Abstract
Background:
Perianal fistula is a complicated disorder and most difficult to manage. New treatment methods would help surgeons to achieve a better outcome in patients with perianal fistula. Human amniotic membrane (HAM) has positive effects on wound healing in several conditions. The present study aimed to further determine the effect of HAM on wound healing of perianal fistula in rabbits.
Methods:
In a prospective experimental study, 14 male rabbits (aged 4-6 months and weighing 3-4 kg) were randomly divided into 2 groups. After 12 weeks, the high type perianal fistula was repaired with endorectal flap (ERF) and ERF plus HAM in the control and case groups, respectively. In all rabbits of the case group, a 1×1 cm width wrap of HAM was applied and fixed around the ERF site. Three weeks later, the repaired site of the perianal fistula was sent for pathologic wound healing scoring. The results were analyzed with the SPSS 21.0 software using Mann-Whitney test.
Results:
Six rabbits of each group survived the study period. There was a statistically significant difference in wound healing between the case and control groups (P<0.001). Wound healing process in the case group occurred better and faster than the control group.
Conclusion:
HAM has an effective role in enhancing the ERF procedure and considered appropriate. A combination of HAM with other methods is recommended.
Keywords: Rectal fistula , Amniotic , Surgical flaps
What’s Known
Human amniotic membrane (HAM) has already been applied in multiple sites of the gastrointestinal tract, such as the duodenum, colon, and rectovaginal fistula.
HAM has shown significant effects on wound healing.
What’s New
Based on histological findings and surgical outcomes, HAM placement has an effective role in healing enhancement of the endorectal flap procedure.
Introduction
An anorectal fistula is one of the inflammatory disorders that involves epithelium of the anal canal and perineum or perianal skin. Perianal fistula has significant implications on patients’ quality of life and its squeal range from minor pain and social hygienic discomfiture to frank sepsis. Anal canal has 1012 to 1014 bacteria/mg faeces and the incidence of an anal fistula that originate from an anal abscess is usually 26-38%1 and the mean age of onset is 40 years old (range 20-60).2 Anorectal abscess is the most common cause of an anorectal fistula. Other causes of anorectal fistulas include Crohn’s disease,3 lymphogranuloma venereum, radiation proctitis, and rectal foreign bodies.4,5
The management of anal fistula is an important challenge in colorectal surgery. The surgery is based on therapy with the final goal of draining the local infection, eradicating the fistulous tract, and avoiding recurrence while preserving native sphincter function.6,7 Biologic dressing to enhance wound healing is usually used. Human amniotic membrane (HAM) includes three elements, namely an epithelial layer, a basement membrane, and a stroma which makes the inner layer of the placenta. HAM has key features, including anti-inflammatory, antimicrobial, antifibrosis, antiscarring, low immunogenicity, and high potency of differentiation. HAM as a biologic matter has been used for wound healing of abdominal adhesiolysis, tenolysis, neurolysis, and injuries of the dura-mater and vagina for many years.8,9
The precise mechanism of HAM remains unidentified. Various mechanisms of anti-inflammatory effect of HAM such as decreasing lipid peroxidation, inducing apoptosis, and inhibiting the chemotactic activity of polymorphonuclear neutrophil (PMN) have been proposed.10 HAM has been investigated and demonstrated significant effects on wound healing of rectovaginal fistula, gastrointestinal tract, colon, and duodenum.10,11 Endorectal flaps are used to protect wound healing with 70-80% success rate. In the present study, for the first time, we aimed to examine the effect of HAM on wound healing process in perianal fistula.
Materials and Methods
Animals and Ethical Considerations
In collaboration with the Animal Laboratory of Shiraz University of Medical Sciences (Shiraz, Iran), 14 rabbits were selected and initially evaluated by a veterinarian surgeon for any underlying problems. The age of the rabbits was 4-6 months, weighing 3-4 kg, and kept in standard cages at 20-24 °C, 55±5% humidity, 12 times per hour ventilation, and 12:12 hours light dark cycle. The rabbits received standard chow and water ad libitum. The Animal Laboratory of Shiraz University of Medical Sciences supervised the procedures, preoperative, and postoperative cares.12 The HAM was provided by Shiraz Burn Research Center, Shiraz Zeinab Hospital (Shiraz, Iran) and primarily evaluated for viral markers (HIV, HCV, and HBV). It was then preserved in gluteraldehyde and frozen at 20 °C. The study was approved by the Research Vice Chancellor and the Ethics Committee of Shiraz University of Medical Sciences (number 7172), Shiraz, Iran.
Experimental Design
Step 1: The rabbits were randomly divided into two groups, namely case group (n=7) and control group (n=7). Following 10-20 minutes intravenous injection of Ketamine-Xylazine (50 mg/kg), the animals underwent surgical high type fistula tract creation by clamp and electrocutery. A short French tube (number 10) was inserted into the fistula and the internal and external orifice of the fistula tract was marsupialized with Vicryl 4-0.
Step 2: Twelve weeks later, after intravenous sedation by Ketamine-Xylazine (50 mg/kg administered 10-20 minutes in advance) followed by preparation and draping, ERF was made in the control rabbits using prolene 4.0 and ERF plus HAM was applied in the case group. A 1×1cm width wrap of HAM was sutured around the ERF site and fixed by Vicryl 4-0 with simple interrupted sutures. Surgical diet was started after 24 hours in both groups. The protocol of anesthesia and all procedures, preoperative care, and postoperative care were similar for all the rabbits.
Step 3 (follow-up analysis of anastomosis): The surviving rabbits were sacrificed after 3 weeks and perianal site surgery specimens were resected and fixed in formaldehyde 10% solution (formalin). All samples were labeled blindly and sent to the Histopathology Department of Faghihi Teaching Hospital, Shiraz, Iran.
Pathological Evaluation
Histopathological assessment was performed after tissue processing and slide preparation with hematoxylin and eosin staining by a single pathologist in the department of histopathology. A modified scoring system for surgical wound healing13 was used to determine healing grade in each sample. Scoring was performed according to multiple factors such as epithelialization, inflammation, neovascularization, necrosis, and granulation tissue formation (table 1).
Table 1.
Modified scoring system for wound healing
| Score tissue | Epithelialization | Collagenization | Inflammation | Neovascularization | Necrosis | Granulation |
|---|---|---|---|---|---|---|
| 1 | None | None | Severe | None | Extensive | None |
| 2 | None | None | Moderate | None | Focal | Immature |
| 3 | Partial | Partial | Mild | <5/HPF | None | Mild mature |
| 4 | Complete, immature | Complete, non-irregular | None | 6-10/HPF | None | Moderately mature |
| 5 | Complete, mature | Complete, regular | None | >10/HPF | None | Fully mature |
HPF: High power field
Statistical Analysis
The statistical SPSS software version 21.0 for Windows (SPSS, Chicago, IL) was used to perform the analyses with Mann-Whitney U test. Two-tailed P values less than 0.05 were considered statistically significant.
Results
Macroscopic Evaluation
After 12 weeks, no surgical site infection was found in all rabbits (case and control groups). Internal orifice perianal fistula sites were resected and prepared for pathologic evaluation.
Pathological Evaluation
Pathological evaluation revealed no significant difference between the case and control groups, possibly due to the small sample size. The mean scores for each histological item are presented in table 2.
Table 2.
The mean of wound healing score in the two groups
| Groups | Case | Control | P value |
|---|---|---|---|
| Epithelialization | 3.57 | 3.00 | 0.35 |
| Collagenization | 4.14 | 3.71 | 0.20 |
| Inflammation | 1.86 | 2.28 | 0.09 |
| Neovascularization | 1.86 | 1.57 | 0.42 |
| Necrosis | 0.28 | 0.71 | 0.24 |
| Granulation | 3.28 | 3.71 | 0.41 |
The pathological evaluation revealed more collagenization, neovascularization, and epithelialization as well as less tissue necrosis, inflammation, and granulation tissue formation in the case group compared with the control group. As shown in figure 1, clinical wound healing was more rapid in the HAM group.
Figure1.
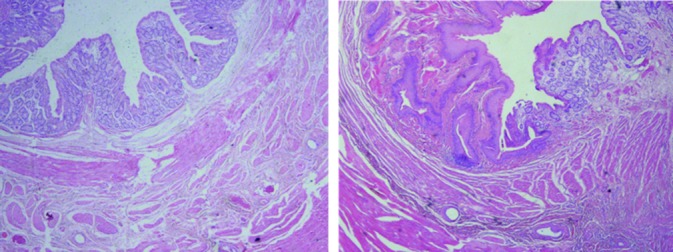
Shows healing of fibromuscular tissue after using HAM (H&E, ×40).
Discussion
The present experimental study was performed as the first investigation to assay the effects of HAM on wound healing and ERF in an animal model. Better histopathological results were found in the HAM group. There was no incident of wound infection, one of the most serious complications, in the control and case groups. One rabbit of each group expired during the study. In the HAM group, no evidence of ERF rejection due to infection was detected.
Various treatment approaches are primary fistulotomy, fibrin sealant injection, draining setons to preserve the sphincter mechanism, endoanal advancement flaps, fibrin glue, fistula plugs, modified Hanley procedure, ligation of the intersphincteric fistula tract, or diversion.14-16 In a study by Uludag et al.,8 HAM had a preventive effect on the colonic anastomosis leakage. In the present study, pathological evaluation revealed more collagenization, neovascularization, epithelialization, and less tissue necrosis and granulation tissue formation in the case group compared with the control group. A range of studies assessed the effect of HAM on wound healing. In a study by Ghahramani et al.,15 dogs were a candidate for duodenorraphy and HAM patch was used for wound repair. In gross evaluation with/without HAM, no difference in duodenal diameter was reported after the operation. However, in pathological evaluations, they revealed less inflammation and a better wound healing with HAM.9 In another study, HAM as a bioprosthesis was used with TachoSil® for the repair of rectovaginal fistula in a rabbit model. After 4 weeks, the histopathological results showed differences in fistula healing between the rabbits. The most rectovaginal fistula repair was related to HAM and TachoSil® did not have additional healing effect (P>0.05).11
In an animal study, Barlas et al.17 evaluated the effectiveness of HAM as an intestinal patch and neomucosal growth was mentioned. They reported that after two weeks, the neomucosa consisted of a thin layer of columnar epithelial cells that covered all the patches. In an experimental study on dogs, Najibpour et al.18 indicated that the use of HAM results in a better colonic anastomosis and wound healing outcome. The left colon of dogs was resected and end-to-end anastomosis was performed in a single layer. HAM patch was used around the anastomotic line and the result showed significantly higher healing score in the HAM group (P=0.01).
Roshanravan et al.19 mentioned better outcomes when HAM was used as a bioprosthesis to repair the rectovaginal fistula in dogs. After 6 weeks, similar to previous and current investigations, there was no difference in the healing of fistula with or without HAM as a gross evaluation. However, the histopathological evaluation revealed significantly higher healing score in the HAM group (P=0.029).
A study on rats revealed that the wound healing process was accelerated due to the HAM effect on duodenal wall structure.20 The rats were subjected to duodenotomy and HAM was used on duodenal wound in one group. No changes were observed regarding the formation of duodenal fistula or peritoneal adherences in the region of duodenal wall repair. Schimidt et al. reported that regeneration of the mucosa and smooth muscle layer was increased and the healing of duodenal wall with HAM was better. 20
Kuriu et al.21 demonstrated that HAM had a curing effect on the intraperitoneal adhesion in rats. They observed severe adhesions between the cecum and surrounding organs after 1 week of surgical trauma without HAM, while it was significantly reduced by HAM treatment. Histological evaluation showed a layered structure that consisted of myofibroblasts attached to the surfaces of HAM grafts. HAM was well absorbed after 10 weeks and it served as a substrate for regenerating mesothelium.
Some studies showed that the use of HAM might play a significant role and a feasible technical approach to prevent post-irradiation colorectal anastomosis leakage.22,23 In a study, animals underwent resection of colorectal segment and end-to-end anastomosis four weeks after irradiation. Then, HAM was applied around the site of anastomosis. A segment of anastomotic sites was obtained 8 weeks later for pathological evaluation and significant epithelialization and neovascularization with HAM demonstrated. The healing score was higher with HAM (P<0.001). Moslemi et al. suggested that the use of HAM could be protective from anastomosis leakage in post irradiation.22
In another animal study, from a total of 10dogs, 5 received 10 Gy of external radiation preoperatively while the other 5 received no radiation. Then, end-to-end anastomosis was done on the bowel remnant and HAM was used around the anastomosis. Tahamtan et al. demonstrated the ulceration of the mucosa with infiltration and granulation tissue formation. They showed that fibrotic tissue was formed between the serosa and HAM graft and suggested that complications of radiotherapy and loop ileostomy could be resolved with the application of HAM and eliminate the need for ileostomy insertion.23
Uludag et al.8 showed that HAM reduced inflammation and increase wound healing process. Additionally, inflammatory cytokines suppressed, tissue growth factor and anti-inflammatory proteins expressed, and fibroblast activity and angiogenesis increased. In the present study, granulation tissue formation was less in the case than the control group. The healing process in rabbits, due to their different immune system, is faster than humans. After 12 weeks, only the marked inner orifice was found and ERF was performed. The external orifice was healed in most rabbits.
The limitation of the present study is related to the small size of the animals. It is recommended to apply this method to large animals and even human beings. It should be noted that risk factors such as diabetes mellitus, obesity, anemia, and smoking could affect the results of human perianal fistula. Evaluation of these risk factors is recommended in future research.
Conclusion
In the present study, the effect of HAM on the ERF procedure was investigated on rabbits. The pathological evaluation revealed no statistically significant difference between the case and control groups. However, HAM placement had an effective role in enhancing the ERF procedure and showed a better histological findings and surgical outcomes in the HAM group.
Acknowledgement
The authors would like to thank the Vice Chancellor of Shiraz University of Medical Sciences for supporting the research (grant number: 7172)
Conflict of Interest:None declared.
References
- 1.Williams JG, Farrands PA, Williams AB, Taylor BA, Lunniss PJ, Sagar PM, et al. The treatment of anal fistula: ACPGBI position statement. Colorectal Dis. 2007;9:18–50. doi: 10.1111/j.1463-1318.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 2.Gurer A, Ozlem N, Gokakin AK, Ozdogan M, Kulacoglu H, Aydin R. A novel material in seton treatment of fistula-in-ano. Am J Surg. 2007;193:794–6. doi: 10.1016/j.amjsurg.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 3.Hasan RM. Incidence of fistula after management of perianal abscess. Journal of Coloproctology. 2016;36:216–9. doi: 10.1016/j.jcol.2016.05.002. [DOI] [Google Scholar]
- 4.Niyogi A, Agarwal T, Broadhurst J, Abel RM. Management of perianal abscess and fistula-in-ano in children. Eur J Pediatr Surg. 2010;20:35–9. doi: 10.1055/s-0029-1241878. [DOI] [PubMed] [Google Scholar]
- 5.Safar B, Sands D. Perianal Crohn’s disease. Clin Colon Rectal Surg. 2007;20:282–93. doi: 10.1055/s-2007-991027. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg JE, Steele SR. Rectal foreign bodies. Surg Clin North Am. 2010;90:173–84. doi: 10.1016/j.suc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Kurer MA, Davey C, Khan S, Chintapatla S. Colorectal foreign bodies: a systematic review. Colorectal Dis. 2010;12:851–61. doi: 10.1111/j.1463-1318.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 8.Uludag M, Citgez B, Ozkaya O, Yetkin G, Ozcan O, Polat N, et al. Effects of amniotic membrane on the healing of primary colonic anastomoses in the cecal ligation and puncture model of secondary peritonitis in rats. Int J Colorectal Dis. 2009;24:559–67. doi: 10.1007/s00384-009-0645-y. [DOI] [PubMed] [Google Scholar]
- 9.Ghahramani L, Jahromi AB, Dehghani MR, Ashraf MJ, Rahimikazerooni S, Rezaianzadeh A, et al. Evaluation of repair in duodenal perforation with human amniotic membrane: An animal model (dog) Adv Biomed Res. 2014;3:113. doi: 10.4103/2277-9175.131029. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 11.Hosseini SV, Aski MH, Al-Hurry AMAH, Hassan ARK, Khazraei H, Zabangirfard Z, et al. Simultaneous application of human amniotic membrane and Tachosil® in the repair of recto-vaginal fistula in an animal model. Comp Clin Path. 2017;26:405–9. doi: 10.1007/s00580-016-2391-1. [DOI] [Google Scholar]
- 12. Consensus Author Guidelines for Animal Use [Internet] USA: International Association of Veterinary Editors. c2010. Available from: [http://www.veteditors.org/consensus-author-guidelines-on-animal-ethics-and-welfare-for-editors. ]
- 13.Zekavat O, Amanat A, Karami M, Paydar S, Gramizadeh B, Zareian-Jahromi M. Wound Healing Studies Using Punica granatum Peel: An Animal Experimental Study. Adv Skin Wound Care. 2016;29:217–25. doi: 10.1097/01.ASW.0000481116.16998.55. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo JA, Naig AL, Johnson EK. Anorectal abscess and fistula-in-ano: evidence-based management. Surg Clin North Am. 2010;90:45–68. doi: 10.1016/j.suc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Whiteford MH, Kilkenny J, 3rd Hyman, N Buie, WD Cohen, J Orsay, C et. Practice parameters for the treatment of perianal abscess and fistula-in-ano (revised) Dis Colon Rectum. 2005;48:1337–42. doi: 10.1007/s10350-005-0055-3. [DOI] [PubMed] [Google Scholar]
- 16.van Koperen PJ, Wind J, Bemelman WA, Slors JF. Fibrin glue and transanal rectal advancement flap for high transsphincteric perianal fistulas; is there any advantage? Int J Colorectal Dis. 2008;23:697–701. doi: 10.1007/s00384-008-0460-x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlas M, Gokcora H, Erekul S, Dindar H, Yucesan S. Human amniotic membrane as an intestinal patch for neomucosal growth in the rabbit model. J Pediatr Surg. 1992;27:597–601. doi: 10.1016/0022-3468(92)90456-h. [DOI] [PubMed] [Google Scholar]
- 18.Najibpour N, Jahantab MB, Hosseinzadeh M, Roshanravan R, Moslemi S, Rahimikazerooni S, et al. The effects of human amniotic membrane on healing of colonic anastomosis in dogs. Annals of Colorectal Research. 2013;1:97–100. doi: 10.17795/acr-16139. [DOI] [Google Scholar]
- 19.Roshanravan R, Ghahramani L, Hosseinzadeh M, Mohammadipour M, Moslemi S, Rezaianzadeh A, et al. A new method to repair recto-vaginal fistula: Use of human amniotic membrane in an animal model. Adv Biomed Res. 2014;3:114.– doi: 10. doi: 10.4103/2277-9175.131033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schimidt LR, Cardoso EJ, Schimidt RR, Back LA, Schiazawa MB, d’Acampora AJ, et al. The use of amniotic membrane in the repair of duodenal wounds in Wistar rats. Acta Cir Bras. 2010;25:18–23. doi: 10.1590/s0102-86502010000100006. [DOI] [PubMed] [Google Scholar]
- 21.Kuriu Y, Yamagishi H, Otsuji E, Nakashima S, Miyagawa K, Yoshikawa T, et al. Regeneration of peritoneum using amniotic membrane to prevent postoperative adhesions. Hepatogastroenterology. 2009;56:1064–8. [PubMed] [Google Scholar]
- 22.Moslemi SM, Joraghi SAM, Roshanravan RM, Ghahramani LM, Mohammadianpanah MM, Hosseinzadeh MM, et al. Effect of Human Amniotic Membrane on Prevention of Colorectal Anastomosis Leakage in Cases with Neoadjuvant Radiotherapy: An Experimental Animal Study. Iran J Med Sci. 2016;41:501–6. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahamtan M, Hosseini SV, Khazraei H, Forozesh M, Bananzadeh A, Mokhtari M, et al. Effect of human amniotic membrane on anastomosis leakage in dog model. Comp Clin Path. 2016;25:1121–6. doi: 10.1007/s00580-016-2313-2. [DOI] [Google Scholar]


