Abstract
Background:
Mentha mozaffarianii, an endemic species from the Labiatae family, is used in Iranian traditional medicine. This study evaluated the acute and repeated oral toxicity of the Mentha mozaffarianii essential oil (MMEO) in rats and mice.
Methods:
To assess the toxicity profile of the MMEO, we administered the essential oil to 48 rats and mice of both sexes by gavage in acute and repeated models. In acute toxicity, the animals were administered the MMEO (2000 mg/kg) and were monitored for 14 days. In the repeated toxicity, the MMEO was administered (100 mg/kg) daily for 4 weeks. On the 28th day, all the animals were scarified and blood and tissue samples were prepared. All the clinical, biochemical, and histopathological changes were assessed and compared with those in the controls.
Statistical significance was determined by one- and two-way analyses of variance, followed by the Tukey test using GraphPad Prism 6.
Results:
In the acute test, there was no mortality; therefore, the oral LD50 value determined in the mice and rats of both sexes was greater than 2000 mg/kg. In the repeated test, the animals received the MMEO and there was no mortality. In the biochemical analysis, there were significant increases in blood glucose, cholesterol, ALT, AST, ALP, and TSH in the female rats and also in BUN in the male rats. The histopathological studies revealed evidence of microscopic lesions in the liver, kidney, stomach, and small intestine tissues of the MMEO group.
Conclusion:
The results indicated that the acute toxicity of the MMEO in the mice and rats was of a low order and it revealed slight tissue damage to several organs when given subchronically at a dose of 100 mg/kg.
Keywords: Mentha , Mentha mozaffarianii , Essential oil , Toxicity , Rat , Mice
What’s Known
Recently, we demonstrated the antinociception and antiseizure effects of the essential oil from the areal part of the Mentha mozaffarianii essential oil. However, the toxicity of Mentha mozaffarianii has not been studied.
What’s New
In the acute model, a single administration of the essential oil at a dose of 2000 mg/kg did not induce mortality in mice and rats. Thus, the LD50 of the essential oil of Mentha mozaffarianii has been estimated higher than 2000 mg/kg.
However, repeated administrations of the essential oil (100 mg/kg) over a period of 28 days induced important damage in mice and rats. Thus, it would be advisable to use this plant’s essential oil at a dose≤100 mg/kg to limit its adverse effects.
Introduction
Lamiaceae, the alternative name of Labiatae, is represented in Iran by 46 genera and 410 species and subspecies. This family is important for flavor and medicinal properties. Lamiaceae plants are also used as culinary and ornamental plants.1 Aromatic medicinal plants from this family have long been used in Iranian traditional medicine. Mentha species (Mentha spp.) are well-known due to their medicinal and commercial use. Indeed, the aerial parts of Mentha spp. are frequently used as an aromatic in food, tea, or cosmetic and perfumery products.2,3Mentha mozaffarianii (M. mozaffarianii) is an Iranian endemic plant known locally as “Pooneh-Koohi” (Mozaffarian, 2006). This plant is among the medicinal plants that serve as antiseptics and analgesics to treat painful menstruation, dyspepsia, arthralgia, fever, headache, common cold, and wounds in Iranian traditional medicine.4,5 Previous studies have shown that M. mozaffarianii possesses antimicrobial6 and antifungal6 effects. Recently, we demonstrated the antinociception and antiseizure effects of the areal parts of the M. mozaffarianii essential oil (MMEO).7 However the toxicity of M. mozaffarianii has never been studied. There is a paucity of scientific research on the safety of herbal medicines through an assessment of their action mechanisms and toxicological profiles.8,9 There are also only a few reports on the toxicity and mutagenic activity of some Mentha spp. at higher concentrations,10,11 which underscores the need for further knowledge regarding the dose and procedure of applications. In addition, the essential oil of the other Mentha spp. such as M. piperita has exhibited nontoxicity in humans and low toxicity in mice.12 Because the biosynthetic pathways followed in the different Mentha spp. are subjected to varying geographical conditions, they can be divided into menthol-rich, carvone-rich, and pulegone/piperitone-rich essential oils.
Given that the toxicity of a plant is affected by the type of its compounds, the present study sought to investigate the acute and subchronic toxicity effects of the oral administration of the MMEO on mice and rats.
Materials and Methods
Plant collection and identification: Leaves of M. mozaffarianii were collected in March 2014 from the Genow protected area, Bandar Abbas, Hormozgan Province, south of Iran. The leaves were identified by R. Asadpour. A voucher specimen was deposited at the Herbarium of the Department of Pharmacognosy, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran (code #1011-AUPF).
Preparation of the essential oil: Dried leaves were submitted to hydrodistillation in a Clevenger-type apparatus for 3 hours. The oil was collected, dried with anhydrous Na2SO4, and transferred to a clean glass vial and kept at a temperature of −18 °C for further biological and analytical tests.
Analysis of the essential oil: Oil sample analysis was performed on an HP-6890 gas chromatograph equipped with a flame injector detector (FID) and a DB-5 capillary column (30 m×0.25 mm×0.25 μm film thickness). The temperature was programmed as follows: 60 °C to 240 °C at 4 °C/min. The carrier gas was N2 at a flow rate of 2.0 mL/min; the injector port and detector temperature were 250 °C and 300 °C, respectively. The sample was injected by splitting, and the split ratio was 1:10. The gas chromatography-mass spectrometry analysis was performed on a Hewlett-Packard 6890/5972 system with a DB-5 capillary column (30 m×0.25 mm×0.25 μm film thickness). The operating conditions were as described above, but the carrier gas was He. Mass spectra were taken at 70 eV. The scan mass range was 40 to 400 m/z at a sampling rate of 1.0 scan/s. Computable data were obtained from the electronic integration of the FID peak areas. The components of the oils were identified by their retention times, retention indices relative to C9-C28n-alkanes, and computer matching with the WILEY275.L library, as well as by comparison of their mass spectra with data already available in the literature. The percentage composition of the identified compounds was computed from the gas-chromatograph peak areas without any correction factors and was calculated relatively. The result of the oil analysis was the average of 3 replicates.
Animals
The toxicity study of the MMEO was carried out using male and female Wistar rats (150-200 g, 8-12 wk) and NMRI mice (18-25 g, 8-12 wk). All the experiments were conducted during the time period between 9.00 a.m. and 16 p.m. with normal room light and temperature (22±1 °C). All the procedures were carried out in accordance with the National Institute of Health (NIH) guidelines for the care and use of laboratory animals and the Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University (approval #22510603941011).
Acute toxicity study: Acute toxicity was evaluated according to the guideline 423 of Economic Cooperation and Development (OECD).13 The test was carried out on 24 mice and rats, comprising 12 females/males (6 cases and 6 controls). The determined dose of the essential oil was the selected dose of 2000 mg/kg of body weight, which was administered by oral gavage. The treated animals were observed for general behavior changes and physical examination was done to observe death, hair coat, mucus membrane/eye/skin color, body temperature, respiratory rate, lacrimation, salivation, and eye prominence. Mortality was observed continuously for 30 minutes and then intermittently for 4 and 24 hours and then twice daily for 14 days after the administration of the MMEO.
Repeated oral toxicity study: The subchronic oral toxicity test was performed in compliance with the OECD guideline No. 407 (OECD, 2008). After the acute test and the previous pharmacological study, the necessary dose for subchronic toxicity was estimated at a single dose of 100 mg/kg. The male/female mice and rats were divided into 2 groups: control (sweet almond oil) and test (MMEO), each of which consisted of 3 animals per each sex. The test group was daily treated with the oral administration of the MMEO at a dose of 100 mg/kg for 28 days. On day 28th, the animals of the control and test groups were sacrificed. During the experimental period, the behavioral parameters, body weight change, and food intakes were recorded weekly.
Biochemical assays: At the end of the treatment in the repeated toxicity model, the experimental animals were subjected to fasting for 12 hours. The rats were sacrificed by decapitation under anesthesia and their heart blood was collected into dry tubes and centrifuged at 3000 g at 4 °C for 15 minutes. The serum collected was stored at 20°C for biochemical analysis (blood glucose, ALT, AST, ALP, total bilirubin, total cholesterol, creatinine, urea, T3, T4, and TSH) using a commercial kit.
Pathological studies: Heart, kidney, liver, lungs, stomach, small intestine, and spleen were the major organs that were removed from the animals for histopathological studies at the end point of the study. The tissues were fixed in 10% buffered formalin and dehydrated in graded series of alcohol, cleared in xylene, and embedded in paraffin wax. Multiple sections from each block were prepared at a thickness of 5 μm and stained with hematoxylin and eosin (H&E) for histopathological studies.
Statistical Analysis
The data are expressed as the mean±standard error of mean. Statistical significance was determined by one- and two-way analyses of variance, followed by the Tukey test (comparison between the test and control groups) using GraphPad Prism 6. Differences were considered significant at a P value less than 0.05.
Results
Chemical composition of the essential oils: The major components in the tested essential oils are presented in table 1. The aerial parts of the MMEO mainly contained piperitone (51.0%).
Table 1.
Gas chromatography-mass spectrometry analysis of the essential oil of the aerial parts of Mentha mozaffarianii
| Compounda | KIb | KIc | Percentage |
|---|---|---|---|
| α-Pinene | 938 | 939 | 0.6 |
| Camphene | 952 | 954 | 0.2 |
| Sabinene | 971 | 975 | 0.5 |
| β-Pinene | 977 | 979 | 1.0 |
| Myrcene | 990 | 991 | 0.3 |
| Ocymene | 998 | 999 | 0.6 |
| Limonene | 1028 | 1029 | 0.4 |
| 1,8-Cineol | 1033 | 1031 | 11.7 |
| Linalool | 1097 | 1097 | 11.1 |
| Menthone | 1149 | 1153 | 1.9 |
| δ-Terpineol | 1162 | 1166 | 0.3 |
| Borneol | 1165 | 1169 | 1.0 |
| 4-Terpineol | 1178 | 1177 | 0.2 |
| α-Terpineol | 1190 | 1189 | 3.4 |
| Pulegone | 1237 | 1237 | 0.3 |
| Piperitone | 1251 | 1253 | 51.0 |
| Thymol | 1290 | 1290 | 1.0 |
| Piperitenone | 1339 | 1343 | 8.6 |
| Piperitenone oxide | 1371 | 1369 | 2.3 |
| Trans-Jasmone | 1390 | 1391 | 1.9 |
| β-Caryophyllene | 1419 | 1419 | 0.8 |
| Bicyclogermacrene | 1500 | 1500 | 0.3 |
| Total | 99.4 | ||
Compounds listed in order of elution.
KI (Kovats index) measured relative to n alkanes (C9 C28) on the non polar DB 5 column under condition listed in the experimental section.
KI: (Kovats index) from literature
Acute Toxicity of the Mentha mozaffarianii Essential Oil
In mice: The MMEO at the dose of 2000 mg/kg had no adverse effect on the behavioral responses of the tested mice up to 14 days of observation. Physical observations indicated no signs of changes in the skin, fur, eyes, mucous membrane, behavior patterns, tremors, salivation, and diarrhea of the mice. There was no mortality observed at the tested dose. In the acute test, there were slight changes in body weight and food consumption in the male mice during the acute test (figures 1A and 1B).
Figure1.
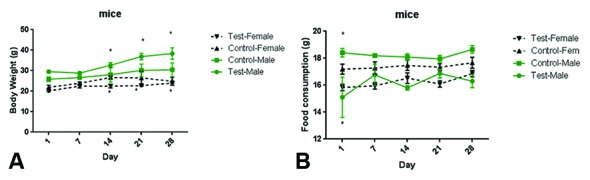
Effects of the acute oral dose (2000 mg/kg) of the Mentha mozaffarianii essential oil on the evolution of bodyweight (A) and food intake (B) in the mice. Data are expressed as mean±SEM; n=3.
In rats: No abnormalities in the general behavior or death were observed on the 1st day in the group of the female rats that were given the MMEO at the dose of 2000 mg/kg of body weight. Abnormalities occurred in the male rats that had taken the MMEO in the form of symptoms such as hypoactivity and drowsiness. In the acute test, there were no changes in body weight and food consumption in the male and female rats compared to the controls. There was no mortality observed at the tested dose, and nor was there any weight loss in the rats. Moreover, there was no mortality in both rats and mice; therefore, the oral LD50 value of the MMEO was greater than 2000 mg/kg.
Clinical Signs, Body Weight Gain, and Food Consumption in the Repeated Oral Administration of the Mentha mozaffarianii Essential Oil
In mice: The daily oral administration of the MMEO (100 mg/kg) for 28 days did not induce any obvious symptom of toxicity in the mice of both sexes. No deaths or obvious clinical signs were found in either group throughout the experimental period. The physical observation of the treated rats throughout the study indicated that none of them showed signs of toxicity in their skin, fur, eyes, or mucus membrane. In addition, there were no behavioral changes, diarrhea, tremors, salivation, sleep, and coma. Normal body weight gain was observed during the study period compared to the control group. The food consumption of the treated mice, measured throughout the study, was not significantly different from the control mice. Nonetheless, at the higher dose (200 mg/kg), some of the animals died on the 5th day.
In rats: All the rats that were given the MMEO at the dose of 100 mg/kg survived, and the normal behavior exhibited on the 1st day continued throughout the 28 days of experiment. Normal body weight gain and food consumption were observed during the study period compared to the control group.
Clinical Biochemistry Analysis
In mice: The blood biochemical studies failed due to problems in sample collection and insufficient blood volumes obtained from the mice.
In rats: The effects of the subchronic administration of the MMEO on some biochemical parameters are presented in table 2. The blood sugar and cholesterol levels in the treated female rats were significantly higher than those in the control group. Moreover, the essential oil had no effect on the serum triglyceride, cholesterol, and blood glucose in the male rats.
Table 2.
Effects of the Mentha mozaffarianii essential oil on some serum biochemical parameters in repeated toxicity in the rats
| Animals Parameters | Rats | |||
|---|---|---|---|---|
| Female | Male | |||
| Test | Control | Test | Control | |
| Blood glucose (mg/dL) | 244.7±14.53** (P=0.0041) | 119.0±15.53 | 455±143.0 | 167±26.50 |
| Total cholesterol (mg/dL) | 90.00±4.16* (P=0.0162) | 72.00±1.73 | 78.00±3.0 | 71.50±4.50 |
| TG (mg/dL) | 75.00±4.04 | 69.33±1.76 | 182.5±67.50 | 83.00±6.00 |
| ALT IU/l | 204.7±1.85** (P=0.0043) | 142.7±10.48 | 233.0±10.00 | 188.5±23.50 |
| AST IU/l | 254.00±5.56**** (P=0.0001) | 54.00±3.055 | 55.50±5.50 | 43.00±9.00 |
| ALP IU/l | 707.0±17.62**** (P=0.0001) | 234.3±21.18 | 471.5±30.50 | 141.6±116.2 |
| BUN (mg/dL) | 64.33±4.25 | 48.33±4.910 | 81.00±8.00* | 38.50±3.50 |
| Cr (mg/dL) | 0.700±0.00 | 0.53±0.66 | 0.85±0.050 | 0.40±0.10 |
| T3 IU/l | 8.073±0.50 | 7.213±0.02 | 9.635±1.22 | 7.350±0.11 |
| T4 IU/l | 1.323±0.02 | 1.730±0.2 | 1.385±0.01 | 1.380±0.13 |
| TSH IU/l | 1.237±0.08** (P=0.0022) | 1.887±0.02 | 1.085±0.18 | 1.145±0.13 |
The essential oil of the aerial parts of Mentha mozaffarianii was given daily by the oral route at the dose of 100 mg/kg over a 28-day period. Serum biochemical parameters were measured at the end of the experimental period. Data are expressed as mean±SEM; n=3.
P<0.05.
P<0.01.
P<0.001.
P<0.0001 compared to the control group
The kidney function parameters (BUN and creatinine) did not reveal any relevant changes following the administration of the essential oil in the female rats, while in the male rats the BUN level was higher than that in the control group (table 2). In the female rats, there was a significant increase in the liver function parameters (ALP, ALT, and AST). No statistically significant differences in the liver function parameters (ALT, AST, and alkaline phosphatase) were noted in the male rats (table 2). The effects of the MMEO on circulating thyroid hormone levels in the rats revealed a significant decrease in the TSH level in the female rats. No statistically significant differences were noted in T3 and T4 in the female/male rats and TSH in the male rats (table 2).
Histopathology: The histopathological examination of the control and treated rats showed normal structure and absence of any gross pathological lesion in the organs. The macroscopic examination of the vital organs of the treated animals revealed no abnormalities in the color or texture when compared with the organs of the control group. Chemically induced histological changes were not seen in the heart, lung, and spleen in the test group. However, mild changes were present in the liver, kidney, and small intestine in the MMEO-treated rats (figures 2, 3 and 4). Apropos of the liver, the parenchymal cells of the liver had a cord-like arrangement of hepatocytes radiating outward from a central vein and sinusoid structure, whereas in some of the MMEO-treated animals the arrangement of the hepatocytes around the central vein and sinusoid structures was disturbed (figure 2). From a clinical perspective, the renal corpuscle is probably the most significant histological feature of the kidney. In these cases, the renal corpuscle was disturbed and most of the tissue contained collecting tubules, while the renal cortex mostly contained convoluted tubules (figure 3). The small intestine wall has 4 layers: outermost serosa, muscularis, submucosa, and innermost mucosa. The intestinal villi constitute a part of the mucosa and have simple columnar epithelia; nevertheless, in the current study, there were no villi in the mucosal layers in some sections (figure 4).
Figure2.
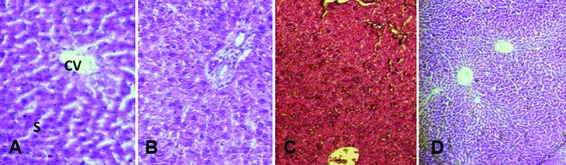
Microscopic photograph of the liver in the female (A) and male (B) test group of rats and female (C) and male (D) test group of mice that received the repeated dose (100 mg/kg) of the Mentha mozaffarianii essential oil for 28 days. A and D: Apropos of the liver, the parenchymal cells of the liver normally have a cord-like arrangement of hepatocytes radiating outward from a central vein (CV) and sinusoid (S) structure. However, in B and C, the arrangements of the hepatocytes around the CV and S are disturbed.
Figure3.
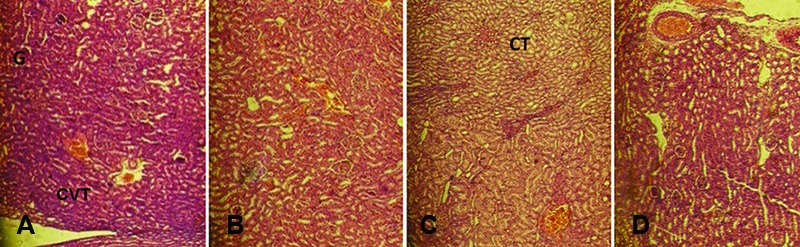
Microscopic photograph of the kidney in the female (A) and male (B) test group of rats and female (C) and male (D) mice that received the repeated dose (100 mg/kg) of the Mentha mozaffarianii essential oil for 28 days. From a clinical perspective, the renal corpuscle (G) is probably the most significant histological feature of the kidney. In these cases, the G was disturbed and most of the tissue contained collecting tubules (CTs), while the renal cortex mostly contained convoluted tubules (CVTs).
Figure4.
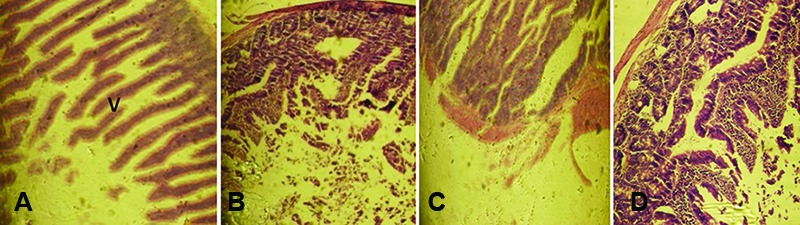
Microscopic photograph of the small intestine in the female (A) and male (B) test group of rats and female (C) and male (D) test group of mice that received the repeated dose (100 mg/kg) of the Mentha mozaffarianii essential oil for 28 days. The intestinal villi (V) are part of the mucosa and have simple columnar epithelia. However, in this study, there were no villi (V) in the mucosal layer (B, C, and D).
Discussion
The main objective of the present study was to examine the acute and repeated toxicity of the MMEO. The results showed that the acute dose of the MMEO had slight toxicity, while the repeated usage of the essential oil had some toxic effects.
M. mozaffarianii Jamzad, an Iranian endemic plant, is commonly used in Iranian traditional medicine for its potent therapeutic effects. As the safety profile of this plant in acute and repeated tests had yet to be determined, we undertook the present study and succeeded in demonstrating the safety profile of this plant in both models of toxicity assessment. Given that we observed no deaths and no serious signs of toxicity in the first 24 hours following the MMEO administration at the dose of 2000 mg/kg, we can classify this essential oil as Category 5 material according to the Globally Harmonized System of Classification and Labeling of Chemicals criteria.14 The criteria for Category 5 are intended to enable the identification of substances that are of relatively low acute toxicity hazard, and the MMEO was anticipated to have an LD50 higher than 2000 mg/kg, which is not hazardous in acute doses in mice and rats. Nevertheless, taking a single dose of the MMEO (2000 mg/kg) induced hypoactivity and drowsiness 4 hours after its administration in the rats. These consequences suggest that rats are more sensitive than mice to the central effects of the essential oil. These results could be explained by the presence in the essential oil of sedative compounds, which could act on the central nervous system. Indeed, preliminary qualitative gas chromatography-mass spectrometry revealed the presence of 22 components, accounting for about 99.4% of the total chromatographically materials. The 3 major identified compounds were piperitone (51%), 1,8-cineol (11.7%), and linalool (11.1%); some of these compounds are known to have depressive activity on the central nervous system.15,16
Since the essential oils of plants can be administered orally in some therapeutic applications, it is advisable that their safety profiles be assessed and recorded in repeated-dose studies.7 Having observed the therapeutic effects at a dose of 100 mg/kg in a previous study, we started the subchronic test at a dose of 100 mg/kg/d. Although this dose appeared to be safe in the 28-day study period, it exhibited some toxic effects in each sex and species at the end of the study period. In repeated treatment, the administration of the essential oil at the dose of 100 mg/kg did not cause significant changes in food consumption and body weight in the rats. Nonetheless, repeated oral administrations to the mice produced a reduction in food intake in both sexes, which may have been brought about by loss of appetite in the animals. These results show the role of animal species in different responses to essential oil toxicity.
The subchronic administration of the oil for 28 days led to an increase in the glucose and cholesterol levels in the female rats. Moreover, the essential oil had no effect on the serum triglyceride level in the rats. A previous subchronic toxicity study of the M. piperita essential oil also revealed a significant rise in total cholesterol, triglyceride, and high-density lipoprotein in rats.17 The difference between our results and those previously reported in the literature may be due to dissimilar components in the different Mentha spp.
It can also be concluded from the results of the present study that the MMEO (100 mg/kg) in repeated doses could be toxic, especially in some organs of female rats. In the female rats, an increase in the serum ALT activity level was associated with histological changes in the liver, revealing the mild hepatotoxicity potential of the MMEO. Our subchronic oral dosage regimen of the essential oil in the rats appeared to adversely affect the kidney and led to an increase in the level of BUN (in the males) and mild histological changes. The difference in the biochemistry profile observed between the males and females may have been due to hormonal variations. These results suggest that females are more susceptible than males. The histological analysis of the different organs showed slight microscopic tissue damage to the liver, kidney, stomach, and small intestine tissues of the MMEO group at the repeated dose of 100 mg/kg. Part of the observed organ toxicity effects could have been related to the presence of pulegone in the MMEO, which accounted for 0.3% of the total chromatographic materials.
There are limited comparison studies as regards the different toxicity levels of herbal essential oils in animal species. The salient advantage of the current study is that we evaluated the toxicity of the MMEO in 2 species of mice and rats of both sexes, producing useful information vis-à-vis the impact of species and sexes on the difference in the toxicity of this essential oil.
Reports on the toxicity of the different Mentha spp. are contradictory. A previous toxicity study on the essential oil of M. piperita L. showed that it was able to cause immediate sedation effects, closed eyes, accelerated breathing, and diarrhea in mice.12 Also reported were changes in the body weight of the animals, secondary to reduced food consumption following the ingestion of the oil. The calculated LD50 value for M. piperita L. is reported to be 1612.45 mg/kg, with confidence limits of 1461.41 mg/kg and 1779.11 mg/kg. In a study, linalool (60.72%) was characterized as the major compound in the essential oil of Mentha piperita L.12 Elsewhere, the acute toxicity investigations of the methanolic extracts of M. suaveolens and M. arvensis revealed a lack of toxicity and reported LD50 values (i.p.) at doses lower than 3000 mg/kg and 1000 mg/kg, respectively.3,18
The essential oil obtained from Iranian and Spanish collections of M. longifolia was reported to have an LD50 value of 470 mg/kg and 437.4 mg/kg in rats and mice, correspondingly.19,20 However, it is deserving of note that LD50 information relating to this herb was not reported by others.21 There is a direct relationship between the components of a plant and its toxicity and different Mentha spp. could have a toxicity range.
The anti-inflammatory, antinociception, antiseizure, and antibacterial effects of the MMEO have been shown in several pharmacological studies.6,7 The current study suggests daily doses of less than 100 mg/kg for the long-term administration of the MMEO in different oral dosage forms. Hence, it is necessary to establish the scientific basis for the therapeutic actions of this folk medicine since it may serve as the source for the development of more effective drugs.
The main limitation of our study is that we examined the changes in blood biochemical parameters in the subchronic toxicity model only in the rats. In future studies, a simultaneous assessment of blood biochemical and histological changes should be undertaken to evaluate the organ toxicity of the MMEO in mice.
Conclusion
The findings of the present study demonstrated that the MMEO was slightly toxic with LD50 greater than 2000 mg/kg. However, the repeated administration of the MMEO over a period of 28 days and at a relatively lower dose induced important damage in the rats and mice. At the dose of 100 mg/kg, there were significant changes in some biochemical parameters and histological analyses. Thus, it would be prudent to use the MMEO at a maximum dose of 100 mg/kg in order to limit its adverse effects. These results confer additional evidence that essential oils, if applied at non-recommended doses, can cause functional damage to critical organs such as the liver, kidney, stomach, and small intestine in animals and probably in humans. Overall, in this study, the mice and rats appeared to tolerate the MMEO at the dose of 2000 mg/kg of body weight well.
Acknowledgement
This work was supported by the Pharmaceutical Sciences Branch of the Islamic Azad University, Tehran, Iran. We would like to thank Ms. Amiri at the Toxicology Lab of Pharmaceutical Sciences Branch for her support.
Conflict of Interest:None declared.
References
- 1.Naghibi F, Mosaddegh M, Mohammadi Motamed M, Ghorbani A. Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iran J Pharm Res. 2010;4:63–79. [Google Scholar]
- 2.Hajlaoui H, Trabelsi N, Noumi E, Snoussi M, Fallah H, Ksouri R, et al. Biological activities of the essential oils and methanol extract of tow cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J Microbiol Biotechnol. 2009;25:2227–38. doi: 10.1007/s11274-009-0130-3. [DOI] [Google Scholar]
- 3.Moreno L, Bello R, Primo-Yufera E, Esplugues J. Pharmacological properties of the methanol extract from Mentha suaveolens Ehrh. Phytother Res. 2002;16:S10–3. doi: 10.1002/ptr.744. [DOI] [PubMed] [Google Scholar]
- 4.Tavakkoli-Khaledi S, Asgarpanah J. Essential Oil Chemical Composition of Mentha mozaffarianii Jamzad Seeds. J Mex Chem Soc. 2016;60:19–22. [Google Scholar]
- 5.Mozaffarian V. A dictionary of Iranian plant names. Tehran: Farhang Mosavar Publ; 2006. [Google Scholar]
- 6.Arman M, Yousefzadi M, Khademi SZ. Antimicrobial activity and composition of the essential oil from Mentha mozaffarianii. Journal of Essential Oil Bearing Plants. 2011;14:131–5. doi: 10.1080/0972060X.2011.10643912. [DOI] [Google Scholar]
- 7.Sam-Dahri H, Mousavi Z, Naderi N, Asgarpanah J. Chemical composition and analgesic activity of the essential oil of Mentha mozaffarianii jamzad leaves. Bulgarian Chemical Communications. 2016;48:641–5. [Google Scholar]
- 8.Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33:179–89. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 9.Chan TY. Monitoring the safety of herbal medicines. Drug Saf. 1997;17:209–15. doi: 10.2165/00002018-199717040-00001. [DOI] [PubMed] [Google Scholar]
- 10.Franzios G, Mirotsou M, Hatziapostolou E, Kral J, Scouras ZG, Mavragani-Tsipidou P. Insecticidal and genotoxic activities of mint essential oils. J Agric Food Chem. 1997;45:2690–4. doi: 10.1021/jf960685f. [DOI] [Google Scholar]
- 11.Gardiner P[Internet] Peppermint (Mentha piperita): Longwood Herbal Task Force. c2000. [cited 2016 May 10] . Available from: [www.longwoodherbal.org/peppermint/peppermint.pdf. ]
- 12.Debbab A, Mosaddak B, Aly A, Hakiki A, Mosaddak M. Chemical characterization and toxicological evaluation of the essential oil of Mentha piperita L. growing in Morocco. Science Study Resources. 2007;8:281–8. [Google Scholar]
- 13. Organisation for Economic Co-operation and Development. Oecd guideline for testing of chemicals. Paris: OECD; 2001. [Google Scholar]
- 14.Delazar A, Delnavazi MR, Nahar L, Moghadam SB, Mojarab M, Gupta A, et al. Lavandulifolioside B: a new phenylethanoid glycoside from the aerial parts of Stachys lavandulifolia Vahl. Nat Prod Res. 2011;25:8–16. doi: 10.1080/14786411003754330. [DOI] [PubMed] [Google Scholar]
- 15.Elisabetsky E, Marschner J, Souza DO. Effects of Linalool on glutamatergic system in the rat cerebral cortex. Neurochem Res. 1995;20:461–5. doi: 10.1007/BF00973103. [DOI] [PubMed] [Google Scholar]
- 16.Linck VM, da Silva AL, Figueiro M, Caramao EB, Moreno PR, Elisabetsky E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine. 2010;17:679–83. doi: 10.1016/j.phymed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Sharafi SM, Rasooli I, Owlia P, Taghizadeh M, Astaneh SD. Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn Mag. 2010;6:147–53. doi: 10.4103/0973-1296.66926. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagetia GC, Baliga MS. Influence of the leaf extract of Mentha arvensis Linn. (mint) on the survival of mice exposed to different doses of gamma radiation. Strahlenther Onkol 2002;178:91–8. doi: 10.1007/s00066-002-0841-y. [DOI] [PubMed] [Google Scholar]
- 19.Raya M, Utrilla M, Navarro M, Jimenez J. CNS activity of Mentha rotundifolia and Mentha longifolia essential oil in mice and rats. Phytother Res. 1990;4:232–4. doi: 10.1002/ptr.2650040606. [DOI] [Google Scholar]
- 20.Jalilzadeh-Amin G, Maham M. Antidiarrheal activity and acute oral toxicity of Mentha longifolia L. essential oil. Avicenna J Phytomed 2015;5:128–37. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 21.Odeyemi OO, Yakubu MT, Masika PJ, Afolayan AJ. Toxicological evaluation of the essential oil from Mentha longifolia L. subsp. capensis leaves in rats. J Med Food. 2009;12:669–74. doi: 10.1089/jmf.2008.0136. [DOI] [PubMed] [Google Scholar]


