Abstract
Individuals stably vary in their responses to rewards, but researchers have not yet determined whether sensitivity to rewarding outcomes translates across social and non-social contexts or whether different forms of reward sensitivity relate to distinct behavioral tendencies. We tested for responsiveness to different types of rewards by assessing individuals’ neural sensitivity to personal vs. vicarious monetary reward outcomes and explored how responses to each related to prosociality and well-being. Forty-six participants underwent functional magnetic resonance imaging (fMRI) scanning while winning money for themselves and observing a friend and stranger win money. All types of reward outcomes engaged the ventral striatum, but neural sensitivity to rewards for the self and for others were uncorrelated across individuals. Further, while sensitivity to rewards for the self or a close friend correlated with individuals’ psychological well-being, only sensitivity to a friend’s rewards correlated with individuals’ prosociality. These findings highlight the value of independently assessing responsiveness to different types of reward and illuminate affective mechanisms that may promote prosocial behavior and well-being.
Keywords: empathy, reward, striatum, prosocial, well-being
Recent research demonstrates that individuals differ in their neural responsiveness to reward and that these differences correlate with individuals’ affective traits and economic decisions (Abe & Greene, 2014; Benningfield et al., 2014; Wu et al., 2014). However, these findings leave two crucial questions unanswered. First, do individual differences in sensitivity to personal reward (such as monetary prizes) translate to other forms of value, such as social or vicarious reward (for example, sharing and enjoying others’ positive outcomes)? Prior work suggests that regions such as the ventral striatum (VS) and ventromedial pre-frontal cortex (VMPFC) show overlapping responses to personal and vicarious rewards, but this has typically only been examined at the group level (Mobbs et al., 2009; Morelli et al., 2015c). Thus, particular individuals might strongly engage VS and VMPFC in response to personal, but not vicarious rewards, or vice versa. If different individuals show differential sensitivity to personal and vicarious rewards, this might suggest that neural reward sensitivity is not ‘global’, but rather can track particular forms of reward.
Second, do different forms of reward sensitivity relate to distinct experiential and behavioral outcomes? For instance, some individuals are more likely to engage in prosocial behaviors than others (Crockett et al., 2014; Peysakhovich et al., 2014). However, the psychological forces that underlie this ‘prosocial phenotype’ remain unclear. One intriguing possibility is that people consistently act kindly towards others because they enjoy sharing others’ rewarding experiences, which may uniquely relate to their vicarious reward sensitivity. A handful of studies have demonstrated that activity in VS and VMPFC accompanies prosocial actions in laboratory settings—for example, donating money to charities or winning money for others in a gambling game (Moll et al., 2006; Harbaugh et al., 2007; Telzer et al., 2010; Sul et al., 2015). However, it is unclear if individual differences in VS and VMPFC activity in response to vicarious reward reliably relate to real-world prosocial behaviors. Past studies have, however, demonstrated connections between vicarious negative affect and subsequent helping behavior in daily life (Rameson, et al., 2012; Morelli et al., 2014).
Here, we examined whether sharing others’ positive emotions likewise correlates with increased everyday prosocial actions and prosocial spending. In addition, the present paper investigates whether neural indices of vicarious reward relate to individual differences in well-being. Previous work suggests that the experience of vicarious reward can benefit individuals. For instance, individuals report increased well-being after acting prosocially, but only if they experienced vicarious affect while doing so (Morelli et al., 2015a). This suggests that vicarious reward may drive not only prosocial acts, but also the hedonic benefits conferred by those acts. More broadly, vicarious reward may boost well-being by allowing individuals to share others’ positive affect (Hicks & Diamond, 2008; Morelli et al., under review).1 Thus, vicarious affect might contribute not only to prosociality, but also to general well-being.
We combined neuroimaging and daily experience sampling to address these unanswered questions. We first assessed neural sensitivity to both personal and vicarious reward. Participants underwent functional magnetic resonance imaging (fMRI) scanning while winning money for themselves (i.e. personal reward) and observing a close friend and stranger win money (i.e. vicarious reward) during a card-guessing game. We employed a relatively large sample for fMRI (n = 46), which allowed us to assess individual differences in neural responses to each type of reward. In light of the prior work, we focused, in particular, on examining neural activity in the VS, VMPFC, and medial pre-frontal cortex (MPFC) and predicted that these reward-related regions would respond to both personal and vicarious reward outcomes. Crucially, however, our approach allowed us to examine whether individuals who responded strongly to one type of reward would likewise exhibit strong responses to the other type or whether individuals might differ in their sensitivity to personal vs. vicarious reward. We further examined whether neural sensitivity to personal and vicarious reward was differentially associated with well-being and prosocial behavior.
Materials and Methods
Participants
To determine the sample size, we calculated the minimum number of participants (i.e. 45) required to detect correlations of r = .35 or higher (p < .05, two-tailed) with 80% power. To meet our goal of 45 participants, we recruited and enrolled 55 participants from fliers and advertisements posted around the Stanford campus. To qualify for the study, participants needed to be a Stanford undergraduate and participate in the study with a close friend. Both members of the dyad were required to be the same gender, perceive a high degree of similarity with their friend (4 or higher on the Inclusion of Other in Self Scale on a 1–7 Likert scale) and report seeing their friend at least three times per week (Aron et al., 1992). In addition, both members of the dyad needed to meet criteria for magnetic resonance imaging (MRI) scanning (i.e. right-handed, no metal, no psychoactive medications and no neurological problems). We excluded five pairs of friends because one member of the dyad completed less than 10 days of daily surveys. One pair withdrew from the study due to an interpersonal conflict, and one pair could not be scheduled for an MRI scanning session. We previously reported on daily data from this sample (Morelli et al., 2015a), but present neuroimaging data from this sample for the first time here.
From the remaining 48 same-gender pairs, we randomly selected one member of each pair to scan. Two of the 48 scanned participants were excluded due to excessive motion during the scanning session. Thus, the final sample consisted of 46 participants, including 23 males and 23 females (mean age = 19.33, s.d. = 1.23). The sample was 13% East Asian, 4% Pacific Islander, 11% Black or African American, 41% White or Caucasian, 13% Hispanic or Latino/a, 2% South Asian, 13% Mixed Race and 3% Other.
Procedure
Participants completed a battery of online trait surveys and a 2-week experience sampling study, and then one member of each friend dyad participated in a scanning session. The other member of the dyad came to the same experimental session, but completed tasks outside the scanner. In addition, participants met an age- and gender-matched confederate (hereafter: stranger) whom they believed was another Stanford University student participating in the study. Participants were told that they would play a card-guessing game while being scanned, as well as observe their friend and the stranger play this same card-guessing game. The experimenter took photos of the participant, their friend and the stranger and then explained the details of the task. After entering the scanner, participants alternated between playing the card-guessing game and watching their friend and the stranger play the same game. After exiting the scanner, participants completed a brief survey to assess their emotional reactions during the scanning session. They were also debriefed and paid in cash for two of their own games selected at random.
Behavioral measures
Positive empathy
We used the Positive Empathy Scale (PES) as a broad proxy of vicarious reward (α = .88) (Morelli et al., under review; Morelli et al., 2015b). The PES consists of seven items: (i) At a surprise party, I become very excited watching someone react to the surprise; (ii) When I see someone else smile, I can’t help but smile too; (iii) If I don’t understand why someone is excited, I try to put myself in their shoes and understand what they’re thinking and feeling; (iv) When someone else is enthusiastic, I can’t help but be enthusiastic too; (v) When someone succeeds at something that I don’t think is very important, I can still understand why they’re happy; (vi) When people talk about their hopes and dreams, I always hope they achieve them; (vii) I often feel excited when I’m with other people who are excited. The PES items were rated on a five-point Likert scale from 1 (does not describe me at all) to 5 (describes me very well). We then averaged the seven items that were averaged together for a measure of positive empathy.
Prosociality
Participants also reported on the extent to which they engaged in several forms of prosocial behavior. This provided a broad assessment of prosocial behavior toward others and did not assess prosocial behavior toward any specific targets (e.g. their close friend participating in the study). The Prosocial Tendencies Measure (PTM) captured participants’ tendency to engage in six types of helping behaviors: (i) helping conducted in front of others (i.e. public), (ii) helping others in emotionally charged situations (i.e. emotional), (iii) helping in crisis or emergency situations (i.e. dire), (iv) helping performed anonymously (i.e. anonymous), (v) helping motivated by concern for others’ needs (i.e. altruism) and (vi) helping others in response to a verbal or non-verbal request (i.e. compliant) (Carlo & Randall, 2002). The PTM items were rated on a five-point Likert scale from 1 (does not describe me at all) to 5 (describes me very well). We calculated the average score for each subscale of the PTM: public (α = .85), emotional (α = .88), dire (α = .84), anonymous (α = .77), altruism (α = .77) and compliant (α = .72). We then conducted a factor analysis with all six subscales using unweighted least squares estimation with oblimin (i.e. oblique) rotation. Although we hypothesized that these items would emerge as a single construct, we allowed for a multiple-factor solution as well. However, the scree plot supported a one-factor solution, and all subscales except the public subscale loaded on one factor. Therefore, we eliminated the public subscale and conducted another factor analysis with the remaining five subscales using unweighted least squares estimation with a one-factor solution. The final factor accounted for 37.61% of the variance, and factor loadings indicated relatively high internal consistency (0.41–0.79, Table 1). We then multiplied each subscale by its factor loading and then averaged across all subscales to create a Prosocial Tendencies Composite.
Table 1.
Factor loadings for Prosocial Tendencies Composite
| Subscale | Prosocial Tendencies Composite |
|---|---|
| Emotional | 0.66 |
| Dire | 0.79 |
| Anonymous | 0.52 |
| Altruistic | 0.41 |
| Compliant | 0.61 |
| Explained Variance | 37.61% |
We also evaluated prosocial spending by asking participants to estimate (in dollars) how much they spent in a typical month on (i) gifts for others (e.g. birthday, holiday), (ii) treating someone to a meal out and (iii) donations to charitable or non-profit organizations (Dunn et al., 2008). We summed all three categories to get a total amount for prosocial spending. We also collected information about personal spending to use as a covariate in analyses, thus ensuring that prosocial spending did not merely reflect participants’ overall financial means or behavior. We asked participants to report (in dollars) how much they spent in a typical month on (i) treating themselves to a meal out, (ii) entertainment, (iii) travel and recreation, (iv) clothing and (v) saving money (Dunn et al., 2008). We summed all categories for the personal spending total.
Well-being
Participants responded to questions about trait loneliness, perceived stress, life satisfaction, positive affect, and negative affect. They also completed the UCLA Loneliness Scale (example items: ‘How often do you feel that there is no one you can turn to?’ and ‘How often do you feel that you are no longer close to anyone?’), reporting how often they experienced each loneliness item on a four-point scale (never, rarely, sometimes or always) (Russell, 1996). Participants also indicated how often their lives felt unpredictable, uncontrollable, and overloaded in the last month (never, almost never, sometimes, fairly often, very often) (10-item Perceived Stress Scale; Cohen et al., 1983). In addition, participants reported how satisfied they felt with their lives (example item: ‘In most ways my life is close to my ideal’) on a scale from 1 (strongly disagree) to 7 (strongly agree) (Satisfaction with Life Scale; Diener, Emmons, Larsen, & Griffin, 1985). Finally, a 20-item version of the Positive and Negative Affect Schedule captured the extent to which participants ‘generally feel’ positive emotions (i.e. interested, excited, strong, enthusiastic, proud, alert, inspired, determined, attentive, or active) and negative emotions (i.e. distressed, upset, guilty, scared, hostile, irritable, ashamed, nervous, jittery, or afraid) (Watson et al., 1988).
For the experience sampling portion of the study, participants answered questions about loneliness, perceived stress, positive emotion and negative emotion each day for 14 consecutive days. We measured daily loneliness with a six-item measure that assessed how alone or isolated individuals felt each day (adapted from the UCLA Loneliness Scale). Participants also rated their daily stress with the four-item version of the Perceived Stress Scale. For both of these scales, participants rated their agreement with each statement using a seven-point scale from 1 (strongly disagree) to 7 (strongly agree). Participants also indicated how much positive emotion (i.e. happy, joyful, excited or elated) and negative emotion (i.e. anxious, stressed, upset or scared) they felt each day (Morelli, et al., 2015a). For both of these scales, participants rated their agreement with each statement using a five-point scale from 1 (not at all) to 5 (extremely). Scores for all measures were calculated each day and then averaged across all 14 days.
To determine whether these measures cohered into a ‘well-being’ factor, we conducted a factor analysis using the average score of all trait measures and the 14-day average of all daily measures (Table 2). We used an unweighted least squares estimation with oblimin rotation and allowed for a multi-factor solution. However, the scree plot and factor loadings supported a one-factor solution. The one-factor solution explained 49.4% of the variance and yielded high internal consistency (-0.45--0.87, Table 2). Based on the factor analysis, we then multiplied each scale by its factor loading and averaged across all subscales to create a Well-Being Composite.
Table 2.
Factor loadings for Well-being Composite
| Subscale | Well-being Composite |
|---|---|
| Trait Loneliness | 0.77 |
| Trait Perceived Stress | 0.69 |
| Trait Negative Affect | 0.65 |
| Trait Positive Affect | −0.45 |
| Trait Life Satisfaction | −0.66 |
| Mean Daily Loneliness | 0.83 |
| Mean Daily Perceived Stress | 0.87 |
| Mean Daily Negative Affect | 0.81 |
| Mean Daily Positive Affect | −0.47 |
| Explained Variance | 49.40% |
Additional measures
In addition to the primary measures listed above, we also collected trait measures that assessed empathy, autism, volunteerism, coping, personality, emotional expressivity, and subjective social status. As previously reported (Morelli et al., 2015a), we also collected daily measures of positive events, stressful events, coping, emotional support, practical support, and empathy.
fMRI task
Our fMRI task included a novel combination of two popular reward tasks, blending elements of the Monetary Incentive Delay task (Knutson et al., 2001) with a card-guessing task (Delgado et al., 2000; Preuschoff et al., 2006; Mobbs et al., 2009; Fareri et al., 2012). Before entering the scanner, the experimenter told participants that they would be (i) playing a card-guessing game and could win real money for themselves (for two randomly selected games) and (ii) observing a close friend, or a stranger play the same game. Each player (self, friend, and stranger) played games in which they could potentially gain money, potentially lose money, or receive no money. Because our main hypotheses focused on the receipt of rewards for the self or others, we restricted our analyses to examining potential gain outcomes. Therefore, we only describe analyses for the subset of 180 trials related to personal reward (i.e. self trials) and vicarious reward (i.e. friend and stranger trials). These 180 trials were separated into three runs of 60 trials each. Within each run, participants played 20 self trials, 20 friend trials, and 20 stranger trials. Trials for each person were spilt into two sets of 10 trials in a row, and all sets of trials were randomly presented during each run. Trials were grouped by person in order to minimize competitiveness and confusion about who was playing.
Before each set of trials, participants saw a prompt to either ‘play’ (for self trials) or ‘observe’ (for friend and stranger trials). For self trials, participants (i) viewed a crosshair during the intertrial interval (ITI), (ii) saw their own photo along with a cue indicating that they could potentially win $5 (i.e. a circle with a line at the top), (iii) guessed whether the card would be above 5 (i.e. up arrow) or below 5 (i.e. down arrow) from a deck of cards from 1–9 (excluding 5) and (iv) saw the actual value of the card (e.g. 8 or 3) and the corresponding amount of money they gained or did not gain (i.e. + 5.00 or $0.00). For other trials, participants (i) viewed a crosshair during the ITI, (ii) saw a friend or stranger’s photo along with a cue indicating that person could potentially win $5, (iii) imitated the other person’s card guess by pressing the button that corresponded with their arrow selection and (iv) saw the actual value of the card and the corresponding amount of money the other person gained or did not gain (Figure 1).
Fig. 1.
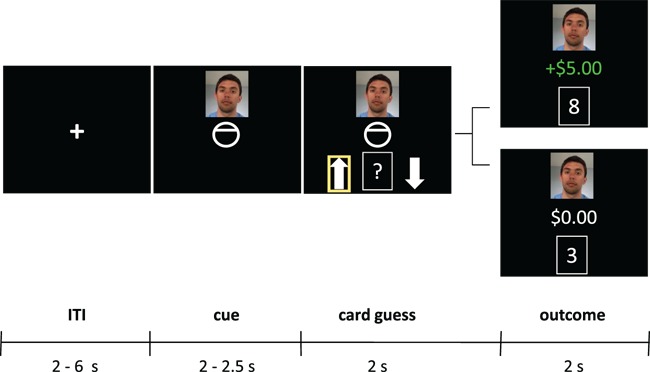
A schematic for the card-guessing game. For self trials, participants (i) viewed a crosshair during the ITI, (ii) saw their own photo and a cue indicating that they could win $5 (iii) guessed whether the card would be above or below 5 and (iv) saw the value of the card and the amount of money they received. The structure was identical for other trials, except participants saw a photo of a friend or stranger and imitated their card guesses.
A correct guess yielded a monetary gain of $5 (reward outcome in green), whereas an incorrect guess resulted in $0 (no reward outcome in white). If participants failed to make or imitate card guesses, participants saw the word ‘MISS’ in red and knew they would lose $2.50 (for not paying attention). Outcomes were pre-programmed (50% reward, 50% no reward) for self and other trials and were presented randomly. Although participants believed they were viewing a friend or stranger’s actual card guesses in real time, these responses were pre-programmed into the game.
fMRI acquisition and data analysis
Acquisition
Scanning was performed on a 3.0 T General Electric MRI scanner with a 32-channel head coil at the Stanford Center for Cognitive and Neurobiological Imaging. The MATLAB Psychophysics Toolbox version 7.9 was used to present the task to participants and record their responses. Participants viewed the task through a mirror system and responded with both hands on a button response box. For each participant, 324 functional T2*-weighted gradient echo pulse sequence image volumes were acquired in each of three functional runs (slice thickness = 2.9 mm, no gap, 46 slices, TR = 2000 ms, TE = 25 ms, flip angle = 77°, interleaved acquisition). High-resolution structural scans were also acquired with a T1-weighted pulse sequence (slice thickness = 0.9 mm, TR = 7.2 ms, TE = 2.8 ms, flip angle = 12°) in between the second and third functional scans.
Data processing and analysis
In SPM8 (Wellcome Department of Imaging Neuroscience, London), all functional and structural images were manually reoriented, realigned, co-registered to the structural scan, spatially normalized, and smoothed (6 mm full width at half maximum Gaussian kernel). First-level effects were estimated using the general linear model. Due to our event-related design, we separately modeled each component of each trial: cue (2–2.5 s), card guess (2 s) and outcome (2 s) (Figure 1). All trial components were modeled as a boxcar spanning their duration and convolved with a canonical (double gamma) hemodynamic response function. Based on the a priori hypotheses, we focused on the outcome phase of self and other reward trials. The model included six regressors of interest: Self Reward (i.e. + $5 for self), Self No Reward ($0 for self), Friend Reward (+ $5 for friend), Friend No Reward ($0 for friend), Stranger Reward (+ $5 for stranger) and Stranger No Reward ($0 for stranger). If participants failed to make a guess (for self trials) or failed to imitate the other person’s guess, these ‘misses’ were modeled as covariates of no interest. In addition, all other parts of the trials (e.g. each cue type and card guess) were included as covariates of no interest. The model also included additional covariates of no interest, such as the six motion parameters from image realignment and regressors modeling time points where in-brain global signal change exceeded 2.5 s.d. of the mean global signal change or where estimated motion exceeded 0.5 mm translation or 0.5° rotation. The time series was high-pass filtered using a cutoff period of 128 s. Serial autocorrelations were modeled as an AR(1) process. Random effects analyses of the group were computed using the contrast images generated for each participant.
Although whole-brain analyses were not central to our hypotheses, we conducted these analyses for exploratory and data sharing purposes (https://neurovault.org/collections/812/). We computed whole-brain random effects contrasts comparing gain to no gain outcomes when participants (i) played the game themselves, (ii) watched their friend play, or (iii) watched a stranger play. We then tested our central hypotheses by interrogating activity in four reward-related regions of interest (ROI): left and right VS, VMPFC, and MPFC (Figure 2). More specifically, we extracted parameter estimates from the whole-brain contrasts using the Marsbar toolbox (http://marsbar.sourceforge.net), averaging across all voxels in each ROI for each of the three contrasts. Due to non-normal distributions in mean parameter estimates across individuals, we also created a rank order based on average activity in each ROI.
Fig. 2.
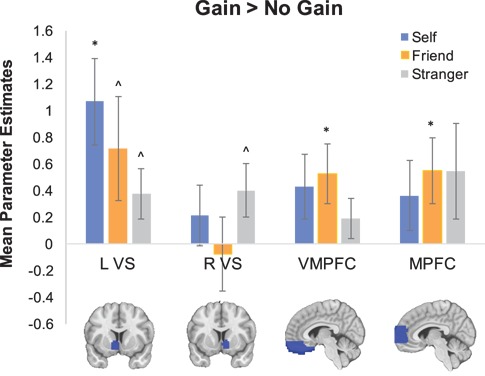
The mean parameter estimates for each ROI extracted from the whole-brain contrasts for each type of reward outcome. * indicates p < 0.05 and ^ indicates p < 0.10, when testing if the mean parameter estimates were above zero.
We constructed the VS ROIs in the Wake Forest University Pickatlas Tool using the Automated Anatomical Labeling Atlas. The VS ROIs were structurally defined and constrained according to the following coordinates: −12 < x < 12, 4 < y < 18, −12 < z < 0. The MPFC and VMPFC ROIs were manually constructed in FSLview in a voxel-by-voxel fashion, informed by meta-analyses and reviews pertaining to MPFC function (Northoff et al., 2006). The MPFC ROI was bounded to the following: −20 < x < 20, 46 < y < 76, −10 < z < 24, and the VMPFC ROI was constrained to the following: −20 < x < 20, 12 < y < 70, −34 < z < −12. All ROIs can be accessed on NeuroVault (https://neurovault.org/collections/812/).
Post-scanner survey
After exiting the scanner, participants completed a brief questionnaire about how they felt when they played and watched the card-guessing games. Participants read a basic description of valence and arousal, along with examples of negative emotion (e.g. sad), positive emotion (e.g. happy), low arousal (e.g. slow, still) and high arousal (e.g., alert, energized). They also learned how to use both scales to capture their emotional states, such as excitement after winning a prize (positive, high arousal) or depression (negative, low arousal). Then, participants indicated how they felt when they saw the cue for self, friend, and stranger trials, using seven-point Likert scales to rate valence (very negative–very positive) and arousal (low arousal–high arousal). In addition, participants rated how similar they felt to the stranger on a scale from 1 (not all) up to 7 (very).
Results
Post-scanner survey
Participants reported feeling positive valence (M = 5.78, s.d. = 0.96) and moderately high arousal (M = 5.46, s.d. = 1.46) prior to playing the game themselves. Participants also reported feeling positive valence (M = 5.35, s.d. = 1.17) and moderate arousal (M = 4.61, s.d. = 1.48) in anticipation of their friend playing the game, but less positive valence (M = 4.43, s.d. = 1.01) and lower arousal (M = 3.78, s.d. = 1.76) in anticipation of the stranger playing. Two paired-samples t-tests revealed that participants felt more positive (t(45) = 2.25, p < 0.05) and more aroused (t(45) = 4.31, p < 0.001) before playing the game themselves, as compared to watching their friend play. In addition, participants felt more positive (t(45) = 7.01, p < 0.001) and more aroused (t(45) = 6.43, p < 0.001) before playing the game themselves, as compared to watching the stranger play. Participants also reported feeling more positive (t(45) = 5.13, p < 0.001) and more aroused (t(45) = 4.61, p < 0.001) before watching a friend vs. stranger play.
Group-level responses to personal and vicarious reward
In an effort to replicate prior work (Mobbs et al., 2009; Morelli et al., 2015c), we tested if reward-related regions were commonly activated by both personal and vicarious reward outcomes. To accomplish this, we extracted parameter estimates from the three whole-brain contrasts in VS, VMPFC, and MPFC. We conducted a series of one-sample t-tests to determine if reward-related regions were generally activated when individuals experienced personal and vicarious reward (Figure 2).
When participants won money (self gain > self no gain), average activity in L VS (t(45) = 3.265, p = 0.002) was significantly above zero and marginally significant for VMPFC (t(45) = 1.786, p = 0.081). When they observed their friend win money (friend gain > friend no gain), average activity for VMPFC (t(45) = 2.336, p = 0.024) and MPFC (t(45) = 2.231, p = 0.031) was significantly above zero and marginally significant for L VS (t(45) = 1.831, p = 0.074). When they observed a stranger win money (stranger gain > no gain), the one-sample t-tests for L VS (t(45) = 1.976, p = 0.054) and R VS (t(45) = 2, p = 0.052) were marginally significant. These results partially replicate past findings, showing reward-related activity in some ROIs at the group level for both personal and vicarious reward. More importantly, these analyses also reveal that neural responses to personal and vicarious reward vary substantially across individuals within the group (see standard error bars in Figure 2), providing further impetus to investigate if this neural variability correlates with individual differences in prosociality and well-being.
Individuals’ sensitivity to personal vs. vicarious reward
We then examined whether neural responses to personal and vicarious reward would track each other within individuals. Intriguingly, individuals’ relative sensitivity to personal rewards did not correlate with their relative sensitivity to their friend's rewards in any of the ROIs (L VS: rs = 0.09, p = 0.57; R VS: rs = 0.14, p = .35; VMPFC: rs = 0.09, p = 0.54 and MPFC: rs = 0.08, p = 0.62) (Figure 3). In addition, individuals’ relative sensitivity to personal rewards did not correlate with their relative sensitivity the stranger's rewards in any of the ROIs (L VS: rs = −0.11, p = 0.46; R VS: rs = .05, p = 0.75; VMPFC: rs = .1, p = 0.53; and MPFC: rs = .19, p = 0.2). These data suggest that sensitivity to personal reward and vicarious reward vary independently. Based on our sample size (n = 46) with 80% power & p < 0.05 (two-tailed), we should have been able to detect a correlation of r = 0.35 or higher. Therefore, these findings do not suggest that no relationship exists between personal and vicarious reward, but rather that a strong relationship does not exist.
Fig. 3.
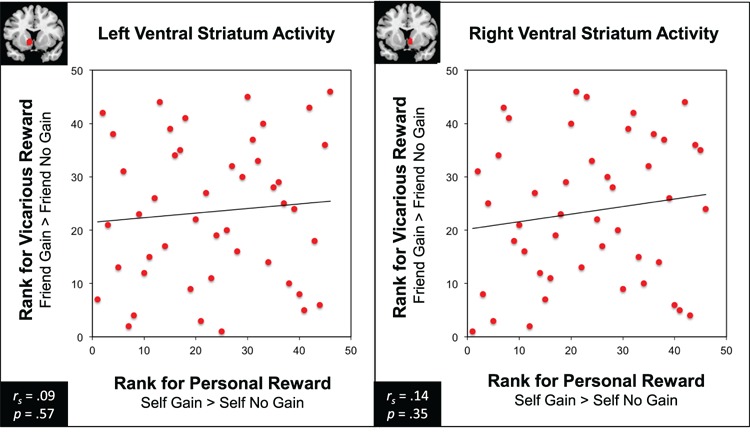
For personal and vicarious reward outcomes, rank order of ventral striatal activity (left and right) was not significantly associated.
Correlations between neural and behavioral measures
In order to verify correspondence between neural and behavioral indices of vicarious reward, we first tested if responses to vicarious reward, but not personal reward, would positively correlate with trait positive empathy. For both friend and stranger reward, we confirmed that VS activity had a significant positive relationship with trait positive empathy (Table 3). We also tested whether participants’ perceived similarity to their friend or the stranger modulated responses to vicarious reward (Mobbs et al., 2009). For the friend, perceived similarity (M = 5.11, s.d. = .9, range: 4–7) did not correlate with activity in L VS (r = .18, p = 0.24) or R VS (r = .11, p = 0.48), marginally correlated with MPFC activity (r = .27, p = 0.08) and significantly correlated with VMPFC activity (r = .3, p = 0.046). For the stranger, perceived similarity (M = 3.07, s.d. = 1.1, range = 1–5) did not significantly correlate with activity in any of these reward-related ROIs.
Table 3.
Correlations between neural responses to vicarious and personal reward outcomes and positive empathy, prosocial tendencies, prosocial spending, and well-being
| Behavioral measures | ||||
|---|---|---|---|---|
| Predictors | Positive empathy | Prosocial tendencies | Prosocial spending | Well-being |
| Stranger Gain > Stranger No Gain | ||||
| L VS | 0.31* | 0.26 † | −0.02 | −0.01 |
| R VS | 0.38* | 0.26 † | 0.09 | 0.03 |
| Ventromedial PFC | 0.05 | 0.15 | −0.09 | 0.1 |
| Medial PFC | .12 | .19 | −0.08 | 0.0 |
| Friend Gain > Friend No Gain | ||||
| L VS | 0.28 † | 0.12 | 0.37* | 0.31* |
| R VS | 0.38** | 0.36* | 0.30* | 0.11 |
| Ventromedial PFC | 0.16 | 0.11 | 0.10 | 0.19 |
| Medial PFC | 0.14 | 0.09 | 0.08 | 0.20 |
| Self Gain > Self No Gain | ||||
| L VS | 0.18 | −0.14 | 0.01 | 0.36* |
| R VS | 0.17 | 0.03 | −0.12 | 0.18 |
| Ventromedial PFC | 0.07 | −0.25† | 0.20 | 0.21 |
| Medial PFC | 0.05 | −0.26† | 0.21 | 0.14 |
Notes. † p < 0.10 * p < 0.05 ** p < 0.01; L = left; R = right; PFC = prefrontal cortex
Additional correlational analyses revealed that VS activity in response to vicarious reward tracked several measures of prosociality. In particular, individuals' VS responses to observing their friend win money (friend gain > friend no gain) correlated with those individuals' trait-level reports of spending money prosocially (Table 3). Even after controlling for personal spending, the partial correlation between L VS activity and prosocial spending remained significant (r = .32, p = .03). The correlation between R VS activity and prosocial spending showed a positive association, but was not significant (r = .23, p = .12). In contrast, neural activity in response to personal reward (self gain > no gain) did not significantly relate to prosocial spending. To more directly test the hypothesis that responses to a friend's rewards would correlate with prosocial spending more strongly than responses to personal reward, we conducted a one-tailed Fisher's z-test comparing these relationships (Fisher, 1921). This analysis revealed significant differences such that neural responses to friend reward and prosocial spending versus personal reward and prosocial spending showed stronger associations in the left VS (Z = 1.77, p < .04) and right VS (Z = 1.98, p < .02).
Similarly, VS responses to friend, but not personal, reward significantly correlated with participants’ tendency to help others in everyday situations (Table 3, Figure 4). Right VS activity in response to friend reward positively correlated with prosocial tendencies, while right and left VS activity in response to stranger reward marginally correlated with prosocial tendencies. As such, we used a second one-tailed Fisher’s z-test to test whether the correlation between right VS activity in response to friend reward and prosocial tendencies was significantly stronger than the correlation between right VS activity in response to personal reward and prosocial tendencies. The difference between these correlations was in the predicted direction and marginally significant (Z = 1.58, p < .06). Unlike prosociality, which uniquely tracked vicarious reward sensitivity, well-being showed a more general positive association with neural responses to both personal and friend reward (Table 3, Figure 4).
Fig. 4.
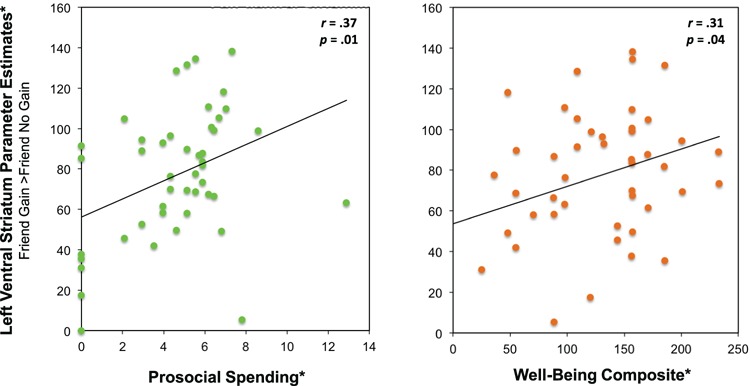
Correlation between neural activity in left VS for friend gain > friend no gain outcomes and (i) prosocial spending (left panel) and (ii) well-being (right panel). *Scale is based on transformed variables.
Discussion
This study offers several key insights concerning neural sensitivity to vicarious reward and its relationship to prosociality and well-being. First, individuals’ neural sensitivity to personal and vicarious reward did not significantly relate to each other, suggesting that each individual has a unique profile of sensitivity to each type of reward. While some people are relatively sensitive to personal, but not vicarious reward, others exhibit the opposite pattern. Prior work demonstrates a link between VS responsiveness to personal reward anticipation and individual characteristics such as extraversion (Wu et al., 2014). The current data suggest that neural responses to personal reward may not globally index individuals’ reward sensitivity. Rather, researchers could characterize individuals based on their responses to multiple reward classes, for instance in both non-social and social settings. By examining these tendencies in tandem, researchers may gain more precise insights into which profiles are most closely linked to helping behaviors and more broadly to well-being.
These findings importantly reveal that individuals’ sensitivity to vicarious reward, but not personal reward, relates to a number of real-world prosocial behaviors. Previous research demonstrates that empathizing with others’ negative emotions can predict prosocial behavior (Batson, 2011). These findings expand on this idea by showing that individuals with greater sensitivity to both types of vicarious reward (i.e. friend and stranger) also reported increases in a variety of everyday helping behaviors. Thus, neural responses to vicarious reward, in general, may track the tendency to behave prosocially across contexts (Peysakhovich et al., 2014; Hubbard et al., 2016). Notably, sensitivity to a friend’s rewarding outcomes showed a stronger relationship with everyday helping behaviors and additionally related to spending money on others. As such, future work should include a wide range of prosocial behaviors and investigate whether sensitivity to a friend’s rewarding outcomes serves as a more general marker of prosocial behavior across contexts.
In addition, different prosocial motivations may influence the quality of one’s social relationships. For example, people’s willingness to sacrifice in order to obtain positive outcomes for others (such as a partner’s happiness) is associated with enhanced relationship quality, whereas willingness to sacrifice in order to avoid others’ negative outcomes is negatively associated with relationship quality (Impett et al., 2005). Future studies might test these ideas more directly by manipulating neural sensitivity to vicarious reward (e.g. a perspective-taking manipulation) and examining immediate effects on prosocial behavior.
The dissociation between personal and vicarious reward sensitivity in predicting behavior and experience did not extend, however, to well-being. Instead, neural sensitivity to rewarding outcomes for the self and a friend correlated with individuals’ reports of psychological well-being. This suggests that although vicarious reward sensitivity tracks people’s tendency to engage in prosocial acts, people sensitive to rewards either for the self or a friend may benefit psychologically, potentially through distinct mechanisms. For example, individuals may feel highly connected to friends after empathizing with their rewarding outcomes, thereby reducing their sense of loneliness (Gable et al., 2012; Morelli et al., under review). In contrast, sensitivity to personal rewards may enhance well-being by increasing the intensity and duration of positive emotional experiences. In line with this idea, savoring personal positive events can enhance positive affect and life satisfaction (Heller et al., 2013; Smith et al., 2014). Future work might test these predictions with manipulations that boost positive empathy or savoring and then assess how sensitivity to each type of reward uniquely predicts subsequent changes in well-being.
Limitations and future directions
Our measures of prosocial behavior relied on self-report, and so might reflect a desire to appear prosocial, rather than actual prosocial behavior. However, two factors mitigate this concern. First, participants completed all self-report measures online, without an experimenter present. Instructions reminded participants that their responses were anonymous and de-identified. Such anonymity encourages people to behave according to their actual preferences, rather than following socially desirable norms. Further, the prosociality measure we employed does not significantly correlate with social desirability, but does correlate with third-party ratings of respondents’ generosity (Carlo & Randall, 2002; Carlo et al., 2003). Even when no precautions are taken to reduce social desirability concerns, most people willingly report selfish patterns of spending (Dunn et al., 2008). Therefore, it seems unlikely that participants altered how much they spent on others to appear more prosocial. To fully rule out this possibility, future work could connect brain responses during vicarious reward to self-reported and other-reported measures of prosociality.
Because participants observed friends receive rewards, they might have imagined indirect benefits from the money their friend earned. Due to this concern, the experimenter explicitly told the participants that their friends’ earnings would not be shared with them. Participants also did not have an opportunity to talk with their friend after learning about their friends’ potential rewards. Therefore, potential indirect benefits are likely minimal. Because we selected for high levels of similarity in friend dyads and found low levels of perceived similarity to strangers, it is difficult to separately assess how similarity modulated neural responses during each type of vicarious reward. Thus, future work might more continuously vary these dimensions within social categories to determine how more fine-grained target characteristics modulate vicarious reward responses.
Finally, the present results do not specify whether neural sensitivity to vicarious reward outcomes captures a broader neural sensitivity to social stimuli. Recent work suggests that behavioral measures of vicarious reward uniquely relate to prosociality and well-being, above and beyond social sensitivity (Andreychik & Migliaccio, 2015; Morelli et al., under review). Future work might investigate whether neural indices of vicarious reward can account for unique variance in prosociality and well-being. Further, it will be critical for future research to experimentally manipulate sensitivity to vicarious rewards and examine subsequent effects on prosociality and well-being, in order to establish that these correlational findings do not stem from an unexamined third variable.
Conclusion
Ultimately, these findings reveal a possible explanation for why some individuals consistently engage in more prosocial behaviors across time and contexts, as well as emotionally benefit from the positive experiences of others. Vicarious reward may serve as an affective engine that can drive prosocial behavior and boost well-being for the self as well as others.
Supplementary Material
Acknowledgements
We thank Molly Arnn and Kiefer Katovich for their help with data collection and coding.
Conflict of interest. None declared.
Funding
National Institute of Mental Health supported this work (#F32MH098504 to S.A.M.).
Footnotes
Morelli, S. A., Kwok, Z., Lieberman, M. D., & Zaki, J. (under review). Positive empathy: Its structure and relation to prosociality, social connection, and well-being.
References
- Abe N., Greene J.D. (2014). Response to anticipated reward in the nucleus accumbens predicts behavior in an independent test of honesty. The Journal of Neuroscience, 34(32), 10564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreychik M.R., Migliaccio N. (2015). Empathizing with others pain versus empathizing with others joy: examining the separability of positive and negative empathy and their relation to different types of social behaviors and social emotions. Basic and Applied Social Psychology, 37(5), 274–91. [Google Scholar]
- Aron A., Aron E.N., Smollan D. (1992). Inclusion of other in the self scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology, 63(4), 596. [Google Scholar]
- Batson C.D.(2011). Altruism in Humans, New York, NY: Oxford University Press. [Google Scholar]
- Benningfield M.M., Blackford J.U., Ellsworth M.E., et al. (2014). Caudate responses to reward anticipation associated with delay discounting behavior in healthy youth. Developmental Cognitive Neuroscience, 7, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo G., Hausmann A., Christiansen S., Randall B.A. (2003). Sociocognitive and behavioral correlates of a measure of prosocial tendencies for adolescents. The Journal of Early Adolescence, 23(1), 107–34. [Google Scholar]
- Carlo G., Randall B.A. (2002). The development of a measure of prosocial behaviors for late adolescents. Journal of Youth and Adolescence, 31(1), 31–44. [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–96. [PubMed] [Google Scholar]
- Crockett M.J., Kurth-Nelson Z., Siegel J.Z., Dayan P., Dolan R.J. (2014). Harm to others outweighs harm to self in moral decision making. Proceedings of the National Academy of Sciences, 111(48), 17320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D., Fiez J.A. (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84(6), 3072–7. [DOI] [PubMed] [Google Scholar]
- Diener E., Emmons R.A., Larsen R.J., Griffin S. (1985). The satisfaction with life scale. Journal of Personality Assessment, 49(1), 71–5. [DOI] [PubMed] [Google Scholar]
- Dunn E.W., Aknin L.B., Norton M.I. (2008). Spending money on others promotes happiness. Science, 319(5870), 1687–8. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Niznikiewicz M.A., Lee V.K., Delgado M.R. (2012). Social network modulation of reward-related signals. The Journal of Neuroscience, 32(26), 9045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. (1921). On the probable error of a coefficient of correlation deduced from a small sample. Metron, 1, 3–32. [Google Scholar]
- Gable S.L., Gosnell C.L., Maisel N.C., Strachman A. (2012). Safely testing the alarm: close others, responses to personal positive events. Journal of Personality and Social Psychology, 103(6), 963. [DOI] [PubMed] [Google Scholar]
- Harbaugh W.T., Mayr U., Burghart D.R. (2007). Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science, 316(5831), 1622–5. [DOI] [PubMed] [Google Scholar]
- Heller A. S., Reekum C. M., Schaefer S. M., et al. (2013). Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychological Science ,24(11), 2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks A.M., Diamond L.M. (2008). How was your day? Couples affect when telling and hearing daily events. Personal Relationships, 15(2), 205–28. [Google Scholar]
- Hubbard J., Harbaugh W.T., Srivastava S., Degras D., Mayr U. (2016). A general benevolence dimension that links neural, psychological, economic, and life-span data on altruistic tendencies. Journal of Experimental Psychology: General, 145(10), 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impett E.A., Gable S.L., Peplau L.A. (2005). Giving up and giving in: the costs and benefits of daily sacrifice in intimate relationships. Journal of Personality and Social Psychology, 89(3), 327. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Meyer M., et al. (2009). A key role for similarity in vicarious reward. Science, 324(5929), 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., Oliveira-Souza R., Grafman J. (2006). Human fronto–mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences ,103(42), 15623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Lee I.A., Arnn M.E., Zaki J. (2015a). Emotional and instrumental support provision interact to predict well-being. Emotion, 15(4), 484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Lieberman M.D., Zaki J. (2015b). The emerging study of positive empathy. Social and Personality Psychology Compass, 9(2), 57–68. [Google Scholar]
- Morelli S.A., Rameson L.T., Lieberman M.D. (2014). The neural components of empathy: predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience, 9(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Sacchet M.D., Zaki J. (2015c). Common and distinct neural correlates of personal and vicarious reward: a quantitative meta-analysis. NeuroImage, 112, 244–53.z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage ,31(1), 440–57. [DOI] [PubMed] [Google Scholar]
- Peysakhovich A., Nowak M.A., Rand D.G. (2014). Humans display a ‘cooperative phenotype' that is domain general and temporally stable. Nature Communications, 5, Article 4939. [DOI] [PubMed] [Google Scholar]
- Preuschoff K., Bossaerts P., Quartz S.R. (2006). Neural differentiation of expected reward and risk in human subcortical structures. Neuron, 51(3), 381–90. [DOI] [PubMed] [Google Scholar]
- Rameson L.T., Morelli S.A., Lieberman M.D. (2012). The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience, 24(1), 235–45. [DOI] [PubMed] [Google Scholar]
- Russell D.W. (1996). UCLA Loneliness Scale (version 3): reliability, validity, and factor structure. Journal of Personality Assessment, 66(1), 20–40. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Harrison P.R., Kurtz J.L., Bryant F.B. (2014). Nurturing the capacity to savor: Interventions to enhance the enjoyment of positive experiences. In A. C. Parks & S. M. Schueller (Eds.), The Wiley Blackwell Handbook of Positive Psychological. Interventions (pp. 42–65) Hoboken: Wiley Blackwell. [Google Scholar]
- Sul S., Tobler P.N., Hein G., et al. (2015). Spatial gradient in value representation along the medial prefrontal cortex reflects individual differences in prosociality. Proceedings of the National Academy of Sciences, 112(25), 7851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. (2010). Gaining while giving: an fMRI study of the rewards of family assistance among White and Latino youth. Social Neuroscience, 5(5–6), 508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Wu C.C., Samanez-Larkin G.R., Katovich K., Knutson B. (2014). Affective traits link to reliable neural markers of incentive anticipation. NeuroImage, 84, 279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


