Abstract
Background:
Cerebral microbleeds can confer a high risk of intracerebral hemorrhage, ischemic stroke, death and dementia, but estimated risks remain imprecise and often conflicting. We investigated the association between cerebral microbleeds presence and these outcomes in a large meta-analysis of all published cohorts including: ischemic stroke/TIA, memory clinic, “high risk” elderly populations, and healthy individuals in population-based studies.
Methods:
Cohorts (with > 100 participants) that assessed cerebral microbleeds presence on MRI, with subsequent follow-up (≥ 3 months) were identified. The association between cerebral microbleeds and each of the outcomes (ischemic stroke, intracerebral hemorrhage, death, and dementia) was quantified using random effects models of (a) unadjusted crude odds ratios and (b) covariate-adjusted hazard rations.
Results:
We identified 31 cohorts (n = 20,368): 19 ischemic stroke/TIA (n = 7672), 4 memory clinic (n = 1957), 3 high risk elderly (n = 1458) and 5 population-based cohorts (n = 11,722). Cerebral microbleeds were associated with an increased risk of ischemic stroke (OR: 2.14; 95% CI: 1.58–2.89 and adj-HR: 2.09; 95% CI: 1.71–2.57), but the relative increase in future intracerebral hemorrhage risk was greater (OR: 4.65; 95% CI: 2.68–8.08 and adj-HR: 3.93; 95% CI:2.71–5.69). Cerebral microbleeds were an independent predictor of all-cause mortality (adj-HR: 1.36; 95% CI: 1.24–1.48). In three population-based studies, cerebral microbleeds were independently associated with incident dementia (adj-HR: 1.35; 95% CI: 1.00–1.82). Results were overall consistent in analyses stratified by different populations, but with different degrees of heterogeneity.
Conclusions:
Our meta-analysis shows that cerebral microbleeds predict an increased risk of stroke, death, and dementia and provides up-to-date effect sizes across different clinical settings. These pooled estimates can inform clinical decisions and trials, further supporting cerebral microbleeds role as biomarkers of underlying subclinical brain pathology in research and clinical settings.
Keywords: Antithrombotic, brain microbleeds, cerebral microbleeds, cerebral small vessel disease, intracerebral hemorrahage, magnetic resonance imaging
Introduction
Cerebral microbleeds (CMBs) are small round hypoin-tense lesions detected on paramagnetic-sensitive MRI sequences, including T2*-weighted gradient-recalled echo (T2*-GRE) and susceptibility-weighted imaging (SWI).1 Although the mechanisms leading to CMBs remain elusive, results from limited histopathological correlation studies, suggest that most MR-visible lesions correspond to focal deposits of blood-breakdown products in perivascular tissue,2,3 likely representing blood leakage from microvasculopathies. CMBs have received enormous attention in the literature—the broad consensus is that they constitute biomarkers of “silent” or “subclinical” small vessel disease in the brain.1,4
In this context, much of the enduring interest in the topic relates to the implicit clinical conundrums created by the high prevalence of CMBs in many different populations.5,6 CMBs are found in up to 5–21% of the general population, 30–40% of patients with ischemic stroke, 60–68% of patients with primary ICH, and 15–25% of memory clinic patients, including Alzheimer’s disease and vascular cognitive impairment.1 In these settings, CMBs generate increasingly common clinical dilemmas due to concern that they may be a marker of future stroke (both ischemic stroke and intracerebral hemorrhage ICH) raising questions regarding optimal antithrombotic therapy.7,8 Available data also suggest that CMBs can contribute to dementia,9,10 and increase overall mortality.11,12
Several single-center cohorts have assessed the relation between CMBs and risk of stroke, dementia, and death, with partly conflicting results and wide confidence intervals (CIs). Accurate estimates of these risks are needed to inform clinical decisions, and potentially allow the incorporation of CMBs as an informative biomarker in clinical trials.13 So far, previous meta-analyses have only focused on patients with a history of ischemic stroke or TIA (but not other settings),7,14 and demonstrated that CMBs presence increases the risk of recurrent stroke (OR: 2.25; 95% CI: 1.70–2.98; p < 0.0001), either hemorrhagic or ischemic.7
Therefore, given new data in the field (through the International META-MICROBLEEDS Initiative15), we systematically reviewed and synthesized in meta-analyses all published longitudinal observational studies testing the association between CMBs with risk of ICH, ischemic stroke, dementia, and death, in the general population, high-risk populations, and in hospital-based settings (stroke/TIA and memory clinics). In addition, in the meta-anaysis syntheses for each outcome in relation to CMBs presence, we provide both unadjusted and adjusted estimates–a unique feature in the literature on this topic, which has not been attempted in the past.
Methods
The study was conducted with reference to the PRISMA,16 the MOOSE17 guidelines, and the Cochrane Handbook for Systematic Reviews of Interventions. A pre-specified summary protocol was developed in-house in January 2016 (not published or registered).
Search strategy and study selection
We searched PubMed for potentially eligible studies between 1 January 1995 and 1 March 2016, using a combination of search terms and Medical Subject Headings (MeSH): ((microbleed*) OR (microhemorrhag*) OR (microhemorrhag*) OR (“dot-like”)) AND (MRI OR SWI OR T2* OR suscept* OR hemosid*) AND ((brain OR cerebr* OR (cerebral small vessel disease) OR (vascular dementia) OR (Alzheimer disease) OR (Alzheimer’s disease) OR cognit* OR dement*)). The systematic literature search was updated on 10 February 2017. All identified citations (comprising titles, abstracts and keywords) were retrieved and imported into ABSTRACKR,18 a collaborative web-based annotation tool which utilizes interactive machine learning components for the citation screening task. We also used snowballing to screen the reference lists of all included articles, relevant review articles, meta-analyses, and author’s own files (including regular PubMed searches updates on the topic for the last six years). To identify recent studies not yet published as full papers, we searched abstract books from the following recent conferences: European Stroke Organization Conference 2014–2016 and International Stroke Conference 2014–2016. The abstracts of all papers identified from the initial searches were reviewed by two authors, who also then reviewed the full text of all eligible studies independently. The final list of included studies was decided upon consensus.
Retrospective or prospective studies (published as full papers or conference abstracts) were eligible for inclusion regardless of language if they characterized CMBs presence on MRI at baseline with subsequent follow-up for the development of future symptomatic stroke, death, or dementia. Other specific inclusion criteria were: (1) studies of at least 100 adult subjects (aged > 18 years); (2) MRI determination of CMBs at baseline using standard criteria; (3) ascertainment of the outcomes of interest after the baseline MRI during follow-up; (4) quantification of the risk for each outcome in relation to the presence CMBs. Studies including only patients with spontaneous ICH were not included in this analysis because of the different clinical significance of CMBs in this setting. For studies with more than one publication describing results among overlapping groups of participants and with the same outcome measure, we included only the dataset with the longest follow-up, or the dataset with the largest number of participants if the follow-up period was identical.
All papers from the same cohort reporting different primary outcomes of interest were included.
Outcome measures
The primary outcomes of interest were: (a) stroke, defined as an acute onset focal neurological deficit of presumed vascular cause lasting at least 24 h or interrupted by death within 24 hours, and diagnosed as (i) ischemic stroke or (ii) spontaneous intracerebral hemorrhage (presumed to be due to small vessel disease) based on standardized brain imaging criteria; (b) death of any cause; and (c) new onset dementia measured by standard criteria in each study, such as diagnostic and statistical manual of mental disorders IV (DSM IV), international classification of disease-10 (ICD-10), CDR, or a mini-mental state examination (MMSE) score of less than 24.
Data extraction and quality assessment
We classified studies as being in ischemic stroke/TIA populations, memory clinic populations, “high risk” elderly populations (i.e. if carried out in people selected for the presence of high risk factor profile at baseline) and asymptomatic individuals in a population-based setting (“general population”). For each study, we extracted information on study design, number, and nature of participants (including mean age and sex), characteristics of MRI sequences used for CMBs rating, duration of follow-up, and number of participants with the outcomes of interest per CMBs presence group. When available, adjusted estimates from multivariable models of the independent association between CMBs and the outcomes were extracted as hazard ratios. Two authors independently extracted data and disagreements were resolved by consensus.
Studies were critically appraised against an 8-item tool published by the Cochrane Methods Bias group.19
Data synthesis and statistical analysis
Data were pooled in a meta-analysis when at least two studies with relevant data per outcome were available. In all analyses, we used a random effects model with DerSimonian-Laird weights.20 First, in unadjusted analyses, we quantified the strength of the association between CMBs presence and each of the outcomes (stroke—ischemic and hemorrhagic, death, and dementia) using odds ratios (OR) and their corresponding 95% CIs, with the inverse variance method for weighting. Second, in adjusted analyses, for each of the outcomes, we pooled the covariate-adjusted HRs as provided from relevant multivariable survival analysis models in included studies, calculating pooled adjusted hazard ratios using the random effects inverse variance method. Meta-analyses were performed both separately by study setting/population, and overall. We assessed statistical heterogeneity using I-squared statistics and visually through inspection of the forest plot. Values of ≤ 25%, 25% to 50%, and ≥ 50% were defined as low, moderate, and high degrees of heterogeneity, respectively. We explored publication bias with funnel plots. For the unadjusted analyses, we used meta-regression to explore whether certain key baseline characteristics of the included patient populations could have affected our results in a random-effect univariable meta-regression analyses. Meta-analyses were performed using Stata13.0 (StataCorp LP, Texas).
Results
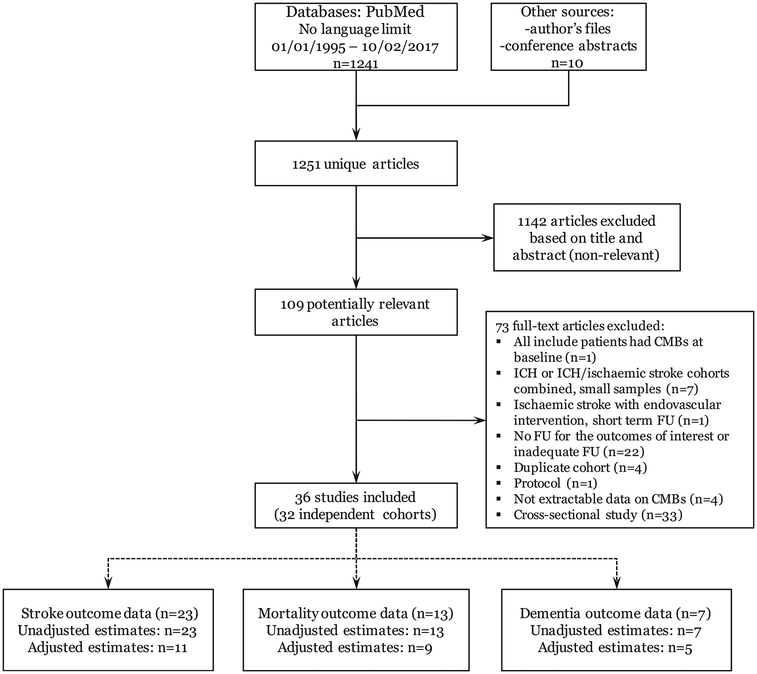
A total of 1251 titles and abstracts were screened, of which 36 met the inclusion criteria and were pooled in meta-analyses (Figure 1). These reported data from 31 independent cohorts. Some cohorts reported different outcomes in separate papers, while some studies contained data on more than one outcome in a single publication. In summary, we included 20 studies of ischemic stroke/TIA patients (19 cohorts, n = 7672),11,21–39 4 studies of memory clinic patients (4 cohorts, n = 1957),40–43 4 studies in high-risk elderly populations (3 cohorts, n = 1458)44–47 and 8 population-based studies of healthy elderly participants (5 cohorts, n = 11,722).12,48–54 Table 1 highlights key baseline and methodological characteristics and outcomes available in the included studies. No evidence of publication bias was identified for any of the outcomes and analyses (Egger’s test p > 0.3). Studies published as conference abstracts (except for the stroke outcomes in the Framingham Heart Study and AGES Reykjavik Study) at the time of the initial literature search were then published as full papers55–58 and identified in our ongoing real-time search strategy as part of the META-MICROBLEEDS Initiative.
Figure 1.
Flow chart of study identification and selection process.
Table 1.
Participant characteristics and methodological aspects of included studies
| Study | Country(study period) | Setting/cohort design | Cases number (%male) | Age (mean) | HTN (%) | DM (%) | Antiplat. (%) | Anticoags (%) | MRI parameters | Average FU (mo) | Person-years of FU | Outcomes | FU method | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence | Field strength (Tesla) | Echo time (ms) | |||||||||||||
| Ischemic stroke/TIA cohorts | |||||||||||||||
| Fan et al.21 | China (1999–2000) | Prospective | 121 (68%) | 68 | 69% | 32% | 80% | 6% | T2*-GRE | 1.5 | 30 | 28 | 227 | Stroke, Death | Telephone In person Notes |
| Imaizumi et al.22 | Japan (1999–2003) | Prospective | 138 (66%) | 66 | 73% | - | 33% | 2% | T2*-GRE | 1.5 | 26 | 22 | - | Stroke | Telephone Notes |
| Boulanger et al.23 | Canada (2002–2004) | Prospective | 236 (55%) | - | 60% | 55% | - | - | T2*-GRE | 3 | 20/45 | 18 | 275 | Stroke, Death | Telephone In person |
| Naka et al.24 | Japan (2002–2004) | Prospective | 183 (63%) | 67 | 70% | 26% | 93 | 2 | T2*-GRE | 1 | 26 | 18 | - | Stroke | In person |
| Huang et al.25 | China (2004) | RCT | 636 (68%) | 60 | 67 | 68 | 100% | 0% | T2*-GRE | 1.5 | NA | 14 | 740 | Stroke | In person |
| Soo et al.26 | China (1999–2004) | Prospective | 908 (58%) | 68 | 68% | 32% | 93% | 3% | T2*-GRE | 1.5 | 30 | 26.6 | 2013 | Stroke, Death (stroke) | In person |
| OXVASC27,28 | UK (2000–2008) | Prospective | 291 (51%) | 66 | 63% | - | 33% | 4% | T2*-GRE | 1.5 | 95/14 | 35 | - | Stroke | In person |
| Thijs et al.29 | Belgium (2003–2005) | Prospective | 487 (61%) | 72 | 64% | 19% | 32% | T2*-GRE | 1.5/3 | 35/26/16 | 26 | 1071 | Stroke | In person Telephone Notes | |
| Song et al.30 | South Korea (2005–2012) | Retrospective (AF) | 550 (59%) | 71 | 77% | 25% | 46% | 96% | T2*-GRE | 3 | 16 | 37 | - | Stroke Death (stroke) | Telephone Notes Coding data |
| Fluri et al.31 | Switzerland (2006–2008) | Prospective (TIA) | 176 (61%) | 71 | 72% | 185 | 77% | 12% | T2*-GRE | 1.5 | 15 | 3 | - | Stroke | In person |
| Imaizumi et al.32 | Japan (2004–2010) | Prospective | 562 | 71 | 64% | - | 75% | 18% | T2*-GRE | 1.5 | 26 | 31 | - | Stroke | Telephone Notes |
| Song et al.11 | South Korea (2004–2010) | Retrospective (AF) | 504 (57%) | 70 | 78% | 25% | - | 97% | T2*-GRE | 3 | 16 | 30 | 1260 | Death | Coding data |
| Kwa et al.33 | Netherlands (2000–2010) | Prospective | 397 (59%) | 68 | 27% | 14% | 25% | 10% | T2*-GRE | 1.5 | 27.6 | 46 | 1509 | Stroke, Death |
Telephone Notes |
| Orken et al.34 | Turkey (2009–2013) | Retrospective (AF) | 204 (57%) | 69 | 88% | 25% | 27% | 100% | T2*-GRE | 1.5 | 15 | 24 | - | Stroke | In person Notes |
| Horstmann et al.35 | Germany (2009–212) | Prospective | 265 (67%) | 65 | 80% | - | 78% | 20% | SWI | 3 | 19.7 | 12 | 265 | Stroke | In person Notes |
| Shoamanesh et al.36,55 | Multicenter | RCT (SPS3) (Lacunar) | 1278 (65%) | 63 | 75% | 36% | 100% | 0% | T2*-GRE | 1.5/3 | - | 40 | - | Stroke, Death | In person Notes |
| Haji et al.37 | USA (2008–2014) | Prospective | 117 | 80 | 87% | - | 67% | 74% | T2*-GRE | 1.5 | 20/15/24 | 28.8 | 322 | Stroke, Death | In person Written survey Notes |
| Lim et al.38 | South Korea (2010–2012) | Prospective (TIA) | 500 (58%) | 65 | 67% | 30% | 91% | 15% | T2*-GRE | - | 15–25 | 3 | 123 | Stroke (early) | In person Telephone |
| Charidimou et al.39 | Japan (2008–2012) | Prospective (AF) | 119 (54%) | 76 | 71% | 28% | 42% | 86% | T2*-GRE | 1.5 | 26 | 17 | - | Stroke | In person Telephone Notes |
| High-risk elderly cohorts | |||||||||||||||
| Altmann-Schneider et al.44 | Netherlands | Prospective (PROSPER) | 434 (56%) | 75 | 64% | 15% | - | - | T2*-GRE | 1.5 | 48 | 84 | - | Death | Coding data |
| van der Holst et al.45 | Netherlands (2006–2014) | Prospective (RUN DMC) | 503 (57%) | 66 | 73% | 13% | - | - | T2*-GRE | 1.5 | 26 | 94 | 3923 | Death | Coding data Notes |
| Miwa et al.46 | Japan (2001–2009) | Prospective (OSACA2) | 524 (58%) | 68 | 83% | 23% | T2*-GRE | 1.5 | 20 | 90 | 3930 | Dementia | In person (MMSE < 24, > 3 decline, or CDR > 1, DSM-III-R) | ||
| van Uden et al.47 | Netherlands (2006–2012) | Prospective (RUN DMC) | 500 (57%) | 66 | T2*-GRE | 1.5 | 20 | 62.4 | Dementia | In person Notes (MMSE < 26, > 3 decline, or MINI) | |||||
| Memory clinic cohorts | |||||||||||||||
| Benedictus et al.40 | Netherlands (2002–2009) | 333 (58%) | 71 | 23% | 6% | 37% | T2*-GRE | I/I.5/3 | - | 36 | - | Stroke Death |
|||
| Prospective (1:2 matched) (MISTRAL) | Coding data Questionnaires to GPs | ||||||||||||||
| Benedictus et al.41 | Netherlands (2000–2013) | Prospective (Amsterdam Dementia Cohort) | 334 (53%) (with SCD) | 62 | 31% | 8% | T2*-GRE | I/I.5/3 | 36 | 1002 | Dementia or MCI | In person (Neuropsychology and MMSE) | |||
| Henneman et al.42 | Netherlands (1993–2006) | Prospective | 1138 (55%) | 66 | 25% | 8% | - | - | T2*-GRE | 1.5 | 15–22 | 31 | 2959 | Death | Questionnaires to GPs Notes |
| Staekenborg et al.43 | Netherlands | Prospective | 152 (27%) (with MCI) | 72 | T2*-GRE | 1 | 22 | 25 | 334 | Dementia | In person (NINCDS-ADRDA and NINDS-AIREN) | ||||
| Asymptomatic elderly general population cohorts | |||||||||||||||
| Nishikawa et al.48 | Japan (2003–2004) | Prospective | 698 (46%) | 67 | 43% | 12% | 18% | T2*-GRE | 1.5 | 23 | 42 | - | Stroke | In person? Admissions/Notes | |
| Bokura et al.49 | Japan (2001–2007) | Prospective | 2238 | 62 | - | - | - | - | T2*-GRE | 1.5 | 25 | 43 | - | Stroke | Patient questionnaires Telephone |
| Akoudad et al.50 | Netherlands (2005–2013) | Prospective (Rotterdam study) | 4759 (45%) | 64 | 61% | 9% | 27% | T2*-GRE | 1.5 | 31 | 59 | 23319 | Stroke | Automated linkage of GP medical records and coding data | |
| Akoudad et al.12 | Netherlands (2005–2009) | Prospective (Rotterdam study) | 4841(45%) | 64 | T2*-GRE | 1.5 | 31 | 62 | Death | GP automatic updates Coding data Notes |
|||||
| Akoudad et al.51 | Netherlands (2002–2014) | Prospective (Rotterdam study) | 3257 (45%) (dementiafree) | 60 | T2*-GRE | 1.5 | 31 | 58 | Dementia | GP automatic updates Coding data In person (NINCDS-ADRDA, DSMMD-3) |
|||||
| Romero et al.52,53,57,58 | USA | Prospective (Framingham heart study) | 1296 (46%) (dementia-free) I963 (46%) |
72 | 50% 56% |
13% 14% |
30% 29% |
4% 4% |
T2*-GRE | 1.5 | 26 | 80 86 |
8687 14129 |
Dementia Stroke Deatha | In person Notes |
| AGES Reykjavik Study54,56 | Iceland (2002–2015 | Prospective (AGES Reykjavik Study) | 1982 (42%) 4206 (41%) |
76 | 80% | 11% | 21% | 7% | T2*-GRE | 1.5T | 50 ms | 111.6 | 43904 38620 |
Dementia Stroke Death | Icelandic National Roster maintained by
Statistics Iceland Linkage of adjudicated stroke registries and hospital medical records with study database |
MINI: mini international neuropsychiatric interview; SCD: subjective cognitive decline; MRI: magnetic resonance imaging; T2*-GRE: T2*-weighted gradient-recalled echo; SWI: susceptibility-weighted imaging.
First row corresponds to characteristics of participants included in the dementia outcome analysis; second row corresponds to the stroke/mortality outcomes.
Note: National Institute on Neurological and Communicative Diseases and Stroke-Alzheimer Disease and Related Disorders Association. VaD was diagnosed by National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche´ et l’Enseignement en Neurosciences (NINDS-AIREN).
CMBs and risk of ICH and ischemic stroke
Nineteen studies of ischemic stroke/TIA patients (n = 7672),11,21–39 one memory clinic cohort (n = 333),40 and five population-based studies (n = 13,864)48–50,52 examined the relation between CMBs presence and risk of ICH and ischemic stroke.
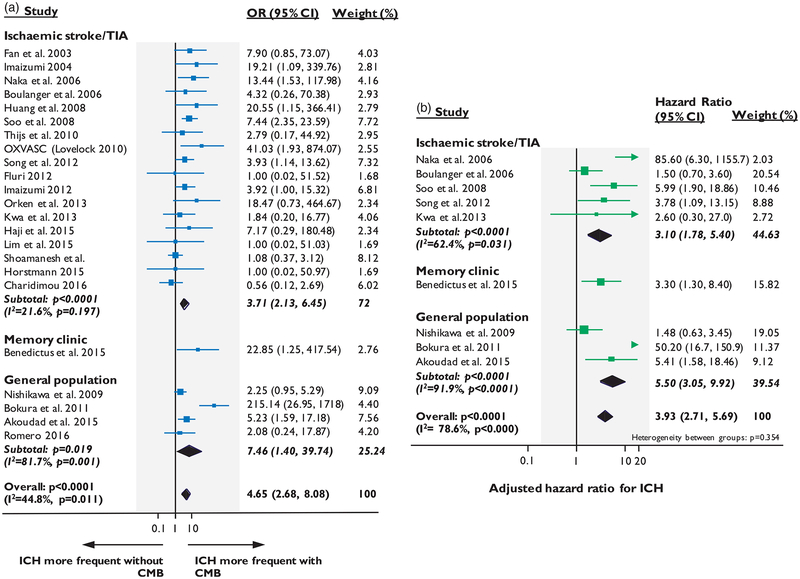
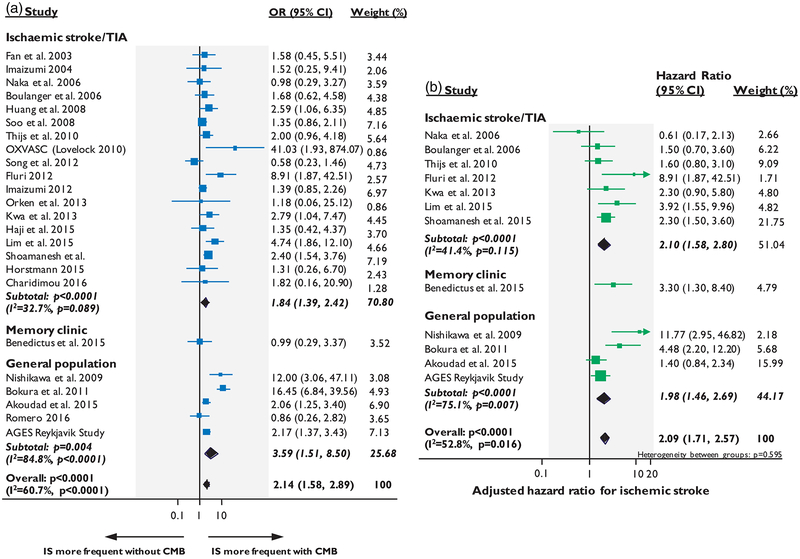
In the pooled analyses of patients with ischemic stroke/TIA, CMBs presence (vs. no CMBs) was associated with an increased crude risk of ICH (OR: 3.71; 95% CI: 2.13–6.45, p < 0.0001) (Figure 2(a)) and recurrent ischemic stroke (OR: 1.84; 95% CI: 1.39–2.42, p < 0.0001) (Figure 3(a)) during follow-up. Nine cohorts (n = 4715) provided adjusted estimates for CMBs and the risk of future stroke: five for symptomatic ICH (n = 2274)23,24,26,33,59 and seven for ischemic stroke (n = 3257). 23,24,29,31,33,36,38 In the pooled analysis of these cohorts that provided adjusted estimates, CMBs presence was independently associated with increased risk of ICH (adj-HR: 3.10; 95% CI: 1.78–5.40, p < 0.0001) (Figure 2(b)), but the increase in the risk for future ischemic stroke was relatively lower (adj-HR: 2.10; 95% CI: 1.58–2.80, p < 0.0001), with intermediate degree of statistical heterogeneity (Figure 3(b)).
Figure 2.
Forest plots of the association between CMBs presence and risk of spontaneous ICH during follow-up. Meta-analysis performed using a random effects model, with crude odds ratios pooled in (a) and adjusted-hazard ratios pooled in (b). Weights are shown by the point estimate area.
Figure 3.
Forest plots of the association between CMBs presence and risk of ischemic stroke. Meta-analysis performed using a random effects model, with crude odds ratios pooled in (a) and adjusted-hazard ratios pooled in (b). Weights are shown by the point estimate area.
In the meta-analysis of stroke-free individuals from large population-based studies, CMBs presence was associated with incident ICH (OR: 7.46; 95% CI:1.40–39.74, p = 0.019) and ischemic stroke risk (OR: 3.59 95% CI: 1.51–8.50, p = 0.004), but with high degree of statistical heterogeneity. Four of these population-based cohorts provided adjusted estimates (n = 7695),48–50 while for the fifth one (i.e. Framingham Heart study),53 the number of incident stroke events was too low to allow for multivariable survival analysis. In a subgroup analysis of these studies, CMBs remained an independent predictor of incident ICH (adj-HR: 5.50; 95% CI: 3.05–9.92, p < 0.0001) and, with lower effect size, ischemic stroke (adj-HR: 1.98; 95% CI: 1.46–2.69, p < 0.0001), again with high statistical heterogeneity.
The overall meta-analysis combining data from all populations yielded a significant association of CMBs presence with future ICH and ischemic stroke in both crude and adjusted analyses, with moderate degree of statistical heterogeneity (Figures 2 and 3). The relative risks were overall higher for ICH than ischemic stroke.
CMBs and mortality
Six studies in ischemic stroke/TIA patients (n = 3257),11,21,23,33,37 two memory clinic cohorts (n = 1471),40,42 two studies in high-risk elderly populations (n = 937),44,45 and three population-based studies (n = 8768)12,53,54 investigated the relation between CMBs and all-cause mortality.
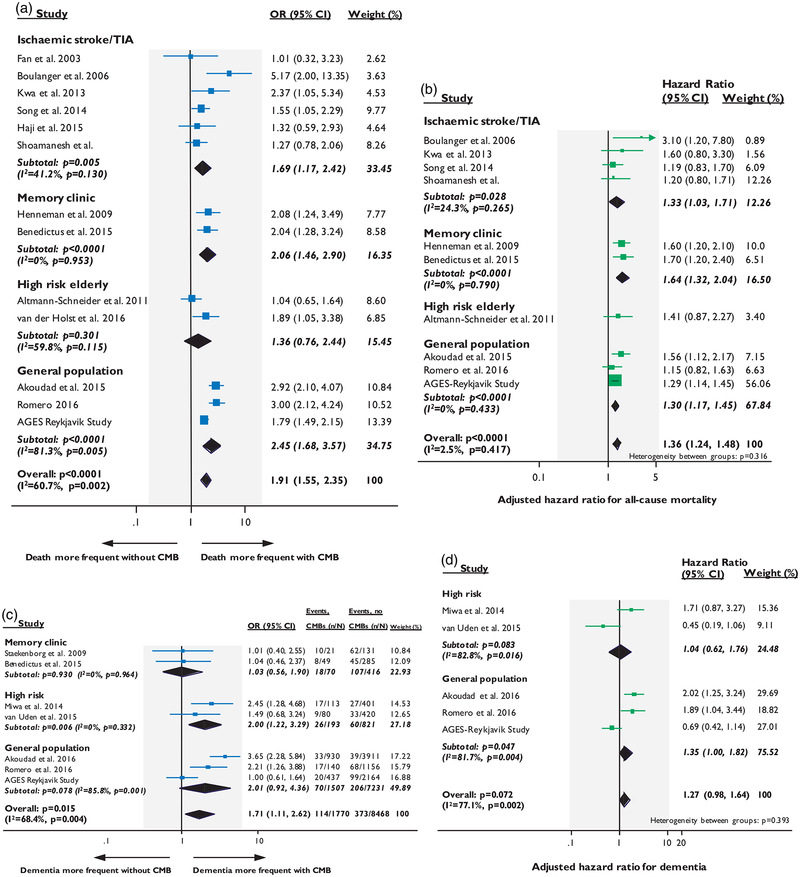
In ischemic stroke/TIA cohorts, CMBs presence was associated with all-cause mortality both in the crude analysis (OR: 1.69; 95% CI: 1.17–2.42, p = 0.005) and in adjusted meta-analysis of four studies (n = 2415)11,23,33 providing relevant data (adj-HR: 1.33; 95% CI: 1.03–1.71, p = 0.028). A similar effect size was found in the two studies of memory clinic cohorts in both unadjusted and adjusted analyses (Figure 4(a) and (b)). There was no relation between CMBs and mortality in high-risk elderly cohorts (Figure 4(a)). When studies from the four population-based studies were pooled, CMBs presence was associated with an increased risk of death during follow-up (OR: 2.45; 95% CI: 1.68–3.57, p < 0.0001, with high statistical heterogeneity) and remained an independent predictor in adjusted meta-analysis (adj-HR: 1.30; 95% CI:1.17–1.45, p < 0.0001, with no evidence of statistical heterogeneity) (Figure 4(a) and (b)).
Figure 4.
Forest plots of the association between CMBs presence and mortality (a–b) and dementia (c–d). Meta-analysis were performed using random effects models, with crude odds ratios for all-cause mortality pooled in (a) and adjusted-hazard ratios pooled in (b). Crude odds ratios for all-cause dementia were pooled in (c) and adjusted-hazard ratios pooled in (d). Weights are shown by the point estimate area.
In the overall meta-analysis including all studies across different populations, CMBs presence was an independent predictor of all-cause mortality during follow-up (adj-HR: 1.36; 95% CI: 1.24–1.48, p < 0.0001, with no evidence of statistical heterogeneity) (Figure 4(b)).
CMBs and risk of incident dementia
Two memory clinic cohorts (n = 486),41,43 two studies in high-risk elderly populations (n = 1024)46,47 and three population-based studies (n = 6535)51,52,54 provided prospective data on the relation between CMBs presence and incident dementia overall. The studies used different but validated methods to assign a dementia diagnosis during follow-up (Table 1).
In the two memory clinic studies, CMBs presence was not associated with dementia during follow-up in the crude analysis (Figure 4(c)). Of note, these two studies also included patients with mild cognitive impairment at baseline and no adjusted estimates could be extracted. In the two studies in high-risk elderly populations, CMBs presence at baseline was associated with 2-fold risk of dementia in the crude meta-analysis (OR: 2.00; 95% CI: 1.22–3.29, p = 0.006), but this effect was not sustained in the adjusted meta-analysis (adj-HR: 1.04; 95% CI: 0.62–1.76, p = 0.083, with high statistical heterogeneity) (Figure 4(d)).
Meta-analysis of the three population-based studies (Rotterdam Study,51 Framingham Heart study52 and AGES Reykjavik Study54), which included dementia-free participants at baseline, yielded a trend toward crude association between CMBs presence and incident dementia, with high degree of statistical heterogeneity (OR: 2.01; 95% CI: 0.92–4.36, p = 0.078) (Figure 4(c)). However, in adjusted meta-analysis (Figure 4(d)), CMBs were independently associated with marginally increased risk of all-cause incident dementia, but with high statistical heterogeneity (adj-HR: 1.35; 95% CI:1.00–1.82, p = 0.047). Data on dementia subtype were limited and hence not pooled in a meta-analysis.
Discussion
CMBs are often incidentally detected on MRI in various populations and clinical settings raising clinical dilemmas about the optimal management of patients.4 In this systematic review, we brought together data on clinical relevance of CMBs involving > 22,000 participants in total. Our meta-analyses provide evidence that CMBs are an important indicator of future disease, including ICH, ischemic stroke, death, and dementia, but with different effect sizes, degree of certainty, and generalizability. The current paper thus provides the most up-to-date estimates, including-for the first time-adjusted analyses, on the clinical relevance of CMBs based on the totality of evidence from longitudinal cohorts. Few evidence-based guidelines exist on how to best manage patients with incidentally found CMBs,4 partly due to the paucity of evidence from large prospective cohorts and the lack of randomized trials. Accordingly, data from comprehensive meta-analyses are thus the most informative available approach for providing actionable information.
CMBs were significantly associated with an increased risk of stroke, both ICH and ischemic stroke, reinforcing the notion that they are a marker of subclinical cerebrovascular disease. In patients with a previous ischemic stroke/TIA, we found that the presence of CMBs conferred a ≈4-fold increased risk of subsequent ICH and ≈2-fold higher risk of recurrent ischemic stroke. These results are in line with previous meta-analyses on the topic,14 but we have increased our sample size by > 40%, and statistical power by including more outcome events, resulting in more precise estimates. It could be argued that this association between CMBs and future stroke in confounded by shared vascular risk factors, such as age and hypertension with both CMBs and future stroke.1 Indeed, this has been a valid criticism of all crude, unadjusted meta-analyses on CMBs. In the adjusted pooled analyses, however, CMBs presence remained a significant predictor of future stroke risk after taking into account potential confounders, including vascular risk factors, in studies providing relevant data. Of note, we observed an approximate 3-fold increase of the independent risk of ICH in the presence of CMBs and a doubling of the independent risk for recurrent ischemic stroke. Two points deserve special notice in these adjusted estimates. First, it seems that CMBs increase the risk of subsequent stroke relatively higher towards ICH rather than ischemic stroke, but more data on absolute risk ratios are needed. Secondly, the overall independent risk of ICH conferred by CMBs reported here (when various other risk factors are accounted for), is in general lower than previously assumed based on individual estimates from small studies or unadjusted meta-analyses (OR/RR ≈6–8).4,7,14 It is possible that the independent ICH risk when > 5 CMBs are detected might also be lower than reported when various confounders are taken into account. This finding can have implications for anticoagulation use in patients with CMBs, a thorny clinical dilemma.
Of note, the abovementioned overall considerations also apply for stroke-free individuals from large population-based studies included in our analysis. We found that CMBs are also associated with an increased risk of incident stroke, in particular ICH, in community-dwelling elderly without a prior stroke history. However, the elevated adjusted-HR for future ICH (≈5-fold) in population-based studies represented a relatively low absolute event rate: no more than ≈2–4 incident ICHs per 1000 person-years among CMB-positive participants.50 There was high statistical heterogeneity in the pooled estimates, likely reflecting the low even rate, different baseline characteristics of included populations, and methodological variation of the studies (Table 1). These studies found a consistent association between CMBs and risk of stroke and provide valuable epidemiological data to strengthen the notion that these lesions mark progression of silent cerebrovascular pathology. Nevertheless, the clinical relevance of CMBs in healthy elderly populations is uncertain and likely limited, since routine MRI screening is not generally performed in this setting.
CMBs presence was significantly associated with an increased risk of death during follow-up. This relationship was consistent in all included populations and settings, with similar effect size and no heterogeneity and maintained in adjusted analyses. The association with mortality could be plausibly partly mediated by an increased risk of stroke and dementia in patients with CMBs11 but this requires further research. The association with mortality likely reflects CMBs capacity as a surrogate marker for severe diffuse vascular pathology and frailty, as well as disease-associated vascular risk factors, rather than a direct causal relationship.1
We found limited data on the relation of CMBs to new-onset dementia risk during follow-up. Most available studies to date have been cross-sectional, were carried out in small patient populations and evaluated cognitive function using different instruments.9 A meta-analysis reported that CMBs were associated with cognitive dysfunction in two studies (OR: 3.06; 95% CI: 1.59–5.89) and lower cognitive function in three other studies (standardized mean difference: −1.06, 95% CI: 2.10 to −0.02) based on the MMSE or the Montreal cognitive assessment scale (MoCA).9 Another meta-analysis found no significant difference in the cognitive performance of Alzheimer’s disease patients with versus without CMBs.60 This is in line with the absence of any longitudinal relation between CMBs and incident dementia in memory clinic patients in our analysis, since presentation to a memory clinic indicates roughly the same of the mix of neurodegeneration and vascular injury, and has similar risk for progressing to dementia. The most pertinent and epidemiologically robust data on CMBs effect on dementia risk are those from general population samples. In the three major population-based studies (Rotterdam Study,51 Framingham Heart study52 and AGES Reykjavik Study54) pooled in our analysis, CMBs presence was independently associated with incident dementia risk, but the association was marginal statistically and with considerable degree of heterogeneity. However, our analysis primarily focused on the presence/absence of CMBs. In the recent publication of AGES Reykjavik study, having ≥ 3 CMBs was associated with a higher incidence of dementia.56 Whether the mechanism of the link between CMBs and dementia is direct and independent of other pathologies in the ageing brain, or simply reflect more severe small vessel damage, remains speculative.51 Most likely, CMBs represent a surrogate of diffuse cerebral micro-vascular damage, and hence their presence influences dementia risk only indirectly.51
Several limitations of our study are important to consider. First, our crude meta-analyses used unadjusted OR which are prone to bias introduced by the different populations and methodology (including MRI parameters for CMBs detection) in included studies. For example, imaging protocols and CMBs analysis were similar but not entirely uniform; most studies were performed at 1.5 T with echo times within a narrow optimal range, making this factor unlikely to influence our conclusions. To account for various confounding effects, we also present pooled adjusted estimates. However, covariate-adjusted HR was not available in all studies resulting in residual confounding. The largest studies with adequate outcome events were more likely to present multivariable analyses. Of note, in all adjusted analyses, the sample size was > 1500 subjects, which is the pre-specified sample of large ongoing studies in the field.61 Second, given the variability in follow-up time between studies, calculation of absolute outcome event rates was not possible. We acknowledge that there is likely substantial heterogeneity among the various subjects classified as CMBs positive, including different CMBs burden and distribution per subject. In turn, the CMBs distribution reflects different types of cerebral small vessel diseases with intrinsically distinct risk for the outcomes we studied. Cerebral amyloid angiopathy is typically associated with multiple CMBs in strictly lobar brain regions, whereas non-amyloid-related microangiopathies (including the vascular risk factor driven process of arteriolosclerosis) commonly lead to CMBs in deep distribution.62 Finally, given the strong association of vascular risk factors, other small vessel disease MRI markers and antithrombotic drugs during follow-up both with CMBs and with the clinical outcomes we studied, it would be important to dissect the modifying effect of these risk factors on the reported associations.
Despite limitations, our comprehensive meta-analysis significantly illuminates the understanding of the clinical relevance of CMBs in terms of future stroke, death, and dementia risk. It generally supports that the discovery of CMBs should prompt detailed screening for risk factors of stroke and dementia and recommendations regarding aggressive measures of prevention. The pooled estimates presented, based on large sample sizes, can inform clinical decision-making guidelines on increasingly common dilemmas posed by CMBs, clinical trials in the field and patient counseling.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to acknowledge NIA grant AG054076 and NINDS grant NS017950 and the Framingham study (supported by the National Heart, Lung, and Blood Institute (contract no. N01-HC-25195 and no. HHSN268201500001I)) for sharing data.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoamanesh A, Kwok CS and Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 2011; 32: 528–534. [DOI] [PubMed] [Google Scholar]
- 3.van Veluw SJ, Biessels GJ, Klijn CJ, et al. Heterogeneous histopathology of cortical microbleeds in cerebral amyl-oid j iopathy. Neurology 2016; 86: 867–871. [DOI] [PubMed] [Google Scholar]
- 4.Smith EE, Saposnik G, Biessels GJ, et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e44–e71. [DOI] [PubMed] [Google Scholar]
- 5.Fisher M Cerebral microbleeds: where are we now? Neurology 2014; 83: 1304–1305. [DOI] [PubMed] [Google Scholar]
- 6.Cordonnier C, Al-Shahi Salman R and Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007; 130: 1988–2003. [DOI] [PubMed] [Google Scholar]
- 7.Charidimou A, Kakar P, Fox Z, et al. Cerebral micro-bleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke 2013; 44: 995–1001. [DOI] [PubMed] [Google Scholar]
- 8.Charidimou A, Shakeshaft C and Werring DJ. Cerebral microbleeds on magnetic resonance imaging and anticoagulant-associated intracerebral hemorrhage risk. Front Neurol 2012; 3: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei C, Lin S, Tao W, et al. Association between cerebral microbleeds and cognitive function: a systematic review. J Neurol Neurosurg Psychiatry 2013; 84: 693–697. [DOI] [PubMed] [Google Scholar]
- 10.Charidimou A, Jager HR and Werring DJ. Cerebral microbleed detection and mapping: principles, methodological aspects and rationale in vascular dementia. Exp Gerontol 2012; 47: 843–852. [DOI] [PubMed] [Google Scholar]
- 11.Song TJ, Kim J, Song D, et al. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology 2014; 83: 1308–1315. [DOI] [PubMed] [Google Scholar]
- 12.Akoudad S, Ikram MA, Koudstaal PJ, et al. Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol 2013; 28: 815–821. [DOI] [PubMed] [Google Scholar]
- 13.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson D, Charidimou A, Ambler G, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology 2016; 87: 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charidimou A, Soo Y, Heo JH, et al. A call for researchers to join the META-MICROBLEEDS Consortium. Lancet Neurol 2016; 15: 900. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 18.Byron CW, Small K, Brodley EC, et al. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. In: Proceedings of the ACM International Health Informatics Symposium (IHI), Miami, FL, USA, January 28–30, 2012, pp.819–824. ACM New York, NY, USA. [Google Scholar]
- 19.Cochrane_Methods_Bias_Group. Tool to assess risk of bias in cohort studies, http://bmgcochraneorg/sites/bmgcochraneorg/files/uploads/Tool to Assess Risk of20Bias in Cohort Studiespdf (accessed 18 August 2015).
- 20.DerSimonian R and Laird N. Meta-analysis in clinical trials. Controll Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21.Fan YH, Zhang L, Lam WW, et al. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke 2003; 34: 2459–2462. [DOI] [PubMed] [Google Scholar]
- 22.Imaizumi T, Horita Y, Hashimoto Y, et al. Dotlike hemosiderin spots on T2*-weighted magnetic resonance imaging as a predictor of stroke recurrence: a prospective study. J Neurosurg 2004; 101: 915–920. [DOI] [PubMed] [Google Scholar]
- 23.Boulanger JM, Coutts SB, Eliasziw M, et al. Cerebral microhemorrhages predict new disabling or fatal strokes in patients with acute ischemic stroke or transient ischemic attack. Stroke 2006; 37: 911–914. [DOI] [PubMed] [Google Scholar]
- 24.Naka H, Nomura E, Takahashi T, et al. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. Am J Neuroradiol 2006; 27: 830–835. [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Cheng Y, Wu J, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol 2008; 7: 494–499. [DOI] [PubMed] [Google Scholar]
- 26.Soo YO, Yang SR, Lam WW, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol 2008; 255: 1679–1686. [DOI] [PubMed] [Google Scholar]
- 27.Lovelock CE and Rothwell PM. Cerebral microbleeds are associated with a greater risk of future intracerebral haemorrhage than ischaemic stroke: population-based study and meta-analysis. In: European stroke conference, Hamburg, Germany, 24–27 May 2011, p.34 Cerebrovascular Diseases 2011; 31 (Suppl. 2). [Google Scholar]
- 28.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 2010; 41: 1222–1228. [DOI] [PubMed] [Google Scholar]
- 29.Thijs V, Lemmens R, Schoofs C, et al. Microbleeds and the risk of recurrent stroke. Stroke 2010; 41: 2005–2009. [DOI] [PubMed] [Google Scholar]
- 30.Song TJ, Kim J, Lee HS, et al. The frequency of cerebral microbleeds increases with CHADS(2) scores in stroke patients with non-valvular atrial fibrillation. Eur J Neurol 2013; 20: 502–508. [DOI] [PubMed] [Google Scholar]
- 31.Fluri F, Jax F, Amort M, et al. Significance of micro-bleeds in patients with transient ischaemic attack. Eur J Neurol 2012; 19: 522–524. [DOI] [PubMed] [Google Scholar]
- 32.Imaizumi T, Inamura S, Kohama I, et al. Antithrombotic drug uses and deep intracerebral hemorrhages in stroke patients with deep cerebral microbleeds. J Stroke Cerebrovasc Dis 2013; 22: 869–875. [DOI] [PubMed] [Google Scholar]
- 33.Kwa VI, Algra A, Brundel M, et al. Microbleeds as a predictor of intracerebral haemorrhage and ischaemic stroke after a TIA or minor ischaemic stroke: a cohort study. BMJ Open 2013; 3: BMJ Open 2013 3: 1–6. doi: 10.1136/bmjopen-2013-002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orken DN, Uysal E, Timer E, et al. New cerebral micro-bleeds in ischemic stroke patients on warfarin treatment: two-year follow-up. Clin Neurol Neurosurg 2013; 115: 1682–1685. [DOI] [PubMed] [Google Scholar]
- 35.Horstmann S, Mohlenbruch M, Wegele C, et al. Prevalence of atrial fibrillation and association of previous antithrombotic treatment in patients with cerebral microbleeds. Eur J Neurol 2015; 22: 1355–1362. [DOI] [PubMed] [Google Scholar]
- 36.Shoamanesh A, Pearce LA, Bazan C, et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Annals of Neurology 2017; 82: 196–207. DOI: 10.1002/ana.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haji S, Planchard R, Zubair A, et al. The clinical relevance of cerebral microbleeds in patients with cerebral ischemia and atrial fibrillation. J Neurol 2016; 263: 238–244. [DOI] [PubMed] [Google Scholar]
- 38.Lim JS, Hong KS, Kim GM, et al. Cerebral microbleeds and early recurrent stroke after transient ischemic attack: results from the korean transient ischemic attack expression registry. JAMA Neurol 2015; 72: 301–308. [DOI] [PubMed] [Google Scholar]
- 39.Charidimou A, Inamura S, Nomura T, et al. Cerebral microbleeds and white matter hyperintensities in cardioembolic stroke patients due to atrial fibrillation: single-centre longitudinal study. J Neurol Sci 2016; 369: 263–267. [DOI] [PubMed] [Google Scholar]
- 40.Benedictus MR, Prins ND, Goos JD, et al. Microbleeds, mortality, and stroke in Alzheimer disease: the MISTRAL study. JAMA Neurol 2015; 72: 539–545. [DOI] [PubMed] [Google Scholar]
- 41.Benedictus MR, van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke 2015; 46: 2661–2664. [DOI] [PubMed] [Google Scholar]
- 42.Henneman WJ, Sluimer JD, Cordonnier C, et al. MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke 2009; 40: 492–498. [DOI] [PubMed] [Google Scholar]
- 43.Staekenborg SS, Koedam EL, Henneman WJ, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke 2009; 40: 1269–1274. [DOI] [PubMed] [Google Scholar]
- 44.Altmann-Schneider I, Trompet S, de Craen AJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke 2011; 42: 638–644. [DOI] [PubMed] [Google Scholar]
- 45.van der Holst HM, van Uden IW, Tuladhar AM, et al. Factors associated with 8-year mortality in older patients with cerebral small vessel disease: the Radboud University Nijmegen Diffusion Tensor And Magnetic Resonance Cohort (RUN DMC) Study. JAMA Neurol 2016; 73: 402–409. [DOI] [PubMed] [Google Scholar]
- 46.Miwa K, Tanaka M, Okazaki S, et al. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology 2014; 83: 646–653. [DOI] [PubMed] [Google Scholar]
- 47.van Uden IW, van der Holst HM, Tuladhar AM, et al. White matter and hippocampal volume predict the risk of dementia in patients with cerebral small vessel disease: the RUN DMC Study. J Alzheimers Dis 2015; 49: 863–873. [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa T, Ueba T, Kajiwara M, et al. Cerebral micro-bleeds predict first-ever symptomatic cerebrovascular events. Clin Neurol Neurosurg 2009; 111: 825–828. [DOI] [PubMed] [Google Scholar]
- 49.Bokura H, Saika R, Yamaguchi T, et al. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke 2011; 42: 1867–1871. [DOI] [PubMed] [Google Scholar]
- 50.Akoudad S, Portegies ML, Koudstaal PJ, et al. Cerebral microbleeds are associated with an increased risk of stroke: the Rotterdam Study. Circulation 2015; 132: 509–516. [DOI] [PubMed] [Google Scholar]
- 51.Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 2016; 73: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero JR, Preis SR, Beiser A, et al. Cerebral micro-bleeds and cognition: the Framingham Heart Study. Stroke 2015; 46: ATP412. [Google Scholar]
- 53.Romero JR, Preis SR, Beiser A, et al. Cerebral micro-bleeds as predictors of mortality: the Framingham Heart Study. Stroke 2017; 48: 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding D, Sigurðsson S, Jónsson PV, et al. Cerebral micro-bleeds are associated with cognitive decline and a higher incidence of dementia: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. In: 5th international CAA conference, Boston, MA, USA, 8–10 Septeber, 2016. [Google Scholar]
- 55.Shoamanesh A, Pearce LA, Bazan C, et al. Microbleeds in the secondary prevention of small subcortical strokes trial: stroke, mortality, and treatment interactions. Ann Neurol 2017; 82: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding J, Sigurethsson S, Jonsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology 2017; 88: 2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romero JR, Beiser A, Himali JJ, et al. Cerebral micro-bleeds and risk of incident dementia: the Framingham Heart Study. Neurobiol Aging 2017; 54: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero JR, Preis SR, Beiser A, et al. Cerebral micro-bleeds as predictors of mortality: the Framingham Heart Study. Stroke 2017; 48: 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song TJ, Kim J, Lee HS, et al. The frequency of cerebral microbleeds increases with CHADS(2) scores in stroke patients with non-valvular atrial fibrillation. Eur J Neurol 2013; 20: 502–508. [DOI] [PubMed] [Google Scholar]
- 60.Sepehry AA, Rauscher A, Hsiung GY, et al. Microbleeds in Alzheimer’s disease: a neuropsychological overview and meta-analysis. Canad J Neurol Sci 2016; 43: 753–759. [DOI] [PubMed] [Google Scholar]
- 61.Charidimou A, Wilson D, Shakeshaft C, et al. The clinical relevance of microbleeds in stroke study (CROMIS-2): rationale, design, and methods. Int J Stroke 2015; 10(Suppl A100): 155–161. [DOI] [PubMed] [Google Scholar]
- 62.Charidimou A, Pantoni L and Love S. The concept of sporadic cerebral small vessel disease: a road map on key definitions and current concepts. Int J Stroke 2016; 11: 6–18. [DOI] [PubMed] [Google Scholar]