Cariplo Foundation [Regione Lombardia POR FESR 2014-2020/Innovazione e competitività, progetto VIPCAT], Italy, CSIR-India (to A.M.), UGC (to T.B.), NSF (CHE-1654122, to P.L.) and computing time from the Center for Research Computing at the University of Pittsburgh and NSF XSEDE are gratefully acknowledged.
Author contributions
A.M. conceived the idea and proved it experimentally along with mechanistic control experiments. A.M., A.D., and T.B. performed the substrate scope of the protocol. D.M. supervised the experimental work. M.B. and G.Z. provided analytical reagents. G.L. and P.L. did the computational studies. All the authors contributed to the final version of the manuscript.
Data Availability
The data that support the findings of this study are included in the article and Supplementary Information
Competing interests
A provisional patent has been filed on this work.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giuseppe Zanoni, Email: gz@unipv.it.
Peng Liu, Email: pengliu@pitt.edu.
Debabrata Maiti, Email: dmaiti@iitb.ac.in.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-06018-2.
References
- 1.Corey, E. J. & Cheng, X. M. The Logic of Chemical Synthesis (Wiley, New York, 1995).
- 2.Fishman A, Tao Y, Wood TK. Toluene 3-monooxygenase of Ralstonia pickettii PKO1 is a para-hydroxylating enzyme. J. Bacteriol. 2004;186:3117–3123. doi: 10.1128/JB.186.10.3117-3123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whited GM, Gibson DT. Toluene-4-monooxygenase, a three-component enzyme system that catalyzes the oxidation of toluene to p-cresol in Pseudomonas mendocina KR1. J. Bacteriol. 1991;173:3010–3016. doi: 10.1128/jb.173.9.3010-3016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, et al. Structural classification and properties of ketoacyl synthases. Protein Sci. 2011;20:1659–1667. doi: 10.1002/pro.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beerhues L. Benzophenone synthase from cultured cells of Centaurium erythraea. FEBS Lett. 1996;383:264–266. doi: 10.1016/0014-5793(96)00265-7. [DOI] [PubMed] [Google Scholar]
- 6.Sommer H, Fürstner A. Hydroxyl-assisted carbonylation of alkenyltin derivatives: development and application to a formal synthesis of tubelactomicin A. Org. Lett. 2016;18:3210–3213. doi: 10.1021/acs.orglett.6b01431. [DOI] [PubMed] [Google Scholar]
- 7.Wu XF, Neumann H, Beller M. Palladium-catalyzed carbonylative coupling reactions between Ar-X and carbon nucleophiles. Chem. Soc. Rev. 2011;40:4986–5009. doi: 10.1039/c1cs15109f. [DOI] [PubMed] [Google Scholar]
- 8.Moragas T, Correa A, Martin R. Metal-catalyzed reductive coupling reactions of organic halides with carbonyl-type compounds. Chem. Eur. J. 2014;20:8242–8258. doi: 10.1002/chem.201402509. [DOI] [PubMed] [Google Scholar]
- 9.Shintani R, Fu GC. Highly enantioselective desymmetrization of anhydrides by carbon nucleophiles: reactions of grignard reagents in the presence of (−)-sparteine. Angew. Chem. Int. Ed. 2002;41:1057–1059. doi: 10.1002/1521-3773(20020315)41:6<1057::AID-ANIE1057>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Rovis T. A unique catalyst effects the rapid room-temperature cross-coupling of organozinc reagents with carboxylic acid fluorides, chlorides, anhydrides, and thioesters. J. Am. Chem. Soc. 2004;126:15964–15965. doi: 10.1021/ja044113k. [DOI] [PubMed] [Google Scholar]
- 11.Wotal AC, Weix DJ. Synthesis of functionalized dialkyl ketones from carboxylic acid derivatives and alkyl halides. Org. Lett. 2012;14:1476–1479. doi: 10.1021/ol300217x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong YT, Barchuk A, Krische MJ. Branch-selective intermolecular hydroacylation: hydrogen-mediated coupling of anhydrides to styrenes and activated olefins. Angew. Chem. Int. Ed. 2006;45:6885–6888. doi: 10.1002/anie.200602377. [DOI] [PubMed] [Google Scholar]
- 13.Cherney AH, Kadunce NT, Reisman SE. Catalytic asymmetric reductive acyl cross-coupling: synthesis of enantioenriched acyclic α,α-disubstituted ketones. J. Am. Chem. Soc. 2013;135:7442–7445. doi: 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]
- 14.Hallett JP, Pollet P, Liotta CL, Eckert CA. Reversible in situ catalyst formation. Acc. Chem. Res. 2008;41:458–467. doi: 10.1021/ar700106a. [DOI] [PubMed] [Google Scholar]
- 15.Bechara WS, Pelletier G, Charette AB. Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides. Nat. Chem. 2012;4:228. doi: 10.1038/nchem.1268. [DOI] [PubMed] [Google Scholar]
- 16.Sartori G, Maggi R. Use of solid catalysts in Friedel−Crafts acylation reactions. Chem. Rev. 2006;106:1077–1104. doi: 10.1021/cr040695c. [DOI] [PubMed] [Google Scholar]
- 17.Dong Z, Ren Z, Thompson SJ, Xu Y, Dong G. Transition-metal-catalyzed C–H alkylation using alkenes. Chem. Rev. 2017;117:9333–9403. doi: 10.1021/acs.chemrev.6b00574. [DOI] [PubMed] [Google Scholar]
- 18.Newton CG, Wang SG, Oliveira CC, Cramer N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem. Rev. 2017;117:8908–8976. doi: 10.1021/acs.chemrev.6b00692. [DOI] [PubMed] [Google Scholar]
- 19.Hummel JR, Boerth JA, Ellman JA. Transition-metal-catalyzed C–H bond addition to carbonyls, imines, and related polarized π bonds. Chem. Rev. 2017;117:9163–9227. doi: 10.1021/acs.chemrev.6b00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arockiam PB, Bruneau C, Dixneuf PH. Ruthenium(II)-catalyzed C–H bond activation and functionalization. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. [DOI] [PubMed] [Google Scholar]
- 21.Leow D, Li G, Mei TS, Yu JQ. Activation of remote meta-C–H bonds assisted by an end-on template. Nature. 2012;486:518. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XC, et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature. 2015;519:334. doi: 10.1038/nature14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann N, Ackermann L. meta-selective C–H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc. 2013;135:5877–5884. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]
- 24.Li J, et al. N-acyl amino acid ligands for Ruthenium(II)-catalyzed meta-C–H tert-alkylation with removable auxiliaries. J. Am. Chem. Soc. 2015;137:13894–13901. doi: 10.1021/jacs.5b08435. [DOI] [PubMed] [Google Scholar]
- 25.Li J, et al. Ruthenium(II)-catalysed remote C–H alkylations as a versatile platform to meta-decorated arenes. Nat. Commun. 2017;8:15430. doi: 10.1038/ncomms15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Z, Wang J, Dong G. Simple amine-directed meta-selective C–H arylation via Pd/Norbornene catalysis. J. Am. Chem. Soc. 2015;137:5887–5890. doi: 10.1021/jacs.5b02809. [DOI] [PubMed] [Google Scholar]
- 27.Kuninobu Y, Ida H, Nishi M, Kanai M. A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem. 2015;7:712. doi: 10.1038/nchem.2322. [DOI] [PubMed] [Google Scholar]
- 28.Gemoets HPL, Laudadio G, Verstraete K, Hessel V, Noël T. A modular flow design for the meta-selective C-H arylation of anilines. Angew. Chem. Int. Ed. 2017;56:7161–7165. doi: 10.1002/anie.201703369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisht R, Chattopadhyay B. Formal Ir-catalyzed ligand-enabled ortho and meta borylation of aromatic aldehydes via in situ-generated imines. J. Am. Chem. Soc. 2016;138:84–87. doi: 10.1021/jacs.5b11683. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Cai L, Ji H, Yang L, Li G. Pd(II)-catalysed meta-C–H functionalizations of benzoic acid derivatives. Nat. Commun. 2016;7:10443. doi: 10.1038/ncomms10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Ji H, Cai L, Li G. Pd(ii)-catalyzed remote regiodivergent ortho- and meta-C-H functionalizations of phenylethylamines. Chem. Sci. 2015;6:5595–5600. doi: 10.1039/C5SC01737H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maji A, Bhaskararao B, Singha S, Sunoj RB, Maiti D. Directing group assisted meta-hydroxylation by C-H activation. Chem. Sci. 2016;7:3147–3153. doi: 10.1039/C5SC04060D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murai S, et al. Efficient catalytic addition of aromatic carbon-hydrogen bonds to olefins. Nature. 1993;366:529. doi: 10.1038/366529a0. [DOI] [Google Scholar]
- 34.Rouquet G, Chatani N. Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013;52:11726–11743. doi: 10.1002/anie.201301451. [DOI] [PubMed] [Google Scholar]
- 35.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhl N, Hopkinson MN, Wencel-Delord J, Glorius F. Beyond directing groups: transition-metal-catalyzed C-H activation of simple arenes. Angew. Chem. Int. Ed. 2012;51:10236–10254. doi: 10.1002/anie.201203269. [DOI] [PubMed] [Google Scholar]
- 37.Tobisu M, Chatani N. Remote control by steric effects. Science. 2014;343:850. doi: 10.1126/science.1250335. [DOI] [PubMed] [Google Scholar]
- 38.Aïssa C, Fürstner A. A rhodium-catalyzed C−H activation/cycloisomerization tandem. J. Am. Chem. Soc. 2007;129:14836–14837. doi: 10.1021/ja0746316. [DOI] [PubMed] [Google Scholar]
- 39.Gensch T, Hopkinson MN, Glorius F, Wencel-Delord J. Mild metal-catalyzed C-H activation: examples and concepts. Chem. Soc. Rev. 2016;45:2900–2936. doi: 10.1039/C6CS00075D. [DOI] [PubMed] [Google Scholar]
- 40.Akai S, Peat AJ, Buchwald SL. Regioselective, directed meta acylation of aromatic compounds. J. Am. Chem. Soc. 1998;120:9119–9125. doi: 10.1021/ja981508t. [DOI] [Google Scholar]
- 41.Fang P, Li M, Ge H. Room temperature palladium-catalyzed decarboxylative ortho-acylation of acetanilides with α-Oxocarboxylic acids. J. Am. Chem. Soc. 2010;132:11898–11899. doi: 10.1021/ja105245f. [DOI] [PubMed] [Google Scholar]
- 42.Xiao F, et al. Palladium-catalyzed oxidative sp2 C−H bond acylation with alcohols. Org. Lett. 2011;13:1614–1617. doi: 10.1021/ol200017a. [DOI] [PubMed] [Google Scholar]
- 43.Liu PM, Frost CG. Ruthenium-catalyzed C–H functionalization of arylpyrazoles: regioselective acylation with acid chlorides. Org. Lett. 2013;15:5862–5865. doi: 10.1021/ol402936c. [DOI] [PubMed] [Google Scholar]
- 44.Song H, Chen D, Pi C, Cui X, Wu Y. Palladium(II)-catalyzed direct regioselectively oxidative acylation of azobenzenes with toluene derivatives. J. Org. Chem. 2014;79:2955–2962. doi: 10.1021/jo5000219. [DOI] [PubMed] [Google Scholar]
- 45.Hoque ME, Bisht R, Haldar C, Chattopadhyay B. Noncovalent interactions in Ir-catalyzed C–H activation: L-shaped ligand for para-selective borylation of aromatic esters. J. Am. Chem. Soc. 2017;139:7745–7748. doi: 10.1021/jacs.7b04490. [DOI] [PubMed] [Google Scholar]
- 46.Maji A. Experimental and computational exploration of para-selective silylation with a hydrogen-bonded template. Angew. Chem. Int. Ed. 2017;56:14903–14907. doi: 10.1002/anie.201708449. [DOI] [PubMed] [Google Scholar]
- 47.Patra T. Palladium-catalyzed directed para C-H functionalization of phenols. Angew. Chem. Int. Ed. 2016;55:7751–7755. doi: 10.1002/anie.201601999. [DOI] [PubMed] [Google Scholar]
- 48.Dey A, Maity S, Maiti D. Reaching the south: metal-catalyzed transformation of the aromatic para-position. Chem. Commun. 2016;52:12398–12414. doi: 10.1039/C6CC05235E. [DOI] [PubMed] [Google Scholar]
- 49.Bag S, et al. Remote para-C–H functionalization of arenes by a D-shaped biphenyl template-based assembly. J. Am. Chem. Soc. 2015;137:11888–11891. doi: 10.1021/jacs.5b06793. [DOI] [PubMed] [Google Scholar]
- 50.Tojo, G. & Fernandez, M. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice (Springer, New York, 2006).
- 51.Littke AF, Fu GC. A versatile catalyst for Heck reactions of aryl chlorides and aryl bromides under mild conditions. J. Am. Chem. Soc. 2001;123:6989–7000. doi: 10.1021/ja010988c. [DOI] [PubMed] [Google Scholar]
- 52.Shrestha B, et al. Ni-catalyzed regioselective 1,2-dicarbofunctionalization of olefins by intercepting Heck intermediates as imine-stabilized transient metallacycles. J. Am. Chem. Soc. 2017;139:10653–10656. doi: 10.1021/jacs.7b06340. [DOI] [PubMed] [Google Scholar]
- 53.Thapa S, et al. Ni-catalysed regioselective 1,2-diarylation of unactivated olefins by stabilizing Heck intermediates as pyridylsilyl-coordinated transient metallacycles. Chem. Sci. 2018;9:904–909. doi: 10.1039/C7SC04351A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Line NJ, Witherspoon BP, Hancock EN, Brown MK. Synthesis of ent-[3]-Ladderanol: development and application of intramolecular chirality transfer [2+2] cycloadditions of allenic ketones and alkenes. J. Am. Chem. Soc. 2017;139:14392–14395. doi: 10.1021/jacs.7b09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y, Smith KB, Brown MK. Copper-catalyzed borylacylation of activated alkenes with acid chlorides. Angew. Chem. Int. Ed. 2017;56:13314–13318. doi: 10.1002/anie.201707323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Werner EW, Sigman MS. Operationally simple and highly (E)-styrenyl-selective Heck reactions of electronically nonbiased olefins. J. Am. Chem. Soc. 2011;133:9692–9695. doi: 10.1021/ja203164p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCammant MS, Shigeta T, Sigman MS. Palladium-catalyzed 1,3-difunctionalization using terminal alkenes with alkenyl nonaflates and aryl boronic acids. Org. Lett. 2016;18:1792–1795. doi: 10.1021/acs.orglett.6b00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue W, Qu ZW, Grimme S, Oestreich M. Copper-catalyzed cross-coupling of silicon pronucleophiles with unactivated alkyl electrophiles coupled with radical cyclization. J. Am. Chem. Soc. 2016;138:14222–14225. doi: 10.1021/jacs.6b09596. [DOI] [PubMed] [Google Scholar]
- 59.Kowalczyk M, Lupton DW. Cascade olefin isomerization/intramolecular Diels-Alder reaction catalyzed by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2014;53:5314–5317. doi: 10.1002/anie.201402067. [DOI] [PubMed] [Google Scholar]
- 60.Ungureanu A, Levens A, Candish L, Lupton DW. N-heterocyclic carbene catalyzed synthesis of ë-sultones via à,á-unsaturated sulfonyl azolium intermediates. Angew. Chem. Int. Ed. 2015;54:11780–11784. doi: 10.1002/anie.201504633. [DOI] [PubMed] [Google Scholar]
- 61.Zhang C, Lupton DW. Enantioselective N-heterocyclic carbene catalyzed synthesis of functionalized indenes. Org. Lett. 2017;19:4456–4459. doi: 10.1021/acs.orglett.7b01981. [DOI] [PubMed] [Google Scholar]
- 62.Garve LKB, Werz DB. Pd-catalyzed three-component coupling of terminal alkynes, arynes, and vinyl cyclopropane dicarboxylate. Org. Lett. 2015;17:596–599. doi: 10.1021/ol503609d. [DOI] [PubMed] [Google Scholar]
- 63.Harnett JJ, Doris E. Stereoselective titanium mediated trimerisation of methyl vinyl ketone: a novel carbocyclisation reaction. Synth. Commun. 1998;28:2685–2688. doi: 10.1080/00397919808004838. [DOI] [Google Scholar]
- 64.Kikuchi S, Saito K, Akita M, Inagaki A. Nonradical light-controlled polymerization of styrene and vinyl ethers catalyzed by an iridium–palladium photocatalyst. Organometallics. 2018;37:359–366. doi: 10.1021/acs.organomet.7b00783. [DOI] [Google Scholar]
- 65.Vita MV, Waser J. Azidation of β-keto esters and silyl enol ethers with a benziodoxole reagent. Org. Lett. 2013;15:3246–3249. doi: 10.1021/ol401229v. [DOI] [PubMed] [Google Scholar]
- 66.Orcel U, Waser J. Palladium-catalyzed vicinal amino alcohols synthesis from allyl amines by in situ tether formation and carboetherification. Angew. Chem. Int. Ed. 2015;54:5250–5254. doi: 10.1002/anie.201500636. [DOI] [PubMed] [Google Scholar]
- 67.Ruan J, Li X, Saidi O, Xiao J. Oxygen and base-free oxidative Heck reactions of arylboronic acids with olefins. J. Am. Chem. Soc. 2008;130:2424–2425. doi: 10.1021/ja0782955. [DOI] [PubMed] [Google Scholar]
- 68.Surry DS, Buchwald SL. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Böhm HJ, et al. Fluorine in medicinal chemistry. ChemBioChem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 70.Leroux FR, Manteau B, Vors JP, Pazenok S. Trifluoromethyl ethers—synthesis and properties of an unusual substituent. Beilstein J. Org. Chem. 2008;4:13. doi: 10.3762/bjoc.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are included in the article and Supplementary Information