Abstract
Narcissists are prone to risky decision-making, but why? This study tested—via behavioral and event-related potential (ERP) measures—two accounts: deficiencies in error monitoring and deficiencies in action updating. High and low narcissists were engaged in a monetary gambling task by choosing between a high-risk and a low-risk option while the electroencephalogram (EEG) was being recorded. Two ERP components relevant to outcome evaluation—feedback-related negativity (FRN) and P3—were analyzed, with the FRN serving as an index of error monitoring and the P3 as an index of action updating. Generally, high and low narcissists differed in the high-risk condition but not in the low-risk condition. At the behavioral level, high (vs low) narcissists made riskier decisions following high-risk decision outcomes, which was in line with past findings; at the neurophysiological level, while no FRN difference emerged between high and low narcissists, the outcome valence effect (positive vs negative) on the P3 was stronger among low narcissists than high narcissists following high-risk decision outcomes. One possible interpretation of the results is that narcissism is associated with reduced action updating. The findings contribute to the understanding of narcissistic decision-making and self-regulation.
Keywords: narcissism, decision-making, economic risk, event-related potential (ERP), feedback-related negativity (FRN), P3
Introduction
We adopt a social neuroscience perspective to examine the relation between narcissism and decision-making. Past research has established that individuals high on narcissism (also referred to as narcissists) are prone to risky decision-making (Campbell et al., 2004; Foster et al., 2011) due to overconfidence, focus on reward or heightened benefit perception (Lakey et al., 2008; Foster et al., 2009b). We aim to untangle the mechanism underlying narcissists’ risky decision-making through an ERP paradigm.
Narcissism
Narcissism is characterized, in part, by grandiose self-views (Morf et al., 2011; Thomaes et al., 2016). Narcissists maintain, or further elevate, their self-views via self-regulation (Morf and Rhodewalt, 2001; Campbell and Green, 2007). Of interest, narcissism moderates responses to rewards and threats (Campbell and Campbell, 2009; Thomaes and Sedikides, 2016). For example, narcissists make self-serving attributions for successful outcomes and focus on the rewarding side of risky choices while neglecting their potential costs (Campbell and Foster, 2007; Lakey et al., 2008). At the same time, narcissists discount negative feedback (e.g. by derogating the evaluator) and even show excessive self-enhancement in dismissing warnings about high risk (Kernis and Sun, 1994; Morf et al., 2011). Indeed, narcissists self-regulate in a riskier manner when perceived threat rises (Jordan and Audia, 2012). Narcissistic self-regulation has been described as ‘better risky than sorry’ (Morf and Horvath, 2010, p. 129), a strategy that could end in suboptimal decision-making. Evidence does indicate that high (compared to low) narcissists make suboptimal decisions (Sedikides and Campbell, 2017). For example, they escalate gambling and suffer losses (Campbell et al., 2004; Lakey et al., 2008), invest on volatile stock markets and lose money (Foster et al., 2009a; Foster et al., 2011) and are prone to financial and health risk-taking (Buelow and Brunell, 2014; Brunell and Buelow, 2015). They are also prone to impulsive buying (Cai et al., 2015), increased road rage (Britt and Garrity, 2006) and higher levels of binge drinking (Wood, 2010).
Researchers have addressed several precursors of narcissistic risk-taking using behavioral measures. One literature stream has highlighted antecedents of risk-taking behavior, including stronger approach motivation, overconfidence, heightened risk acceptance and myopic focus on reward (Campbell et al., 2004; Lakey et al., 2008; Foster et al., 2009a; Foster et al., 2011). Relevant to the present investigation, another literature stream has emphasized cognitive limitations in decision making (Koechlin and Hyafil, 2007; Cokely and Kelley, 2009; Mell et al., 2009). One key factor underlying decision-making is the ability to learn from outcome feedback (Cohen et al., 2011; Ruff and Fehr, 2014), particularly in the outcome evaluation stage (Ernst and Paulus, 2005). During that stage, decision-makers evaluate the consequences of their decisions and adjust their behavior patterns according to outcome feedback (Hastie, 2001; Ernst and Paulus, 2005; Doya, 2008). This stage helps decision-makers to explore action-outcome contingencies in the current context (i.e. learning from their current decisions), thus revising their strategies in order to improve decision-making (Ernst and Paulus, 2005; Kahnt et al., 2009). Two fundamental cognitive components are involved in outcome evaluation: error monitoring (i.e. monitoring negative outcome feedback) and action updating (i.e. updating mental model according to outcome feedback; Steinhauser and Yeung, 2010; Toplak et al., 2010). Deficiencies in either component may culminate in suboptimal decisions.
Indirect evidence suggests that narcissists may have access to error monitoring. For example, they do not necessarily think that negative feedback is incorrect; instead, they may accept that it is accurate (Kernis and Sun, 1994; Morf and Rhodewalt, 2001). Also, narcissists have insight into their undesirable side. Specifically, they are aware of their narcissistic characteristics (e.g. arrogance) or behaviors (e.g. bragging), and know that others see them less favorably than they see themselves (Carlson et al., 2011; Carlson, 2013). And yet they still act out their narcissism unencumbered by intrapersonal or interpersonal barriers (Carlson, 2013; Roberts et al., 2017). Similarly, in decision-making contexts, narcissists are slow to adjust their decisions to outcome feedback (Audia and Brion, 2007; Jordan and Audia, 2012). Based on this indirect evidence, we would not expect for error monitoring to underlie narcissists’ risky decision-making. Action updating, though, is a viable candidate. To find out, we conducted an ERP study of high and low narcissists during outcome evaluation in a monetary gambling task.
ERP components
Feedback-related negativity (FRN) and P3 are the two most well-researched ERP components of outcome evaluation (for a review, see San Martín, 2012). Drawing from the relevant literature, we propose that the FRN and P3 are associated with error monitoring and action updating, respectively.
FRN is a negative-going ERP component that peaks at approximately 250 ms following outcome presentation (Miltner et al., 1997; Gehring and Willoughby, 2002; Muller et al., 2005). Although the distribution of the FRN varies across studies, the difference wave between positive and negative feedback consistently reaches its peak at the frontal-central region (Holroyd and Krigolson, 2007). Error monitoring is the metacognitive process by which individuals detect and signal their errors (e.g. negative outcome feedback; see Yeung and Summerfield, 2012). FRN reflects the error monitoring function of the anterior cingulate cortex (ACC; Miltner et al., 1997; Simons, 2010; Hauser et al., 2014), such that the FRN is typically greater in response to negative than positive outcome (San Martín, 2012). Specifically, FRN may reflect the feedback learning process in which errors act as a guiding signal for behavioral adaptation (Luu et al., 2003; Cohen et al., 2011; Walsh and Anderson, 2012).
Following the FRN, P3 is a centro-parietal positive-going component that peaks at about 300–600 ms after outcome presentation (Polich & Criado, 2006 ; Polich, 2007). P3 is associated with the mental updating process. According to the context updating hypothesis (Donchin and Coles, 1988), when the current stimulus is useful in maintaining or updating the memory representation of the environment, the mental model will then be updated, with the P3 amplitude being proportional to model revision. Large (vs small) magnitude outcomes induce a greater P3 (San Martín, 2012), given that they signal higher environmental motivational significance and are thus more relevant to the updating process (Nieuwenhuis et al., 2005). P3 has been associated with action updating (Chase et al., 2011; San Martín et al., 2013). For example, a greater P3 indicates a stronger proclivity to switch between different behavioral strategies (Zhang et al., 2013; Zhang et al., 2014), and greater P3 sensitivity to outcome information is associated with better behavioral adjustment (San Martín et al., 2013).
Overview
We were concerned with the relation between cortical electrical signals following outcome presentation and subsequent behavioral output in a risk decision-making scenario among high vs low narcissists. We employed EEG recording in a trial-by-trial monetary gambling task to investigate the ERP signals (including the FRN and P3). We aimed to uncover the cognitive mechanisms underlying risk preference in narcissists. We expected that high narcissists would take more risks, due to their deficiencies in action updating, but not in error monitoring, during outcome evaluation. In particular, at the behavioral level, we expected that high (relative to low) narcissists would make more risky choices in the monetary gambling task. At the neurophysiological level, we expected that high and low narcissists would differ on the P3, but not on the FRN, in response to outcome feedback.
Materials and methods
Participants
The study comprised two sessions. In the first session, we administered the 40-item forced-choice Narcissism Personality Inventory (NPI; Raskin and Terry, 1988) to 229 Zhejiang University undergraduates. Each item consists of a narcissistic and a non-narcissistic statement. Sample items are the following: ‘I am more capable than other people’ (narcissistic statement) vs ‘there is a lot that I can learn from other people’ (non-narcissistic statement); and ‘I am an extraordinary person’ (narcissistic statement) vs ‘I am much like everybody else’ (non-narcissistic statement). For each item, participants indicated whether the narcissistic or non-narcissistic statement described them better. We coded the narcissistic statement choice as 1 and the non-narcissistic statement choice as 0 (α = 0.84). The NPI has been used successfully in Chinese samples (Cai et al., 2012; Luo et al., 2014; Cai et al., 2015). A power analysis (G*Power 3.1; Faul et al., 2007) suggested that 46 participants would ensure 90% statistical power even in case of small-to-medium effect sizes (cf. Vazire, 2016). We recruited 49 participants (38 male, 11 female; Mage = 22.61 years, SDage = 0.95 years) based on their NPI scores. Thus, in the second session, we tested 25 high narcissism participants (19 male, 6 female; Mage = 22.32 years, SDage = 0.85 years) and 24 low narcissism participants (19 male, 5 female; Mage = 22.92 years, SDage = 0.97 years). High narcissists (M = 25.20, SD = 3.52) and low narcissists (M = 3.71, SD = 1.37) differed significantly on their NPI scores, t(47) = 28.36, P < 0.001, d = 8.07. (For similar procedures involving selection of high and low scorers on a personality scale, see Li and Yang, 2013; Li et al., 2012; Luo et al., 2014.)
All participants were free of regular use of any substance that might influence the central nervous system, and none had a history of neurological disease. All had normal vision (with correction) and were right-handed. Finally, all participants completed a written informed consent prior to commencement of the study and were remunerated with 80–100 Chinese renminbi (RMB; approximately £10–12; see below for details). The Institutional Review Board at the Institute of Psychology, Chinese Academy of Sciences approved the experimental protocol.
Procedure
Participants engaged in a monetary gambling task on computer. They learned the rules as follows: ’The task consists of many identical rounds. In each round, you need to make a forced-choice between two options, that is, 9 and 99. The number of each option indicates the amount of credits you would receive or lose in this round, depending on the outcome feedback (win or loss) which you will receive immediately after you make the choice. The credits would accumulate throughout the task and would determine your payoff at the end of the experiment.’ We encouraged participants to respond in a manner that would maximize the reward. We rewarded them initially with 80 Chinese RMB, and instructed them that the total bonus would be 80 RMB plus the cumulative outcome (ranging from 0 to 20 RMB) of the experiment.
We conducted stimulus display and behavioral data acquisition in the gambling task using E-Prime software 2.0 (Psychology Software Tools, Inc.). During the task, participants sat comfortably in an electrically-shielded room approximately 80 cm from a computer screen. The formal task consisted of 2 blocks of 160 trials each (Figure 1). Each trial began with the presentation of a central fixation point. After 1200 ms, 2 white rectangles (2.5° × 2.5°) appeared on each side of the fixation point, displaying one of two numbers (options): 9 and 99. Participants were allotted 4000 ms to make decision between the two options by pressing the F or J keys on a keyboard with their left or right index finger, respectively. The selected option was then highlighted by a thick red outline for 500 ms. Thereafter both numbers disappeared, leaving the rectangles on the screen for a random interval between 800 and 1200 ms. Lastly, the outcome feedback was presented in the chosen rectangle for 1000 ms. There were two kinds of outcome feedback: ‘+’ and ‘−’. The ‘+’symbol (positive outcome) indicated that participants won as many points as they chose in that trial, whereas the ‘−‘ symbol (negative outcome) indicated the reverse. The amplitudes of both the FRN and P3 are sensitive to event probability (Holroyd et al., 2004; San Martín, 2012). In order to control the event probability across conditions, we provided outcome feedback in a pseudorandom sequence, and every participant received exactly 160 of each outcome (positive/negative), which was unbeknownst to participants.
Fig. 1.
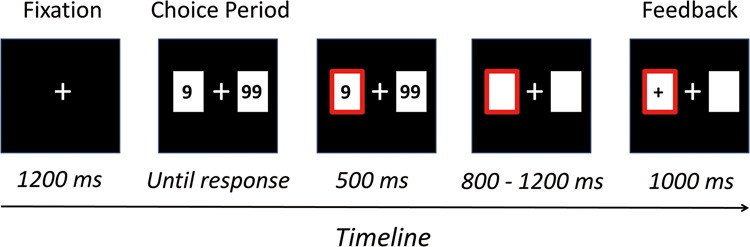
Schematic depiction of a single trial setting. On this exemplar trial, participant chooses the small option (‘9’) and receives positive feedback (‘+’).
Electrophysiological recording
We recorded brain electrical activity at 32 scalp sites using tin electrodes mounted in an elastic cap (Brain Products), with the reference on the left and right mastoids. We recorded the vertical electrooculogram (EOG) with electrode placed above the left eye. We maintained all interelectrode impedance below 5 kΩ. We amplified the EEG and EOG using a 0.05–100 Hz bandpass and continuously sampled at 500 Hz/channel for offline analysis.
We conducted the EEG analysis using the Brain Vision Analyzer software (Brain Products). In each trial, we corrected the EEG for blinks and eye movements using the independent components analysis approach. After 0.05–30 Hz bandpass digital filtering, we segmented the EEG for each trial, beginning 200 ms before outcome feedback onset and continuing for 1000 ms. We baseline corrected the data by subtracting the average activity of that channel during the baseline period from each sample. We excluded from further analysis any trial in which EEG voltages exceeded a threshold of ±80 μV during the recording epoch. We constructed the ERP waveforms by averaging epoch of the remaining trial in each condition for each participant. After data preprocessing, we determined the trials that survived as artifact-free (overall mean value: 271.33 [84.79%]), which were balanced across conditions (‘+9’: 62.55, ‘+99’: 64.14, ‘-9’: 73.63, ‘-99’: 71.00; F[3, 144] = 1.407, P = 0.243, ƞp2 = 0.028). The number of trials in each condition was sufficient for FRN and P3 analyses, as per relevant literature (Cohen and Polich, 1997; Marco-Pallares et al., 2011).
ERP analysis
We determined the time windows for ERP measurement by visual inspection of grand-averaged waveforms. Accordingly, we calculated the FRN amplitude as the mean value within the 250–350 ms window following outcome presentation. We calculated the P3 as the mean value in the 350–450 ms time window following outcome presentation. In order to increase the stability of the ERP results (Luck and Gaspelin, 2017), we selected multiple (rather than one) electrodes for data analysis on each ERP component, based on visual inspection of its scalp distribution (Figures 2 and 3). Thus, we used the arithmetic mean values of electrodes Fz and Cz for further analyses of the FRN amplitude and used the arithmetic mean values of electrodes Cz and Pz for the P3 amplitude. These selections were also consistent with previous findings showing that the FRN and P3 are maximal in the scalp’s fronto-central and centro-parietal areas (Nieuwenhuis et al., 2005; Holroyd and Krigolson, 2007).
Fig. 2.
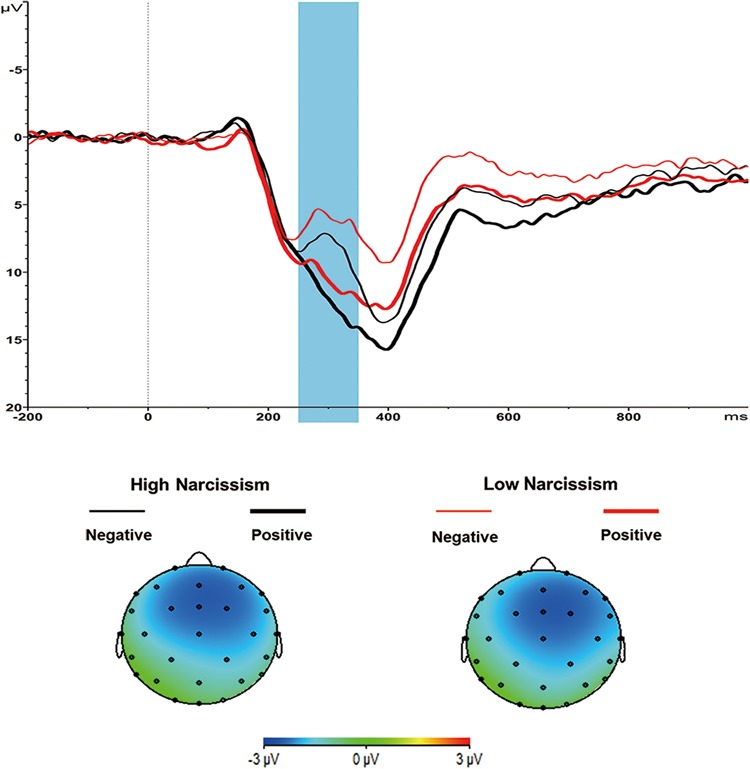
Grand-average ERPs evoked by large outcome presentation at the Fz site, where the FRN reached its maximum. The time point 0 indicates the onset of outcome presentation. The shaded blue area indicates the 250–350 ms time window for the calculation of the mean value of the FRN. The scalp topographies of the difference (Negative–Positive) for large outcomes are presented beneath.
Fig. 3.
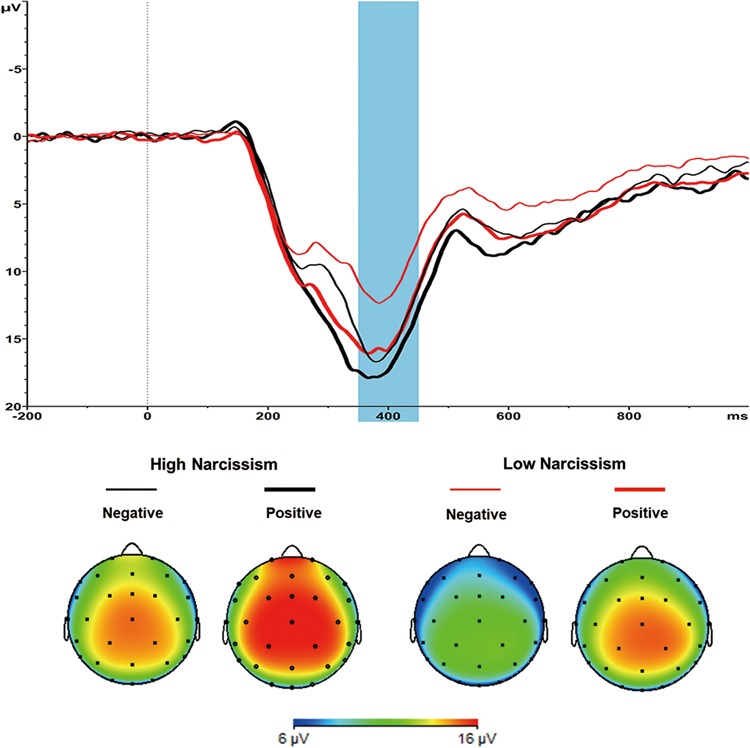
Grand-average ERPs evoked by large outcome presentation at the Cz site, where the P3 reached its maximum. The time point 0 indicates the onset of outcome presentation. The shaded blue area indicates the 350–450 ms time window for the calculation of the mean value of the P3. The scalp topographies of positive and negative condition for large outcomes are presented beneath.
Data analysis
For all analyses, we reported the results of descriptive statistics as mean ± SD. We set the significance level at P = 0.050 and used Greenhouse–Geisser corrections when appropriate. Also, we conducted simple effect comparisons via Least Significant Difference test and analyzed significant interactions using simple-effects models. Finally, we provided partial eta-squared (ƞp2) values to demonstrate effect size where appropriate.
Results
Behavior
While option 9 is low risk, option 99 is high risk; in the latter, a gain leads to a large reward but a loss leads to a large cost. This operational definition of risk is consistent with the theory that defines economic risk as the amount of outcome variance (Rothschild and Stiglitz, 1970). We expected that high (compared to low) narcissists would be more likely to choose the high-risk option regardless of current outcome. To this end, we analyzed the probability of choosing the high-risk choice (‘99’) on the next trial in a 2 (narcissism: high, low) × 2 (valence: positive, negative) × 2 (magnitude: small, large) mixed ANOVA, where outcome valence and magnitude were within-subjects independent variables stemmed from the current trial.
Replicating past findings (Gehring and Willoughby, 2002; Zhang et al., 2013), we obtained a magnitude main effect, F(1, 47) = 47.012, P < 0.001, ƞp2 = 0.500. Participants chose more high-risk options following a large outcome (58.1% ± 20.5%) than a small outcome (36.0% ± 19.7%). This effect points to consistency in risk preference; that is, participants were more likely to make risky decisions after a high-risk trial. More interestingly, this effect was moderated by narcissism, F(1, 47) = 6.931, P = 0.011, ƞp2 = 0.129. High narcissists made more high-risk choices following a large outcome (64.5% ± 21.5%) than a small outcome (34.1% ± 18.7%), t(24) = 6.454, P < 0.001, d = 1.29, but this pattern was weaker for low narcissists (51.5% ± 17.5% vs 37.9% ± 20.8%, respectively), t(23) = 3.130, P = 0.005, d = 0.64. No other effect was significant, Fs < 3.38, Ps > 0.07. The results indicated that high narcissists (relative to low narcissists) made more high-risk choices on the next trial after they received the outcome of a high-risk choice, irrespective of outcome valence (positive vs negative).
ERP
FRN
We analyzed FRN in a 2 (narcissism: high, low) × 2 (valence: positive, negative) × 2 (magnitude: small, large) ANOVA. Consistent with prior findings (Yeung and Sanfey, 2004; Goyer et al., 2008; Zhang et al., 2013), we obtained a valence main effect, F(1, 47) = 30.490, P < 0.001, ƞp2 = 0.393. Negative outcomes elicited a greater FRN than positive outcomes (7.25 ± 5.12 μV vs 9.44 ± 5.31 μV). Also consistent with prior findings (Wu and Zhou, 2009; Gu et al., 2011; Kreussel et al., 2012), we obtained a magnitude main effect, F(1, 47) = 31.034, P < 0.001, ƞp2 = 0.398. Small outcomes elicited a greater FRN than large outcomes (6.61 ± 4.35 μV vs 10.09 ± 6.42 μV). In addition, we found a Valence × Magnitude interaction, F(1, 47) = 19.782, P < 0.001, ƞp2 = 0.296. Large negative outcomes (‘-99’) elicited a greater FRN than large positive outcomes (‘+99’) (8.33 ± 6.19 μV vs 11.87 ± 7.10 μV), t(48) = 6.774, P < 0.001, d = 0.53 (Figure 2), but this effect was weaker in the case of small negative outcomes (‘-9’) and small positive outcomes (‘+9’) (6.18 ± 4.94 μV vs 7.03 ± 4.25 μV), t(48) = 1.789, P = 0.080, d = 0.18 (Supplementary Figure S1). Crucially, narcissism neither showed a main effect nor interacted with any of the aforementioned effects, Fs < 1.86, Ps > .18.
P3
We analyzed P3 in a 2 (narcissism: high, low) × 2 (valence: positive, negative) × 2 (magnitude: small, large) ANOVA. In line with previous findings (Wu and Zhou, 2009; Polezzi et al., 2010; Zhou et al., 2010; Gu et al., 2011), we obtained a valence main effect, F(1, 47) = 47.210, P < 0.001, ƞp2 = 0.501. Positive outcomes elicited a greater P3 than negative outcomes (11.76 ± 5.47 μV vs 10.37 ± 5.75 μV). Also in accordance with previous findings (Wu and Zhou, 2009; Bellebaum et al., 2010; Polezzi et al., 2010; Gu et al., 2011), we obtained a magnitude main effect, F(1, 47) = 26.002, P < 0.001, ƞp2 = 0.356. Large outcomes elicited a greater P3 than small outcomes (13.86 ± 7.30 μV vs 8.27 ± 4.89 μV). Further, we found a Valence × Magnitude interaction, F(1, 47) = 5.820, P = 0.020, ƞp2 = 0.110. Large positive outcomes (‘+99’) elicited a greater P3 than large negative outcomes (‘-99’) (14.92 ± 7.40 μV vs 12.80 ± 7.52 μV), t(48) = 4.697, P < 0.001, d = 0.28, but this effect was not significant in the case of small positive outcomes vs small negative outcomes (‘+9’ vs ‘-9’) (8.60 ± 4.90 μV vs 7.94 ± 5.27 μV), t(48) = 1.652, P = 0.105, d = 0.13.
Most importantly, we obtained a significant Narcissism × Valence × Magnitude interaction, F(1, 47) = 5.422, P = 0.024, ƞp2 = 0.103. We conducted separate analyses of P3 responses for large outcomes and small outcomes. The analysis for large outcomes yielded a significant Narcissism × Valence interaction (Figure 3), F(1, 47) = 6.384, P = 0.015, ƞp2 = 0.120. Large positive outcomes (‘+99’) induced a significantly greater P3 than large negative outcomes (‘−99’) among low narcissists (14.35 ± 7.18 μV vs 11.14 ± 5.17 μV), t(23) = 5.023, P < 0.001, d = 1.02, but this effect was weaker among high narcissists (15.46 ± 7.71 μV vs 14.40 ± 9.07 μV), t(24) = 1.863, P = 0.075, d = 0.37. That is, P3 amplitude was less sensitive to large outcome valence among high compared to low narcissists. In contrast, the Narcissism × Valence interaction in the case of small outcomes was not significant, F(1, 47) = 0.820, P = 0.370, ƞp2 = 0.017 (Supplementary Figure S2). No other effect reached significance, Fs < 1.74, Ps > 0.19.
ERP and behavior
We proceeded to examine the relation between ERP and behavior. In regard to P3, narcissism was linked to the processing of large outcomes (resulting from the high-risk option) than of small outcomes (resulting from the low-risk option); that is, high narcissists displayed a smaller P3 valence difference to large outcomes and were more likely to persist with high-risk choices than low narcissists, although the two groups did not differ in FRN. To explore further the relevance of narcissism, we regressed narcissism (1 = high narcissism, 0 = low narcissism), P3 valence difference (indexed by mean-centered amplitude difference in response to positive vs negative feedback), and their product to the probability of risky decisions following large outcomes. High narcissism predicted riskier decisions, β = 0.296, t = 2.048, P = 0.046. Also, greater P3 valence difference tended to predict less risky decisions, β = −0.337, t = −1.713, P = 0.094. The Narcissism × P3 valence difference interaction was marginally significant, β = 0.352, t = 1.842, P = 0.072. For low narcissists, stronger P3 valence difference tended to be associated with less risky decisions, β = −0.393, t = −2.006, P = 0.057; for high narcissists, however, P3 valence difference did not predict risky decision-making, β = 0.169, t = 0.824, P = 0.418.
Discussion
We addressed the role of narcissism in decision-making, using behavioral and electrophysiological measures. High and low narcissists made choices between a high-risk and a low-risk option in a monetary gambling task. Results indicate that narcissism played a role in the high-risk but not in low-risk condition. In line with prior findings (Campbell et al., 2004; Lakey et al., 2008; Foster et al., 2011), high (vs low) narcissists were more prone to risky decision-making after they received outcome feedback pertaining to the high-risk option (i.e. large outcome magnitude). Meanwhile, the ERP results showed that high and low narcissists did not differ on the FRN but on the P3. Specifically, the impact of outcome valence (positive/negative) on P3 amplitude was stronger among low narcissists in the high-risk condition. Further, greater P3 valence difference among low narcissists was marginally associated with a smaller likelihood of making risky decisions on the next trial.
Our findings reveal not only an association between narcissism and risky decision-making under high-risk circumstances but also a potential mechanism underlying this association. We have proposed the following two possibilities: deficiency in error monitoring and deficiency in action updating. Our results provide no evidence for the error monitoring account. However, the results suggest that compared to low narcissists, high narcissists exhibited a weaker capacity of action updating after receiving a high-risk decision outcome. That is, even though high narcissists were able to detect their errors similar with low narcissists, they might have had more difficulties in updating their mental model in memory. Consequently, they were less likely to change their maladaptive behavior patterns (i.e. risky choices) following feedback. This interpretation implies that compared to low narcissists, high narcissists have more problems with feedback learning, thus providing an explanation for why narcissists have trouble learning from external feedback despite understanding it (Carlson, 2013; Jordan and Audia, 2012).
We consider the P3 as an index of action updating. This consideration was supported by our main ERP and behavioral findings under high-risk circumstances. First, a between-group analysis shows that compared to high narcissists, low narcissists displayed stronger changes of P3 amplitude in response to large positive vs negative outcomes, and were also more likely to change from high-risk to low-risk strategy following large outcomes. Second, a within-group analysis indicates that the P3 amplitude difference between large positive and negative outcomes was marginally predictive of risky decision-making among low narcissists, but not among high narcissists. Thus, both between-group and within-group findings suggest that, relative to high narcissists, low narcissists are more likely to utilize outcome information to guide their decisions in the high-risk condition.
Given that P3 has been associated with various cognitive functions, in particular, attention (Polich and Criado, 2006; Schirmer et al., 2007), it is possible that attention allocation played a role in the ways in which narcissism, action updating and risky behavior interrelated. Indeed, effective learning and action updating entail differential attention allocation to positive and negative outcomes in line with their reinforcement values (Stanisor et al., 2013; Leong et al., 2018). Although we have attributed behavioral and ERP differences between high and low narcissists to action updating, attention allocation may have also played a role. Follow-up research may examine the potential influence of other factors, such as emotion and motivation, given that they may also modulate the P3 amplitude (Nieuwenhuis et al., 2005; Polezzi et al., 2010).
The results also shed light on self-regulation models of narcissism (Morf and Rhodewalt, 2001; Campbell and Campbell, 2009). Error monitoring and action updating are essential in the self-regulation process (Luu et al., 2003; Morf & Horvath, 2010; Heatherton, 2011). Effective self-regulation requires the capabilities of monitoring conflicts and further updating thoughts, feelings and actions to resolve conflicts. Our findings hint at the possibility that high narcissists’ maladaptive self-regulation (i.e. risky) is due to their insufficiency in action updating. This could help explain why narcissistic self-regulatory efforts are often counterproductive (Morf and Rhodewalt, 2001). Successful self-regulation depends on prefrontal cortex (PFC) exerting top–down control over subcortical regions involved in reward and emotion (Delgado et al., 2008; Kober et al., 2010; Heatherton and Wagner, 2011). Future research could examine whether regions related to self-regulation (e.g. PFC) are relatively inactive among high narcissists when they are engaged in a gambling task (and presumably enacting risky decision-making).
We next turn to limitations and additional research directions. First, our decision-making paradigm only involved an economic scenario. As narcissists also manifest unique self-regulatory patterns to interpersonal feedback (Thomaes et al., 2016), future research could test the replicability of our findings in social decision-making tasks. Second, we have only detected a marginal relation between ERP components and behavioral indices, as well as marginal moderation by narcissism. Future samples will need to be more high-powered. Third, the FRN may overlap with the P3 in some situations (Foti et al., 2011), and we are not sure if this was the case in our study, thus confounding the results. Finally, we did not assess self-report of risk preference and outcome experience. With 20/20 hindsight, our interpretation of the ERP results could have been strengthened, if self-report data also showed that high narcissists were less sensitive to outcome feedback compared to low narcissists. Despite these limitations, the current study has contributed to the understanding of the relation between narcissism and decision making, suggesting the possibility that high narcissists’ decisional risk proneness is associated with their weaker ability in action updating (rather than error monitoring) compared with low narcissists.
Funding
This work was supported by the National Natural Science Foundation of China [31571148, 31300871, 31571124], the Major Program of the Chinese National Social Science Foundation [17ZDA324] and the Chinese Academy of Sciences Key Laboratory of Behavioral Science, Institute of Psychology [Y5CX052003].
References
- Audia P. G., Brion S. (2007). Reluctant to change: self-enhancing responses to diverging performance measures. Organizational Behavior and Human Decision Processes ,102(2), 255–69. doi: 10.1016/j.obhdp.2006.01.007. [DOI] [Google Scholar]
- Bellebaum C., Polezzi D., Daum I. (2010). It is less than you expected: the feedback-related negativity reflects violations of reward magnitude expectations. Neuropsychologia ,48(11), 3343–50. doi: 10.1016/j.neuropsychologia.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Britt T. W., Garrity M. J. (2006). Attributions and personality as predictors of the road rage response. British Journal of Social Psychology ,45(1), 127–47. doi: 10.1348/014466605X41355. [DOI] [PubMed] [Google Scholar]
- Brunell A. B., Buelow M. T. (2015). Narcissism and performance on behavioral decision-making tasks. Journal of Behavioral Decision Making ,30(1), 3–14. doi: 10.1002/bdm.1900. [DOI] [Google Scholar]
- Buelow M. T., Brunell A. B. (2014). Facets of grandiose narcissism predict involvement in health-risk behaviors. Personality and Individual Differences ,69, 193–98. doi: 10.1016/j.paid.2014.05.031. [DOI] [Google Scholar]
- Cai H., Kwan V. S., Sedikides C. (2012). A sociocultural approach to narcissism: The case of modern China. European Journal of Personality ,26(5), 529–35. doi: 10.1002/per.852. [DOI] [Google Scholar]
- Cai H., Shi Y., Fang X., Luo Y. L. (2015). Narcissism predicts impulsive buying: phenotypic and genetic evidence. Frontiers in Psychology ,6, 881. doi: 10.3389/fpsyg.2015.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. K., Campbell S. M. (2009). On the self-regulatory dynamics created by the peculiar costs and benefits of narcissism: a contextual reinforcement model and examination of leadership. Self and Identity ,8, 214–32. doi: 10.1080/15298860802505129. [DOI] [Google Scholar]
- Campbell W.K., Foster J.D. (2007). The narcissistic self: background, and extended agency model, and ongoing controversies. In: Sedikides C., Spencer S., editors. Frontiers in Social Psychology: The Self, Philadelphia, PA: Psychology Press, 115–38. [Google Scholar]
- Campbell W. K., Goodie A. S., Foster J. D. (2004). Narcissism, confidence, and risk attitude. Journal of Behavioral Decision Making ,17(4), 297–311. doi: 10.1002/bdm.475. [DOI] [Google Scholar]
- Campbell W.K., Green J.D. (2007). Narcissism and interpersonal self-regulation. In: Wood J.V., Tesser A., Holmes J.G., editors. Self and Relationships, New York, NY: Psychology Press, 73–94. [Google Scholar]
- Carlson E. N. (2013). Honestly arrogant or simply misunderstood? Narcissists’ awareness of their narcissism. Self and Identity ,12(3), 259–77. doi: 10.1080/15298868.2012.659427. [DOI] [Google Scholar]
- Carlson E. N., Vazire S., Oltmanns T. F. (2011). You probably think this paper's about you: narcissists' perceptions of their personality and reputation. Journal of Personality and Social Psychology ,101(1), 185–201. doi: 10.1037/a0023781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H. W., Swainson R., Durham L., Benham L., Cools R. (2011). Feedback-related negativity codes prediction error but not behavioral adjustment during probabilistic reversal learning. Journal of Cognitive Neuroscience ,23(4), 936–46. doi: 10.1162/jocn.2010.21456 [DOI] [PubMed] [Google Scholar]
- Cohen J., Polich J. (1997). On the number of trials needed for P300. International Journal of Psychophysiology ,25(3), 249–55. doi: 10.1016/S0167-8760(96)00743-X. [DOI] [PubMed] [Google Scholar]
- Cohen M. X., Wilmes K., Vijver I. (2011). Cortical electrophysiological network dynamics of feedback learning. Trends in Cognitive Sciences ,15(12), 558–66. doi: 10.1016/j.tics.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Cokely E. T., Kelley C. M. (2009). Cognitive abilities and superior decision making under risk: a protocol analysis and process model evaluation. Judgment and Decision Making ,4(1), 20–33. doi:10.81125/ jdm81125. [Google Scholar]
- Delgado M. R., Gillis M. M., Phelps E. A. (2008). Regulating the expectation of reward via cognitive strategies. Nature Neuroscience ,11(8), 880–81. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E., Coles M. G. H. (1988). Is the P300 component a manifestation of context updating?. Behavioral and Bran Sciences ,11(3), 355–72. doi: 10.1017/S0140525X00058027. [DOI] [Google Scholar]
- Doya K. (2008). Modulators of decision making. Nature Neuroscience ,11(4), 410–16. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Ernst M., Paulus M. P. (2005). Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry ,58(8), 597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A. G., Buchner A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods ,39(2), 175–91. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Foster J. D., Misra T. A., Reidy D. E. (2009b). Narcissists are approach-oriented toward their money and their friends. Journal of Research in Personality ,43(5), 764–69. doi: 10.1016/j.jrp.2009.05.005. [DOI] [Google Scholar]
- Foster J. D., Reidy D. E., Misra T. A., Goff J. S. (2011). Narcissism and stock market investing: correlates and consequences of cocksure investing. Personality and Individual Differences ,50(6), 816–21. doi: 10.1016/j.paid.2011.01.002. [DOI] [Google Scholar]
- Foster J. D., Shenesey J. W., Goff J. S. (2009a). Why do narcissists take more risks? Testing the roles of perceived risks and benefits of risky behaviors. Personality and Individual Differences ,47(8), 885–9. doi: 10.1016/j.paid.2009.07.008. [DOI] [Google Scholar]
- Foti D., Weinberg A., Dien J., Hajcak G. (2011). Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping ,32(12), 2207–16. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. J., Willoughby A. R. (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science ,295(5563), 2279–82. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goyer J. P., Woldorff M. G., Huettel S. A. (2008). Rapid electrophysiological brain responses are influenced by both valence and magnitude of monetary rewards. Journal of Cognitive Neuroscience ,20(11), 2058–69. doi: 10.1162/jocn.2008.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R., Lei Z., Broster L., Wu T., Jiang Y., Luo Y. J. (2011). Beyond valence and magnitude: a flexible evaluative coding system in the brain. Neuropsychologia ,49(14), 3891–97. doi: 10.1016/j.neuropsychologia.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie R. (2001). Problems for judgment and decision making. Annual Review of Psychology ,52, 653–83. doi: 10.1146/annurev.psych.52.1.653. [DOI] [PubMed] [Google Scholar]
- Hauser T. U., Iannaccone R., Stämpfli P., et al. (2014). The feedback-related negativity (FRN) revisited: new insights into the localization, meaning and network organization. Neuroimage ,84, 159–68. doi: 10.1016/j.neuroimage.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Heatherton T. F. (2011). Neuroscience of self and self-regulation. Annual Review of Psychology ,62, 363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T. F., Wagner D. D. (2011). Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences ,15(3), 132–9. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C. B., Krigolson O. E. (2007). Reward prediction error signals associated with a modified time estimation task. Psychophysiology ,44(6), 913–7. doi: 10.1111/j.1469-8986.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Holroyd C. B., Larsen J. T., Cohen J. D. (2004). Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology ,41(2), 245–53. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Jordan A. H., Audia P. G. (2012). Self-enhancement and learning from performance feedback. Academy of Management Review ,37(2), 211–31. doi: 10.5465/amr.2010.0108. [DOI] [Google Scholar]
- Kahnt T., Park S. Q., Cohen M. X., Beck A., Heinz A., Wrase J. (2009). Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. Journal of Cognitive Neuroscience ,21(7), 1332–45. doi: 10.1162/jocn.2009.21092. [DOI] [PubMed] [Google Scholar]
- Kernis M. H., Sun C. R. (1994). Narcissism and reactions to interpersonal feedback. Journal of Research in Personality ,28(1), 4–13. doi: 10.1006/jrpe.1994.1002. [DOI] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E. F., et al. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America ,107(33), 14811–6. doi: 10.1073/pnas.1007779107.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E., Hyafil A. (2007). Anterior prefrontal function and the limits of human decision-making. Science ,318(5850), 594–8. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Kreussel L., Hewig J., Kretschmer N., Hecht H., Coles M. G., Miltner W. H. (2012). The influence of the magnitude, probability, and valence of potential wins and losses on the amplitude of the feedback negativity. Psychophysiology ,49(2), 207–19. doi: 10.1111/j.1469-8986.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- Lakey C. E., Rose P., Campbell W. K., Goodie A. S. (2008). Probing the link between narcissism and gambling: the mediating role of judgment and decision-making biases. Journal of Behavioral Decision Making ,21(2), 113–37. doi: 10.1002/bdm.582. [DOI] [Google Scholar]
- Leong Y. C., Radulescu A., Daniel R., DeWoskin V., Niv Y. (2018). Dynamic interaction between reinforcement learning and attention in multidimensional environments. Neuron ,93(2), 451–63. doi: 10.1016/j.neuron.2016.12.040.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Yang J. (2013). Low self-esteem elicits greater mobilization of attentional resources toward emotional stimuli. Neuroscience Letter ,548, 286–90. doi: 10.1016/j.neulet.2013.05.071. [DOI] [PubMed] [Google Scholar]
- Li H., Zeigler-Hill V., Luo J., Yang J., Zhang Q. (2012). Self-esteem modulates attentional responses to rejection: evidence from event-related brain potentials. Journal of Research in Personality ,46(5), 459–64. doi: 10.1016/j.jrp.2012.02.010. [DOI] [Google Scholar]
- Luck S. J., Gaspelin N. (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn't). Psychophysiology ,54(1), 146–57. doi: 10.1111/psyp.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Wu T., Broster L. S., et al. (2014). The temporal course of the influence of anxiety on fairness considerations. Psychophysiology ,51(9), 834–42. doi: 10.1111/psyp.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y. L., Cai H., Sedikides C., Song H. (2014). Distinguishing communal narcissism from agentic narcissism: a behavior genetics analysis on the agency–communion model of narcissism. Journal of Research in Personality ,49, 52–8. doi: 10.1016/j.jrp.2014.01.001. [DOI] [Google Scholar]
- Luu P., Tucker D. M., Derryberry D., Reed M., Poulsen C. (2003). Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science ,14(1), 47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J., Cucurell D., Munte T. F., Strien N., Rodriguez-Fornells A. (2011). On the number of trials needed for a stable feedback-related negativity. Psychophysiology ,48(6), 852–60. doi: 10.1111/j.1469-8986.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- Mell T., Wartenburger I., Marschner A., Villringer A., Reischies F.M., Heekeren H.R. (2009). Altered function of ventral striatum during reward-based decision making in old age. Frontiers in Human Neuroscience ,3, 34. doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner W. H. R., Braun C. H., Coles M. G. H. (1997). Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a "generic" neural system for error detection. Journal of Cognitive Neuroscience ,9(6), 788–98. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Morf C.C., Horvath S. (2010). Self-regulation processes and their signatures. In: Hoyle R., editor. Handbook of Personality and Self-Regulation, Malden, MA: Blackwell, 117–44. [Google Scholar]
- Morf C.C., Horvath S., Torchetti L. (2011). Narcissistic self-enhancement: tales of (successful?) self-portrayal. In: Alicke M.D., Sedikides C., editors. Handbook of Self-Enhancement and Self-Protection, New York, NY: Guilford, 399–424. [Google Scholar]
- Morf C. C., Rhodewalt F. (2001). Unraveling the paradoxes of narcissism: a dynamic self-regulatory processing model. Psychological Inquiry ,12(4), 177–96. doi: 10.1207/S15327965PLI1204_1. [DOI] [Google Scholar]
- Muller S. V., Moller J., Rodriguez-Fornells A., Munte T. F. (2005). Brain potentials related to self-generated and external information used for performance monitoring. Clinical Neurophysiology ,116(1), 63–74. doi: 10.1016/j.clinph.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Aston-Jones G., Cohen J. D. (2005). Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin ,131(4), 510–32. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Polezzi D., Sartori G., Rumiati R., Vidotto G., Daum I. (2010). Brain correlates of risky decision-making. Neuroimage ,49(2), 1886–94. doi: 10.1016/j.neuroimage.2009.08.068. [DOI] [PubMed] [Google Scholar]
- Polich J. (2007). Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology ,118(10), 2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J., Criado J. R. (2006). Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology ,60(2), 172–85. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Raskin R., Terry H. (1988). A principal-components analysis of the Narcissistic Personality Inventory and further evidence of its construct validity. Journal of Personality and Social Psychology ,54(5), 890–902. doi: 10.1037/0022-3514.54.5.890. [DOI] [PubMed] [Google Scholar]
- Roberts T., Woodman T., Sedikides C. (2017). Pass me the ball: narcissism in performance settings. International Review of Sport and Exercise Psychology, Advance online publication. doi: 10.1080/1750984X.2017.1290815. [DOI] [Google Scholar]
- Rothschild M., Stiglitz J. E. (1970). Increasing risk: I. A definition. Journal of Economic Theory ,2(3), 225–43. doi: 10.1016/0022-0531(70)90038-4. [DOI] [Google Scholar]
- Ruff C.C., Fehr E. (2014). The neurobiology of rewards and values in social decision making. Nature Reviews Neuroscience ,15(8), 549–62. doi: 10.1038/nrn3776. [DOI] [PubMed] [Google Scholar]
- San Martín R. (2012). Event-related potential studies of outcome processing and feedback-guided learning. Frontiers in Human Neuroscience ,6, 304. doi: 10.3389/fnhum.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martín R., Appelbaum L. G., Pearson J. M., Huettel S. A., Woldorff M. G. (2013). Rapid brain responses independently predict gain-maximization and loss-minimization during economic decision-making. Journal of Neuroscience ,33(16), 7011–19. doi: 10.1523/JNEUROSCI.4242-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer A., Simpson E., Escoffier N. (2007). Listen up! Processing of intensity change differs for vocal and nonvocal sounds. Brain Research ,1176, 103–12. doi: 10.1016/j.brainres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Sedikides C., Campbell W. K. (2017). Narcissistic force meets systemic resistance: the energy clash model. Perspectives on Psychological Science ,12(3), 400–21. doi: 10.1177/1745691617692105. [DOI] [PubMed] [Google Scholar]
- Simons R. F. (2010). The way of our errors: theme and variations. Psychophysiology ,47(1), 1–14. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Stanisor L., Togt C, Pennartz C. M., Roelfsema P. R. (2013). A unified selection signal for attention and reward in primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America ,110(22), 9136–41. doi: 10.1073/pnas.1300117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser M., Yeung N. (2010). Decision processes in human performance monitoring. Journal of Neuroscience ,30(46), 15643–53. doi: 10.1523/JNEUROSCI.1899-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes S., Brummelman E., Sedikides C. (2016). Narcissism: a social-developmental perspective. In: Zeigler-Hill V., Shackelford T., editors. The SAGE Handbook of Personality and Individual Differences, New York, NY: Sage, 1–40. [Google Scholar]
- Thomaes S., Sedikides C. (2016). Thin images reflected in the water: narcissism and girls' vulnerability to the thin-ideal. Journal of Personality ,84(5), 633–45. doi: 10.1111/jopy.12187. [DOI] [PubMed] [Google Scholar]
- Toplak M. E., Sorge G. B., Benoit A., West R. F., Stanovich K. E. (2010). Decision-making and cognitive abilities: a review of associations between Iowa Gambling Task performance, executive functions, and intelligence. Clinical Psychology Review ,30(5), 562–81. doi: 10.1016/j.cpr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Vazire S. (2016). Editorial. Social Psychological and Personality Science ,7(1), 3–7. doi: 10.1177/1948550615603955. [DOI] [Google Scholar]
- Walsh M. M., Anderson J. R. (2012). Learning from experience: event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neuroscience & Biobehavioral Reviews ,36(8), 1870–84. doi: 10.1016/j.neubiorev.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A.M. (2010). Narcissism and binge drinking: exploring the role of overconfidence and confidence-based risk-taking. Electronic Theses and Dissertations, Paper 1741.
- Wu Y., Zhou X. L. (2009). The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research ,1286, 114–22. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yeung N., Sanfey A. G. (2004). Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience ,24(28), 6258–64. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N., Summerfield C. (2012). Metacognition in human decision-making: confidence and error monitoring. Philosophical Transactions of the Royal Society B ,367(1594), 1310–21. doi: 10.1098/rstb.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Gu R., Broster L. S., et al. (2014). Linking brain electrical signals elicited by current outcomes with future risk decision-making. Frontiers in Behavioral Neuroscience ,8, 84. doi: 10.3389/fnbeh.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Gu R., Wu T., et al. (2013). An electrophysiological index of changes in risk decision-making strategies. Neuropsychologia ,51(8), 1397–407. doi: 10.1016/j.neuropsychologia.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Yu R., Zhou X. (2010). To do or not to do? Action enlarges the FRN and P300 effects in outcome evaluation. Neuropsychologia ,48(12), 3606–13. doi: 10.1016/j.neuropsychologia.2010.08.010. [DOI] [PubMed] [Google Scholar]


