Abstract
A large body of research indicates that psychopathic individuals lie chronically and show little remorse or anxiety. Yet, little is known about the neurobiological substrates of dishonesty in psychopathy. In a sample of incarcerated individuals (n = 67), we tested the hypothesis that psychopathic individuals show reduced activity in the anterior cingulate cortex (ACC) when confronted with an opportunity for dishonest gain, reflecting dishonest behavior that is relatively unhindered by response conflict. During functional magnetic resonance imaging, incarcerated offenders with different levels of psychopathy performed an incentivized prediction task wherein they were given real and repeated opportunities for dishonest gain. We found that while incarcerated offenders showed a high rate of cheating, levels of psychopathic traits did not influence the frequency of dishonesty. Higher psychopathy scores predicted decreased activity in the ACC during dishonest decision-making. Further analysis revealed that the ACC was functionally connected to the dorsolateral prefrontal cortex, and that ACC activity mediated the relationship between psychopathic traits and reduced reaction times for dishonest behavior. These findings suggest that psychopathic individuals behave dishonestly with relatively low levels of response conflict and that the ACC may play a critical role in this pattern of behavior.
Keywords: anterior cingulate cortex, cognitive conflict, deception, dishonesty, honesty, psychopathy
Introduction
Psychopathy is a complex personality disorder characterized by the severe disruption of moral behavior due to interpersonal and emotional detachment (Hare and Neumann, 2008). Psychopathy is believed to affect ∼1% of the general population and 15–25% of the prison inmates (Hare, 1991, 2003; Kiehl, 2006). Classical clinical observations suggest that morally unacceptable behavior is more common among psychopaths than non-psychopaths (Cleckley, 1941, 2015). One remarkable example is the level of dishonesty observed in psychopathic individuals; psychopaths frequently lie for enjoyment (Lilienfeld and Fowler, 2006), a phenomenon that Ekman (1985) termed ‘duping delight’. Psychopaths also lie with minimal guilt or anxiety. As Cleckley (1941, 2015) observed, ‘The psychopath shows a total disregard for truth and is to be trusted no more in his accounts of the past than in his promises for the future of his statement of present intentions’.
An important question is: what makes psychopathic individuals behave dishonestly? Is there a distinct neural process that facilitates, or at least reflects, dishonesty in psychopathy? Several previous neuroimaging studies have investigated the neural substrates of deception in psychopathic individuals (Nunez et al., 2005; Fullam et al., 2009; Glenn et al., 2017; Shao and Lee, 2017). These studies reported associations between regional brain activity during deception and various aspects of psychopathy. Although these studies shed light on the relationship between deception and psychopathy, an important limitation was that the study participants were being asked to lie for the purposes of the study. Thus, deception described in these previous studies involved neither temptation nor morally questionable behavior.
We have developed an ecologically valid (dis)honesty task in which participants are given repeated opportunities to gain money by lying about their accuracy in a prediction task (Greene and Paxton, 2009; Abe and Greene, 2014). More specifically, participants report on their accuracy in predicting the outcomes of random coin-flips and gain money for (self-reported) accuracy. In some cases participants do not reveal their predictions in advance, thus giving them the opportunity to gain money by lying. Participants who behaved dishonestly, as indicated by improbably high self-reported accuracy, exhibited increased activity in control-related regions such as the lateral prefrontal cortex when lying and when refraining from lying (Greene and Paxton, 2009). Our follow-up study further revealed that dishonest moral decisions were associated with increased activity in the lateral prefrontal cortex and anterior cingulate cortex (ACC) (Abe and Greene, 2014). Other studies using a similar experimental paradigm combined with event-related potentials or near-infrared spectroscopy have also consistently shown the involvement of control-related regions for dishonest behavior (Ding et al., 2013; Hu et al., 2015).
Among the control-related regions described earlier, the ACC’s activity is likely to be related to cognitive conflict in response to an opportunity for dishonest gain. The ACC is reliably recruited in tasks producing high levels of cognitive conflict such as the Stroop task (MacDonald et al., 2000) and responding to moral dilemmas (Greene et al., 2004). Additionally, ACC activity predicts greater prefrontal activity and behavioral adjustments in cognitively demanding tasks (Kerns et al., 2004). Although some studies have argued that ACC function is preserved in psychopathy (Blair, 2005; Glenn et al., 2010), past literature has demonstrated decreases in task-related activity in the ACC across a variety of experimental paradigms (Kiehl et al., 2001; Birbaumer et al., 2005; Cope et al., 2014). These findings suggest that dishonesty in psychopathy is at least partly related to diminished cognitive conflict when opportunities for dishonesty gain arise, and this may be reflected in ACC activation in given such opportunities. We therefore hypothesized that higher psychopathic traits would be associated with increased dishonesty, reduced reaction times (RTs) for dishonest decisions, and reduced ACC engagement.
To test this hypothesis, we recruited a large sample of incarcerated offenders scoring low, medium, and high on the Hare Psychopathy Checklist-Revised (PCL-R) (Hare, 1991, 2003) to complete functional magnetic resonance imaging (fMRI) scanning during our incentivized prediction task. The use of incarcerated control participants in our study has the advantage of avoiding confounding psychopathy with criminality and factors related to criminal lifestyle (Koenigs et al., 2011). To our knowledge, this study is the first attempt to characterize the neural bases of dishonest behavior with real stakes in a group of incarcerated psychopaths.
Materials and methods
Participants
These results are based on data from 67 adult males incarcerated in a medium-security North American correctional facility. Participants gave written informed consent in accordance with a protocol approved by the Ethical and Independent Review Services. Intelligence quotient (IQ) was estimated using the Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (Wechsler, 1997; Ryan et al., 1999). The Wide Range Achievement Test Word Reading subtest was used to assess reading level (Wilkinson, 1993). All participants completed the Structural Clinical Interview and underwent an interview and file review to assess history of central nervous system abnormalities and drug or alcohol abuse (First et al., 2002). The exclusion criteria were as follows: age < 18 years or > 55 years, non-fluency in English, reading level lower than a fourth grade level, IQ score < 70, a history of seizures, prior head injury with loss of consciousness > 30 min, any Axis I diagnosis as per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994), lifetime history of a psychotic disorder or family history of a psychotic disorder in a first degree relative, and current alcohol or drug use. Participants were paid at a rate consistent with the facility hourly labor wage ($1 USD/h). Participants were eligible for bonus pay up to ∼$5/day based on their performance during the experimental tasks as per prison regulations.
Subjects were divided into three groups to isolate differences due to psychopathy. Individuals with a PCL-R total score ≥ 30 were assigned to a high-psychopathy group (n = 18), those with a score 21–29 were assigned to a medium-psychopathy group (n = 17), and those with a score < 20 were assigned to a low-psychopathy group (n = 32) (i.e. the control group in this study). In this study, the PCL-R was used as both a continuous measure (i.e. in a regression analysis) and a dichotomous diagnosis (i.e. for between-group comparisons). Use of the PCL-R as a continuous measure is well established (e.g. Decety et al., 2013; Philippi et al., 2015; Fede et al., 2016).
Our analyses also required the classification of participants as honest, dishonest or ambiguous based on self-reported accuracy in the opportunity condition of the coin-flip task. Consistent with prior methods (Greene and Paxton, 2009; Abe and Greene, 2014), 43 participants reporting improbably high levels of accuracy at the individual level (binomial test, all P-values < 0.001) were classified as dishonest (mean ‘accuracy’ = 90.5%). This conservative threshold was used to ensure a sufficient number of cheat trials per participants classified as dishonest. Of these, nine were high-psychopathy offenders, 12 were medium-psychopathy offenders and 22 were low-psychopathy offenders. The 20 lowest-accuracy participants (binomial test, all P-values > 0.05) were classified as honest (mean accuracy = 53.3%). Of these, nine were high-psychopathy offenders, four were medium-psychopathy offenders, and seven were low-psychopathy offenders. Note that we are not claiming that there was no dishonest responding at all within this group. Rather, our classification is based on the observation that the level of dishonesty in this group is sufficiently low that it cannot be detected with confidence, even at the group level. The remaining four participants (one medium-psychopathy offender and three low-psychopathy offenders) were classified as ambiguous (mean accuracy = 65.9%). While it is clear that at least some individuals in the ambiguous group behaved dishonestly (174/264 trials, group binomial test, P < 0.001), we classified these individuals as ‘ambiguous’ because none of them met our conservative threshold for dishonest behavior at the individual level (Table 1).
Table 1.
Cross tabulation of participant classification and mean self-reported accuracy (% trials claimed as wins)
| Categorical classification of level of cheating |
|||||
|---|---|---|---|---|---|
| Dishonest | Ambiguous | Honest | Total | ||
| High | 9 (88.2%) | 0 | 9 (55.4%) | 18 (71.8%) | |
| Psychopathic traits | Medium | 12 (92.2%) | 1 (68.1%) | 4 (52.0%) | 17 (81.4%) |
| Low | 22 (90.6%) | 3 (65.1%) | 7 (51.4%) | 32 (79.6%) | |
| Total | 43 (90.5%) | 4 (65.9%) | 20 (53.3%) | 67 (77.9%) | |
Cognitive task
To measure dishonesty, we used a coin-flip prediction task in which participants had opportunities to gain money dishonestly by lying about the accuracy of their predictions (Figure 1) (Greene and Paxton, 2009; Abe and Greene, 2014). We used a cover story to justify the obvious opportunity for dishonest gain. This study was presented as a study of paranormal abilities to predict the future, aimed at testing the hypothesis that individuals are better able to predict the future when their predictions are (i) private and (ii) financially incentivized. Thus, participants were implicitly led to believe that the opportunity for dishonest gain was a known but unintended byproduct of the experimental design, and that they were expected to behave honestly. We note that in employing this cover story, participants were deceived about the experimenters’ interests, but not about the economic structure of the task. Participants were not presented with the cover story until after they had been recruited in order to avoid self-selection for participants with interests in parapsychology. Participants were given a thorough explanation of the task procedure and were familiarized with the coin-flip task in practice trials.
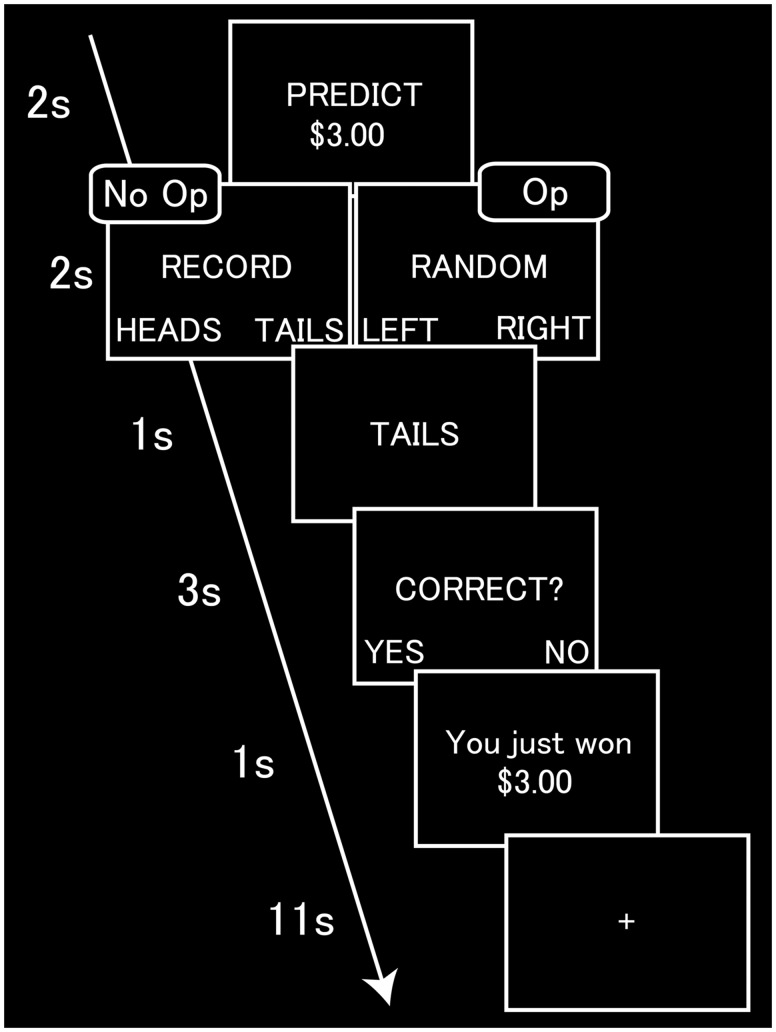
Fig. 1.
Task sequence of the coin-flip task. The participant observed the trial’s monetary value and privately predicted the outcome of the upcoming coin-flip. The participant then recorded this prediction by pressing 1 of 2 buttons (no-opportunity condition) or pressed a button randomly (opportunity condition). The participant then observed the outcome of the coin-flip, indicated whether the prediction was accurate, and observed the amount of money won/lost based on the recorded prediction (no-opportunity condition) or self-reported accuracy (opportunity condition). Trials were followed by a fixation interval. Op, opportunity condition.
In the coin-flip task, participants attempted to predict the outcomes of random computerized coin-flips and were financially rewarded for accuracy or punished for inaccuracy. First, the participant observed the monetary value of the trial and privately predicted the outcome of the upcoming coin-flip (2 s). Then, the participant recorded their prediction by pressing one of two buttons (no-opportunity condition) or pressed one of the buttons randomly (opportunity condition) (2 s). Finally, the participant observed the outcome of the coin-flip (1 s) and indicated whether the prediction was accurate (3 s). The participant then observed the amount of money won/lost based on the recorded prediction (no-opportunity condition) or the reported accuracy (opportunity condition) (1 s) and waited for the next trial (11 s). Thus, in the no-opportunity condition, participants recorded their predictions in advance, such that they did not have an opportunity to cheat by lying about their accuracy. In the opportunity condition, participants made their predictions privately and were rewarded based on their self-reported accuracy, affording them the opportunity to cheat. Each participant completed a total of 210 trials. Values $3, $4, $5, $6 and $7 USD appeared 14 times each in 70 opportunity trials and 70 no-opportunity trials. We included an additional set of 70 low-value opportunity trials with the values $0.02, $0.10, $0.25, $0.35 and $0.50 USD also appearing 14 times each. Data from the latter trials were not analyzed because contrasts involving this condition could not be controlled for monetary value. Rather, low-value opportunity trials were included to provide dishonest participants with additional opportunities to behave honestly at little cost, thus giving them cover for cheating in the regular (higher value) opportunity trials. Net losses were capped at $0 and net winnings were capped at $75 (not including participation payment), although the actual bonus pay was scaled down in accordance with the pay scale permitted by the prison (∼$5/day). Specifically, we let the participants know that the dollar amounts for all of the trials are reduced (the scaling factor: 1/15), although their decisions still had financial consequence throughout the task. Trials were randomized in a series of 7 blocks of 30 trials each. Each block of the coin-flip task lasted ∼10 min. It should be noted that the inmates tested may have suspected that the experiment was related to (dis)honesty as they are not ordinarily given opportunities for dishonest gain by institutional agents such as researchers. Although this is a genuine limitation of this study (or any study attempting to study dishonesty among incarcerated individuals), we emphasize that our focus is on comparisons ‘within’ the inmate population. Thus, our key findings are unlikely to be confounded by level of suspicion.
Image acquisition and data preprocessing
Whole-brain imaging was performed with a 1.5-T Siemens Magnetom Avanto mobile unit equipped with a 12-element head coil. A T2*-weighted echo planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent (BOLD) contrast was used for functional imaging with the following parameters: repetition time = 2000 ms, echo time = 39 ms, flip angle = 75°, acquisition matrix = 64 × 64, field of view = 240 mm and in-plane resolution = 3.75 × 3.75 mm. Twenty-seven axial slices (slice thicknesses = 4 mm and interslice gap = 1 mm) were obtained. Head motion was restricted using padding and a restraint. Visual stimuli were presented via a back-projection system, and participant responses were collected using a magnet-compatible response box. Task presentation was implemented using the Presentation software package (Neurobehavioral Systems, Albany, CA). EPI images were acquired in seven consecutive runs. The first five images from each run were discarded to allow for T1 equilibration effects.
Data pre-processing and statistical analyses were performed using SPM12 (Wellcome Department of Imaging Neuroscience, London, UK). All volumes were spatially realigned to the first volume and the time series for voxels within each slice was temporally realigned to the middle slice. The resulting volumes were normalized to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain (re-sampled voxel size = 2 × 2 × 2 mm). The normalized images were smoothed with an isotropic 8-mm full width at half maximum Gaussian kernel. A high-pass filter of 1/128 Hz was used to remove low-frequency noise, and an AR (1) model was used to correct for temporal autocorrelations.
Statistical analysis
Self-reported accuracy data and RTs were analyzed using standard parametric tests. We first analyzed associations between psychopathic traits and self-reported accuracy using Pearson correlation test and t-test. We then focused on the dishonest group (n = 43) and examined the association between psychopathic traits and RTs for dishonest moral decisions using analysis of variance (ANOVA) and Pearson correlation test. fMRI data were analyzed using an event-related model. All events of interest were modeled through convolution with a canonical hemodynamic response function temporally indexed by participant responses in the context of the general linear model. Parameter estimates (betas) for each condition were calculated for all brain voxels, and the following two contrasts of parameter estimates were computed: opportunity win vs no-opportunity win and opportunity loss vs no-opportunity loss. The first contrast was designed to identify signal differences associated with (but not exclusively associated with) dishonest behavior. The second contrast was meant to identify signal associated with honest behavior in the presence of opportunity for dishonest gain. In the neuroimaging analysis, data from the ambiguous group were excluded because of an extremely low number of individuals classified into the group (i.e. four individuals). In addition, honesty-related activity (opportunity loss vs no-opportunity loss) in the dishonesty group was not analyzed due to a low number of opportunity loss trials in a large number of participants.
Second-level analyses on dishonesty-related activity in 43 dishonest participants were performed using both a regression analysis and a subtraction analysis (i.e. one- and two-sample t-tests). In the regression analysis, contrast images of opportunity win trials vs no-opportunity win trials for all dishonest participants (n = 43) were entered into a series of multiple regression analyses, in which the PCL-R total score was the main predictor. Supplementary analyses were also performed to control for the effects of covariates including participant age and IQ on neural activity associated with dishonest moral decisions. Specifically, each covariate was incorporated as a nuisance variable into a model that examined activity associated with opportunity win trials (relative to no-opportunity win trials), with PCL-R total score as the main predictor. In a one-sample t-test, the contrast images of opportunity win trials vs no-opportunity win trials were pooled for high-psychopathy offenders (n = 9) and low-psychopathy offenders (n = 22), respectively. In the two-sample t-test (i.e. between-group analysis), the contrast images of opportunity win trials vs no-opportunity win trials were directly compared between high-psychopathy offenders and low-psychopathy offenders. Second-level analyses on honesty-related activity (i.e. opportunity loss trials vs no-opportunity loss trials) in 20 honest participants were also performed using both a regression analysis (entire sample) and a subtraction analysis (high- and low-psychopathy groups) (see Supplementary Results).
For whole-brain analyses, statistical maps were assessed at an uncorrected threshold of P < 0.001 at the voxel level, and clusters were considered significant if they passed a cluster-level threshold of P < 0.05 after family wise error (FWE) correction. When necessary, the small volume correction (SVC) method (FWE-corrected, P < 0.05) was applied to a region of interest (ROI) in the ACC, driven by our a priori hypothesis. For this purpose, we used anatomical masks of the bilateral ACC created with the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002) implemented in WFU PickAtlas software (Wake Forest University, Winston-Salem, NC) (Maldjian et al., 2003). We also used MarsBaR software (Brett et al., 2002) to extract percentages of change in BOLD signal in the ACC for additional ROI analyses. The peak voxels of clusters exhibiting reliable effects are reported in MNI coordinates.
Results
Demographic data
Demographic data for the 67 subjects are summarized in Table 2. There were significant differences among the three groups in terms of age [F(2, 64) = 3.54, P = 0.035]; although the medium-psychopathy group was significantly younger than the high- psychopathy group [t(33) = 2.54, P = 0.016, two-tailed] and low-psychopathy group [t(47) = 2.38, P = 0.022, two-tailed], no difference was observed between the high- and low-psychopathy groups [t(48) = 0.09, P = 0.931, two-tailed]. There were no differences among the three groups in terms of IQ [F(2, 64) = 1.39, P = 0.258).
Table 2.
Descriptive population statistics (n = 67)
| Variable | Mean | SD | Percentage |
|---|---|---|---|
| Age | 34.7 | 8.1 | |
| Handedness | |||
| Right | 89.6 | ||
| Left | 9.0 | ||
| Ambidextrous | 1.5 | ||
| Ethnicity | |||
| Hispanic/Latino | 40.3 | ||
| Not Hispanic/Latino | 59.7 | ||
| Race | |||
| White | 44.8 | ||
| American Indian or Alaska Native | 14.9 | ||
| Asian | 0.0 | ||
| Black or African American | 14.9 | ||
| Other | 25.4 | ||
| IQ | 98.7 | 13.0 | |
| Psychopathy (PCL-R) | |||
| Total score | 22.1 | 7.3 | |
| Factor 1 score | 5.9 | 4.1 | |
| Factor 2 score | 13.5 | 3.5 |
IQ, intelligence quotient; PCL-R, Hare Psychopathy Checklist-Revised; SD, standard deviation.
Self-reported accuracy
Self-reported accuracy data are summarized in Table 1. We first analyzed the association between psychopathic traits and the frequency of dishonest behavior. Specifically, we looked for a correlation between PCL-R total scores and self-reported accuracy in the entire sample (n = 67), but no significant correlation was observed (r = −0.18, P = 0.140, two-tailed). A between-group comparison (i.e. high- vs low-psychopathy group) similarly revealed no significant difference [t(48) = 1.42, P = 0.161, two-tailed].
Reaction times
RT data are summarized in Table 3. Because we were interested in RTs for dishonest moral decisions, we focused on data obtained from the dishonest group (n = 43) and tested an association between psychopathic traits and RTs for opportunity win trials. We first conducted a 3 (group: high-, medium- or low-psychopathy) × 2 (condition: opportunity or no-opportunity) × 2 (outcome: win or loss) ANOVA. We excluded data from four inmates with no opportunity loss trials (i.e. self-reported accuracy of 100%). Thus, the ANOVA included data from 39 participants. There were significant main effects of condition [F(1, 36) = 6.56, partial η2 = 0.15, P = 0.015] and outcome [F(1, 36) = 28.25, partial η2 = 0.44, P < 0.001]. There was also a significant two-way interaction between condition and outcome [F(1, 36) = 10.39, partial η2 = 0.22, P = 0.003]. This interaction effect was driven by longer RTs for opportunity loss trials, as compared with those of no-opportunity loss trials [t(38) = 3.48, p = 0.001, two-tailed). There was no significant difference in RTs between opportunity win trials and no-opportunity win trials [t(38) = −0.92, P = 0.365, two-tailed]. This pattern of findings indicates that additional controlled processing was required when dishonest participants forewent opportunities for dishonest gain, consistent with our previous findings (Greene and Paxton, 2009; Abe and Greene, 2014).
Table 3.
RTs (ms) in the dishonest group (n = 43)
| Condition |
|||||
|---|---|---|---|---|---|
| Op Win | Op Loss | No-Op Win | No-Op Loss | ||
| Psychopathic traits | High | 623 ± 57 | 863 ± 164 | 665 ± 97 | 765 ± 76 |
| Medium | 649 ± 74 | 979 ± 210 | 652 ± 73 | 776 ± 123 | |
| Low | 694 ± 52 | 973 ± 166 | 693 ± 51 | 805 ± 84 | |
Data represent the mean ± 95% CI.
Op, opportunity.
We next examined the relationship between psychopathic traits and RTs for the opportunity condition. Based on the previous findings indicating that psychopathic individuals are impulsive decision-makers (Cleckley, 1941, 2015; Kiehl, 2006), we predicted that psychopathic inmates tend to make faster responses when there is an opportunity for dishonest gain, but not when there is no opportunity. As predicted, the PCL-R total scores were inversely (but only marginally) correlated with the RTs for the opportunity condition (r = −0.28, P = 0.068, two-tailed), whereas the PCL-R total scores were not correlated with the RTs for the no-opportunity condition (r = −0.13, P = 0.392, two-tailed). To test for a difference between these two correlations, we compared the correlation coefficients using a test of two overlapping correlations in the same group (Hittner et al., 2003). The direct comparison revealed a statistically marginal difference (z = −1.67, P = 0.095, two-tailed). Thus, these data provide suggestive, but not conclusive, evidence for the predicted relationship between degree of psychopathy and faster responding in the presence of an opportunity for dishonest gain.
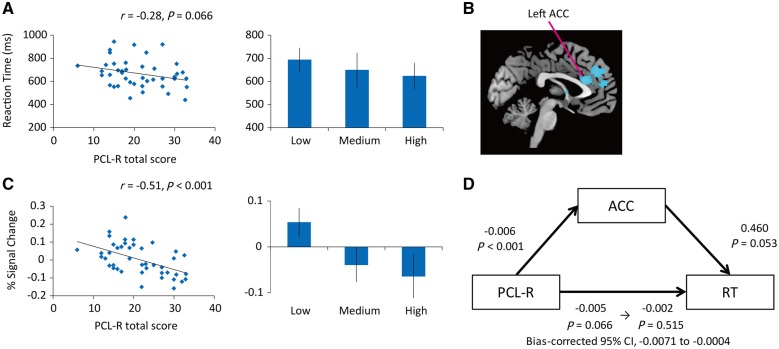
Because we are primarily interested in dishonest behavior, we then considered, in greater detail, this RT effect for the opportunity win trials, i.e. those that included the dishonest behavior. The PCL-R total scores were inversely and marginally correlated with RTs for opportunity win trials (r = −0.28, P = 0.066, two-tailed) (Figure 2A). The correlation between the RT for opportunity win trials and PCL-R factor 2 score was marginal (r = −0.30, P = 0.053, two-tailed), and that between RT for opportunity win trials and PCL-R factor 1 score was non-significant (r = −0.25, P = 0.107, two-tailed). As suggested by the results of the ANOVA, a between-group comparison failed to detect any significant difference in RTs for opportunity win trials between the low- and high-psychopathy groups [t(29) = −1.55, P = 0.133, two-tailed].
Fig. 2.
Psychopathy, RTs, and ACC activity. (A) Scatter plot and correlation of PCL-R total score with RT in opportunity win trials across dishonest participants (r = −0.28, P = 0.066). Histogram bars represent the RT based on the groupwise comparisons (mean ± 95% CI). (B) Results of a whole-brain regression analysis indicating a negative relationship between PCL-R total score and activity in the left ACC during decisions including many dishonest moral decisions (i.e. opportunity win vs no-opportunity win) across dishonest participants. (C) Scatter plot and correlation of PCL-R total score and percent signal change in left ACC (r = −0.51, P < 0.001). To avoid potential issues regarding circularity and double dipping, signal change was extracted from the anatomically delimited left ACC region. Histogram bars represent ACC signal based on groupwise comparisons (mean ± 95% CI). (D) Results of the mediation analysis. Unstandardized regression coefficients and bias-corrected 95% CIs for the indirect effect from a bootstrap-mediation analysis demonstrated that left ACC activity during dishonest moral decision-making mediated the relationship between psychopathic traits (i.e. PCL-R total score) and RT for opportunity win trials among 43 inmates classified as dishonest. ACC, anterior cingulate cortex; PCL-R, Hare Psychopathy Checklist-Revised.
Dishonesty-related activity in inmates classified as dishonest
We first considered dishonesty-related activity (i.e. the opportunity win vs no-opportunity win contrast) in 43 individuals who were classified as dishonest. To examine psychopathy from a dimensional perspective rather than a categorical one, PCL-R total scores were computed as continuous variables in the regression analysis. PCL-R total score was negatively correlated with dishonesty-related activity in several brain regions including the left ACC (Figure 2B and C and Table 4). These results were virtually unchanged when we additionally controlled for age and IQ. We then examined whether the BOLD signal in the left ACC (based on the anatomically defined ROI) showed a similar negative correlation with PCL-R factors 1 and 2 scores. The results revealed that both the factor 1 (r = −0.51, P < 0.001, two-tailed) and factor 2 scores (r = −0.39, P = 0.009, two-tailed) were negatively correlated with left ACC activity, indicating that the reduced ACC activity is specifically related to both factor 1 (interpersonal and affective traits) and factor 2 (lifestyle and antisocial traits).
Table 5.
Results of a PPI analysis with the left ACC as the seed region
| Region (Brodmann area) | Coordinates |
Cluster-level P-value (corrected) | Peak-level P-value (corrected) | Peak-level P-value (uncorrected) | Z value | Cluster size | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Right cerebellum | 26 | −82 | −18 | <0.001 | 0.049 | <0.001 | 4.74 | 421 |
| Right insula | 52 | 18 | 6 | <0.001 | 0.319 | <0.001 | 4.21 | 560 |
| Left inferior frontal gyrus (extending to left DLPFC) (47) | −24 | 44 | 8 | 0.003 | 0.33 | <0.001 | 4.20 | 282 |
| Right thalamus | 4 | 6 | 4 | 0.002 | 0.411 | <0.001 | 4.12 | 286 |
| Left middle occipital gyrus (18) | −26 | −90 | 2 | 0.036 | 0.751 | <0.001 | 3.82 | 158 |
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex.
Table 4.
Results of the regression and subtraction analyses of dishonesty-related brain activation (i.e. op win > no-op win)
| Region (Brodmann area) | Coordinates |
Cluster-level P-value (corrected) | Peak-level P-value (corrected) | Peak-level P-value (uncorrected) | Z value | Cluster size | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Regression: Negative correlation between the PCL-R total score and the dishonesty-related activity (n = 43) | ||||||||
| Left ACC (24)a | −2 | 28 | 20 | 0.036 | 0.086 | <0.001 | 4.56 | 165 |
| Left medial superior frontal gyrus (10) | −14 | 60 | 14 | <0.001 | 0.327 | <0.001 | 4.17 | 458 |
| Left thalamus | −12 | −8 | 4 | 0.004 | 0.415 | <0.001 | 4.08 | 271 |
| Regression: Controlling for age and IQ | ||||||||
| Left ACC (24)a | −2 | 28 | 20 | <0.001 | 0.206 | <0.001 | 4.32 | 693 |
| Right pallidum | 10 | 0 | −2 | 0.004 | 0.362 | <0.001 | 4.13 | 278 |
| Subtraction: Dishonest classifications in the high-psychopathy group (n = 9) | ||||||||
| No suprathreshold activation | ||||||||
| Subtraction: Dishonest classifications in the low-psychopathy group (n = 22) | ||||||||
| No suprathreshold activationb | ||||||||
| Subtraction: Dishonest classifications in the high-psychopathy group vs low-psychopathy group | ||||||||
| No suprathreshold activation | ||||||||
| Subtraction: Dishonest classifications in the low-psychopathy group vs high-psychopathy group | ||||||||
| No suprathreshold activationb | ||||||||
Significant even using the SVC method for the anatomically defined ACC (FWE-corrected, P < 0.05).
The results of ACC were significant when using the SVC method for the anatomically defined ACC (FWE-corrected, P < 0.05).
ACC, anterior cingulate cortex; IQ, intelligence quotient; Op, opportunity; PCL-R, Hare Psychopathy Checklist-Revised; SVC, small volume correction.
In subtraction analyses, participants were selected from the extremes of the PCL-R score distribution to generate psychopathy and control groups. The results of a one-sample t-test for the high-psychopathy group (n = 9) revealed no suprathreshold activation. A one-sample t-test for the low-psychopathy group (n = 22) failed to detect significant activation in the left ACC, but the SVC method (using anatomical masks of the bilateral ACC; FWE-corrected, P < 0.05) revealed significant activation in the left ACC (MNI coordinates: −4, 26, 22; peak-level corrected P value = 0.01).
We then directly compared dishonesty-related activity (i.e. the opportunity win vs no-opportunity win contrast) in the low- and high-psychopathy groups. The high- versus low-psychopathy group contrast did not reveal any significant differences in regional activation, and the opposite contrast again revealed no significant differences in any brain regions. However, differences in the ACC region was significant using a FWE-corrected threshold (P < 0.05) when calculated using the SVC method and anatomical masks of the bilateral ACC (MNI coordinates: −4, 26, 22; peak-level corrected P value = 0.038).
Mediation analysis
We conducted a bootstrap-mediation analysis in which psychopathic traits, percentage change in BOLD signal in the ACC (based on the anatomically defined ROI), and RT for the opportunity win trials were modeled as the independent, mediating, and dependent variables, respectively (see Supplementary Materials and Methods for details). The results revealed a marginally significant total effect of PCL-R total score on RT (total effect = −0.005, P = 0.066), and this effect disappeared after controlling for the effects of mediation factors (direct effect = −0.002, P = 0.515). Furthermore, a bias-corrected 95% CI for the indirect effect size did not include 0 (−0.0071 to −0.0004), indicating a significant indirect effect of psychopathic traits on RTs for dishonest moral decisions via ACC activity (Figure 2D).
Psycho–physiological interaction analysis
We conducted a psycho–physiological interaction (PPI) analysis to identify regions showing increased functional connectivity with the left ACC during dishonest moral decision-making (i.e. the opportunity win vs no-opportunity win contrast) (see Supplementary Materials and Methods for details). We hypothesized that control-related regions including the dorsolateral prefrontal cortex (DLPFC) would be functionally connected with the left ACC. Supporting our hypothesis, we identified increased functional connectivity between the left ACC and several cortical regions including the left DLPFC (Table 5 and Figure 3). We also examined whether the psychopathic traits were negatively correlated with functional coupling between the left ACC and left DLPFC and observed no significant relationship.
Fig. 3.
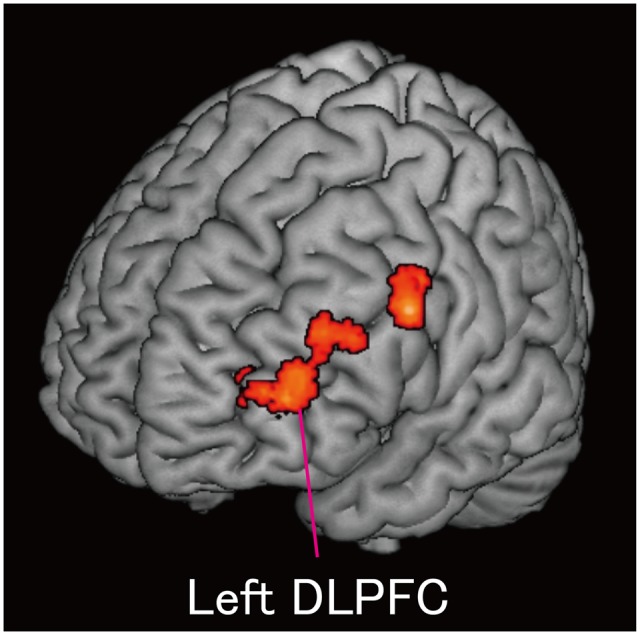
Results of a PPI analysis using the left ACC as the seed region. A functional connectivity analysis demonstrated that the left ACC was functionally connected to the left DLPFC during dishonest moral decision-making (opportunity win vs no-opportunity win). ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex.
Discussion
Using both continuous and between-group comparison approaches, we found that psychopathy was characterized by decreased ACC activity during dishonest moral decision-making, such that the level of ACC activity was a significant mediator of the relationship between psychopathic traits and reduced RTs for dishonest behavior. To the best of our knowledge, this study is the first to examine the neural substrates of dishonesty in psychopathy using the task providing real opportunities for dishonest gain.
In this study, we did not identify a significant association between psychopathic traits and the frequency of dishonest behavior, which appears to be discordant with our a priori prediction. However, this is nevertheless consistent with a high level of dishonesty in psychopathic individuals compared with normal individuals. We emphasize that the proportions of participants classified as dishonest in the high-psychopathy group (i.e. 50%) and in the whole sample (i.e. 64%) were much higher than those reported in previous studies by our group using healthy participants (i.e. ∼40%) (Greene and Paxton, 2009; Abe and Greene, 2014). Thus, it appears that criminal behavior, rather than psychopathic traits per se, is closely linked to the frequency of dishonest behavior, at least in the present context.
We found that psychopathic traits were marginally inversely correlated with RTs for dishonest moral decision-making, and that this relationship was mediated by decreased ACC activation. These observations may provide insight into the mechanisms of psychopathic individuals who behave dishonestly. We propose that when making decisions to behave dishonestly, psychopathic individuals experience reduced response conflict, either because of a lack of moral concern about behaving dishonestly or a relative insensitivity to the strategic dimensions of the choice. Atypical information processing in the ACC in psychopathic individuals may facilitate, or at least reflect, relatively automatic dishonest moral decision-making. We emphasize that this cognitive profile appears to differ not only form those of ordinary people, but also from those of non-psychopathic incarcerated individuals. These results regarding the reduced engagement of the ACC are broadly consistent with the hypothesis that psychopathy involves severe deficits in the integration of socio-affective information during decision making (Cleckley, 1941, 2015; Newman and Lorenz, 2003).
Our results provide additional evidence for a dual-process framework regarding the cognitive underpinnings of honest and dishonest behavior, based on the interplay between controlled and automatic processes. By this we do not wish to imply that such processes operate completely independently. Instead, we regard automatic processes as essential to all decision-making while controlled processes, when engaged, modulate input–output pathways, enabling behavior to be directed away from more automatic responses based on internal goals and situational features (Miller and Cohen, 2001; Dayan, 2012). Within this framework, automatic responses can be more ‘hot’ while controlled processes are more ‘cold’ (Metcalfe and Mischel, 1999), but this need not be the case, as in classic cognitive tasks such as the Stroop task (MacDonald et al., 2000; Kerns et al., 2004) and in the Cognitive Reflection Test (Frederick, 2005). For present purposes, we are agnostic as to whether the more automatic responses observed here are ‘hot’.
Within this framework, there is a qualitative distinction between tasks that do and do not require control, as manifest in classic neuropsychological dissociations (e.g. Perret, 1974; Koenigs et al., 2007). However, in neurologically intact individuals, the distinction between automatic and controlled processing typically manifests as behavior along a continuum, as control can be applied to varying degrees. Across a range of tasks, this variation can be reflected in both RTs (Koechlin et al., 2003) and in the observed engagement of the prefrontal control network, including both ACC and DLPFC (Miller and Cohen, 2001). We regard the “bottom-up” detection of conflict in the ACC, and typically one or both competing processes, as automatic, while we regard the ‘top-down’ modulation of task response by the DLPFC as controlled (Botvinick et al., 2001).
In the case of honest behavior, we previously provided evidence that consistently honest behavior requires no active self-control, whereas both honest and dishonest behaviors in individuals who behave dishonestly are associated with additional controlled processing (Greene and Paxton, 2009). We also provided evidence that responses to anticipated reward are an important determinant of (dis)honest behavior, with relatively weak responses to anticipated rewards predicting ‘graceful’ honesty, but allowing for the possibility that individuals who respond more strongly may resist temptation by force of will (Abe and Greene, 2014). Paralleling this distinction in honest behavior, two distinct processes are considered with respect to dishonest behavior, i.e. ‘willful’ controlled dishonesty and a more automatic, ‘disgraceful’ tendency toward dishonesty. The present results further refine this idea, suggesting that the degree of psychopathy is a modulating factor in determining whether dishonest behavior is more controlled or ‘willful’ (low-psychopathy group) vs more automatic or ‘(dis)graceful’ (high-psychopathy group).
In our PPI analysis, we observed increased functional connectivity between the left ACC and left DLPFC during dishonest moral decision-making (i.e. the opportunity win vs no-opportunity win contrast), consistent with the hypothesis that ACC causes the engagement of the DLPFC, which then serves a self-regulatory function (MacDonald et al., 2000; Kerns et al., 2004). In light of this, as well as our finding of decreased engagement of ACC as a function of higher psychopathy scores, one might expect to see a similar relationship between DLPFC activity and PCL-R scores during dishonest decision-making. Consistent with this, we observed a negative correlation between DLPFC activity during opportunity-win trials for dishonest subjects and total PCL-R scores, though this effect did not survive correction for multiple comparisons (see Supplementary Results).
An alternative interpretation of our current observations is that the effects observed in the ACC simply reflect attentional modulation. Attentional deficits have been proposed as central to psychopathy, with psychopathic individuals having relatively intact top-down attention, but abnormal reflective shifting in bottom-up attention (Moul et al., 2012). Other researchers have suggested that socioemotional deficits in psychopathy are due to situation-specific abnormalities in attention, such that these individuals have difficulty processing affective information when it is peripheral to their primary attentional focus (Newman and Lorenz, 2003). We believe that this attentional account and the cognitive conflict account described above are not mutually exclusive, as differences in attention may produce differences in levels of response conflict.
From these data one might infer that ACC function is impaired in psychopathic individuals, but that need not be the case. On the contrary, it is possible that ACC function is normal in all of the individuals tested and that the observed differences in ACC activity related to RT and psychopathy may simply be downstream consequences of functional differences or deficits elsewhere. Consider, as an analogy, the ACC activity of individuals engaged in the color-naming Stroop test performed in a foreign language. Here, participants may exhibit reduced ACC activity, relative performing a native-language Stroop task, and this may reflect reduced response conflict. But this, of course, need not reflect ACC dysfunction. Rather, the reduced conflict and related ACC activity is best explained by the nature of the decision inputs, whereby the meanings of the unintelligible foreign color words fail to conflict with the colors of the ‘ink’ in which the words are displayed. Likewise, the present ACC effects may reflect normal ACC function, but abnormal inputs to decision involving fewer competing considerations for psychopaths deciding whether or not to behave honestly.
Related to the point described earlier, the absence of amygdala activation in this task does not inform regional dysfunction in psychopathic individuals as anticipated by past literature (Kiehl, 2006; Blair, 2007). In fact, it is possible that decreased responsiveness of the ACC to opportunities for dishonest gain was related to reduced input from the amygdala. Past literature has indicated strong connectivity between the amygdala and anterior portions of the dorsal ACC (Etkin et al., 2011; Shackman et al., 2011). The amygdala is thought to be important for valence representation in stimulus-reinforcement associations in moral socialization (i.e. learning that certain behaviors are harmful to others and should be avoided) (Blair, 2007). Thus, dysfunction within a network involving the ACC and amygdala might make psychopathic individuals less sensitive to the aversive consequences of moral transgressions, and thus less likely to avoid dishonest moral decisions. Here, we emphasize that it is not surprising that no effect was observed in the amygdala in this study. Unlike some other experimental paradigms that have measured dishonesty in an interpersonal context (e.g. Garrett et al., 2016), the coin-flip prediction task does not involve causing harm to a specific person, which healthy people in most contexts find intrinsically aversive. Indeed, our previous studies using the coin-flip prediction task did not elicit amygdala activation (Greene and Paxton, 2009, Abe and Greene, 2014).
Although this study did not yield any striatal effects associated with psychopathy, another potentially critical neural mechanism underlying impulsive dishonest decision-making in psychopaths is heightened ventral striatal subjective value signaling. Unlike the monetary incentive delay (MID) task (Knutson et al., 2001a,b) used in our previous work linking increased striatal responses to dishonest behavior (Abe and Greene, 2014), the coin-flip task itself is not optimized for the detection of striatal effects related to the representation of subjective value. This is partly due to the rapid succession of distinct cognitive events during the coin-flip task (see Materials and Methods), each with distinct implications for subjective value. This is also due to the modulation of subjective value based on whether and to what extent the participant is responding honestly, an endogenous variable that reduces experimental control. To examine more precisely the role of striatal subjective value representations in the dishonest decision-making of psychopaths, it will likely be necessary to use other tasks such as the MID. For example, Buckholtz et al. (2010) used the MID to show that psychopathic traits in a nonclinical sample are associated with heightened reward-related nucleus accumbens activity. More recently, Hosking et al. (2017) used a delay-discounting task to demonstrate that striatal valuation signals are dysregulated during inter-temporal choice in psychopathy. Although past results do not speak directly to this question, the extant evidence suggests that striatal hyper-reactivity may be a key factor in dishonest decision-making in psychopathic individuals. We leave this possibility as a topic for future research.
This study has several limitations. First, we note once again that one of our more suggestive findings, the inverse correlation between psychopathic traits and RTs for dishonest moral decision-making, was only marginally significant. This effect awaits confirmation in future studies. We note, however, that this relationship was significantly mediated by ACC activity, consistent with our hypothesis. Second, our task design did not allow us to identify individual lies; whereas some of the opportunity win trials involved decisions to lie, other opportunity win trials were won honestly. This limitation, however, is more likely to lead to a false negative result than a false positive result, and therefore provides no specific reason to discount the positive results reported here. Finally, although the present experimental paradigm did involve morally questionable behavior in response to real opportunities for financial gain, our paradigm nevertheless employs a highly reduced interpersonal context. Thus, these results should be interpreted cautiously with awareness of their limited generalizability. Despite these limitations, these results provide evidence that dishonesty in psychopathic individuals is cognitively distinct from that of other individuals exhibiting antisocial behavior and that this is related to relatively low levels of decision conflict reflected in reduced engagement of the ACC, as well as a mediating effect of ACC activity on RTs.
Supplementary Material
Acknowledgements
The authors would like to thank Keith Harenski for his assistance with fMRI data collection and Kuniaki Yanagisawa for his helpful comments on the statistical analyses. We also thank the Kiehl lab for collection of the clinical data and the staff and inmates of the New Mexico Corrections Department for their assistance and cooperation.
Funding
This research was supported by the JSPS KAKENHI (JP16H05861), the John D. and Catherine T. MacArthur Foundation (07–89249-000-HCD), and a research grant from the National Institute on Drug Abuse (1R01DA026964-01A1, PI: Kiehl).
Conflict of interest. None declared.
References
- Abe N., Greene J.D. (2014). Response to anticipated reward in the nucleus accumbens predicts behavior in an independent test of honesty. Journal of Neuroscience, 34(32), 10564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Washington, DC: American Psychiatric Press. [Google Scholar]
- Birbaumer N., Veit R., Lotze M., et al. (2005). Deficient fear conditioning in psychopathy. A functional magnetic resonance imaging study. Archives of General Psychiatry, 62(7), 799–805. [DOI] [PubMed] [Google Scholar]
- Blair R.J. (2005). Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology, 17(3), 865–91. [DOI] [PubMed] [Google Scholar]
- Blair R.J. (2007). The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences, 11(9), 387–92. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–52. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. (2002). Region of interest analysis using an SPM toolbox [Abstract]. Neuroimage, 16(Suppl 1), abstract 497. [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., et al. (2010). Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience, 13(4), 419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. (1941). The Mask of Sanity: An Attempt to Reinterpret the so-Called Psychopathic Personality. St Louis, MO: Mosby. [Google Scholar]
- Cleckley H. (2015). The Mask of Sanity: An Attempt to Clarify Some Issues about the so-Called Psychopathic Personality. Brattleboro, VT: Echo Point Books & Media. [Google Scholar]
- Cope L.M., Vincent G.M., Jobelius J.L., Nyalakanti P.K., Calhoun V.D., Kiehl K.A. (2014). Psychopathic traits modulate brain responses to drug cues in incarcerated offenders. Frontiers in Human Neuroscience, 8, 87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P. (2012). How to set the switches on this thing. Current Opinion in Neurobiology, 22(6), 1068–74. [DOI] [PubMed] [Google Scholar]
- Decety J., Skelly L.R., Kiehl K.A. (2013). Brain response to empathy-eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry, 70(6), 638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.P., Gao X., Fu G., Lee K. (2013). Neural correlates of spontaneous deception: a functional near-infrared spectroscopy (fNIRS) study. Neuropsychologia, 51(4), 704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. (1985). Telling Lies: Clues to Deceit in the Maketplace, Politics, and Marriage. New York: Norton. [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fede S.J., Borg J.S., Nyalakanti P.K., et al. (2016). Distinct neuronal patterns of positive and negative moral processing in psychopathy. Cognitive, Affective, and Behavioral Neuroscience, 16(6), 1074–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. (2002). Structurured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research. New York State Psychiatric Institute. [Google Scholar]
- Frederick S. (2005). Cognitive reflection and decision making. Journal of Economic Perspectives, 19(4), 25–42. [Google Scholar]
- Fullam R.S., McKie S., Dolan M.C. (2009). Psychopathic traits and deception: functional magnetic resonance imaging study. British Journal of Psychiatry, 194(3), 229–35. [DOI] [PubMed] [Google Scholar]
- Garrett N., Lazzaro S.C., Ariely D., Sharot T. (2016). The brain adapts to dishonesty. Nature Neuroscience, 19(12), 1727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A.L., Yang Y., Raine A., Colletti P. (2010). No volumetric differences in the anterior cingulate of psychopathic individuals. Psychiatry Research: Neuroimaging, 183(2), 140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A.L., Han H., Yang Y., Raine A., Schug R.A. (2017). Associations between psychopathic traits and brain activity during instructed false responding. Psychiatry Research: Neuroimaging, 266, 123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J.D., Nystrom L.E., Engell A.D., Darley J.M., Cohen J.D. (2004). The neural bases of cognitive conflict and control in moral judgment. Neuron, 44(2), 389–400. [DOI] [PubMed] [Google Scholar]
- Greene J.D., Paxton J.M. (2009). Patterns of neural activity associated with honest and dishonest moral decisions. Proceedings of the National Academy of Sciences of the United States of America, 106(30), 12506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R.D. (1991). Manual for the Hare Psychopathy Checklist-Revised. Toronto, Canada: Multi-Health Systems. [Google Scholar]
- Hare R.D. (2003). Manual for the Hare Psychopathy Checklist-Revised, 2nd ed Toronto, Canada: Multi-Health Systems. [Google Scholar]
- Hare R.D., Neumann C.S. (2008). Psychopathy as a clinical and empirical construct. Annual Review of Clinical Psychology, 4, 217–46. [DOI] [PubMed] [Google Scholar]
- Hittner J.B., May K., Silver N.C. (2003). A Monte Carlo evaluation of tests for comparing dependent correlations. Journal of General Psychology, 130(2), 149–68. [DOI] [PubMed] [Google Scholar]
- Hosking J.G., Kastman E.K., Dorfman H.M., et al. (2017). Disrupted prefrontal regulation of striatal subjective value signals in psychopathy. Neuron, 95(1), 221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Pornpattananangkul N., Nusslock R. (2015). Executive control- and reward-related neural processes associated with the opportunity to engage in voluntary dishonest moral decision making. Cognitive, Affective, and Behavioral Neuroscience, 15(2), 475–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., et al. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303(5660), 1023–6. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A. (2006). A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research, 142(2–3), 107–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A., Smith A.M., Hare R.D., et al. (2001). Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry, 50(9), 677–84. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001a). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. (2001b). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport, 12(17), 3683–7. [DOI] [PubMed] [Google Scholar]
- Koechlin E., Ody C., Kouneiher F. (2003). The architecture of cognitive control in the human prefrontal cortex. Science, 302(5648), 1181–5. [DOI] [PubMed] [Google Scholar]
- Koenigs M., Baskin-Sommers A., Zeier J., Newman J.P. (2011). Investigating the neural correlates of psychopathy: a critical review. Molecular Psychiatry, 16(8), 792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Young L., Adolphs R., et al. (2007). Damage to the prefrontal cortex increases utilitarian moral judgements. Nature, 446(7138), 908–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld S.O., Fowler K.A., (2006). The self-report assessment of psychopathy: problems, pitfalls, and promises In: Patrick C. J., editor. Handbook of Psychopathy, pp. 107–132. New York: Guilford. [Google Scholar]
- MacDonald A.W. 3rd, Cohen J.D., Stenger V.A., Carter C.S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–8. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Metcalfe J., Mischel W. (1999). A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review, 106(1), 3–19. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Moul C., Killcross S., Dadds M.R. (2012). A model of differential amygdala activation in psychopathy. Psychological Review, 119(4), 789–806. [DOI] [PubMed] [Google Scholar]
- Newman J.P., Lorenz A.R. (2003). Response modulation and emotional processing: implications for psychopathy and other dysregulatory psychopathology In Davidson R. J., Scherer K. R., Goldsmith H. H., editors. Handbook of Affective Sciences, pp. 904–29. Oxford, UK: Oxford University Press. [Google Scholar]
- Nunez J.M., Casey B.J., Egner T., Hare T., Hirsch J. (2005). Intentional false responding shares neural substrates with response conflicts and cognitive control. Neuroimage, 25(1), 267–77. [DOI] [PubMed] [Google Scholar]
- Perret E. (1974). The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia, 12(3), 323–30. [DOI] [PubMed] [Google Scholar]
- Philippi C.L., Pujara M.S., Motzkin J.C., Newman J., Kiehl K.A., Koenigs M. (2015). Altered resting-state functional connectivity in cortical networks in psychopathy. Journal of Neuroscience, 35(15), 6068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J.J., Lopez S.J., Werth T.R. (1999). Development and preliminary validation of a Satz-Mogel short form of the WAIS-III in a sample of persons with substance abuse disorders. International Journal of Neuroscience, 98(1–2), 131–40. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R., Lee T.M. (2017). Are individuals with higher psychopathic traits better learners at lying? Behavioural and neural evidence. Translational Psychiatry, 7(7), e1175.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1997). Wechsler Adult Intelligence Scale. New York: Psychological Corporation. [Google Scholar]
- Wilkinson G. (1993). WRAT-III: Wide Range Achievement Test Administration Manual. Wilmington, DE: Wide Range, Inc. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.