Abstract
Background:
Considering the association between cardiac abnormalities and non-alcoholic fatty liver disease (NAFLD), the present study aimed to evaluate the relationship between biopsy-proven NAFLD and functional echocardiographic parameters, including left ventricular (LV) global longitudinal strain (GLS) in asymptomatic individuals.
Methods:
Thirty asymptomatic patients with liver biopsy-proven NAFLD and the same number with no evidence of fatty liver in ultrasonography were enrolled in the study as cases and controls, respectively. The measured echocardiographic parameters included LV ejection fraction (LVEF), LV end-systolic and end-diastolic dimensions (ESD, EDD), LV end-systolic and end-diastolic volumes (ESV, EDV), E/e’ ratio (early-diastolic mitral inflow velocity/early-diastolic myocardial velocity), E/A ratio (early-diastolic mitral inflow velocity/late-diastolic mitral inflow velocity), and GLS. Data were analyzed using the SPSS statistical software (version 18.0) by performing the independent t test, Chi-square, and non-parametric Mann-Whitney U tests. P values <0.05 were considered statistically significant.
Results:
A significant difference in ESD (32.1±1.4 mm vs. 34±1.8 mm), EDD (41.9±1.7 mm vs. 45.2±3.1 mm), and E/e’ ratio (8.4±0.8 vs. 7.4±1.2) was detected among individuals with NAFLD compared with those without NAFLD (P<0.001 for the first two parameters and P=0.002 for the last one). GLS was also significantly lower in NAFLD patients than in controls, but within normal levels (19.3%±2.0 vs. 21.2%±1.4, P<0.001).
Conclusion:
The findings support the presence of subclinical cardiovascular structural and functional changes in patients affected by NAFLD. It also indicates that the use of GLS is more sensitive than LVEF for the detection of LV systolic dysfunction in NAFLD patients.
Keywords: Non-Alcoholic fatty liver disease , Ventricular function , Left , Echocardiography
What’s Known
STE has been recently used in obese adolescents with NAFLD, demonstrating significant decrease of LV global longitudinal systolic strain in obese than lean individuals.
A recent study has shown an association between NAFLD and subclinical myocardial dysfunction using the STE.
What’s New
The selected population in this study is more homogenous and the two study groups are well matched, allowing a more accurate comparison. Additionally, the contributing factors are considered
Due to the paucity of such studies, further work is required.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is now the most frequent chronic liver disease across all age groups worldwide, representing a serious and growing clinical problem due to the increased prevalence of overweight and obesity. It is characterized by fatty infiltration of hepatocytes in the presence of less than 30 g (three units) of alcohol consumption per day for men and 20 g (two units) per day for women without evidence of other causes of liver disease.1,2
NAFLD includes a wide spectrum of liver disease presentation, ranging from simple steatosis to co-existent inflammation with hepatocyte ballooning and necrosis, variable grades of fibrosis, and cirrhosis and hepatocellular carcinoma.1,3 Non-alcoholic steatohepatitis (NASH) is a slowly progressive disease representing the most extreme form of NAFLD (i.e. the ‘inflammatory’ component in addition to steatosis), which carries a higher risk of cardiovascular disease (CVD) and mortality than simple steatosis.4 In addition, NAFLD has been identified as a risk factor for early subclinical abnormalities in cardiac structure and function, including increased risk of coronary artery disease, left ventricular (LV) dysfunction and hypertrophy, heart failure, valvular heart disease and arrhythmias. NAFLD has been shown to exacerbate the systemic/hepatic insulin resistance and atherogenic dyslipidemia and is strongly associated with obesity and hypertension. It is now regarded as the hepatic manifestation of the metabolic syndrome, which makes the affected individuals susceptible to premature atherosclerosis. The release of a variety of pro-inflammatory, pro-coagulant, and pro-fibrogenic mediators in this condition may play important roles in the pathophysiology of cardiac and arrhythmic complications.5-7
Speckle-tracking echocardiography (STE), a new non-invasive ultrasound imaging technique, allows an objective and quantitative evaluation of global and regional myocardial function independently from the angle of insonation and cardiac translational movements based on an analysis of the spatial dislocation of speckles on routine 2-dimensional sonograms. Hence, because of its potential benefits in the measurement of LV function8 along with other systolic and diastolic echocardiographic parameters of LV function measurement, this novel quantitative technique was used in this study to assess the relationship between biopsy-proven NAFLD and subclinical LV myocardial dysfunction. To our knowledge, it has been recently used in obese adolescents with NAFLD, demonstrating that LV global longitudinal systolic strain and early diastolic strain rates were significantly decreased in obese than in lean individuals and in obese individuals with NAFLD than those without NAFLD.9 Another recent study also showed an association between NAFLD and subclinical myocardial dysfunction using the STE.10
Carabay et al. stated that patients with NAFLD without evidence of insulin resistance had a similar myocardial performance in STE as compared with the normal population.11 Therefore, subclinical myocardial systolic dysfunction has been scantily noticed in previous studies, and more studies with more powerful tools are required to reveal this probable relationship, especially in the mild stages of the disease. Consequently, the aim of this study was to evaluate the effect of NAFLD on myocardial performance in asymptomatic patients using a newer and more sensitive echocardiographic technique.
Participants and Methods
Study Population
The present case-control study was approved and performed in accordance with the regulations of Institutional Review Board, Shiraz University of Medical Sciences, Shiraz, Iran. In total, 30 outpatient individuals aged more than 18 years old with NAFLD were enrolled. These individuals referred to the liver biopsy clinic in Shahid Faghihi and Namazi hospitals (Shiraz, Iran) from February 2016 to August 2016. NAFLD was confirmed by liver biopsy in all patients.
The public was invited to participate in the evaluation via a general announcement throughout the premises of both hospitals. Among those who accepted the invitation, 30 healthy individuals were selected as controls. They voluntarily underwent liver ultrasonography that showed no evidence of NAFLD. The control group was matched with the fatty liver group for age, sex, anthropometric features (BMI), lipid profile and lifestyle habits (smoking and physical activity according to patients’ self-report questionnaire data). Bouchard physical activity questionnaire was used for the estimation of physical activity.12 All participants were selected among those with resting heart rate between 60 to 80 beats per minute. The liver enzymes of the case group were measured in the Namazi research laboratory. The results showed that 10 patients had elevated liver enzymes and the remaining 20 patients had normal liver enzymes. All individuals in the control group had normal liver enzymes.
The exclusion criteria were a sedentary lifestyle, history of using hepatotoxic drugs or herbal supplements, and history of viral hepatitis or chronic liver disease (hemochromatosis, Wilson’s disease, autoimmune hepatitis, alpha 1-antitrypsin deficiency, alcohol-induced or drug-induced liver disease, cirrhosis, etc.). Additionally, those with a history of alcohol consumption as well as medications (estrogens, amiodarone, diltiazem, steroids, and tamoxifen), and postmenopausal women were excluded. The presence of hypertension, significant disturbances in sinus rhythm (other than infrequent premature complexes or sinus arrhythmia), and ischemic or valvular heart disease were the other exclusion criteria.
In line with previous studies,10 a sample size of at least 30 participants in each group was considered; providing the study power of 80% and a type I error of 5% according to the following formula:
σ = 2.5, d=1.2, ρ=0.5
The convenience sampling method was used for selecting the patients and normal individuals. The excluded individuals were replaced with others who met the eligibility inclusion criteria.
The present study was approved by the Ethics Committee of Shiraz University of Medical Sciences (Shiraz, Iran). Written informed consent was obtained from the participants after being debriefed about the objectives of the trial and the non-invasive assessment method.
Liver Biopsy
All 30 patients were selected among those who were referred for percutaneous liver biopsy. The hematologic abnormalities and other contraindications of the procedure were assessed after initial liver ultrasonography. Then, liver biopsy was performed by radiologists and samples were sent to the pathology laboratory to determine the presence of NAFLD. The patients with characteristics other than simple steatosis were replaced by those who met both the inclusion and exclusion criteria and their liver biopsy did not show any sign of inflammation or necrosis.
Echocardiographic Examination
All participants underwent a 2-dimensional transthoracic echocardiography, including STE using a Vivid E9 system (GE, Norway). All echocardiographic measurements were performed and analyzed by an echocardiologist who was blinded to the patient group assignment, according to the latest recommendations by the American Society of Echocardiography.13 The same ultrasound machine was used to acquire all echocardiograms. The echocardiographic examinations were done within one week after taking the liver biopsy in NAFLD patients and within one week after performing liver ultrasonography in controls; considering the stability of hemodynamic status at the time of echocardiography using heart rate, blood pressure, and clinical judgment about body fluid balance. The end-systolic and end-diastolic dimensions (ESD, EDD) and end-diastolic ventricular septal thickness were measured via M-mode. The LV ejection fraction (LVEF) and end-systolic and end-diastolic volumes (ESV, EDV) were calculated from the 4- and 2-chamber apical views using the modified Simpson’s biplane method. Left ventricular global longitudinal strain (GLS) was measured using STE at a frame rate of 43 to 60 fps. On 2-dimensional echocardiography, the GLS describes the relative length change of the LV myocardium between end-diastole and end-systole. After optimizing image quality, maximizing frame rate and minimizing foreshortening, peak mid-wall GLS measurement was taken in the three standard apical views and averaged by AFI (automated function imaging) application and demonstrated in Bull’s eye plot (figure 1).
Figure1.
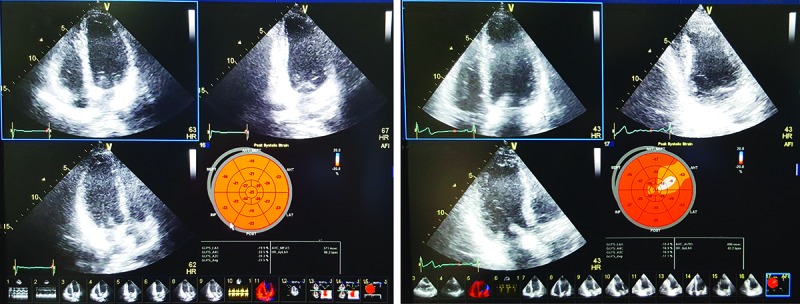
Bull’s eye display of segmental and global peak-systolic longitudinal strain of a normal individual (upper) and a patient with NAFLD (lower).
Previous studies have demonstrated that GLS values are more negative than -16% in healthy individuals and a cut-off point at -16% has been shown to provide important risk stratification and prognostic value.14 Diastolic function was assessed by tissue Doppler imaging (TDI). TDI-derived early-diastolic myocardial velocity (e’) obtained at the medial side of the mitral annulus, E/e’ ratio (a marker of left atrial (LA) pressure), and E/A ratio that determines the severity of diastolic dysfunction. The E/A ratio <1.5 along with both E/e’ ratio <15 and e’ velocity >8 cm/sec was considered as the normal diastolic function; otherwise the participants were characterized to have diastolic dysfunction.
Statistical Analysis
All statistical analyses were performed with the statistical package for social sciences, version 18.0 (SPSS Inc., Chicago, IL, USA). Using the Kolmogrov-Smirnov test, the distribution of values in all variables was studied. The non-parametric Mann-Whitney U test was used for the comparison of E and A velocities in both groups (cases and controls) and variables were expressed as median plus the interquartile range of 25th and 75th percentiles (Q1-Q3). The independent sample t test and Chi-square test were used for normally distributed continuous and categorical parameters, respectively. All continuous variables were expressed as mean±SD (standard deviation) and categorical variables were expressed as number (n) and percentage (%). A two-sided P value <0.05 was considered statistically significant.
Results
Sixty individuals were enrolled in the present study. The mean age of the participants was 38.4±5.0 and 36.9±4.5 years old in the case and control groups, respectively, ranging from 28 to 44 years in both groups. Among the patients with NAFLD, 53.3% were male and 46.7% were female, with a similar sex distribution in the control group.
The participants that had diastolic dysfunction in the NAFLD and control groups were 16.7% and 10%, respectively (P=0.440). The E/e’ ratio was significantly higher in the NAFLD group (8.4±0.8 vs. 7.4±1.2, P=0.002). The difference in E/A ratio between the groups was not significant (P=0.440). ESD and EDD had a significant difference between the case and control groups (32.1±1.4 mm vs. 34±1.8 mm and 41.9±1.7 mm vs. 45.2±3.1, respectively, both P values were <0.001). GLS was also significantly lower in NAFLD patients than in controls, but within normal values (19.3%±2 vs. 21.2%±1.4, P<0.001). However, the difference in ventricular septal thickness between the groups was not statistically significant (P=0.450). LVEF was also statistically insignificant between the groups (P=0.753). Table 1 shows the distribution of individuals with abnormal values of lipid profile components in each group. The demographic and echocardiographic characteristics of the participants are summarized in table 2.
Table 1.
Distribution of individuals with normal serum lipid profile components in the NAFLD and control groups
| Serum lipid profile components | NAFLD group (n=30) | Control group (n=30) | P value |
|---|---|---|---|
| Triglyceride (<150 mg/dL) | 20 (66.67%) | 19 (63.34%) | 0.999 |
| Total cholesterol (<200 mg/dL) | 18 (60%) | 18 (60%) | >0.999 |
| HDL (>40 mg/dl in female and>50 mg/dL in male) | 22 (73.34%) | 24 (80%) | 0.760 |
| LDL (<130 mg/dL) | 19 (63.34%) | 21 (70%) | 0.784 |
HDL: High density lipoprotein; LDL: Low density lipoprotein
Table 2.
Comparison of demographic characteristics and echocardiographic data in the NAFLD and control groups
| Variable | NAFLD group (n=30) | Control group (n=30) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 38.4±5 | 36.9±4.5 | 0.108 |
| Male (n, %) | 16 (53.3%) | 15 (50%) | 0.790 |
| BMI (kg/m2) | 25.84±2.16 | 25.73±2.29 | 0.848 |
| Smoking history (yes: n, %) | 3 (10%) | 5 (16.7%) | 0.440 |
| Echocardiographic data | |||
| Diastolic dysfunction (yes: n, %) | 5 (16.7%) | 3 (10%) | 0.440 |
| E velocity (m/sec) | 1.03 (0.8-1.3) | 1.12 (0.8-1.4) | 0.230 |
| A velocity (m/sec) | 0.92 (0.6-1.2) | 0.97 (0.6-1.3) | 0.470 |
| e’ velocity (cm/sec) | 11.5±1.2 | 14.8±1.6 | <0.001 |
| E/e’ ratio | 8.4±0.8 | 7.4±1.2 | 0.002 |
| E/A ratio | 0.9±0.3 | 1±0.S3 | 0.440 |
| Ventricular septal thickness (mm) | 8.6±0.8 | 8.8±1.1 | 0.450 |
| ESD (mm) | 32.1±1.4 | 34±1.8 | <0.001 |
| EDD (mm) | 41.9±1.7 | 45.2±3.1 | <0.001 |
| ESV (mL) | 39.6±8.55 | 41.3±7.6 | 0.419 |
| EDV (mL) | 90.36±13.08 | 95.7±11.1 | 0.093 |
| LVEF (%) | 56.7±4.6 | 57.1±5.2 | 0.753 |
| GLS (%) | 19.3±2 | 21.2±1.4 | <0.001 |
E and A velocities are expressed as median (Q1 Q3), other variables as mean±SD or number. BMI: Body mass index; E: Early diastolic mitral inflow velocity; e’: Early diastolic myocardial velocity; A: Late diastolic mitral inflow velocity; ESD/V: End systolic dimension per volume; EDD/V: End diastolic dimension per volume; LVEF: Left ventricular ejection fraction; GLS: Global longitudinal strain
Discussion
In the present study, the cardiac structure and function in patients with NAFLD were examined which showed significant differences from the normal population. The end-systolic and end-diastolic dimensions, but not volumes, were significantly lower in participants with NAFLD than those without; however, all variables were still within the normal range. This result is inconsistent with the findings of Sert et al. who demonstrated higher ESD and EDD in obese adolescents with NAFLD than the normal population.15 In addition, ventricular septal thickness was not different between the case and control groups, which is also incongruent with most of the previous studies that revealed higher values of septal thickness and LV mass in both children and adults with fatty liver.16-18 The selection of patients among those with more mild stages of the disease may justify the inconsistency between our results and the above-mentioned studies.
In an echocardiographic assessment, Petta et al. showed that after adjusting for cardiometabolic confounders, diastolic posterior wall thickness, LV mass, relative wall thickness, and left atrial volume were linked to severe liver fibrosis. Therefore, the morphological and functional cardiac alterations in NAFLD are more pronounced according to the severity of fibrosis.19 Fallo et al. showed that NAFLD patients had a similar prevalence of LV hypertrophy compared to those without NAFLD who were matched with the cases by sex, age, and blood pressure levels.20 Furthermore, in a study by Singh et al., relative wall thickness was not significantly different between the groups and was normal for age in most patients.9 The diversity of selected populations in previous studies in terms of matched items between the groups does not justify the observed differences between studies in determining the effects of NAFLD on LV geometry. Even in similarly matched groups, there is still a striking difference between the results.
The patients with NAFLD were characterized by significantly lower e’ velocity and higher E/e’ ratio in comparison with age- and sex-matched individuals without NAFLD; suggesting the adverse effects of NAFLD on diastolic indices, which is in concordance with the previous studies.10,15-18,20,21 Using multivariate analysis, Goland et al. and Kim et al. demonstrated that the e’ velocity on TDI was the only independent parameter associated with NAFLD.16,22 Both E/A and E/e’ ratios are reliable markers of LV diastolic function. In the present study, E/e’ ratio was shown to be more sensitive than E/A ratio for the detection of diastolic dysfunction in NAFLD, as identified in other conditions.23,24 GLS, an indicator of systolic function, was decreased in patients with NAFLD compared with those without NAFLD; indicating greater subclinical systolic dysfunction in NAFLD patients. This result is similar to those stated by Singh et al. and VanWagner et al. on decreased value of GLS in adolescents and adults with NAFLD.9,10 The presence of LV systolic dysfunction in patients with NAFLD has been mentioned in previous studies using TDI-derived S’ velocity.16-18,22 However, the comparison of LVEF between the two groups did not reveal any significant difference, illustrating that the use of this conventional tool would result in missing the early stages of LV systolic dysfunction. Similarly, a recent study has revealed slightly smaller values of LVEF only in men with NAFLD.25
Although the pathogenesis of cardiac dysfunction in NAFLD and especially LV systolic dysfunction is still unclear, insulin resistance, abnormal lipid profile, and low-grade inflammation have been suggested to be contributing factors. In patients with NAFLD, the increase in free fatty acids may lead to myocardial lipid deposition, with consequent alterations in LV performance. Furthermore, hepatic steatosis is associated with hepatic insulin resistance causing hyperglycemia and compensatory hyperinsulinemia, which in turn may worsen both systemic and cardiac insulin resistance and subsequent myocardial dysfunction.9,26,27
Despite the above-mentioned advantages of the present investigation, in a highly understudied population for cardiac alterations in NAFLD, this study has some limitation. One limitation is related to the small sample size due to case selection among biopsy-proven NAFLD patients as well as excluding comorbidities and confounding factors to enhance the study power. In addition, most of the enrolled patients were young-aged adults, and therefore the results cannot be generalized to other age groups in the population. Moreover, the potential long-term abnormalities and their clinical consequences were not determined.
Conclusion
The findings of this study support the presence of subclinical cardiovascular structural and functional changes in NAFLD patients. However, further studies with larger and less heterogeneous sample volumes are required to elucidate the relationship between cause and effect as well as the underlying mechanism of cardiac dysfunction in NAFLD.
Acknowledgement
The present article was extracted from the thesis written by Ehsan Samiee and financially supported (grant #93-7380) by Shiraz University of Medical Sciences.
Conflict of Interest:None declared.
References
- 1.Temple JL, Cordero P, Li J, Nguyen V, Oben JA. A Guide to Non-Alcoholic Fatty Liver Disease in Childhood and Adolescence. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17060947. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abd El-Kader SM, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol. 2015;7:846–58. doi: 10.4254/wjh.v7.i6.846. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–71. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 4.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–40. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Bonci E, Chiesa C, Versacci P, Anania C, Silvestri L, Pacifico L. Association of Nonalcoholic Fatty Liver Disease with Subclinical Cardiovascular Changes: A Systematic Review and Meta-Analysis. Biomed Res Int. 2015;2015:213737. doi: 10.1155/2015/213737. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724–45. doi: 10.3748/wjg.v20.i7.1724. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Lu HY. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol. 2014;20:8407–15. doi: 10.3748/wjg.v20.i26.8407. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotte MJ, Germans T, Russel IK, Zwanenburg JJ, Marcus JT, van Rossum AC, et al. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006;48:2002–11. doi: 10.1016/j.jacc.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 9.Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, Magkos F, et al. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr. 2013;162:1160–8, 8 e1. doi: 10.1016/j.jpeds.2012.11.024. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62:773–83. doi: 10.1002/hep.27869. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karabay CY, Kocabay G, Kalayci A, Colak Y, Oduncu V, Akgun T, et al. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26:325–31. doi: 10.1097/MEG.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 12.Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G. A method to assess energy expenditure in children and adults. Am J Clin Nutr. 1983;37:461–7. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Burrage M, Leano R, et al. Left Ventricular Global Longitudinal Strain (GLS) Is a Superior Predictor of All-Cause and Cardiovascular Mortality When Compared to Ejection Fraction in Advanced Chronic Kidney Disease. PLoS One. 2015;10:e0127044. doi: 10.1371/journal.pone.0127044. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sert A, Aypar E, Pirgon O, Yilmaz H, Odabas D, Tolu I. Left ventricular function by echocardiography, tissue Doppler imaging, and carotid intima-media thickness in obese adolescents with nonalcoholic fatty liver disease. Am J Cardiol. 2013;112:436–43. doi: 10.1016/j.amjcard.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 16.Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, et al. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949–55. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 17.Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457–63. [PubMed] [Google Scholar]
- 18.Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, et al. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59:461–70. doi: 10.1002/hep.26610. [DOI] [PubMed] [Google Scholar]
- 19.Petta S, Argano C, Colomba D, Camma C, Di Marco V, Cabibi D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62:928–33. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, et al. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646–53. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–95. doi: 10.2337/dc11-1820. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim NH, Park J, Kim SH, Kim YH, Kim DH, Cho GY, et al. Non-alcoholic fatty liver disease, metabolic syndrome and subclinical cardiovascular changes in the general population. Heart. 2014;100:938–43. doi: 10.1136/heartjnl-2013-305099. [DOI] [PubMed] [Google Scholar]
- 23.Lee SW, Park MC, Park YB, Lee SK. /E’ ratio is more sensitive than E/A ratio for detection of left ventricular diastolic dysfunction in systemic lupus erythematosus. Lupus. 2008;17:195–201. doi: 10.1177/0961203307087303. [DOI] [PubMed] [Google Scholar]
- 24.Lee SW, Choi EY, Jung SY, Choi ST, Lee SK, Park YB. E/E’ ratio is more sensitive than E/A ratio for detection of left ventricular diastolic dysfunction in patients with systemic sclerosis. Clin Exp Rheumatol. 2010;28:S12–7. [PubMed] [Google Scholar]
- 25.Trovato FM, Martines GF, Catalano D, Musumeci G, Pirri C, Trovato GM. Echocardiography and NAFLD (non-alcoholic fatty liver disease) Int J Cardiol. 2016;221:275–9. doi: 10.1016/j.ijcard.2016.06.180. [DOI] [PubMed] [Google Scholar]
- 26.Bugianesi E. Nonalcoholic fatty liver disease (NAFLD) and cardiac lipotoxicity: Another piece of the puzzle. Hepatology. 2008;47:2–4. doi: 10.1002/hep.22105. [DOI] [PubMed] [Google Scholar]
- 27.Di Sessa A, Umano GR, Miraglia Del Giudice E, Santoro N. From the liver to the heart: Cardiac dysfunction in obese children with non-alcoholic fatty liver disease. World J Hepatol. 2017;9:69–73. doi: 10.4254/wjh.v9.i2.69. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


