Abstract
The energy expending and glucose sink properties of brown adipose tissue (BAT) make it an attractive target for new obesity and diabetes treatments. Despite decades of research, only recently have mechanistic studies started to provide a more complete and consistent picture of how activated brown adipocytes handle glucose. Here, we discuss the importance of intracellular glycolysis, lactate production, lipogenesis, lipolysis, and beta‐oxidation for BAT thermogenesis in response to natural (temperature) and artificial (pharmacological and optogenetic) forms of sympathetic nervous system stimulation. It is now clear that together, these metabolic processes in series and in parallel flexibly power ATP‐dependent and independent futile cycles in brown adipocytes to impact on whole‐body thermal, energy, and glucose balance.
Keywords: brown adipose tissue thermogenesis, fatty acid metabolism, glucose metabolism, positron emission tomography, uncoupling protein 1
Subject Categories: Metabolism
Glossary
- 123/125I‐BMIPP
[123/125I]‐b‐Methyl‐p‐iodophenyl‐pentadecanoic acid
- 18F‐FDG PET
18Flurodeoxyglucose positron emission tomography
- 18F‐FTHA
18F‐Fluroheptadonic acid
- ACL
ATP citrate lyase
- AGPAT2
1‐Acylglycerol‐3‐phosphate O‐acyltransferase 2
- ARC
Arcuate nucleus
- ATGL
Adipose triglyceride lipase
- BAT
Brown adipose tissue
- CCK
Cholecystokinin
- CPT1
Carnitine palmitoyltransferase 1
- DGAT2
Diacylglycerol acyltransferase 2
- EPAC1
Exchange protein activated by cyclic AMP 1
- ErbB3/4
Epidermal growth factor receptor 3/4
- FASN
Fatty acid synthase
- FGF21
Fibroblast growth factor 21
- G6PDX
Glucose‐6‐phosphate dehydrogenase, X‐linked
- GD1
Glycerol‐3‐phosphate dehydrogenase 1
- GLUT1/4
Glucose transporter 1/4
- GPAT3
Glycerol‐3‐phosphat‐O‐acyltransferase 3
- GYS1/2
Glycogen synthase 1/2
- HK2
Hexokinase 2
- HSL
Hormone‐sensitive lipase
- IMM
Inner mitochondrial membrane
- LDH
Lactate dehydrogenase
- MCC
Malonyl coenzyme A carboxylase
- MCT
Monocarboxylate transporter 1
- MPC1/MPC
Mitochondrial pyruvate carriers 1/2
- mTORC2
Mammalian target of rapamycin complex 2
- NRG4
Neuregulin 4
- NTS
Nucleus tractus solitarius
- PCK1
Phosphoenolpyruvate carboxykinase 1
- PGD
6‐Phosphogluconate dehydrogenase
- PKA
Protein kinase
- PKM
Pyruvate kinase M
- POMC
Pro‐opiomelanocortin
- RYR2
Ryanodine receptor 2
- SERCA2b
Sarco/ER Ca2+‐ ATPase 2b
- SLC25A1
Mitochondrial citrate transporter
- SPECT
Single‐photon emission computed tomography
- TCA
Tricarboxylic acid
- TGR5
Takeda G‐protein receptor 5
- TRPV1
Transient receptor potential vanilloid receptor 1
- UCP1/2
Uncoupling protein 1/2
- WAT
White adipose tissue
Lessons from the brain
Human functional neuroimaging was first introduced in the early 1980s on the basis that metabolically active brain regions can be visualized by 18flurodeoxyglucose positron emission tomography (18F‐FDG PET) 1. What ensued was a heated and lengthy debate about precisely how and where glucose is handled by brain cells to power neuronal activity, with glycolytic and oxidative processes at presynaptic and postsynaptic sites as well as in astrocytes being tabled 2, 3, 4, 5. Only recently, however, have optogenetic and pharmacological small‐animal 18F‐FDG PET imaging studies unequivocally confirmed neuronal 6 and glial 7 contributions to the brain 18F‐FDG PET signal, respectively. Despite these issues, there is at least unanimous agreement that during brain activity, glucose is metabolized to produce ATP required for the function of active transporters and ion pumps essential for synaptic transmission 8.
18F‐FDG PET imaging of brown adipose tissue
Human functional imaging of thermogenic brown adipose tissue (BAT) was first introduced in the late 2000s again using 18F‐FDG PET 9, 10, 11. It followed from careful retrospective analysis of clinical 18F‐FDG PET imaging data that pointed to the existence of metabolically active BAT mainly in the supraclavicular area of a small proportion of adults 12, 13, 14 (Fig 1), and that increased in prevalence during the winter months 15. Through the use of 18F‐fluroheptadonic acid (18F‐FTHA) 16 and 11C‐acetate PET imaging 17, respectively, human BAT was subsequently shown to consume large amounts of circulating fatty acids and to be highly oxidative during temperature‐induced thermogenesis 18. Prior to these seminal studies, it had generally been thought that BAT only exists in the interscapular region in human infants 19. Coupled with the knowledge from animal work that increasing energy expenditure by stimulating BAT thermogenesis promotes a negative whole‐body energy balance 19, nothing short of a biomedical revolution was heralded.
Figure 1. The distribution of thermogenic adipose tissue depots in mice and humans.
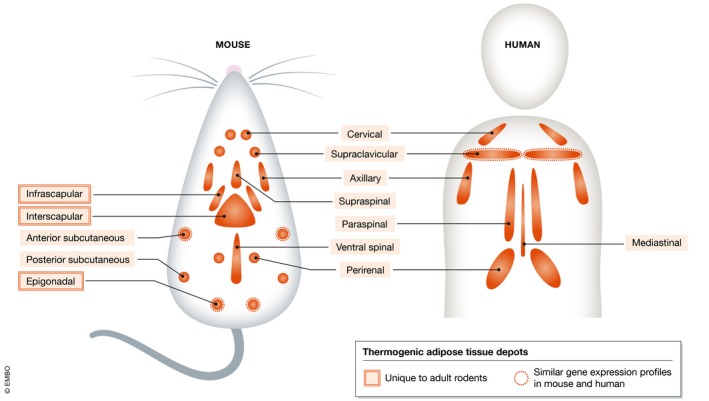
Molecular imaging techniques such as positron emission tomography (PET) and single‐photon emission computed tomography (SPECT) have allowed for the identification of various thermogenic adipose tissue depots in animals and humans. This is principally because these depots consume large amounts of glucose and fatty acids. Cervical, supraclavicular, axillary, and spinal depots are shared between species, whereas scapular and gonadal depots are unique to adult rodents. Gene expression profiling has revealed that the molecular signature of interscapular brown adipose tissue (BAT) of mice is unique, whereas that of the browned anterior subcutaneous and epigonadal adipose tissue depots of mice are more similar to the supraclavicular BAT depot of humans.
BAT glucose uptake and thermogenesis do not go hand in hand
While in the last decade, it has become clear that BAT can potentially be harnessed to independently manage hyperglycemia 20 and hyperlipidemia 21 as well as obesity 22, 23 in humans, a new but somewhat familiar controversy was gaining traction in the background: Can 18F‐FDG PET imaging really be considered a reliable technique to measure BAT thermogenic activity? The demonstration that BAT 18F‐FDG uptake is directly proportional to the degree of non‐shivering thermogenesis measured by indirect calorimetry first suggested that this could be the case 18, 22, 23, 24. However, several findings arose which challenged this notion. For example, like the small molecule drug Mirabegron which activates beta‐3 adrenergic receptors in brown adipocytes to stimulate 18F‐FDG uptake 23, insulin does the same through the insulin receptor 25. Consequently, insulin‐resistant individuals accumulate less 18F‐FDG in BAT but show normal non‐shivering thermogenesis as well as BAT fatty acid uptake and oxidative metabolism 26.
More definitive data eventually came again from small‐animal 18F‐FDG PET imaging studies performed on mice that lack uncoupling protein 1 (UCP1) 27, 28, 29. This inner mitochondrial membrane (IMM) protein is most highly expressed in brown adipocytes and is essential for both forms of non‐shivering thermogenesis, i.e., temperature‐induced 30 and diet‐induced 31. It was found that stimulation of sympathetic nerves innervating BAT by acute cold exposure results in BAT 18F‐FDG uptake in female UCP1 knockout mice as it does in wild‐type mice 27. In line with this, in subsequent studies, BAT 18F‐FDG uptake in response to a beta‐3 adrenergic receptor agonist in UCP1 knockout mice was fully retained despite defective BAT thermogenesis upon the same pharmacological treatment 28, 29. Furthermore, independent experiments on isolated brown adipocytes lacking UCP1 have all corroborated the in vivo imaging results 28, 32, 33. These findings clearly show that BAT glucose uptake can be dissociated from UCP1‐mediated thermogenesis.
However, evidence in support of the contrary does exist. Acute noradrenaline treatment of UCP1 knockout mice did not further promote BAT 2‐[3H]deoxyglucose uptake compared to vehicle treatment 34. Similarly, unlike for female UCP1 knockout mice, cold exposure of male UCP1 knockout mice did not stimulate BAT 18F‐FDG uptake 27. Optogenetic stimulation of BAT following siRNA‐mediated knockdown of Ucp1 in mice further failed to regulate glycemia 35. Because this intervention resulted in markedly lower blood glucose levels in mice with normal BAT, it was inferred that brown adipocyte glucose utilization is secondary to UCP1‐mediated thermogenesis 35. However, this conclusion was not supported by any direct measurements of glucose uptake by BAT. The discrepancies between the in vivo studies with beta‐3 adrenergic receptor agonist and noradrenaline treatments may be due to the different housing temperatures of mice prior to scanning 28, 29, 34. It will be of interest to determine the causes underlying the gender differences in UCP1 knockout mice in terms of temperature‐induced BAT glucose uptake, i.e., whether they are at the level of sympathetic nerve or brown adipocyte responsivity, although the latter is not supported by data from male and female UCP1 knockout mice treated with a beta‐3 adrenergic receptor agonist 29.
The retained glucose uptake by brown adipocytes upon adrenergic stimulation in the absence of UCP1‐mediated thermogenesis can be explained by the fact that the two processes occur in parallel rather than in series. This differs from the situation in hippocampal neurons for instance where electrical stimulation leads to GLUT4 trafficking to the presynaptic membrane, where it promotes glucose uptake secondary to increases in the intracellular AMP:ATP ratio sensed by AMP kinase 36. Instead, upon beta‐3 adrenergic receptor activation of brown adipocytes and the subsequent rise in intracellular cyclic AMP concentrations, the EPAC1 signaling arm causes GLUT1 trafficking to the plasma membrane and glucose uptake, while simultaneously the PKA signaling arm causes UCP1‐mediated thermogenesis through fatty acids released upon lipolysis 37, 38, 39. Further, despite the fact that UCP1‐mediated thermogenesis in brown adipocytes decreases ATP production by dissipating the proton gradient across the IMM 40, this does not appreciably influence AMP kinase activity 32. Thus, unlike neurons, activated brown adipocytes do not take up glucose as a result of increased AMP kinase activity in response to energy demands placed on the cell.
Insight into BAT function guides technique and drug development
In light of the false negatives and positives that occur with 18F‐FDG PET imaging described above and the exposure it causes to ionizing radiation, other techniques are now being considered to visualize human BAT thermogenic activity, such as infrared thermal 41 and near‐infrared optoacoustic 42 imaging, that both have potential to supersede the current gold standard. The studies highlighted above also raise a fundamental question concerning BAT fuel utilization: If so much glucose is taken up by activated brown adipocytes during thermogenesis to the point of visualization with 18F‐FDG PET, what exactly happens to it? The answer has strong implications for the design of new strategies to treat hyperglycemia aimed at taking advantage of the glucose sink property of BAT. Elegant work performed decades ago in which rats were administered either 14C‐glucose 43 or tritiated water 44 first revealed that a substantial degree of lipogenesis takes place in BAT following acclimation to cold. These findings have now been extended by those from more recent cell‐based, animal and human studies that have finally provided us with a more detailed and complete picture of glucose metabolism in brown adipocytes (Fig 2) and that will be discussed below.
Figure 2. The metabolic fate of glucose in brown adipocytes during thermogenesis.
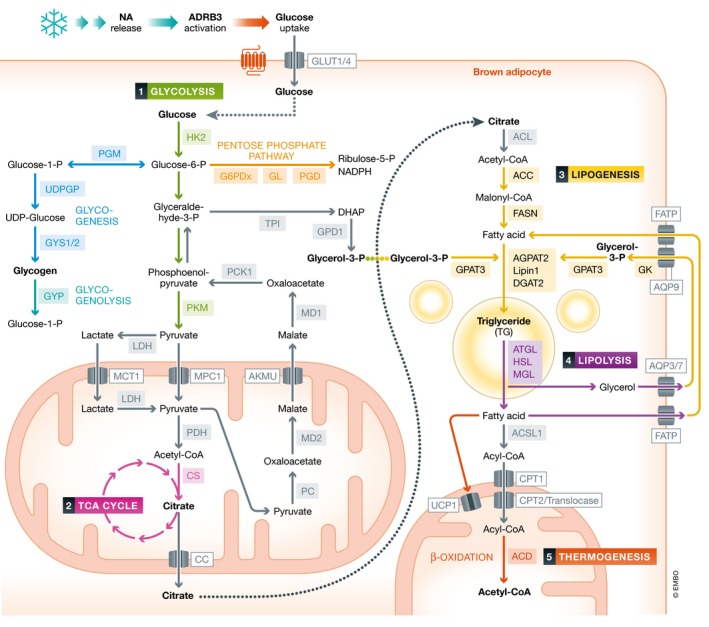
Upon beta‐3 adrenergic receptor activation following noradrenaline release by sympathetic nerve endings in response to cold, glucose is taken up by brown adipocytes via glucose transporters 1 and 4 (GLUT1/4). Glucose then undergoes glycolysis to generate dihydroxyacetone phosphate (DHAP), pyruvate, and lactate in the cytosol. Simultaneously, glucose‐6‐phosphate (glucose‐6‐P) feeds into the pentose phosphate pathway to generate ribulose‐5‐P and NADPH which is used for lipogenesis and also into glycogen synthesis and breakdown pathways. Pyruvate and/or lactate are next transported into the mitochondria via monocarboxylate transporter 1 (MCT1) and for pyruvate, the pyruvate carrier 1 (PC1), while DHAP is converted into glycerol‐3‐P by glycerol‐3‐phosphate dehydrogenase 1 (GPD1). Once inside the mitochondria, pyruvate is converted into acetyl‐CoA by pyruvate dehydrogenase (PDH). If lactate is indeed transported into mitochondria, it would be converted by LDH back into pyruvate. Acetyl‐CoA then undergoes partial breakdown in the TCA cycle into citrate by citrate synthase (CS) which is then exported by the citrate carrier (CC) into the cytosol. Citrate then feeds into a lipogenic pathway after being converted back into acetyl‐CoA by ATP citrate lyase (ACL). Acetyl‐CoA carboxylase (ACC), fatty acid synthase (FASN), glycerol‐3‐phosphat‐O‐acyltransferase 3 (GPAT3), AGPAT2, lipin 1, and diacylglycerol O‐acyltransferase 2 (DGAT2) then contribute to the generation of triglycerides (TG) from fatty acids and glycerol‐3‐P. These then rapidly undergo lipolysis in cytosolic lipid droplets through the action of adipose triglyceride lipase (ATGL), hormone‐sensitive lipase (HSL), and monoacylglycerol lipase (MGL). The liberated fatty acids either activate UCP1 to generate heat or are next converted into acyl‐CoA by long chain acyl‐CoA synthase 1 (ACSL1) and then transported into the mitochondria via the sequential action of carnitine palmitoyltransferase 1 (CPT1), translocase, and CPT2. Once inside the mitochondria, acyl‐CoA undergoes β‐oxidation by acyl‐CoA deyhyrogeneses (ACD) to generate the necessary proton gradient across the inner mitochondrial membrane. The liberated glycerol from lipolysis is phosphorylated by glycerol kinase (GK) into glycerol‐3‐P to facilitate fatty acid re‐esterification. Similarly, pyruvate carboxylase (PC) generates oxaloacetate (OA) from pyruvate, which is then converted into malate (MA) by malate dehydrogenase 2 (MD2). MA is exported outside of the mitochondria into the cytosol by the alpha‐ketoglutarate malate uniporter (AKMU) where it is reconverted back into OA by MD1. Finally, phosphoenolpyruvate carboxykinase 1 (PCK1) generates phosphoenolpyruvate which feeds G3P synthesis required for fatty acid re‐esterification. Fatty acids and glycerol released from lipolysis may enter an extracellular loop via fatty acid transport protein1 (FATP) and aquaporin 3/7/9 (AQP 3/7/9) as part of the fatty acid recycling pathway. Abbreviations: HK2, hexokinase 2; PGM, phosphoglucomutase; G1P, glucose‐1‐phosphate; G6PDx, glucose‐6‐phosphate dehydrogenase X‐type; GL, gluconolactolase GYP, glycogen phosphorylase; PGD, 6‐phosphogluconate dehydrogenase; UDP, uracil‐diphosphate; UDPGP, UDP glucose pyrophosphorylase; PK, pyruvate kinase; and TPI, triosephosphate isomerase. Some reactions have been omitted for clarity.
The significance of glucose uptake and glycolysis for BAT thermogenesis
Early estimates on the contribution of glucose as an energy source for BAT thermogenesis were in the range of 2–16% 45, 46, 47. They were based on glucose and oxygen consumption rates of isolated rat brown adipocytes upon acute adrenergic stimulation 46, 47 or on arteriovenous differences in blood glucose and oxygen across BAT of anesthetized cold‐acclimated rats 45. To specifically address the requirement of BAT glucose uptake and subsequent glycolysis for thermogenesis, loss‐of‐function experiments were performed on cultured immortalized mouse brown adipocytes 33. The increase in oxygen consumption by these cells upon acute adrenergic stimulation was prevented by siRNA‐mediated knockdown of glucose transporters Glut1 and Glut4. Similar results were obtained with siRNA‐mediated knockdown of hexokinase 2 (Hk2) and pyruvate kinase M (Pkm), the first and last enzymes in glycolysis, respectively. This is consistent with infrared thermal imaging data from differentiated human brown adipocytes showing that the GLUT4 inhibitor indinavir dose‐dependently reduces thermogenesis 48, and with the acute cold intolerance of mice genetically engineered to have defective glucose uptake and glycolysis in brown adipocytes through inactivation of the serine/threonine kinase mTORC2, a downstream target of EPAC1 39. Remarkably, adeno‐associated virus‐mediated overexpression of Hk2 in the BAT of these mice restored its glucose uptake and glycolytic capacity as well as their cold tolerance 39. Therefore, while quantitatively speaking glucose may only modestly contribute to fueling BAT thermogenesis compared to fatty acids 33, 45, 46, 47, 49, it is nevertheless essential. Furthermore, 18F‐FDG uptake increases in BAT when fatty acid uptake and oxidation are diminished in UCP2‐deficient mice 50, suggesting that when necessary, glucose can make a larger contribution to fueling BAT thermogenesis in the short term. This is in line with findings from differentiated T37i cells (a brown adipocyte cell line) acutely treated with a beta‐3 adrenergic receptor agonist, in which glucose oxidation doubled when fatty acid oxidation was pharmacologically inhibited 49.
Offshoots of glycolysis
Glucose‐6‐phosphate generated at the first step of glycolysis can also proceed to the biosynthetic pentose phosphate pathway (PPP). A transcriptome analysis revealed that mRNA levels of G6pdx, Pgl, and Pgd whose protein products produce ribulose‐5‐phosphate from glucose‐6‐phosphate as part of the PPP (Fig 2) increased in the BAT of mice upon chronic cold exposure 51. The significance of this is unclear, but the extra NADPH produced from the PPP may be used for lipogenesis (see below). Recruitment of the PPP may also provide the nucleotides and amino acids for cold‐induced cell proliferation and differentiation in BAT and the dramatic changes in gene expression and protein translation that take place in activated brown adipocytes to boost thermogenic capacity. The glycogen production rate is also enhanced in BAT during cold acclimation reflected by increased Gys1/2 mRNA expressions 51. This provides another avenue for the deposition of excess glucose in brown adipocytes. Complementing these findings, glycogen phosphorylase mRNA expression also increases in BAT of rats upon acute and chronic cold exposure, which generates an on‐demand intracellular source of glucose from glycogen 52.
The different routes taken by pyruvate in brown adipocytes during thermogenesis
Fatty acid and glycerol production
Clues about what happens to the pyruvate generated during glycolysis in activated brown adipocytes have been obtained from another more comprehensive transcriptome study comparing BAT samples from mice chronically housed at thermoneutrality (30°C) with those at standard room (22°C) and extreme cold (4°C) temperatures 53. An astonishing degree of differential gene expression was reported between thermoneutrality and room temperature that did not generally change much further in extreme cold. In particular, a wide array of lipogenic gene products was upregulated 53. Of interest here was that mRNA expression of the mitochondrial pyruvate carriers 1 and 2 (Mpc1 and Mpc2) and the mitochondrial citrate transporter (Slc25a1) increased. These two sets of transcripts translate into proteins that provide gateways for the entrance of cytosolic pyruvate into mitochondria and for the exit of citrate generated during the TCA cycle back out, respectively (Fig 2). It is in the cytosolic compartment that ATP citrate lyase (ACL) then generates acetyl coenzyme A which feeds into lipogenesis through the catalytic activities of malonyl coenzyme A carboxylase (MCC) and fatty acid synthase (FASN) (Fig 2). Importantly, mRNA and protein expression of these three enzymes markedly increase from thermoneutrality to room/extreme cold temperatures 53.
The transcription of genes involved in glycerol metabolism also increases in BAT of mice when the temperature drops for prolonged time periods, such as glycerol kinase and Agpat2 53. These two enzymes produce glycerol‐3‐phosphate from glycerol and phosphatidic acid from lysophosphatidic acid, respectively (Fig 2). Interestingly, analysis of the mitochondrial proteome in BAT of mice chronically exposed to cold revealed a two‐ to threefold increase in glycerol kinase and AGPAT2 protein expressions 54. Considering that AGPAT2 is an ER‐localized enzyme, this may be due to the establishment of ER–mitochondrial contacts during thermogenesis 55. In line with the findings in mice, an increase in Gpd1, Pck1, and Gpat3 mRNA expression in BAT of rats occurs upon acute and chronic cold exposure 52. These three enzymes catalyze the production of glycerol‐3‐phosphate from dihydroxyacetonephosphate generated during glycolysis, phosphoenolpyruvate from oxaloacetate generated from mitochondrial pyruvate carboxylase, and lysophosphatidic acid from glycerol‐3‐phosphate, respectively (Fig 2). Correspondingly, AGPAT enzymatic activity is approximately five‐fold higher in BAT of rats following cold acclimation 56. The mRNA expression of Lipin1 in BAT of mice also increases upon acute cold exposure 57, the protein product of which produces diacylglycerol from phosphatidic acid 57. Importantly, the significance of glycerol‐metabolizing enzymes for thermogenesis was demonstrated by the acute cold intolerance of mice lacking lipin 1 specifically in WAT and BAT 57.
Like glycerol kinase, the aforementioned glycerol‐metabolizing enzymes could also be important in brown adipocytes for fatty acid re‐esterification as part of a dynamic steady state with lipolysis. This so‐called fatty acid recycling has long been known to be a UCP1‐independent ATP‐consuming form of thermogenesis in white adipocytes 58. In these cells, GPD and PCK1 play a dominant role in glycerol‐3‐phosphate production due to the absence of appreciable amounts of glycerol kinase 58, 59. The increased oxygen consumption by epididymal white adipose tissue (WAT) but not BAT explants from UCP1 knockout mice chronically treated with a beta‐3 adrenergic receptor agonist suggests that fatty acid recycling does not play a significant thermogenic role in BAT in response to this particular pharmacological stimulus 60. Rather, the regulation of Ucp1 transcription by glycerol kinase by as yet unknown mechanisms in brown adipocytes may contribute to regulating whole‐body thermal and energy balance 61.
Beige adipocyte thermogenesis can compensate for diminished brown adipocyte thermogenesis
Sanchez‐Gurmaches et al 53 further went on to show that while signaling of the serine/threonine kinase Akt2 in brown adipocytes is required for the global changes in gene expression in response to cold acclimation, it is dispensable for acute cold tolerance. This is most likely because the inguinal WAT of mice lacking Akt2 specifically in BAT underwent compensatory browning, which involves the formation of brite/beige adipocytes. These thermogenic cells intersperse in WAT and have a unique gene expression profile 62. They can arise either through chronic activation of beta‐1 adrenergic receptors in dedicated precursor cells in response to cold exposure or by transdifferentiation of existing white adipocytes due to chronic pharmacological activation of beta‐3 adrenergic receptors 63. Besides UCP1‐mediated thermogenesis 64, inguinal beige adipocytes can also generate heat upon alpha‐1 and beta‐3 adrenergic receptor activation from calcium cycling into and out of the endoplasmic reticulum (ER) through the ER Ca2+‐ATPase SERCA2b and the ryanodine receptor RYR2, respectively 65. As calcium cycling is fueled almost entirely by ATP generated from glycolysis, inguinal beige adipose tissue functions as another glucose sink that favorably regulates glycemia 65. Furthermore, upon chronic cold exposure, inguinal beige adipocytes initiate another UCP1‐independent, ATP‐dependent thermogenic futile cycle between creatine and phosphocreatine in the mitochondrial intermembrane space 66. Creatine kinase‐mediated phosphocreatine production from creatine also operates in epididymal beige adipocytes following chronic beta‐3 adrenergic receptor agonist treatment 67. Notably, fatty acid recycling upon chronic cold exposure and beta‐3 adrenergic receptor agonist treatment in inguinal 68 and epididymal 60 beige adipocytes, respectively, may intersect with creatine and phosphocreatine cycling to accept the high energy phosphate from phosphocreatine for the required ATP. Thus, adipocytes from distinct adipose tissue depots can exhibit numerous forms of thermogenic futile cycling depending on the environmental, pharmacological, and genetic conditions found.
Lactate production
Another direction of pyruvate metabolism in cells particularly under anaerobic conditions is toward lactate production through the catalytic activity of lactate dehydrogenase (LDH) (Fig 2). In the brain, this also takes place when oxygen levels are normal, which is referred to as aerobic glycolysis 3. The mismatch between an excess of glucose but normal oxygen consumption in activated brown adipocytes 25, 45, 46, 47 is indicative that these cells utilize aerobic glycolysis that achieves expedience at the expense of energetic efficiency. Indeed, early studies revealed that rat brown adipocytes release lactate upon acute adrenergic stimulation 69 and that intact BAT does the same in cold‐acclimated rats 45.
Lactate metabolism was recently shown to occur in response to optogenetic stimulation of BAT thermogenesis in mice 35, which more realistically mimics cold exposure than pharmacological approaches. One‐hour photostimulation of BAT sympathetic nerve endings expressing the blue light‐activated photoreceptor channelrhodopsin 2 not only raised BAT noradrenaline concentrations and temperature measured by telemetry but also precipitously decreased blood glucose levels. This was prevented by local siRNA‐mediated knockdown of Glut1 in BAT and inhibition of glycolysis with 2‐deoxyglucose. Similar results were obtained from local pharmacological inhibition of LDH in BAT and siRNA‐mediated knockdown of monocarboxylate transporter 1 (Mct1), which transports both lactate and pyruvate from the cytosol into mitochondria (Fig 2). These remarkable findings confirm the importance of glucose uptake and glycolysis for BAT thermogenesis in the acute setting. They also provide new causal evidence that intracellular lactate metabolism significantly contributes to this process. However, despite the recent discovery that in most peripheral tissues (including WAT), lactate is not merely a metabolic end‐product but actively feeds into the TCA cycle 70, it remains to be shown whether this is also the case in BAT. If LDH can be found in brown adipocyte mitochondria as part of a lactate oxidation complex characterized in neurons 71, this uncertainty might be resolved. Also, it is unclear whether the improved glycemia from optogenetic stimulation of BAT results from the release of a glucoregulatory endocrine factor such as FGF21 from brown adipocytes 72. This pleiotropic peptide is as effective in clearing blood glucose in mice as optogenetic BAT stimulation, through enhancing glucose uptake by WAT and liver 73. Regardless of the mechanism, the fact that optogenetic stimulation of BAT can be achieved non‐invasively due to the skin‐penetrating properties of blue light 35 opens up new therapeutic potential.
Lipogenesis is quickly followed by lipolysis in brown adipocytes during thermogenesis
Up to here, we have discussed the generation of triglyceride components from glucose (i.e., fatty acids and glycerol metabolites) in brown adipocytes during thermogenesis. To specifically address the mechanism of triglyceride formation in activated BAT, differentiated/immortalized mouse brown adipocytes were acutely treated with a selective beta‐3 adrenergic receptor agonist following siRNA‐mediated knockdown of diacylglycerol acyltransferases 1 or 2 (Dgat1/2) 74. These ER‐localized enzymes catalyze the addition of a final fatty acid to diacylglycerol which is the rate‐limiting reaction in triglyceride formation (Fig 2). It was found that the incorporation of 14C‐glucose‐derived carbons into fatty acid and glycerol moieties of triglycerides into specialized cytosolic lipid droplet pools occurred entirely via the action of DGAT2. Furthermore, these de novo generated triglycerides in wild‐type cells rapidly underwent lipolysis such that the released 14C‐labeled free fatty acids return back to the mitochondria to be oxidized into 14CO2. Accordingly, this was fully prevented by pharmacological inhibition of ACL, ATGL, and CPT1. Induction of lipogenesis and fatty acid oxidation also occurs in mouse BAT under cold conditions 75. Such simultaneous catabolism of glucose and fatty acids in brown adipocytes goes against the fundamental tenets of the Randle cycle, which stipulates that each occurs individually by inhibiting the other. It may be that in this cell type, however, there is a unique partitioning of inhibitory glucose and fatty acid metabolites away from their protein targets such as malonyl‐CoA from CPT1 75. Alternatively/additionally, post‐translational modifications may render these proteins resistant to inhibitory allosteric modulation 75, such as that of acetyl‐CoA on pyruvate dehydrogenase. Nevertheless, the in vitro findings provide perhaps the most in‐depth and detailed account of the metabolic fate of glucose in brown adipocytes. Future studies implementing hyperpolarized 13C spectroscopy with 13C‐labeled glucose, as is typically applied to study brain energetics, can provide a more global view of metabolites generated from glucose in BAT in the in vivo setting as well as in isolated brown adipocytes 76. This can build upon the results from a previous hyperpolarized 13C spectroscopy study that only revealed changes in the distribution of endogenous 13C‐labeled species in rat BAT upon acute cold exposure and in isolated brown adipocytes upon acute adrenergic stimulation, without tracking the fate of glucose 76. Interestingly, untargeted metabolomics of differentiated T37i cells acutely treated with a beta‐3 adrenergic receptor agonist revealed that 13C‐labeled glucose feeds into the PPP, glycolysis, and TCA cycle but only into the glycerol moiety of triglycerides 49. The reason for a lack of fatty acid synthesis from glucose—as has been amply described for activated BAT/primary brown adipocytes 43, 44, 45, 46, 47, 74—is unclear but might be an issue inherent to T37i cells.
The importance of lipolysis for BAT thermogenesis
The above‐mentioned study by Irshad et al 74 revealed that lipolysis is required for the full oxidation of glucose in brown adipocytes but did not address its role in UCP1‐mediated thermogenesis. Initial pharmacological experiments performed on cultured differentiated mouse brown and brite/beige adipocytes with ATGL and hormone‐sensitive lipase (HSL) inhibitors suggested that lipolysis is mandatory for thermogenesis 37. This was supported by subsequent in vivo findings in rats 52 and humans 77 treated with nicotinic acid, a GPR109a agonist that opposes beta‐3 adrenergic receptor signaling and thus inhibits lipolysis and severely impairs temperature‐induced thermogenesis. However, nicotinic acid also markedly reduced circulating glucose and fatty acid uptake by BAT in these studies 52, 77 so it remains unclear to what extent its effect on intracellular lipolysis impacts on thermogenesis.
These observations generally supported the model that free fatty acids released by lipolysis upon stimulation of sympathetic nerves innervating BAT act as activators of UCP1. This was in fact postulated early on, based on the observations that removal of intracellular fatty acids by activation of mitochondrial beta‐oxidation inhibited uncoupled respiration of brown adipocytes 78, and nanomolar concentrations of fatty acids could override the inhibitory action of purine nucleotides on UCP1 79. The latter was confirmed more recently by detailed patch‐clamp electrophysiological studies of brown fat IMM preparations 80. The patch‐clamp electrophysiological approach also revealed that UCP1 acts as a thermogenic symporter that transports protons bound to fatty acids from the intermembrane space into the mitochondrial matrix 80. This insight was gained using fatty acid analogues in the external solution (representing the intermembrane space) with varying dissociation constants. The large UCP1‐mediated proton conductance across the IMM was only recorded for fatty acid analogues that bind to protons at physiological pH 80.
The unexpected finding that genetically interfering with BAT ATGL function does not affect cold sensitivity in mice 81, 82 suggests that while in the normal genetic landscape, fatty acids derived from intracellular lipolysis act as UCP1 activators and provide a major source of energy for BAT thermogenesis, the fatty acid sources can be redundant. Indeed, it was proposed from the studies of Shin et al 81 and Scheiber et al 82 that circulating fatty acids derived from WAT lipolysis can fully compensate for defective BAT lipolysis 81, 82. This is because fasted mice with defective lipolysis in both WAT and BAT, as opposed to in BAT alone, are in fact cold‐intolerant 81, 82. Interestingly, the provision of food to these mice can preserve body temperature upon acute 81, 82 and chronic 82 exposure to cold, whereas for mice with defective glycolysis in WAT and BAT, this is not the case—at least in the acute setting 39. Together, these results suggest that liproprotein lipase on capillaries near brown adipocytes acting on circulating triglyceride‐rich lipoproteins can provide exogenous fatty acids from ingested food 83, 84. They further attest that intracellular glucose but not fatty acid metabolism is indispensable for rapid thermogenesis in brown adipocytes. Notably, when fatty acids cannot be obtained from lipid droplets within the brown adipocyte, increased glucose uptake occurs, which improves glycemic control 80. Here, we have another example of the therapeutic potential of increasing glucose metabolism in BAT through blocking intracellular lipolysis.
The higher production of the electron donating reducing equivalents NADH and FADH2 from exogenous fatty acids compared with glucose in brown adipocytes may be more important for acclimation to cold 50. This is consistent with the finding that exogenous fatty acids seem to feed into a separate and larger lipid droplet pool than that replenished by glucose in brown adipocytes, which is not as readily regulated by acute beta‐3 adrenergic receptor activation 74. Accordingly, mice with reduced BAT fatty acid uptake due to a lack of fatty acid transport protein 1 (FATP1) 85 or cluster of differentiation 36 (CD36) 86 in brown adipocytes initially handle the cold when given access to food but eventually succumb to it after prolonged exposure 85. The avid uptake of circulating fatty acids by activated BAT has recently been underscored by a [123/125I]‐b‐methyl‐p‐iodophenyl‐pentadecanoic acid (123/125I‐BMIPP) single‐photon emission computed tomography (SPECT) imaging study in mice, which led to the identification of several new bona fide BAT depots with a similar distribution pattern to that in man 87 (Fig 1).
Mitochondrial beta‐oxidation: a final common pathway?
Intracellular fatty acids are activated for mitochondrial entrance by acyl‐CoA synthases (ACS) and are then converted into acylcarnitine by CPT1 for transport across the IMM by translocase. CPT2 then regenerates acyl‐CoA from acylcarnitine in the mitochondrial matrix in preparation for beta‐oxidation (Fig 2). While long‐chain ACS 1 (ACSL1) 88, CPT1b 89, CPT2 90, and various acyl‐CoA dehydrogenases 91, 92, and thus beta‐oxidation, have been shown to be essential for mice to mount a proper acute thermogenic response to cold, the role of fatty acids derived from glucose remains unclear. One way this can be addressed explicitly is by deleting ACL or DGAT2 from BAT. Interestingly, this has already been achieved in principle by crossing adiponectin‐Cre mice with floxed Acl counterparts 39, 81, 82, but the BAT and cold tolerance of their offspring were not assessed in this particular study 93. Lipogenesis from exogenous acetate may, however, compensate for that from exogenous glucose in this model 93. This is because isolated inguinal and epididymal white adipocytes lacking ACL were found to incorporate considerably more 13C acetate into acetyl‐CoA and malonyl‐CoA than wild‐type cells 93.
From a therapeutic perspective, the negative role of mitochondrial acyl‐CoA thioesterases (ACOT) in regulating BAT thermogenesis deserves consideration. These enzymes generate acetyl‐CoA and free fatty acids from acyl‐CoA in the cytosol and the mitochondrial matrix. Consequently, blocking ACOT action would be expected to have the dual effect of increasing cytosolic acyl‐CoA availability for mitochondrial import and for beta‐oxidation by preventing its breakdown in the mitochondrial matrix. Consistent with this notion, genetic inactivation of ACOT11 was first shown to protect mice from developing obesity and insulin resistance from a high‐fat diet through increased energy expenditure 94. This was associated with enhanced fatty acid oxidation in brown adipocytes 94. Subsequently, genetic inactivation of ACOT13 in mice was found to improve cold tolerance 95 and to increase energy expenditure 95, 96. Interestingly, this seems to be through enhanced oxidation of fatty acids released by intracellular lipolysis in brown adipocytes 96. Thus, compounds that inhibit ACOT11/13 might be useful drugs to treat obesity and associated insulin resistance by promoting BAT thermogenesis.
BAT crosstalk with other tissues
In this Review, we have focused on how intracellular glucose metabolism in brown adipocytes regulates systemic homeostasis. Analogous to the previously long‐held view that WAT is simply an energy storage organ, it is becoming increasingly evident that BAT does not function in isolation just to dissipate chemical energy as heat. Instead, BAT has been shown to interact extensively with other tissues through neural and endocrine pathways in both health and disease (Fig 3). This crosstalk involves some of the intracellular catabolic and anabolic processes mentioned here. For instance, the predisposition to developing obesity from chronic overnutrition may have its origins in aberrant liver to BAT communication 97. This involves increased hepatic glucokinase activity which, via vagal afferents to the hindbrain nucleus tractus solitarius (NTS), decreases sympathetic regulation of BAT thermogenesis (Fig 3) 97. Additionally, the impaired thermoregulation that occurs with aging associates with decreased acylcarnitine release from the liver 98. In healthy young mice, these lipid species are generated by the action of CPT1/2 in hepatocytes from free fatty acids derived from WAT lipolysis upon acute cold exposure. They are then channeled to BAT where they are ultimately metabolized in the TCA cycle to promote thermogenesis (Fig 3) 98.
Figure 3. BAT crosstalk with other organs in health and disease.
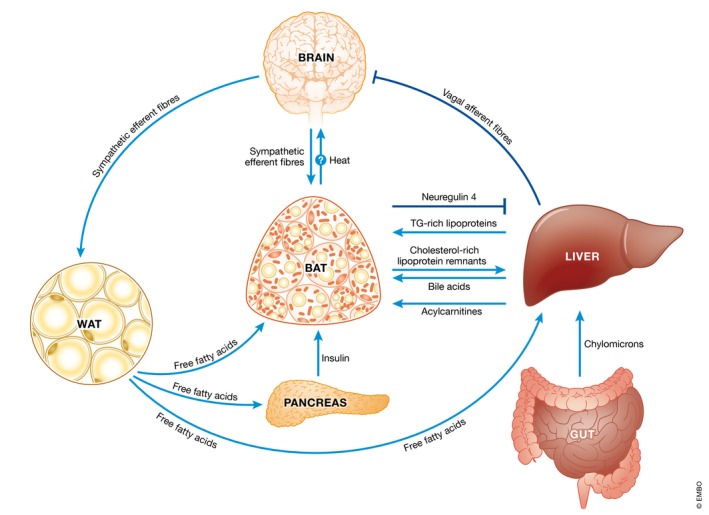
Here we provide examples of crosstalk between BAT and other organs in health and disease states. During a high‐fat meal, cholesterol in the form of chylomicrons is delivered first to the liver and then in the form of triglyceride‐rich lipoproteins to BAT. Cholesterol‐rich lipoprotein remnants are then released from the action of lipoprotein lipase and are in turn delivered via the bloodstream back to the liver where hepatocytes convert cholesterol into bile acids. These dietary cholesterol‐derived circulating bile acids then return to BAT to promote thermogenesis. There may also be a line of communication from BAT to the brain during feeding due to the heat‐sensing properties of hypothalamic pro‐opiomelanocortin neurons. During chronic consumption of a high‐carbohydrate diet, increased glucose kinase activity in the liver results in the activation of vagal afferents which inhibit sympathetic efferents to BAT resulting in decreased thermogenesis in those susceptible to weight gain. Similarly, during aging decreased acylcarnitine released from the liver produced by free fatty acids released from WAT lipolysis results in diminished BAT thermogenesis and cold intolerance. BAT itself releases neuregulin 4 to protect against fatty liver and diabetes from chronic consumption of a high‐fat diet. Fatty acids released by WAT lipolysis also promote insulin release from the pancreas which then causes glucose, fatty acid and triglyceride‐rich lipoprotein uptake by BAT to sustain thermogenesis.
In the reverse direction, BAT communicates with the liver to prevent excessive lipid accumulation in hepatocytes and insulin resistance in the face of a high‐fat diet 99. This is through the constitutive release of the extracellular domain of the transmembrane protein neuregulin 4 (NRG4) following proteolytic cleavage at Ser53. The circulating NRG4 fragment is then thought to bind to epidermal growth factor receptor 3 and 4 (ErbB3/4) in hepatocytes to limit the expression of lipogenic genes such as Fasn (Fig 3) 99. Interestingly, while mRNA expression of Nrg4 in BAT increases upon both acute and chronic cold exposure, it is not involved in the acute maintenance of body temperature 99. This underscores the fact that BAT can regulate different physiological functions independently from thermogenesis.
Box A: In need of answers.
To what extent do nutrients and metabolites other than glucose and fatty acids fuel BAT thermogenesis?
What is the overall metabolic fate of glucose in brown adipocytes during thermogenesis? Is there a difference with beige adipocytes? Is there a species difference?
Is BAT glucose and fatty acid uptake and metabolism differentially required for acute and chronic tolerance to cold? Is there a difference in BAT fuel utilization for diet‐induced thermogenesis?
Can chronic activation of BAT reduce circulating blood glucose in hyperglycemic humans by acting as a glucose sink as it does in rodents?
Can the full benefits of BAT glucose uptake on glycemia be obtained without invoking BAT thermogenesis?
The fatty acids released by WAT lipolysis upon acute cold exposure or beta‐3 adrenergic receptor agonist treatment also stimulates insulin release from pancreatic beta‐cells 100. Remarkably, this was shown to be required for the normal uptake of glucose, fatty acids and triglyceride‐rich lipoproteins by activated BAT in mice and, consequently, thermogenesis. As such, mice lacking ATGL in BAT do not release insulin in response to acute cold exposure or beta‐3 adrenergic receptor agonist treatment and mice treated with an insulin receptor antagonist or lacking insulin receptors in brown adipocytes are cold intolerant 100.
Communication also exists between the gut and BAT especially during diet‐induced thermogenesis. An elaborate example of this involves the delivery of dietary cholesterol via the liver to BAT (Fig 3) 101. Upon acute cold exposure or beta‐3 adrenergic receptor agonist treatment, LPL in BAT capillaries causes the breakdown of triglycerides in triglyceride‐rich lipoproteins and the uptake of the fatty acids by brown adipocytes. Consequently, cholesterol‐rich lipoprotein remnants are released from BAT into the circulation. These are then directed to the liver where the cholesterol is converted into bile acids in hepatocytes via the alternative pathway through the action of CYP7B1‐genetic inhibition or promotion of which decreases and increases BAT thermogenesis, respectively 101. Interestingly, gut microbiota may add an additional layer to this crosstalk as they dramatically change in diversity upon acute cold exposure to regulate hepatic Cyb7b1 mRNA expression, circulating bile acid species and BAT thermogenesis 102. The clinical potential of bile acids is supported by the finding that acute administration of chenodeoxycholate to human subjects increases whole‐body energy expenditure associated with increased BAT 18FDG uptake 103. This was proposed to be mediated by the Takeda G‐protein receptor (TGR5) based on pharmacological experiments on cultured differentiated human brown adipocytes 103.
Lastly, the finding that anorexigenic hypothalamic arcuate nucleus (ARC) pro‐opiomelanocortin (POMC) neurons are heat‐sensitive 104 opens up the possibility of a BAT‐brain axis in feeding control. This is consistent with the thermostatic regulation of feeding by BAT which was originally proposed to act as a negative feedback pathway during diet‐induced thermogenesis 105. Indeed, CRISPR‐Cas‐9‐mediated deletion of the heat‐activated transient receptor potential vanilloid 1 receptor (TRPV1) in ARC POMC neurons prevents the reduced food intake caused by exercise in mice, which itself robustly increases core body as well as ARC temperatures 104.
Conclusions and future directions
The use of 18F‐FDG PET imaging has led to tremendous strides in the translational fields of experimental neuroscience and metabolism not only as a functional technique, but also in motivating research into the energetics of neurons and brown adipocytes, respectively. Despite this, questions about why glucose takes such an inefficient route from mitochondria to lipid droplets and back in brown adipocytes during thermogenesis still remain. One obvious explanation could be that in itself, this contributes to heat production 106. Another could be that fatty acid recycling, as part of this route, may contribute to the sensitivity of metabolic control 107. Alternatively, it could serve as a backup mechanism to maintain BAT triglycerides stores. Indeed, during chronic caloric restriction, BAT glucose uptake and metabolism increases markedly even under unstimulated conditions 108.
Much work has been performed on how BAT is fueled during temperature‐induced thermogenesis. It is less clear how BAT is fueled during diet‐induced thermogenesis. Studies originally suggested contributions from circulating glucose 109, 110. This could provide the ATP from glycolysis required for creatine and phosphocreatine cycling in brown adipocytes that has recently been shown in mice to be an essential component of diet‐induced thermogenesis and to prevent weight gain on a high‐fat diet 111. Additionally, circulating fatty acid uptake by BAT was shown to be directly proportional to BAT thermogenesis in response to a mixed carbohydrate‐rich meal in humans 112. Interestingly, despite initially being thought to drive diet‐induced thermogenesis 113, BAT sympathetic nerve function does not seem to be involved in both rodents 114, 115 and humans 112. In this context, WAT sympathetic nerve function and the formation of beige adipocytes may play a more dominant role than that of BAT through insulin receptor signaling in ARC neurons 116. Another important key point is that species differences may exist concerning BAT fuel utilization and intracellular mechanisms of thermogenesis. This is accentuated by the fact that human brown adipocytes have a gene expression profile closer to mouse beige adipocytes than classical (interscapular) brown adipocytes 62, 117, 118, 119. Indeed, unlike rodent BAT, human BAT consumes a high amount of glucose at thermoneutrality as recently determined by microdialysis 118. This technique also revealed that human BAT uptake of the amino acid glutamate increases upon acute cold exposure which may fuel thermogenesis by feeding into the TCA cycle through the action of glutamate dehydrogenase 118. In line with this, the concentration of glutamate markedly increases in rodent BAT upon acute and chronic cold exposure as does the enzymatic activity of glutamate dehydrogenase 119, 120. Overall, the explosion of knowledge in brown adipocyte biology has generated new hope for the development of novel treatments for the devastating consequences of metabolic disease.
Author contributions
MKH produced the original draft of the manuscript which MK substantially revised.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank Dr. Annett Hoffmann for help with producing the figures. MKH is particularly grateful to Prof. Matthias Blüher for continued support. MKH receives funding from the German Research Foundation Collaborative Research Centre 1052 in Obesity Mechanisms (Project A8). MK receives funding from Else Kröner‐Fresenius‐Stiftung and the German Research Foundation (Grant KL 973/12‐1).
EMBO Reports (2018) 19: e46404
See the Glossary for abbreviations used in this article.
Contributor Information
Mohammed K Hankir, Email: hankir_m@klinik.uni-wuerzburg.de.
Martin Klingenspor, Email: mk@tum.de.
References
- 1. Phelps ME, Kuhl DE, Mazziota JC (1981) Metabolic mapping of the brain's response to visual stimulation: studies in humans. Science 211: 1445–1448 [DOI] [PubMed] [Google Scholar]
- 2. Fox PT, Raichle ME, Mintun MA, Dence C (1988) Nonoxidative glucose consumption during focal physiologic neural activity. Science 241: 462–464 [DOI] [PubMed] [Google Scholar]
- 3. Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91: 10625–10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall CN, Klein‐Flügge MC, Howarth C, Attwell D (2012) Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 32: 8940–8951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Díaz‐García CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G (2017) Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab 26: 361–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thanos PK, Robison L, Nestler EJ, Kim R, Michaelides M, Lobo MK, Volkow ND (2013) Mapping brain metabolic connectivity in awake rats with μPET and optogenetic stimulation. J Neurosci 33: 6343–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zimmer ER, Parent MJ, Souza DG, Leuzy A, Lecrux C, Kim HI, Gauthier S, Pellerin L, Hamel E, Rosa‐Neto P (2017) [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci 20: 393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yellen G (2018) Fueling thought: management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol 217: 2235–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525 [DOI] [PubMed] [Google Scholar]
- 10. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (2009) Cold‐activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508 [DOI] [PubMed] [Google Scholar]
- 11. Saito M, Okamatsu‐Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio‐Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K et al (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms‐Hagen J, von Schulthess Gustav K (2002) Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 29: 1393–1398 [DOI] [PubMed] [Google Scholar]
- 13. Cohade C, Osman M, Pannu HK, Wahl RL (2003) Uptake in supraclavicular area fat (“USA‐Fat”): description on 18F‐FDG PET/CT. J Nucl Med 44: 170–176 [PubMed] [Google Scholar]
- 14. Yeung Henry WD, Grewal RK, Gonen M, Schöder H, Larson SM (2003) Patterns of (18)F‐FDG uptake in adipose tissue and muscle: a potential source of false‐positives for PET. J Nucl Med 44: 1789–1796 [PubMed] [Google Scholar]
- 15. Cohade C, Mourtzikos KA, Wahl RL (2003) “USA‐Fat”: prevalence is related to ambient outdoor temperature‐evaluation with 18F‐FDG PET/CT. J Nucl Med 44: 1267–1270 [PubMed] [Google Scholar]
- 16. DeGrado TR, Coenen HH, Stocklin G (1991) 14(R, S)‐[18F]fluoro‐6‐thia‐heptadecanoic acid (FTHA): evaluation in mouse of a new probe of myocardial utilization of long chain fatty acids. J Nucl Med 32: 1888–1896 [PubMed] [Google Scholar]
- 17. Brown M, Marshall DR, Sobel BE, Bergmann SR (1987) Delineation of myocardial oxygen utilization with carbon‐11‐labeled acetate. Circulation 76: 687–696 [DOI] [PubMed] [Google Scholar]
- 18. Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122: 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359 [DOI] [PubMed] [Google Scholar]
- 20. Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM et al (2014) Brown adipose tissue improves whole‐body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chondronikola M, Volpi E, Børsheim E, Porter C, Saraf MK, Annamalai P, Yfanti C, Chao T, Wong D, Shinoda K et al (2016) Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 23: 1200–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123: 3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cypess AM, Weiner LS, Roberts‐Toler C, Franquet Elía E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A et al (2015) Activation of human brown adipose tissue by a β3‐adrenergic receptor agonist. Cell Metab 21: 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM et al (2013) Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 123: 3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S et al (2011) Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279 [DOI] [PubMed] [Google Scholar]
- 26. Blondin DP, Labbé SM, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte ÉE, Haman F, Richard D, Carpentier AC (2015) Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 64: 2388–2397 [DOI] [PubMed] [Google Scholar]
- 27. Jeanguillaume C, Metard G, Ricquier D, Legras P, Bouchet F, Lacoeuille F, Hindre F (2013) Visualization of activated BAT in mice, with FDG‐PET and its relation to UCP1. Adv Mol Imag 3: 19–22 [Google Scholar]
- 28. Hankir MK, Kranz M, Keipert S, Weiner J, Andreasen SG, Kern M, Patt M, Klöting N, Heiker JT, Brust P et al (2017) Dissociation between brown adipose tissue 18F‐FDG uptake and thermogenesis in uncoupling protein 1‐deficient mice. J Nucl Med 58: 1100–1103 [DOI] [PubMed] [Google Scholar]
- 29. Olsen JM, Csikasz RI, Dehvari N, Lu L, Sandström A, Öberg AI, Nedergaard J, Stone‐Elander S, Bengtsson T (2017) β3‐Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: mediation through the mTOR pathway. Mol Metab 6: 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP (1997) Mice lacking mitochondrial uncoupling protein are cold‐sensitive but not obese. Nature 387: 90–94 [DOI] [PubMed] [Google Scholar]
- 31. von Essen G, Lindsund E, Cannon B, Nedergaard J (2017) Adaptive facultative diet‐induced thermogenesis in wild‐type but not in UCP1‐ablated mice. Am J Physiol Endocrinol Metab 313: E515–E527 [DOI] [PubMed] [Google Scholar]
- 32. Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T (2005) Beta‐adrenoceptors, but not alpha‐adrenoceptors, stimulate AMP‐activated protein kinase in brown adipocytes independently of uncoupling protein‐1. Diabetologia 48: 2386–2395 [DOI] [PubMed] [Google Scholar]
- 33. Winther S, Isidor MS, Basse AL, Skjoldborg N, Cheung A, Quistorff B, Hansen JB (2018) Restricting glycolysis impairs brown adipocyte glucose and oxygen consumption. Am J Physiol Endocrinol Metab 314: E214–E223 [DOI] [PubMed] [Google Scholar]
- 34. Inokuma K, Ogura‐Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M (2005) Uncoupling protein 1 is necessary for norepinephrine‐induced glucose utilization in brown adipose tissue. Diabetes 54: 1385–1391 [DOI] [PubMed] [Google Scholar]
- 35. Jeong JH, Chang JS, Jo YH (2018) Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Sci Rep 8: 6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ashrafi G, Wu Z, Farrell RJ, Ryan TA (2017) GLUT4 mobilization supports energetic demands of active synapses. Neuron 93: 606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, Fromme T, Schweizer S, Schöttl T, Klingenspor M (2014) Taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured primary brown and brite/beige adipocytes. EMBO Rep 15: 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olsen JM, Sato M, Dallner OS, Sandström AL, Pisani DF, Chambard JC, Amri EZ, Hutchinson DS, Bengtsson T (2014) Glucose uptake in brown fat cells is dependent on mTOR complex 2‐promoted GLUT1 translocation. J Cell Biol 207: 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Albert V, Svensson K, Shimobayashi M, Colombi M, Muñoz S, Jimenez V, Handschin C, Bosch F, Hall MN (2016) mTORC2 sustains thermogenesis via Akt‐induced glucose uptake and glycolysis in brown adipose tissue. EMBO Mol Med 8: 232–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pettersson B, Vallin I (1976) Norepinephrine‐induced shift in levels of adenosine 3′:5′‐monophosphate and ATP parallel to increased respiratory rate and lipolysis in isolated hamster brown‐fat cells. Eur J Biochem 62: 383–390 [DOI] [PubMed] [Google Scholar]
- 41. Law J, Morris DE, Izzi‐Engbeaya C, Salem V, Coello C, Robinson L, Jayasinghe M, Scott R, Gunn R, Rabiner E et al (2018) Thermal imaging is a noninvasive alternative to PET/CT for measurement of brown adipose tissue activity in humans. J Nucl Med 59: 516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dubikovskaya E, Karampinos DC, Holzapfel C, Hauner H, Klingenspor M, Ntziachristos V (2018) Non‐invasive measurement of brown fat metabolism based on optoacoustic imaging of hemoglobin gradients. Cell Metab 27: 689–701 [DOI] [PubMed] [Google Scholar]
- 43. Steiner G, Cahill GF Jr (1964) Brown and white adipose tissue metabolism in cold‐exposed rats. Am J Physiol 207: 840–844 [DOI] [PubMed] [Google Scholar]
- 44. Trayhurn P (1979) Fatty acid synthesis in vivo in brown adipose tissue, liver and white adipose tissue of the cold‐acclimated rat. FEBS Lett 104: 13–16 [DOI] [PubMed] [Google Scholar]
- 45. Ma SW, Foster DO (1986) Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo . Can J Physiol Pharmacol 64: 609–614 [DOI] [PubMed] [Google Scholar]
- 46. Isler D, Hill HP, Meier MK (1987) Glucose metabolism in isolated brown adipocytes under beta‐adrenergic stimulation. Quantitative contribution of glucose to total thermogenesis. Biochem J 245: 789–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saggerson ED, McAllister TW, Baht HS (1988) Lipogenesis in rat brown adipocytes. Effects of insulin and noradrenaline, contributions from glucose and lactate as precursors and comparisons with white adipocytes. Biochem J 251: 701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A, Govendir MA, Emmett L, Greenfield JR (2016) Brown adipose tissue exhibits a glucose‐responsive thermogenic biorhythm in humans. Cell Metab 23: 602–609 [DOI] [PubMed] [Google Scholar]
- 49. Held NM, Kuipers EN, van Weeghel M, van Klinken JB, Denis SW, Lombès M, Wanders RJ, Vaz FM, Rensen PCN, Verhoeven AJ et al (2018) Pyruvate dehydrogenase complex plays a central role in brown adipocyte energy expenditure and fuel utilization during short‐term beta‐adrenergic activation. Sci Rep 8: 9562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caron A, Labbé SM, Carter S, Roy MC, Lecomte R, Ricquier D, Picard F, Richard D (2017) Loss of UCP2 impairs cold‐induced non‐shivering thermogenesis by promoting a shift toward glucose utilization in brown adipose tissue. Biochimie 134: 118–126 [DOI] [PubMed] [Google Scholar]
- 51. Hao Q, Yadav R, Basse AL, Petersen S, Sonne SB, Rasmussen S, Zhu Q, Lu Z, Wang J, Audouze K et al (2016) Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. Am J Physiol Endocrinol Metab 308: E380–E392 [DOI] [PubMed] [Google Scholar]
- 52. Labbé SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, Richard D (2015) In vivo measurement of energy substrate contribution to cold‐induced brown adipose tissue thermogenesis. FASEB J 29: 2046–2058 [DOI] [PubMed] [Google Scholar]
- 53. Sanchez‐Gurmaches J, Tang Y, Jespersen NZ, Wallace M, Martinez Calejman C, Gujja S, Li H, Edwards YJK, Wolfrum C, Metallo CM et al (2018) Brown fat AKT2 is a cold‐induced kinase that stimulates ChREBP‐mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab 27: 195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M (2009) Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab 10: 324–335 [DOI] [PubMed] [Google Scholar]
- 55. Golic I, Velickovic K, Markelic M, Stancic A, Jankovic A, Vucetic M, Otasevic V, Buzadzic B, Korac B, Korac A (2014) Calcium‐induced alteration of mitochondrial morphology and mitochondrial‐endoplasmic reticulum contacts in rat brown adipocytes. Eur J Histochem 58: 2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Darnley AC, Carpenter CA, Saggerson ED (1988) Changes in activities of some enzymes of glycerolipid synthesis in brown adipose tissue of cold‐acclimated rats. Biochem J 253: 351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nadra K, Médard JJ, Mul JD, Han GS, Grès S, Pende M, Metzger D, Chambon P, Cuppen E, Saulnier‐Blache JS et al (2012) Cell autonomous lipin 1 function is essential for development and maintenance of white and brown adipose tissue. Mol Cell Biol 32: 4794–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ball EG, Jungas RL (1961) On the action of hormones which accelerate the rate of oxygen consumption and fatty acid release in rat adipose tissue in vitro . Proc Natl Acad Sci USA 47: 932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ballard FJ, Hanson RW, Leveille GA (1967) Phosphoenolpyruvate carboxykinase and the synthesis of glyceride‐glycerol from pyruvate in adipose tissue. J Biol Chem 242: 2746–2750 [PubMed] [Google Scholar]
- 60. Granneman JG, Burnazi M, Zhu Z, Schwamb LA (2003) White adipose tissue contributes to UCP1‐independent thermogenesis. Am J Physiol Endocrinol Metab 285: E1230–E1236 [DOI] [PubMed] [Google Scholar]
- 61. Lasar D, Rosenwald M, Kiehlmann E, Balaz M, Tall B, Opitz L, Lidell ME, Zamboni N, Krznar P, Sun W et al (2018) Peroxisome proliferator activated receptor gamma controls mature brown adipocyte inducibility through glycerol kinase. Cell Rep 22: 760–773 [DOI] [PubMed] [Google Scholar]
- 62. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jiang Y, Berry DC, Graff JM (2017) Distinct cellular and molecular mechanisms for β3 adrenergic receptor‐induced beige adipocyte formation. Elife 6: e30329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J (2013) UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 5: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 65. Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P (2017) UCP1‐independent signaling involving SERCA2b‐mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23: 1454–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik‐Bogoslavski D, Hasenfuss SC et al (2015) A creatine‐driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bertholet AM, Kazak L, Chouchani ET, Bogaczyńska MG, Paranjpe I, Wainwright GL, Bétourné A, Kajimura S, Spiegelman BM, Kirichok Y (2017) Mitochondrial patch clamp of beige adipocytes reveals UCP1‐positive and UCP1‐negative cells both exhibiting futile creatine cycling. Cell Metab 25: 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP (2006) UCP1‐independent thermogenesis in white adipose tissue of cold‐acclimated Ucp1‐/‐ mice. J Biol Chem 281: 31894–31908 [DOI] [PubMed] [Google Scholar]
- 69. Fain JN, Loken SC (1969) Response of trypsin‐treated brown and white fat cells to hormones. Preferential inhibition of insulin action. J Biol Chem 244: 3500–3506 [PubMed] [Google Scholar]
- 70. Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Zhan L, Yanxiang Guo J et al (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551: 115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA (2008) Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE 3: e2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F (2011) Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 286: 12983–12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M et al (2009) Acute glucose‐lowering and insulin‐sensitizing action of FGF21 in insulin‐resistant mouse models–association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab 297: E1105–E1114 [DOI] [PubMed] [Google Scholar]
- 74. Irshad Z, Dimitri F, Christian M, Zammit VA (2017) Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J Lipid Res 58: 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu XX, Lewin DA, Forrest W, Adams SH (2002) Cold elicits the simultaneous induction of fatty acid synthesis and beta‐oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J 16: 155–168 [DOI] [PubMed] [Google Scholar]
- 76. Zancanaro C, Nano R, Marchioro C, Sbarbati A, Boicelli A, Osculati F (1994) Magnetic resonance spectroscopy investigations of brown adipose tissue and isolated brown adipocytes. J Lipid Res 35: 2191–2199 [PubMed] [Google Scholar]
- 77. Blondin DP, Frisch F, Phoenix S, Guérin B, Turcotte ÉE, Haman F, Richard D, Carpentier AC (2017) Inhibition of intracellular triglyceride lipolysis suppresses cold‐induced brown adipose tissue metabolism and increases shivering in humans. Cell Metab 25: 438–447 [DOI] [PubMed] [Google Scholar]
- 78. Hittelman KJ, Lindberg O, Cannon B (1969) Oxidative phosphorylation and compartmentation of fatty acid metabolism in brown fat mitochondria. Eur J Biochem 11: 183–192 [DOI] [PubMed] [Google Scholar]
- 79. Locke RM, Rial E, Scott ID, Nicholls DG (1982) Fatty acids as acute regulators of the proton conductance of hamster brown‐fat mitochondria. Eur J Biochem 129: 373–380 [DOI] [PubMed] [Google Scholar]
- 80. Fedorenko A, Lishko PV, Kirichok Y (2012) Mechanism of fatty‐acid‐dependent UCP1 uncoupling in brown fat mitochondria. Cell 151: 400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AKG, Jackson R, Rumore D, Xue B, Shi H et al (2017) Lipolysis in brown adipocytes is not essential for cold‐induced thermogenesis in mice. Cell Metab 26: 764–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M, Kolb D, Hoeks J, Kershaw EE, Sedej S et al (2017) Cold‐induced thermogenesis depends on ATGL‐mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab 26: 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C et al (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205 [DOI] [PubMed] [Google Scholar]
- 84. Khedoe PP, Hoeke G, Kooijman S, Dijk W, Buijs JT, Kersten S, Havekes LM, Hiemstra PS, Berbée JF, Boon MR et al (2015) Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J Lipid Res 56: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A, Stahl A (2006) Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55: 3229–3237 [DOI] [PubMed] [Google Scholar]
- 86. Putri M, Syamsunarno MR, Iso T, Yamaguchi A, Hanaoka H, Sunaga H, Koitabashi N, Matsui H, Yamazaki C, Kameo S et al (2015) CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem Biophys Res Commun 457: 520–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang F, Hao G, Shao M, Nham K, An Y, Wang Q, Zhu Y, Kusminski CM, Hassan G, Gupta RK et al (2018) An adipose tissue atlas: an image‐guided identification of human‐like BAT and beige depots in rodents. Cell Metab. 27: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA (2010) Adipose acyl‐CoA synthetase‐1 directs fatty acids toward beta‐oxidation and is required for cold thermogenesis. Cell Metab 12: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ji S, You Y, Kerner J, Hoppel CL, Schoeb TR, Chick WS, Hamm DA, Sharer JD, Wood PA (2008) Homozygous carnitine palmitoyltransferase 1b (muscle isoform) deficiency is lethal in the mouse. Mol Genet Metab 93: 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee J, Ellis JM, Wolfgang MJ (2015) Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress‐induced inflammation. Cell Rep 10: 266–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP (1998) Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest 102: 1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tolwani RJ, Hamm DA, Tian L, Sharer JD, Vockley J, Rinaldo P, Matern D, Schoeb TR, Wood PA (2005) Medium‐chain acyl‐CoA dehydrogenase deficiency in gene‐targeted mice. PLoS Genet 1: e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhao S, Torres A, Henry RA, Trefely S, Wallace M, Lee JV, Carrer A, Sengupta A, Campbell SL, Kuo YM et al (2016) ATP‐citrate lyase controls a glucose‐to‐acetate metabolic switch. Cell Rep 17: 1037–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang Y, Li Y, Niepel MW, Kawano Y, Han S, Liu S, Marsili A, Larsen PR, Lee CH, Cohen DE (2012) Targeted deletion of thioesterase superfamily member 1 promotes energy expenditure and protects against obesity and insulin resistance. Proc Natl Acad Sci USA 109: 5417–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kang HW, Ozdemir C, Kawano Y, LeClair KB, Vernochet C, Kahn CR, Hagen SJ, Cohen DE (2013) Thioesterase superfamily member 2/Acyl‐CoA thioesterase 13 (Them2/Acot13) regulates adaptive thermogenesis in mice. J Biol Chem 288: 33376–33386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Okada K, LeClair KB, Zhang Y, Li Y, Ozdemir C, Krisko TI, Hagen SJ, Betensky RA, Banks AS, Cohen DE (2016) Thioesterase superfamily member 1 suppresses cold thermogenesis by limiting the oxidation of lipid droplet‐derived fatty acids in brown adipose tissue. Mol Metab 5: 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H (2012) Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab 16: 825–832 [DOI] [PubMed] [Google Scholar]
- 98. Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, Lee S, Jiang L, Huck I, Kershaw EE et al (2017) Global analysis of plasma lipids identifies liver‐derived acylcarnitines as a fuel source for brownfat thermogenesis. Cell Metab 26: 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X (2014) The brown fat‐enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 20: 1436–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Heine M, Fischer AW, Schlein C, Jung C, Straub LG, Gottschling K, Mangels N, Yuan Y, Nilsson SK, Liebscher G et al (2018) Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated brown adipose tissue in mice. Cell Metab 10.1016/j.cmet.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 101. Worthmann A, John C, Rühlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, Heine M, Schlein C, Evangelakos I, Mineo C et al (2017) Cold‐induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 23: 839–849 [DOI] [PubMed] [Google Scholar]
- 102. Ziętak M, Kovatcheva‐Datchary P, Markiewicz LH, Ståhlman M, Kozak LP, Bäckhed F (2016) Altered microbiota contributes to reduced diet‐induced obesity upon cold exposure. Cell Metab 23: 1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, Schaart G, Kouach M, Charton J, Deprez B et al (2012) The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 22: 418–426 [DOI] [PubMed] [Google Scholar]
- 104. Jeong JH, Lee DK, Liu SM, Chua SC Jr, Schwartz GJ, Jo YH (2018) Activation of temperature‐sensitive TRPV1‐like receptors in ARC POMC neurons reduces food intake. PLoS Biol 16: e2004399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Glick Z (1982) Inverse relationship between brown fat thermogenesis and meal size: the thermostatic control of food intake revisited. Physiol Behav 29: 1137–1140 [DOI] [PubMed] [Google Scholar]
- 106. Masoro EJ (1963) Role of lipogenesis in nonshivering thermogenesis. Fed Proc 22: 868–873 [PubMed] [Google Scholar]
- 107. Newsholme EA, Arch JR, Brooks B, Surholt B (1983) The role of substrate cycles in metabolic regulation. Biochem Soc Trans 11: 52–56 [DOI] [PubMed] [Google Scholar]
- 108. Wetter TJ, Gazdag AC, Dean DJ, Cartee GD (1999) Effect of calorie restriction on in vivo glucose metabolism by individual tissues in rats. Am J Physiol 276: E728–E738 [DOI] [PubMed] [Google Scholar]
- 109. Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, Phelps ME, Weber WA (2006) Impact of animal handling on the results of 18F‐FDG PET studies in mice. J Nucl Med 47: 999–1006 [PubMed] [Google Scholar]
- 110. Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD (2013) Brown adipose tissue activity after a high‐calorie meal in humans. Am J Clin Nutr 98: 57–64 [DOI] [PubMed] [Google Scholar]
- 111. Kazak L, Chouchani ET, Lu GZ, Jedrychowski MP, Bare CJ, Mina AI, Kumari M, Zhang S, Vuckovic I, Laznik‐Bogoslavski D et al (2017) Genetic depletion of adipocyte creatine metabolism inhibits diet‐induced thermogenesis and drives obesity. Cell Metab 26: 660–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. U DM, Saari T, Raiko J, Kudomi N, Maurer SF, Lahesmaa M, Fromme T, Amri EZ, Klingenspor M, Solin O et al (2018) Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab 28: P207–216.E3 [DOI] [PubMed] [Google Scholar]
- 113. Glick Z, Raum WJ (1986) Norepinephrine turnover in brown adipose tissue is stimulated by a single meal. Am J Physiol 251: R13–R17 [DOI] [PubMed] [Google Scholar]
- 114. Chen M, Chen H, Nguyen A, Gupta D, Wang J, Lai EW, Pacak K, Gavrilova O, Quon MJ, Weinstein LS (2010) G(s)alpha deficiency in adipose tissue leads to a lean phenotype with divergent effects on cold tolerance and diet‐induced thermogenesis. Cell Metab 11: 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sakaguchi T, Arase K, Fisler JS, Bray GA (1989) Effect of a high‐fat diet on firing rate of sympathetic nerves innervating brown adipose tissue in anesthetized rats. Physiol Behav 45: 1177–1182 [DOI] [PubMed] [Google Scholar]
- 116. Dodd GT, Andrews ZB, Simonds SE, Michael NJ, DeVeer M, Brüning JC, Spanswick D, Cowley MA, Tiganis T (2017) A hypothalamic phosphatase switch coordinates energy expenditure with feeding. Cell Metab 26: 375–393 [DOI] [PubMed] [Google Scholar]
- 117. Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH (2015) Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 21: 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Weir G, Ramage LE, Akyol M, Rhodes JK, Kyle CJ, Fletcher AM, Craven TH, Wakelin SJ, Drake AJ, Gregoriades ML et al (2018) Substantial metabolic activity of human brown adipose tissue during warm conditions and cold‐induced lipolysis of local triglycerides. Cell Met 27: 1348–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. López‐Soriano FJ, Alemany M (1987) Effect of cold‐temperature exposure and acclimation on amino acid pool changes and enzyme activities of rat brown adipose tissue. Biochim Biophys Acta 925: 265–271 [DOI] [PubMed] [Google Scholar]
- 120. Lu X, Solmonson A, Lodi A, Nowinski SM, Sentandreu E, Mills EM, Tiziani S (2017) The early metabolomic response of adipose tissue during acute cold exposure in mice. Sci Rep 7: 3455 [DOI] [PMC free article] [PubMed] [Google Scholar]


