Abstract
Background:
Diazinon (DZN) is an organophosphate pesticide commonly used for pest control in agriculture. It may engender a variety of negative effects in non-target species, including humans and animals. The objective of the present study was to evaluate the ameliorative properties of captopril (CAP), as a thiol containing an angiotensin-converting enzyme inhibitor, against DZN-induced oxidative stress.
Methods:
Twenty-eight male Wistar rats were divided randomly into 4 groups. All the rats were treated orally via gavage once a day for 7 weeks: control (corn oil), CAP (10 mg/kg), DZN (10 mg/kg), and CAP+DZN combination (as mentioned above). Oxidative stress indices in blood serum, liver and kidney homogenates (malondialdehyde [MDA], total thiol groups, and total antioxidant capacity), and erythrocyte hemolysis (superoxide dismutase [SOD] and glutathione peroxidase) were evaluated. Statistical analysis was performed using GraphPad Prism software, version 6.0 (GraphPad, San Diego, CA, USA), by ANOVA, followed by the Tukey post hoc analysis.
Results:
The MDA content and SOD activity increased significantly in the DZN group compared with those in the control group. Treatment with CAP in the DZN-exposed group significantly decreased (P<0.05) the MDA concentration and the SOD activity. The total thiol groups were decreased in the DZN group and elevated again by CAP treatment.
Conclusion:
The co-administration of CAP and DZN was able to attenuate lipid peroxidation and enzyme changes caused by DZN.
Keywords: Captopril , Diazinon , Lipid peroxidation , Antioxidants
What’s Known
Main effect of the subchronic or chronic intoxication with diazinon is oxidative stress.
Evaluation of the epigenetic effect of 5-Aza-CdR on solid tumor has been limited and further investigation is required.
What’s New
Current study is the first to study the ameliorative properties of captopril as a thiol containing an angiotensin-converting enzyme inhibitor against diazinon-induced oxidative stress.
Introduction
Pesticides are used broadly in agricultural, industrial, and household settings to control unwanted insects and disease vectors. Among the pesticides, organophosphate insecticides (OPIs) have been used worldwide and detected in soil and water as well as in vegetables, grains, and other food products.1 Because of their extensive use and easy accessibility, the toxicity of OPIs is an important global health problem. Occupational exposures to these pesticides occur from digestive system and skin absorption or via inhalation.2 Diazinon (DZN; diethoxy-[(2-isopropyl-6-methyl-4-pyrimidinyl) oxy]-thioxophosphorane), as one of the most commonly used OPIs, is a synthetic chemical substance with broad-spectrum insecticide activity.3 The main clinical effect of acute intoxication with DZN is the irreversible inhibition of acetylcholinesterase activity in blood and the nervous system, which at high doses could lead to death. However, the main mechanism of subchronic or chronic exposure is oxidative stress.4 Oxidative stress is commonly defined as an imbalance between reactive oxygen species (ROS) and antioxidants at the cellular or individual level. Oxidative damage is one result of such an imbalance and includes the oxidative modification of cellular macromolecules, cell death, and structural tissue damage.5 Both the increased production of ROS, generated during the metabolism of OPIs by cytochrome P450s, and the debilitation of the antioxidant system lead to OPI-induced oxidative stress.6 Oxidative stress has been described in OPI intoxications in both animals and humans.3,6,7 Lipid peroxidation, a complex process resulting from ROS reactions in biological membranes, seems to be one of the molecular mechanisms of the toxicity of some OPIs.8
Numerous studies have shown that treatment with antioxidant substances such as vitamins E and C,5,9 N-acetyl-cysteine,10 and crocin and safranal11 can decrease the oxidative stress and the mortality rate related to OPI-induced toxicity. Captopril (CAP), an inhibitor of the angiotensin-converting enzyme, is commonly used in the treatment of hypertension and most forms of heart failure.12 Moreover, CAP has been considered a free-radical scavenger due to its terminal sulfhydryl group.13 CAP treatment increased antioxidant enzymes and nonenzymatic antioxidant defenses in several mouse tissues.14 CAP has been shown to decrease serum lipid peroxide concentrations in diabetic patients15 and also to enhance antioxidant capacity in lead-exposed rats.13 In addition, CAP caused hepatocyte protection against paraquat-induced oxidative stress.16
Given the suggested antioxidant and free-radical scavenging activities for CAP, we investigated the in vivo effects of CAP on DZN-induced oxidative stress. Little attention has been given to the chronic low-dose effects of pesticides, which may not have clinically recognizable symptoms but could affect the overall health of an animal. Therefore, the present study was undertaken to evaluate the possible protective effects of CAP on oxidative stress and antioxidant status after 7 weeks’ exposure to a sublethal dose of DZN in rats.
Materials and Methods
Animals
Twenty-eight male Wistar rats (200-250 g) were obtained from the Animal House of Avicenna Research Institute, Mashhad, Iran. The animals were housed in plastic cages covered by wood chips, fed a standard laboratory diet and water ad libitum, and exposed to a 12 h light: 12 h dark cycle, at a room temperature of 18 to 22 °C. The experiment was approved by the Animal Welfare Committee of Ferdowsi University of Mashhad.17 After 1 week of acclimatization, the rats were divided into different groups.
Animal Treatment Schedule
The rats were randomly divided into 4 groups using the block randomization method (n=7).18 The compounds were administrated in the afternoon (between 14:00 and 16:00 PM) to non-fasted rats. All the rats were treated for 7 weeks.
Group 1. control group: Corn oil at a dose of 10 mg/kg/d was given through gavage once a day.
Group 2. CAP-treated group: CAP (Sigma-Aldrich, Germany) (10 mg/kg) was administered orally through gavage once a day.
Group 3. DZN-treated group: DZN (Sigma-Aldrich, Germany) at a dose of 10 mg/kg/d in corn oil was given through gavage.
Group 4. CAP+DZN-treated group: CAP (10 mg/kg/d) was administered orally through gavage before the oral administration of DZN (10 mg/kg/d).
The selection of the DZN dose was based on previously published studies and also our experience in our previous investigation,1,19,20 which determined that the toxic dose of DZN was able to induce oxidative stress in a subchronic study.
The dose of CAP was chosen based on previous research, such that it had the submaximal tolerable and effective concentration of CAP (the concentration without any mortality in a subchronic study).21-23
Sample Preparation
Blood Sampling
At the end of the experiment (wk 7), the rats were anaesthetized with carbon dioxide. Blood samples were taken from the animals’ hearts (5 mL) and divided into 2 parts. Next, 1.5 mL of the blood sample was anticoagulated with EDTA for the preparation of erythrocyte hemolysate in order to determine the effects of DZN on the activities of antioxidant enzymes and 3.5 mL of the blood sample was transferred into plane tubes and centrifuged for 10 minutes at 1800×g for serum separation in order to measure malondialdehyde (MDA), total thiol molecules, and total antioxidant capacity (TAC) as biomarkers of oxidative stress in blood serum. The clear non-hemolyzed sera and the hemolysate samples were stored at −20 °C until measurement.
Preparation of Erythrocyte Hemolysate
Immediately after collection, the blood samples, which were maintained in EDTA, were centrifuged at 800×g for 15 minutes at 4 °C. The plasma and buffy coats were removed by aspiration. The sediment, which contained blood cells, was washed 3 times by re-suspending in isotonic phosphate-buffered saline, followed by re-centrifugation and removal of the supernatant fluid and the buffy coats. Thereafter, the remaining crude red cells were lysed in added ice-cold distilled water at the same volume to prepare erythrocyte hemolysate.
Tissue Sampling
The right kidney and liver were taken quickly, cleaned free of extraneous material, and perfused immediately with sodium phosphate buffer (pH 7.4). The tissue samples were minced, cut into small pieces, and then dried on a filter paper and homogenized (10% w/v) in ice-cold 1.15% KCl-0.01 M sodium, potassium phosphate buffer (pH 7.4) with a Silent Crusher M. type homogenizer (Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). The homogenate was centrifuged at 18000 g for 20 minutes at 4 °C, and the resultant supernatant was used for the determination of oxidative stress markers (MDA and total thiol groups).
Biochemical Analysis
Malondialdehyde Assay
Lipid peroxidation was evaluated using the thiobarbituric acid reactive substances (TBARS) methods as described by Placer et al.24 The method evaluates serum MDA reactive products, which comprise the last products of the lipid breakdown caused by oxidative stress. The optical density was determined at 532 nm using a spectrophotometer. The concentration of the serum MDA was reported as nmol/mL. Moreover, for the tissues the method of Fernández J et al.25 was used. The absorbance of the test sample was read at 548 nm. The nmol of MDA per mL was calculated using 1.56×105 as the extinction coefficient.
Total Thiol Molecule Assay
Total thiol molecules were measured spectrophotometrically at 412 nm using 5,5’-Dithiobis(2-nitrobenzoic acid) as the reagent according to the method described by Hu and Dillard.26
Total Antioxidant Capacity Assay
TAC in blood serum was determined based on the absorbance of a colored radical cation which would form during the oxidative stress procedure.27 For the serum TAC measurement, a commercial test kit (TAC test kit [NX2332], Randox Laboratories Ltd., GB) was used.
Antioxidant Enzymes
The superoxide dismutase (SOD) activity was measured using a modified method of iodophenyl nitrophenol phenyl tetrazolium chloride (INT) (Ransod test kit [SD125], Randox Laboratories Ltd., UK). This method employs xanthine and xanthine oxidase to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyl tetrazolium chloride (INT) to form a red dye. The SOD activity is then measured by the degree of the inhibition of this reaction. One unit of SOD causes a 50% inhibition in the rate of reduction in INT under the assay conditions. The SOD activity was expressed as IU/mL.
The glutathione peroxidase (GSH-Px) activity was determined using commercially available kits (Ransel test kit [RS505], Randox Laboratories Ltd., UK) based on the method proposed by Paglia and Valentine.28 GSH-Px catalyzes the oxidation of glutathione (GSH) by cumene hydroperoxide. In the presence of GSH reductase and nicotinamide adenine dinucleotide phosphate (NADPH), the oxidized GSH is immediately converted into a reduced form with the concomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340 nm is measured. The activity of GSH-Px was exhibited as IU/L.
Statistical Analysis
The statistical analyses were performed using GraphPad Prism software, version 6.0 (GraphPad, San Diego, CA, USA), by one-way analysis of variance (ANOVA), followed by the Tukey post hoc analysis. The results are expressed as mean±standard deviation (SD). Values less than 0.05 were considered significant.
Results
Lipid Peroxidation Concentration
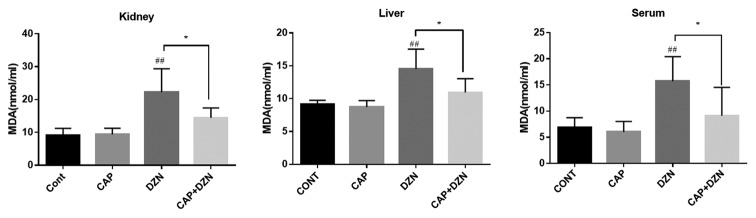
Figure 1 shows the effects of DZN on the MDA level, as a marker of lipid peroxidation, in the blood serum, kidney, and liver of the rats. The DZN treatment significantly increased (P<0.01) the MDA level in the serum. In addition, a significant increase in the MDA levels in the kidney (P<0.001) and liver (P=0.001) tissues were evident in the DZN group compared to those in the control group. In comparison to the DZN group, the concurrent CAP and DZN exposure significantly decreased the MDA content in the serum, kidney, and liver (P=0.02, P<0.01, and P=0.01, respectively). The MDA content of the CAP+DZN group was comparable to that of the control group (table 1).
Figure1.
Effects of diazinon (DZN), captopril (CAP), and their combination on malondialdehyde (MDA) in the rats’ kidney, liver, and serum. Values are expressed as mean±SD (n=7). The diazinon group was significantly different from the vehicle group##P<0.001 while captopril was able to ameliorate the condition * P<0.05 as compared to the DZN-treated group.
Table 1.
Effects of diazinon and captopril on the MDA and total thiol groups in the serum, kidney, and liver of the rats
| Effects | Vehicle (olive oil) (n=7) | Diazinon (n=7) | Captopril (n=7) | Diazinon+Captopril (n=7) |
|---|---|---|---|---|
| MDA, nmol/L | ||||
| Serum | 6.863±1.873 | 15.73±4.689## | 6.034±2.005 | 9.109±5.420* |
| Kidney | 9.123±2.08 | 22.25±7.090## | 9.370±1.923 | 14.46±2.997* |
| Liver | 9.121±0.636 | 14.52±3.017## | 8.717±0.993 | 10.96±2.078* |
| Total thiol groups, mmol/L | ||||
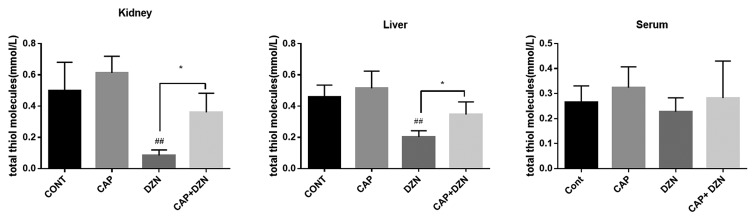
| Serum | 0.2643±0.066 | 0.2277±0.055 | 0.3241±0.082 | 0.2824±0.147 |
| Kidney | 0.4971±0.183 | 0.08429±0.035## | 0.6129±0.107 | 0.3600±0.122* |
| Liver | 0.4571±0.078 | 0.2029±0.039## | 0.5143±0.110 | 0.3457±0.081* |
All values are expressed as mean±SD (one-way ANOVA, followed by the Tukey test).
P<0.001 as compared to the vehicle group;
P<0.05 as compared to the diazinon-treated group; MDA: Malondialdehyde
Total Thiol Molecules
The results of total thiol molecules in the different tissues and blood serum are presented in figure 2. The DZN exposure significantly decreased (P<0.001) the total thiol groups in the liver and kidney, when compared to those in the control group. The concurrent CAP and DZN exposure significantly decreased the total thiol groups in the kidney and liver of the animals (P<0.01 and P=0.01, respectively) (table 1).
Figure2.
Effects of diazinon (DZN), captopril (CAP), and their combination on total thiol molecules in the rats’ kidney, liver, and serum. Values are expressed as mean±SD (n=7) (one-way ANOVA, followed by the Tukey test). While the DZN group was significantly different from the control group as regards the kidney and liver ##P<0.001, the reduced thiol content in serum was not significant compared to that in the control group. CAP given to the rats was able to significantly increase the total thiol molecules *P<0.05 in both liver and kidney tissues.
Total Antioxidant Capacity
No significant difference was seen in the TAC level of the serum samples between the different groups (figure 3).
Figure3.
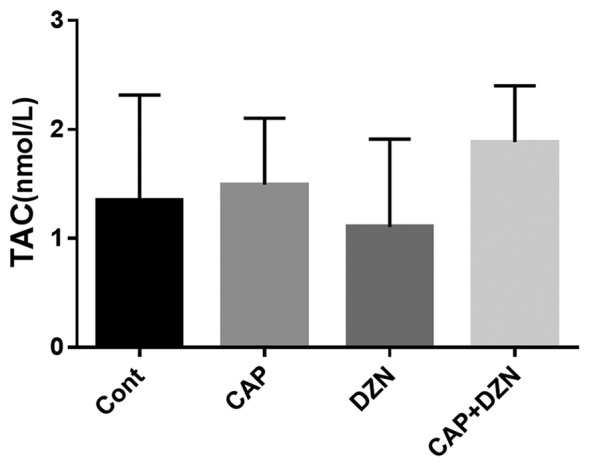
Effects of diazinon (DZN), captopril (CAP), and their combination on total antioxidant capacity (TAC) in the rats’ serum. There was no significant difference between the treatment groups.
Antioxidant Enzymes
The DZN administration significantly increased (P<0.001) the SOD activity in the erythrocyte hemolysate of the DZN-group when compared to that in the control group. However, treatment with CAP in the CAP+DZN group significantly decreased the SOD activity when compared with that in the DZN group (P<0.001). The SOD activity in the erythrocytes of the CAP+DZN group was comparable to that of the control group (figure 4). A significant increase in the GSH-Px activity in the CAP+DZN group was observed when compared to that in the DZN group (P<0.001) (figure 4, table 2).
Figure4.
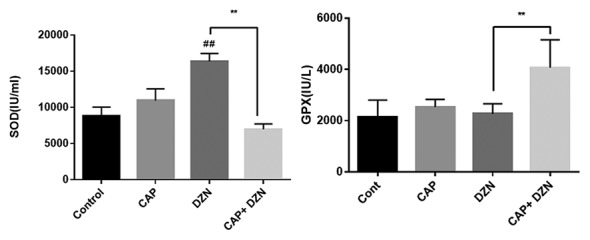
Effects of diazinon (DZN), captopril (CAP), and their combination on the superoxide dismutase (SOD) activity (A) and the glutathione peroxidase (GPX) activity (B) in the rats’ erythrocytes. SOD was enhanced significantly in the DZN group compared to that in the control group (A). CAP administration to the DZN-treated group significantly raised the GPX level (B). Data are expressed as mean±SD (n=7) (one-way ANOVA, followed by the Tukey test), ##P<0.001 as compared to the vehicle group; **P<0.001 as compared to the DZN-treated group.
Table 2.
Effects of diazinon and captopril on the SOD and GPX enzymes in the serum of the rats
| Effects | Serum SOD (IU/L) | P value | Serum GPX (IU/L) | P value |
|---|---|---|---|---|
| Vehicle (olive oil) (n=7) | 8829±1212 | 2136±668.9 | ||
| Diazinon (n=7) | 16383±1084## | P<0.001 | 2269±395.2 | |
| Captopril (n=7) | 10953±1624 | 2529±302.9 | ||
| Diazinon+Captopril (n=7) | 6970±760.7* | P<0.001 | 4070±1085* | P<0.001 |
All values are expressed as mean±SD (one-way ANOVA, followed by the Tukey test).
P<0.001 as compared to the vehicle group;
P<0.001 as compared to the diazinon-treated group; SOD: Superoxide dismutase; GPX: Glutathione peroxidase
Discussion
A large number of studies have been conducted on the toxicity of DZN in rats. However, the current study is the first to determine the protective effects of CAP, as an antioxidant, against DZN toxicity. According to our results, treatment with DZN (10 mg/kg for 7 wk) promoted the MDA content in the liver, kidney, and serum of rats. Lipid peroxidation is involved in many toxic effects such as increased membrane rigidity, release of the amino acid tyrosine into the extracellular medium, osmotic fragility, decreased cellular and subcellular components, increased ROS formation, and lipid fluidity.1 The MDA induction seen in the present study is in agreement with some studies previously published1,8,29-31 insofar as they reported increased lipid peroxidation in rats exposed to DZN. High lipid peroxidation may be due to the oxidation of molecular oxygen to produce superoxide radicals. This reaction is also the source of hydrogen peroxide, which produces MDA by triggering the peroxidation of unsaturated fatty acids in the membrane.32
There are contradictory data concerning the effects of OPIs on antioxidants. The present study demonstrated that DZN significantly augmented the activity of SOD in erythrocytes. DZN chronic toxicity could induce erythrocyte mitochondrial superoxide production. Thus, the increased activity of SOD in erythrocytes might be due to the adaptive response to the generated superoxide radicals. Buyukokuroglu et al.33 also proved a significant elevation in the SOD activity during intoxication with DZN. Furthermore, Akturk et al.34 reported that the single-dose administration of DZN significantly enhanced the activity of SOD in the adult rat heart. The authors suggested that the increased activity of SOD reflected the activation of the compensatory mechanism through the effects of DZN on cells and that its extent depended on the magnitude of the oxidative stress and hence on the dose of a stressor. On the other hand, Abdou and El Mazoudy35 showed significant decreases in the activity of serum SOD when rats were intoxicated with different doses of DZN for 3 weeks. In addition, Altuntas et al.,8 having found that the activity of SOD was respectively decreased with increasing DZN concentrations, suggested that the inhibition of SOD was not directly mediated only by DZN but also by the increased generation of ROS.
In the present study, the difference in the GSH-Px activity was not significant between the DZN group and the control group. El-Shenawy et al.3 reported a significant decrease in the GSH-Px activity and a reduction in the GSH content in DZN-treated mice. In addition, the investigators indicated that the decrease in the GSH-Px activity induced by DZN might be attributable to a direct inhibitory oxidative effect on the enzyme. They also confirmed that the drop in the GSH content could also represent an important inhibitory issue for this enzyme. However, other researchers have found that several OPIs can augment the activity of GSH-Px through compensatory mechanisms.36,37 In agreement with the present study, Akturk et al.34 found that the difference in GSH-Px was not significant following the administration of DZN to rats.
Shadnia and his colleagues10 indicated that exposure to DZN decreased total thiol molecules and TAC in rats. In their study, DZN at a dose of 25 mg/kg was given through gavage for 4 weeks.
The renal toxicity of DZN was investigated by Mahmoud-Shah et al.1 In their investigation, the animals were treated with DZN as a treatment group and with corn oil as a control group. The selected doses of DZN in that study were 10, 15, and 30 mg/kg of body weight daily and for 8 weeks. The authors noted that MDA was significantly enhanced and GSH was decreased and mentioned that DZN mediated renal oxidative stress and toxicity. Similar to their results, according to our findings, MDA significantly increased in the kidney and the total thiol content decreased.
Numerous studies have demonstrated that treatment with antioxidant substances such as vitamins E and C,34 -acetyl-cysteine,10 crocin and safranal,11 and selenium38 can decrease oxidative stress and mortality rates related to OPI-induced toxicity. CAP, a well-known antihypertensive agent, has also been suggested as an antioxidant and free-radical scavenger due to its structural thiol group.13,39 Administration of CAP to the DZN-exposed rats led to a reduction in the MDA concentrations in the serum, liver, and kidney. The reduction in the MDA levels may have been due to the direct radical scavenging effect of CAP. Chiming in with the present study, the administration of CAP to lead-exposed rats reduced lipid peroxidation in the liver.13 Our data are also concordant with those in the studies by Ghazi-Khansari et al.12 and Pourahmad et al.,16 who showed the protective effects of CAP against paraquat-induced oxidative stress in rats. Their studies confirmed that CAP was able to prevent hepatocyte ROS formation induced by the intracellular hydrogen peroxide generating system, suggesting that this angiotensin-converting enzyme inhibitor might easily cross the cell membrane and cope with the intracellular ROS formation. The activity of SOD decreased significantly in the CAP+DZN group compared with the DZN group. The SOD activity in the erythrocytes of the CAP+DZN group was comparable to that of the control group. CAP may exert its effect by inhibiting membrane lipid peroxidation mediated by superoxide anion.12 Our results suggested that CAP might reduce the SOD activity through the elimination of superoxide radicals. Similar to our results, a study designed to examine the in vivo and in vitro effects of CAP on nicotine-induced endothelial dysfunction in rats concluded that chronic CAP treatment prevented the reduction of SOD activities in the serum and aorta caused by nicotine. These results indicate that CAP has some antioxidant features.22 Akturk et al.34 reported that the single-dose administration of DZN significantly boosted the activity of SOD in the adult rat heart. They found that the administration of vitamins E and C was somewhat effective in restoring the activity of SOD. In the present study, the concurrent administration of DZN and CAP significantly increased the GSH-Px activity in comparison with the DZN, CAP, and control groups. Golik et al.40 provided evidence that in hypertensive patients on long-term CAP treatment, the GSH-Px activity was increased. Ghazi-Khansari et al.12 and Gurer et al.13 showed a rise in the GSH content after treatment with CAP, respectively in paraquat and lead toxicity. GSH, the main endogenous nonenzymatic antioxidant, plays an important role in protecting cells against oxidative stress and it also acts as a substrate in the enzymatic reaction of GSH-Px.3 An elevation in GSH, induced by CAP, was able to increase the GSH-Px activity in the CAP+DZN group compared to that in the DZN group. Nevertheless, it is difficult to explain the increase in the GSH-Px activity in the CAP+DZN group by comparison with that in the CAP group.
CAP increased the antioxidant enzyme levels in the rats receiving DZN. Nonetheless, it could not significantly attenuate the total antioxidant capacity volume. Using supplementary tests such as western blot analysis could help to clarify the oxidative stress status even more desirably.
Conclusion
Ours findings demonstrated that the in vivo subchronic administration of DZN resulted in the elevation of MDA levels and changes in the activities of antioxidant enzymes in the rats’ erythrocytes, confirming that ROS may be involved in the toxic effects of DZN. Our findings also showed that CAP had protective effects in the rats exposed to DZN. Moreover, CAP was able to diminish lipid peroxidation by restoring endogenous antioxidant enzymes.
Acknowledgement
This study was supported by a research fund, granted by Ferdowsi University of Mashhad. The authors wish to thank the technicians who kindly helped us with sample collection.
Conflict of Interest:None declared.
References
- 1.Shah MD, Iqbal M. Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem Toxicol. 2010;48:3345–53. doi: 10.1016/j.fct.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi SK, Satyanarayan PV, Ravishankar D, Tripathi S. A study on oxidative stress and antioxidant status of agricultural workers exposed to organophosphorus insecticides during spraying. Indian J Occup Environ Med. 2009;13:131–4. doi: 10.4103/0019-5278.58916. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Shenawy NS, El-Salmy F, Al-Eisa RA, El-Ahmary B. Amelioratory effect of vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pestic Biochem Physiol. 2010;96:101–7. doi: 10.1016/j.pestbp.2009.09.008. [DOI] [Google Scholar]
- 4.Lukaszewicz-Hussain A. Role of oxidative stress in organophosphate insecticide toxicity–Short review. Pestic Biochem Physiol. 2010;98:145–50. doi: 10.1016/j.pestbp.2010.07.006. [DOI] [Google Scholar]
- 5.Mozhdeganloo Z, Jafari AM, Koohi MK, Heidarpour M. Methylmercury-induced oxidative stress in rainbow trout (Oncorhynchus mykiss) liver: ameliorating effect of vitamin C. Biol Trace Elem Res. 2015;165:103–9. doi: 10.1007/s12011-015-0241-7. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A, Sharma B. Pesticides induced oxidative stress in mammalian systems: a review. Int J Biol Med Res. 2010;1:90–104. [Google Scholar]
- 7.Mashali AA, Nounou HA, Sharara GM, Manal H, Aziz A. Role of oxidative stress and apoptosis into acute organophosphorus intoxicated patients. J Med Res Inst. 2005;26:255–63. [Google Scholar]
- 8.Altuntas I, Kilinc I, Orhan H, Demirel R, Koylu H, Delibas N. The effects of diazinon on lipid peroxidation and antioxidant enzymes in erythrocytes in vitro. Hum Exp Toxicol. 2004;23:9–13. doi: 10.1191/0960327104ht408oa. [DOI] [PubMed] [Google Scholar]
- 9.Mozhdeganloo Z, Moghadam Jafari A, Koohi MK, Heidarpour M. Permethrin-induced oxidative damage in liver of rainbow trout (Oncorhynchus mykiss) and its attenuation by vitamin C. Iran J Vet Res. 2016;17:31–5. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadnia S, Dasgar M, Taghikhani S, Mohammadirad A, Khorasani R, Abdollahi M. Protective Effects of alpha-Tocopherol and N-Acetyl-Cysteine on Diazinon-Induced Oxidative Stress and Acetylcholinesterase Inhibition in Rats. Toxicol Mech Methods. 2007;17:109–15. doi: 10.1080/15376510600860318. [DOI] [PubMed] [Google Scholar]
- 11.Hariri AT, Moallem SA, Mahmoudi M, Memar B, Hosseinzadeh H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: protective effects of crocin and safranal. Food Chem Toxicol. 2010;48:2803–8. doi: 10.1016/j.fct.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Ghazi-Khansari M, Mohammadi-Bardbori A. Captopril ameliorates toxicity induced by paraquat in mitochondria isolated from the rat liver. Toxicol In Vitro. 2007;21:403–7. doi: 10.1016/j.tiv.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Gurer H, Neal R, Yang P, Oztezcan S, Ercal N. Captopril as an antioxidant in lead-exposed Fischer 344 rats. Hum Exp Toxicol. 1999;18:27–32. doi: 10.1177/096032719901800104. [DOI] [PubMed] [Google Scholar]
- 14.de Cavanagh EM, Inserra F, Ferder L, Fraga CG. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol. 2000;278:R572–7. doi: 10.1152/ajpregu.2000.278.3.R572. [DOI] [PubMed] [Google Scholar]
- 15.Ha H, Kim KH. Amelioration of diabetic microalbuminuria and lipid peroxidation by captopril. Yonsei Med J. 1992;33:217–23. doi: 10.3349/ymj.1992.33.3.217. [DOI] [PubMed] [Google Scholar]
- 16.Pourahmad J, Hosseini MJ, Bakan S, Ghazi-Khansari M. Hepatoprotective activity of angiotensin-converting enzyme (ACE) inhibitors, captopril and enalapril, against paraquat toxicity. Pestic Biochem Physiol. 2011;99:105–10. doi: 10.1016/j.pestbp.2010.11.006. [DOI] [Google Scholar]
- 17.Ghasemi M, Dehpour AR. Ethical considerations in animal studies. J Med Ethics Hist Med. 2009;2:12. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 18.Suresh K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4:8–11. doi: 10.4103/0974-1208.82352. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Kalender S, Ogutcu A, Uzunhisarcikli M, Acikgoz F, Durak D, Ulusoy Y, et al. Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology. 2005;211:197–206. doi: 10.1016/j.tox.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Sargazi S, Moghadam-Jafari A, Heidarpour M. Protective effect of tert butyl hydroquinone on diazinon-induced oxidative stress in brain and heart of male rats. Zahedan Journal of Research in Medical Sciences. 2016;18 doi: 10.17795/zjrms-7356. [DOI] [Google Scholar]
- 21.Aldahmash BA, El-Nagar DM. Antioxidant effects of captopril against lead acetate-induced hepatic and splenic tissue toxicity in Swiss albino mice. Saudi J Biol Sci. 2016;23:667–73. doi: 10.1016/j.sjbs.2016.05.005. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo HL, Zang WJ, Lu J, Yu XJ, Lin YX, Cao YX. The protective effect of captopril on nicotine-induced endothelial dysfunction in rat. Basic Clin Pharmacol Toxicol. 2006;99:237–45. doi: 10.1111/j.1742-7843.2006.pto_494.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhu B, Sun Y, Sievers RE, Browne AE, Pulukurthy S, Sudhir K, et al. Comparative effects of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia-reperfusion. J Am Coll Cardiol. 2000;35:787–95. doi: 10.1016/S0735-1097(99)00592-6. [DOI] [PubMed] [Google Scholar]
- 24.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16:359–64. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 25.Fernández J, Pérez-Álvarez JA, Fernández-López JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chemistry. 1997;59:345–53. doi: 10.1016/S0308-8146(96)00114-8. [DOI] [Google Scholar]
- 26.Hu M, Dillard C. Plasma SH and GSH measurement. Methods Enzymol. 1994;233:87. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 27.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 28.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 29.Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, Hajigholamali M. The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol Mech Methods. 2012;22:638–47. doi: 10.3109/15376516.2012.716090. [DOI] [PubMed] [Google Scholar]
- 30.Amirkabirian N, Teimouri F, Esmaily H, Mohammadirad A, Aliahmadi A, Abdollahi M. Protection by pentoxifylline of diazinon-induced toxic stress in rat liver and muscle. Toxicol Mech Methods. 2007;17:215–21. doi: 10.1080/15376510600943783. [DOI] [PubMed] [Google Scholar]
- 31.Gokalp O, Buyukvanlı B, Cicek E, Ozer MK, Koyu A, Altuntas I, et al. The effects of diazinon on pancreatic damage and ameliorating role of vitamin E and vitamin C. Pestic Biochem Physiol. 2005;81:123–8. doi: 10.1016/j.pestbp.2004.11.001. [DOI] [Google Scholar]
- 32.Uner N, Oruc EO, Canli M, Sevgiler Y. Effects of cypermethrin on antioxidant enzyme activities and lipid peroxidation in liver and kidney of the freshwater fish, Oreochromis niloticus and Cyprinus carpio (L. ) Bull Environ Contam Toxicol 2001;67:657–64. doi: 10.1007/s001280174. [DOI] [PubMed] [Google Scholar]
- 33.Buyukokuroglu ME, Cemek M, Yurumez Y, Yavuz Y, Aslan A. Antioxidative role of melatonin in organophosphate toxicity in rats. Cell Biol Toxicol. 2008;24:151–8. doi: 10.1007/s10565-007-9024-z. [DOI] [PubMed] [Google Scholar]
- 34.Akturk O, Demirin H, Sutcu R, Yilmaz N, Koylu H, Altuntas I. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat heart and ameliorating role of vitamin E and vitamin C. Cell Biol Toxicol. 2006;22:455–61. doi: 10.1007/s10565-006-0138-5. [DOI] [PubMed] [Google Scholar]
- 35.Abdou HM, ElMazoudy RH. Oxidative damage, hyperlipidemia and histological alterations of cardiac and skeletal muscles induced by different doses of diazinon in female rats. J Hazard Mater. 2010;182:273–8. doi: 10.1016/j.jhazmat.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed RS, Seth V, Pasha ST, Banerjee BD. Influence of dietary ginger (Zingiber officinales Rosc) on oxidative stress induced by malathion in rats. Food Chem Toxicol. 2000;38:443–50. doi: 10.1016/S0278-6915(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 37.Datta C, Gupta J, Sarkar A, Sengupta D. Effects of organophosphorus insecticide phosphomidon on antioxidant defence components of human erythrocyte and plasma. Indian J Exp Biol. 1992;30:65–7. [PubMed] [Google Scholar]
- 38.Kaur R, Sandhu HS. In vivo changes in antioxidant system and protective role of selenium in chlorpyrifos-induced subchronic toxicity in bubalus bubalis. Environ Toxicol Pharmacol. 2008;26:45–8. doi: 10.1016/j.etap.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Chopra M, Beswick H, Clapperton M, Dargie HJ, Smith WE, McMurray J. Antioxidant effects of angiotensin-converting enzyme (ACE) inhibitors: free radical and oxidant scavenging are sulfhydryl dependent, but lipid peroxidation is inhibited by both sulfhydryl- and nonsulfhydryl-containing ACE inhibitors. J Cardiovasc Pharmacol. 1992;19:330–40. doi: 10.1097/00005344-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Golik A, Weissgarten J, Evans S, Cohen N, Zaidenstein R, Modai D, et al. Changes in red blood cell glutathione and glutathione-dependent enzymes on long-term treatment with captopril and enalapril. Clin Chim Acta. 1995;240:89–94. doi: 10.1016/0009-8981(95)06121-3. [DOI] [PubMed] [Google Scholar]