The lymphatic system consists of a network of lymph capillaries and vessels deputed to drain the interstitial fluid (containing floating macromolecules, such as nutrients, cellular debris and tissue-infiltrating pathogens) and to transport tissue-patrolling lymphocytes and dendritic cells (DCs) to draining lymph nodes (drLNs).1 Knowledge of the mechanisms associated with cellular transmigration into the lymphatic system could offer the opportunity to manipulate the immune response in a wide range of diseases, such as autoimmune diseases and cancer.2,3 In this research highlight, we discuss a recent study that sheds light on our understanding of the molecules involved in DCs docking to lymphatic endothelial cells, a critical step prior to their subsequent endothelial transmigration into lymphatic vessels. DC docking occurs through the interaction between hyaluronan (hyaluronic acid, HA) molecules on the plasma membrane and lymphatic-vessel endothelial protein (LYVE-1).4
Peripheral lymphatic capillaries are blind-ended structures composed of a wall of overlapping endothelial cells surrounded by minimal basal membrane; cells are connected by button-like junctions and loose flap-like openings.1 Tight junction and adherence junction proteins, such as VE-cadherin, ZO-1, ESAM, claudin 5 and occludin, are present in button-like junctions, whereas CD31 and lymphatic hyaluronan receptor LYVE-1 are observed in the flap-like openings.1 Transmigration of immune cells across endothelial cells of lymph vessels was considered a passive mechanism until the last decade when new insights into the presence of functionalized junctions through which fluids and leukocytes enter in the lymphatic vessels have been reported.1 Indeed, Baluk et al 5 reported that endothelial cells can modify the expression of adhesion proteins to allow the entry of different leucocyte types, such as DCs.
DCs are the sentinels of our organism and mainly reside in peripheral tissues, especially in skin and mucosae. DCs sample the environment through dendrites, searching for self and non-self molecules that bind Toll-like receptors and trigger the expression of activation markers and the upregulation of CC-chemokine receptor 7 (CCR7).6 The transit of DCs from tissue to lymph occurs through three main passages: (a) DCs migrate across the basal lamina surrounding lymphatic vessels via a process that is driven by haptotactic gradients of CCL21 (ligand of CCR7) and other chemokines. Then, DCs move through gaps and portals in the basement membrane, proceeding with amoeboid movements. (b) DCs transmigrate across endothelial cells via a process that involves either integrin-independent migration (steady state vessels) or integrin-dependent adhesion/migration (ICAM-1 and VCAM-1 in inflamed vessels). (c) DCs move through lymph capillaries and vessels. Cell migration is driven by the fluid flow from initial capillaries downstream to larger vessels and then to lymph nodes (Figure 1).1 When DCs finally reach drLNs, they instruct T cells regarding the cellular response to be activated.7
Figure 1.
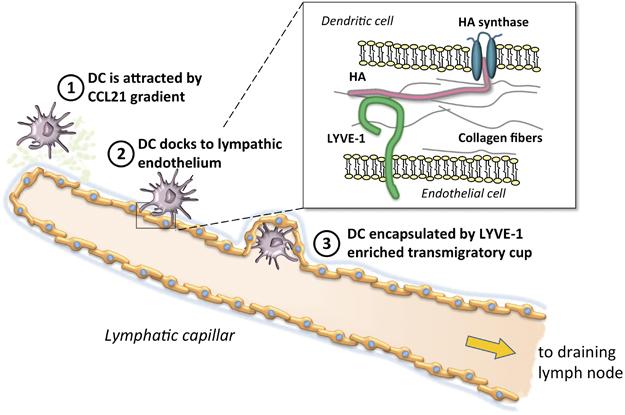
Schematic representation of the major steps in DC migration to the lymphatic system. (1) Myeloid DCs in inflamed tissues are attracted to the regional lymphatic system in response to a CCL21 gradient (light green spots), released by endothelial cells, and move through gaps and portals in the basement membrane (light blue/gray); (2) DCs employ hyaluronan (HA) to anchor themselves to the basolateral surface of lymphatic endothelial cells; (3) DCs are enveloped by LYVE-1+ transmigratory cups to complete their migration into lymphatic capillary to reach draining lymph nodes. (Inset) HA (in pink) is synthesized at the inner face of the DC plasma membrane by hyaluronan synthases (in blue), and the growing HA polymer is extruded through the membrane to the outer surface. Endothelial cells express the LYVE-1 (in green) surface receptor for HA.
LYVE-1 was first described in 1999 as a receptor of HA because it is similar to a previously known HA receptor, CD44, a glycoprotein involved in cell–cell interactions, cell adhesion and migration.8 LYVE-1 is expressed on lymphatic endothelium and is used to identify and characterize lymphatic vessels in healthy and pathological inflamed tissues. LYVE-1 binds HA, a mucopolysaccharide directly synthetized on the plasma cell membrane and released in the extracellular matrix in large quantities. Although macromolecules are freely transported in lymph vessels, LYVE-1-mediated HA uptake could be important in lymph nodes and spleen synusoids. Here HA is degraded, and LYVE-1 exerts a coreceptor function in these sites.9 Because CD44 binds HA and this interaction mediates CD44+ cell migration, researchers have wondered if LYVE-1 regulates HA binding for HA+ cell adhesion and migration.
To address this issue, Johnson and collaborators studied the lymphatic system in the skin, which hosts large numbers of dendritic cells, such as dermal DCs and Langerhans cells. After skin sensitization with oxazolone (a chemical compound that mobilizes antigen-presenting cells), DCs migrate in the lymphatic vessels and reach drLNs.4 To investigate DC docking to lymphatic endothelial cells and their influx in lymphatic vessels, the authors exploited several approaches to demonstrate the fundamental role of LYVE-1: HA interaction in DC adhesion and transmigration. In two models of skin contact hypersensitivity in Lyve1 −/− mice, the number of skin-derived CD11c+ DCs was reduced in drLNs 6 h post sensitization followed by a rebound after 48 h. All subsets of migratory CD11c+MHCII+FITC+ DCs, including CD11b+ DCs, CD103+ DCs, EpCAM+ DCs and langerin+ DCs, were similarly affected. This result was associated with a reduction in DC percentages in lymphatic vessels (85 vs 22%) rather than a defect in intraluminal migration. The key role of LYVE-1 in controlling DC migration was further confirmed by an ex vivo crawl-out assay used to measure the number of DCs that migrated from skin explants into culture medium. Similar conclusions were drawn by blocking different LYVE-1 epitopes with commercial and in-house-generated antibodies. These in vivo experiments revealed that the administration of LYVE-1 blocking antibodies to oxazolone-sensitized Lyve1+/+ mice prevented the accumulation of CD11c+DCs in drLNs. Notably, confocal images displayed an accumulation of fluorescent-labeled DCs outside skin lymphatic capillaries, and less than 20% of cells were detected inside the lumen (versus 80% in control mice). Moreover, the administration of LYVE-1 blocking antibodies to mice (vaccinated with the influenza virus A/NT/60/68 nucleoprotein peptide delivered by dermal DCs to drLNs) reduced the number of CD8+ T proliferating cells in drLNs. These data suggested that non-functional LYVE-1 exhibited downstream effects on the induction of T cell responses.
Furthermore, intradermal injection of HA-depleted DCs into sensitized mice resulted in a significant reduction of DCs that reached drLNs, demonstrating the importance not only of LYVE-1 but also of HA expression in DCs trafficking. Ultimately, the authors investigated the interaction of LYVE-1 and HA in in vitro adhesion and transmigration assays, in which fluorescent-labeled LPS-matured DCs were incubated with mouse dermal lymphatic endothelial cells (mLECs). Indeed, blocking LYVE-1 reduced DC adhesion and transmigration through mLEC layers. DC docking was visualized by confocal microscopy, and a ring-like structure formed on mLEC. DCs were anchored to LYVE-1+ mLEC and were enveloped as a cup in both in vitro and ex vivo experiments.
There is an unmet need for innovative therapeutic options to modulate the altered immune response in inflammatory and autoimmune diseases, and the identification of new molecules targeting specific key points in the immune activation processes is extremely useful in this field. A critical step is represented by T cell recruitment from the bloodstream into the inflamed tissue, a process mediated by several molecules. For most of these processes, specific therapies have been explored. For example, natalizumab is a humanized monoclonal antibody against the cell adhesion molecule α4-integrin and is used for the treatment of multiple sclerosis10 and Crohn's disease;11 blocking the interaction between α4-integrin and VCAM-1 prevents the migration of activated T cells across the blood brain barrier. Furthermore, interfering with the egress of effector T cells from secondary lymphoid organs into the bloodstream is an attractive key point. Indeed, modulation of the sphingosine 1-phosphate (S1P) pathway is the target of fingolimod, a compound analog of sphingosine and that interacts with the S1P receptor expressed on the lymphocyte surface. Because S1P receptors are involved in pseudopodia formation and migration, fingolimod blocks the egress of effector T cells from secondary lymphoid organs.2
In this view, the study by Johnson et al. 4 provides new insights into the molecular mechanisms involved in an earlier event of the immune response, that is, DCs trafficking from inflamed tissues into lymphatic vessels and to secondary lymphoid organs. LYVE-1 could represent a novel molecular target for new immunomodulatory drugs in inflammatory conditions, autoimmunity, and even cancer, where the efficacy of vaccination with antigen-pulsed DCs could be enhanced by improving DC migration in the lymphatic system.
Acknowledgements
The work is supported by the Italian Ministry of Health (WFR PE-2011-02346818).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jackson DG. Lymphatic regulation of cellular trafficking. J Clin Cell Immunol. 2014;5:258–267. doi: 10.4172/2155-9899.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 3.Seyfizadeh N, Muthuswamy R, Mitchell DA, Nierkens S, Seyfizadeh N. Migration of dendritic cells to the lymph nodes and its enhancement to drive anti-tumor responses. Crit Rev Oncol Hematol. 2016;107:100–110. doi: 10.1016/j.critrevonc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LA, Banerji S, Lawrance W, Gileadi U, Prota G, Holder KA, et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat Immunol. 2017;18:762–770. doi: 10.1038/ni.3750. [DOI] [PubMed] [Google Scholar]
- 5.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo E, Nitschké M, Halin C. Dendritic cell interactions with lymphatic endothelium. Lymphat Res Biol. 2013;11:172–182. doi: 10.1089/lrb.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 8.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson DG. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc Med. 2003;13:1–7. doi: 10.1016/S1050-1738(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 10.Singer BA. The role of natalizumab in the treatment of multiple sclerosis: benefits and risks. Ther Adv Neurol Disord. 2017;10:327–336. doi: 10.1177/1756285617716002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandzar S, Gupta S, Platt MO. Crohn's disease: a review of treatment options and current research. Cell Immunol. 2013;286:45–52. doi: 10.1016/j.cellimm.2013.11.003. [DOI] [PubMed] [Google Scholar]


