Abstract
Patients with HIV routinely use medicinal cannabinoids to treat neuropathic pain, anxiety, and human immunodeficiency virus (HIV)–associated wasting. However, Δ9-tetrahydrocannabinol (THC), the primary psychoactive cannabinoid in cannabis, suppresses T-cell function and secretion of interferons, both critically important in the antiviral immune response. Interferon-α (IFNα), a key cytokine in T-cell activation and peripheral control of HIV infection, can potentiate responsiveness to interleukin-7 (IL-7), a crucial homeostatic cytokine for peripheral T-cell maintenance. The objective of this investigation was to compare the response of T cells to stimulation by IFNα and IL-7 in T cells from healthy and HIV+ donors in the absence and presence of THC. To compare T-cell responses between healthy and HIV+ donors signaling through IFNα receptor, IFNα-induced expression of IL-7α receptor (IL-7Rα), cognate signaling through IL-7R, and on IL-7–mediated T-cell proliferation were measured by flow cytometry and real-time quantitative polymerase chain reaction. CD8+ T cells from HIV+ donors showed a diminished response to IFNα-induced phosphorylated signal transducer and activator of transcription-1 activation compared with CD8+ T cells from healthy donors, whereas CD4+ T cells from HIV+ donors and healthy donors were comparable. Treatment with IFNα promoted IL-7R expression and potentiated IL-7–induced STAT5 phosphorylation to augment IL-7–mediated proliferation by T cells from healthy and HIV+ donors. Finally, HIV+ donors exhibited reduced sensitivity to THC-mediated suppression by IFNα- and IL-7–mediated stimulation compared with healthy donors. These results further support THC as being immune suppressive while identifying putatively beneficial aspects of cannabinoid-based therapies in HIV+ patients.
Introduction
Approximately 37 million people globally are infected with human immunodeficiency virus (HIV) (Karim, 2017), of whom 1.1 million reside in the United States (Hess et al., 2017). CD4+ T-cell leukocytopenia is a hallmark of HIV infection, with CD4+ T cells being a primary cellular target for HIV (Dragic et al., 1996; Sousa et al., 2002). The perturbation of CD4+ T cells causes a loss of adaptive immune integrity, including loss of cytotoxic T-lymphocyte (CD8+ T-cell) function (Roederer et al., 1995), culminating in AIDS (Koot et al., 1993). Since the mid-1990s, the standard of care after HIV diagnosis has been antiretroviral therapy (ART) (Autran et al., 1997; Iacob et al., 2017). ART facilitates CD4+ T-cell restoration and, by extension, CD8+ T-cell restoration (Oxenius et al., 2000). However, 15%–20% of patients with HIV continue to have T-cell deficiencies despite undergoing successful ART (Kelley et al., 2009; Serrano-Villar et al., 2014).
One reported T-cell deficiency in patients with HIV exhibiting a low CD4+ T-cell nadir is reduced expression of interleukin-7 (IL-7) receptor (IL-7R) (MacPherson et al., 2001; Nguyen et al., 2016; Hartling et al., 2017). IL-7 is a crucial cytokine for T-cell integrity as it drives both differentiation and peripheral maintenance of T cells (Tan et al., 2001). Likewise, IL-7 enhances the expansion of T cells from HIV+ donors (Bazdar and Sieg, 2007; Levy et al., 2009; Sereti et al., 2009). Clinically, the use of IL-7 in patients with HIV reversed T-cell leukocytopenia and restored gut lumen integrity (i.e., leaky-gut syndrome) (Sereti et al., 2014). The mechanisms underlying IL-7R expression deficiency in HIV-infected T cells is poorly understood, but is correlated with the phenomenon termed “T-cell exhaustion,” which is associated with chronic and extensive antigenic stimulation (Lang et al., 2005).
IL-7R expression is tightly regulated, with type 1 interferons playing a critical role in that regulation through a type 1 interferon–inducible promoter region called an interferon (IFN)-sensitive responsible element (Mazzucchelli and Durum, 2007). Type 1 IFNs are composed of IFNα and IFNβ and possess antiviral activity (García-Sastre and Biron, 2006). Plasmacytoid dendritic cells (pDCs) are the primary source of IFNα secretion (Colonna et al., 2004) and have a direct influence on maintaining T-cell integrity during HIV infection. Typically, the circulating numbers of pDCs and CD4+ T cells are correlated in a positive manner (Donaghy et al., 2001). During chronic HIV infection, there is a reduction of pDC number and function, resulting in a decreased capacity to produce IFNα (Chehimi et al., 2002). IFNα has also been shown to suppress HIV expansion (Poli et al., 1989) and provided protection for CD4+ T cells from HIV-mediated depletion in a humanized mouse model (Lapenta et al., 1999). Furthermore, pDCs promoted T-cell activation and protection against certain viral infections when using an Fc-fused IL-7 (Kang et al., 2017).
In addition to the complications arising from chronic HIV infection, patients with HIV routinely use medicinal cannabinoids to treat HIV-associated wasting, as an appetite stimulant; and neuropathic pain, from the use of some HIV reverse transcriptase inhibitors as part of ART regimens; and generally reduce anxiety (Abrams, 2000; Prentiss et al., 2004; Haney et al., 2007; Ellis et al., 2009). The primary psychoactive cannabinoid in cannabis, Δ9-tetrahydrocannabinol (THC), and synthetic THC, like dronabinol (i.e., marinol), exhibit potent anti-inflammatory activity and are also immunosuppressive (Klein et al., 1998; Tanasescu and Constantinescu, 2010). It is well established that THC can suppress T-cell responses to viral infections (Reiss, 2010; Eisenstein and Meissler, 2015), including HIV (Roth et al., 2005). Additionally, pDC secretion of IFNα is acutely sensitive to THC-mediated suppression, and pDCs from HIV+ donors show increased sensitivity to THC-mediated suppression than pDCs from healthy donors (Henriquez et al., 2017).
The objective of this investigation was to compare the response of T cells to stimulation by IFNα and IL-7 in T cells from healthy and HIV+ donors in the absence and presence of THC. Specifically, studies were conducted to determine whether in vitro stimulation of T cells by IFNα would drive the expression of IL-7Rα, thereby potentiating IL-7–mediated signaling and proliferation. Furthermore, the effect of THC on the stimulation of T cells by IFNα and IL-7 was evaluated. Last, the responses to IFNα and IL-7, in the absence and presence of THC in T cells from healthy and HIV+ donors, were compared.
Materials and Methods
Peripheral Blood Mononuclear Cell Isolation and Cell Identification.
Leukocyte packs were purchased from the Gulf Coast Regional Blood Center (Houston, TX). Blood was diluted with Gibco Hanks’ balanced salt solution from Thermo Fisher Scientific (Waltham, MA) and layered on Ficoll Paque Plus (GE Healthcare Life Sciences, Pittsburgh, PA) in SepMate tubes by StemCell Technologies (Vancouver, BC, Canada). Leukocytes were resuspended in Gibco complete RPMI (C-RPMI) media from Thermo Fisher Scientific containing 5% Human AB Serum (Sigma-Aldrich, St. Louis, MO), 1% Penicillin-Streptomycin (Thermo Fisher Scientific), and 0.1% β-mercaptoethanol. T cells were identified using antibodies by BioLegend (San Diego, CA) as CD3+ cells and as either CD4+ or CD8+. Memory cells were identified as CD45RO+ and nonmemory cells were identified as CD45RO−.
T-Cell Purification by Magnetic Activated Cell Sorting.
T cells were isolated using magnetic activated cell sorting (MACS) CD3-T-cell isolation kits from BioLegend. In short, after peripheral blood mononuclear cell (PBMC) isolation, the cell concentration was determined using a Coulter Particle-Counter (Beckman-Coulter Inc., Brea, CA), and the appropriate volume of non–T-cell antibody cocktail was incubated with PBMCs followed by washing with MACS buffer [1× phosphate-buffered saline (PBS), 0.5% bovine serum albumin, and 2 mM EDTA] and incubation with magnetic beads. Labeled PBMCs were then passed through a MACS magnet, and T cells were collected in the flow through.
Treatment with Cannabinoids or Vehicle Control.
THC was supplied by the National Institute on Drug Abuse. Purified T cells or whole PBMCs were treated with either THC or vehicle control (VC; 0.03% ethanol) prepared in C-RPMI medium. Cells were incubated at 37°C and 5% CO2 for 30 minutes before being stimulated (below).
Stimulation of T Cells.
After treatment with THC or VC, PBMCs or isolated T cells were stimulated as follows: 1) to measure the phosphorylation of STAT1, cells were stimulated with 100 U/ml universal IFNα (PBL Assay Science, Piscataway, NJ) for 30 minutes before harvesting for phospho-protein detection (below); 2) to measure IFNα-induced IL-7Rα mRNA and protein expression, cells were treated with 100 U/ml IFNα for 48 hours before harvesting and measurement of respective endpoints (below); 3) IL-7–induced phosphorylation of STAT5 on day 0 or 48 hours after IFNα stimulation (100 U/ml) was measured by stimulating cells with 10 ng/ml IL-7 for 15 minutes before harvesting for phospho-protein detection (below); and 4) for measuring IL-7–augmented proliferation of T cells (below), cells were stimulated with 100 U/ml IFNα, 2.5 µg/ml mouse antihuman CD3 antibody (BioLegend), and 2.5 µg/ml mouse antihuman CD28 antibody for 48 hours followed by stimulation with 10 ng/ml IL-7 or VC (sterile endotoxin-free water; InvivoGen, San Diego, CA), and cells were incubated for another 48 hours before harvesting.
Gene Expression Analysis.
RNA was isolated using RNeasy Kits (Qiagen, Hilden, Germany) per the manufacturer instructions. Briefly, T cells were lysed using lysing buffer containing β-mercaptoethanol and stored at −20°C. Lysates were purified and treated with DNAse from an ST Total RNA Isolation Kit (Promega, Madison, WI). RNA concentrations were determined by Nanodrop (Thermo Fisher Scientific). Reverse-transcription polymerase chain reaction (PCR) was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). cDNA was stored at −80°C. Gene analysis was determined by real-time quantitative PCR using Life Technologies TaqMan Probes for IL-7Rα [Hs00902334_m1; Thermo Fisher Scientific (through Compendia Bioscience, Ann Arbor, MI)] with 18S ribosomal RNA as the loading control.
Phospho-Protein and IL-7Rα Detection.
PBMCs were washed and T cells were stained as described above. Phosphorylated signal transducer and activator of transcription (pSTAT) 1 and pSTAT5 levels were determined using Phosflow antibodies and the harsh detergent method by BD Biosciences (San Jose, CA). In brief, cells were fixed using BD Biosciences Cytofix buffer for 10 minutes at 37°C, permeabilized with 1× BD Phosflow Perm Buffer IV, stained for 1 hour under continuous motion in BD FACS Buffer (1× PBS, 1% bovine serum albumin, and 0.1% sodium azide) containing 7% Human AB Serum (Sigma-Aldrich), washed once with 0.5× BD Phosflow Perm Buffer, washed twice with BD FACS Buffer, and then immediately analyzed by flow cytometry. IL-7Rα surface expression was determined by surface staining with mouse antihuman antibodies (BioLegend).
Detection of T-Cell Proliferation.
Prior to activation (above), PBMCs were treated with violet CellTrace dye (Thermo Fisher Scientific). In brief, the dye was resuspended in dimethylsulfoxide and diluted in warm sterile PBS (0.02% dimethylsulfoxide). PBMCs were incubated in the PBS/dye mixture at 37°C for 20 minutes and washed with C-RPMI medium. Cells were then centrifuged, washed with RPMI, and resuspended in C-RPMI before stimulation (above). T-cell proliferation for all T-cell populations was determined by dye dilution and presented as the division index (DI), which was generated by the FlowJo version 10 (FlowJo, LLC, Ashland, OR) proliferation tool. Specifically, the DI is the average number of cell divisions that a cell in the original population has undergone and includes all cells in the population.
HIV+ Donor Recruitment and Data Management.
HIV+ donors voluntarily enrolled via the Mid-Michigan HIV Consortium (MMHC) under the Institutional Review Board–approved protocol (Institutional Review Board protocol no. 11-202) and into the MMHC Registry. HIV+ donors were males between the ages of 31 and 71 years, with an average age of 54.4 years, who had CD4+ counts above 500 counts/ml blood, CD4/CD8 ratios >1, did not use medicinal cannabinoids, had HIV viral burdens below the detectable limit (<5 HIV mRNA copies/ml blood), were not coinfected with any strain of hepatitis, and were recruited from clinics attended by author P.G. The status of medicinal cannabinoid use was determined by self-reporting and was verified via plasma detection of THC metabolites using the THC ELISA (enzyme-linked immunosorbent assay) Forensic Kit (Neogen Corporation, Lansing, MI). HIV+ donors received the standard of care and were not asked to change any lifestyle habits to participate. All subject, questionnaire, and abstracted medical record data of the MMHC are managed using the Research Electronic Data Capture (REDCap) (Vanderbilt University, Nashville, TN), which supports 21 Code of Federal Regulations Part 11 compliance for clinical research and trials data and Health Insurance Portability and Accountability Act of 1996 guidelines.
Data Analysis.
Prism 5.0 by GraphPad Software, Inc. (La Jolla, CA) was used for all data analyses. Where appropriate, samples were normalized to VC + IFNα/IL-7, which was considered to be a 100% maximum response for each individual donor, and the appropriate statistical test was performed (see figure legends).
Results
CD4+ and CD8+ T Cells from Healthy and HIV+ Donors Have Comparable Composition of Memory and Nonmemory Cells.
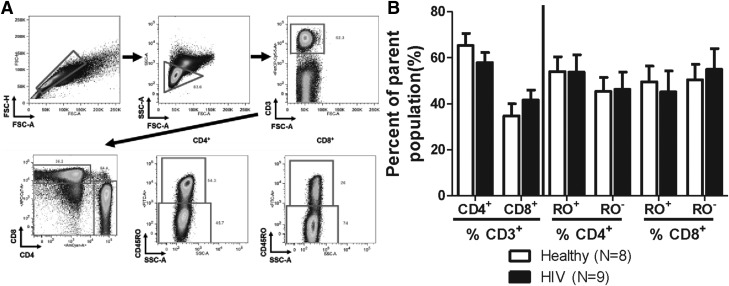
As indicated in the Materials and Methods, HIV+ donors for this study were chosen based on the following criteria: 1) not using medicinal or recreational cannabinoids; 2) CD4+ T-cell counts (>500 counts/µl); 3) CD4/CD8 ratios (>1); 4) not coinfected with hepatitis (A, B, or C); and 5) currently receiving ART with nondetectable viral burdens. It is noteworthy that the number of CD4+ and CD8+ T cells provides only a partial view of the overall T-cell repertoire. T cells were also evaluated for CD45RO expression, which identifies memory T cells (Fig. 1A). No significant differences were observed in the composition of memory and nonmemory CD4+ or CD8+ T cells between healthy and HIV+ donors in this study (Fig. 1B).
Fig. 1.
Healthy and HIV+ donors have comparable T-cell composition. (A) T cells were identified as CD3+ lymphocytes, and then classified as helper or cytotoxic T lymphocytes based upon the surface expression of CD4 and CD8, respectively. Memory T cells were identified as CD45RO+ and nonmemory T cells were identified as CD45RO− for both CD4+ and CD8+ T cells. (B) HIV+ donors possessed CD4+ and CD8+ T-cell numbers that were comparable to those of the healthy donors, and comparable memory (CD45RO+)/nonmemory (CD45RO−) cell composition. FSC-A, forward scatter area; RO−, CD45RO−; RO+, CD45RO+; SSC-A, side scatter area.
IFNα-Induced Phosphorylation of STAT1 in CD4+ and CD8+ T Cells from Healthy Donors Was More Sensitive to THC-Mediated Suppression than T Cells from HIV+ Donors.
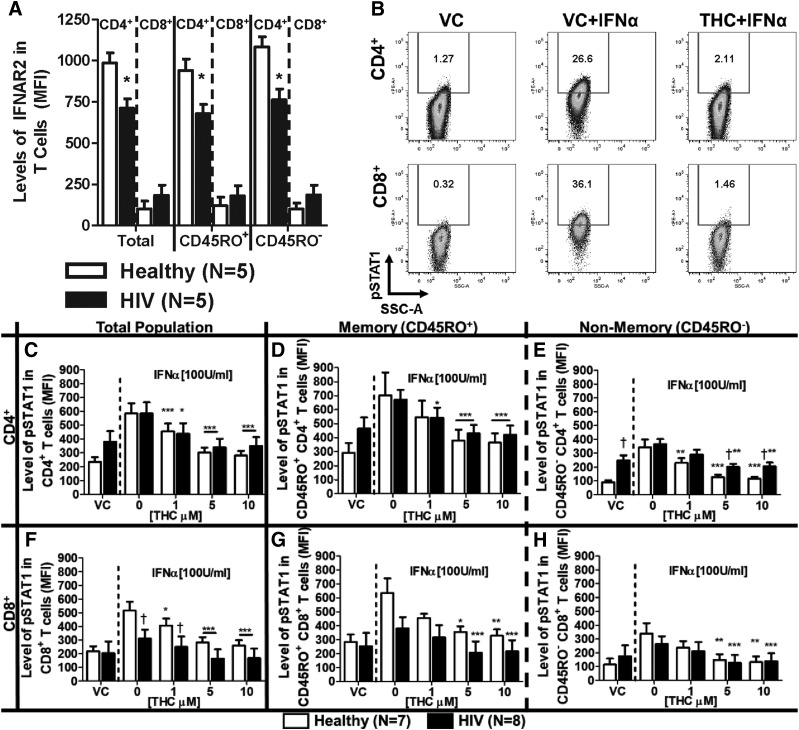
The expression of IFNα receptor (IFNΑR) is known to be diminished in patients with chronic HIV infection (Hardy et al., 2009). Moreover, T cells from HIV+ donors who have not undergone ART have an altered response to IFNα compared with the response to T cells from healthy donors (Hardy et al., 2009). Our results confirm these findings such that CD4+ T cells had diminished expression of IFNAR2 in our cohort of HIV+ donors (Fig. 2A). By contrast, no differences were observed in the expression of IFNAR2 comparing CD8+ T cells from healthy and HIV+ donors.
Fig. 2.
T cells from healthy and HIV+ donors exhibit different profiles of IFNΑR2 expression and IFNα-induced STAT1 phosphorylation, which is suppressed by THC. PBMCs from healthy and HIV+ donors were isolated by Ficoll Paque density gradient centrifugation and used for the determination of either IFNΑR2 surface expression or IFNα-induced STAT1 phosphorylation (pSTAT1). (A) IFNAR2 expression was quantified by flow cytometry using the mean fluorescence intensity (MFI). For pSTAT1, PBMCs were treated with either vehicle (0.03% EtOH) or THC (1, 5, or 10 µM) in 0.03% EtOH for 30 minutes before stimulation with 100 U/ml IFNα for 30 minutes. (B) Representative experiment of IFNα-mediated pSTAT1 induction and THC (10 µM)-mediated suppression in T cells from a healthy donor. The effects of THC on IFNα-pSTAT1 induction in: total (C), memory (D) and nonmemory (E) CD4+ T cells; and total (F), memory (G), and nonmemory (H) CD8+ T cells. For IFNΑR2 expression, asterisks indicate statistically significant differences (*P < 0.05) of MFI in HIV compared with type-matched T cells from healthy donors. For pSTAT1, asterisks indicate statistically significant differences of the treatment with the HIV status–matched VC (0 THC) (*P < 0.05; **P < 0.01; ***P < 0.001). Daggers indicate statistically significant differences of treatment-matched groups between T cells from healthy and HIV+ donors (†P < 0.05) (two-way analysis of variance with Bonferroni multiple-comparisons post-test). SSC-A, side scatter area.
Presently, it is unknown how T cells from HIV+ donors receiving ART respond to IFNα or THC. To address this, the phosphorylation of STAT1 in response to treatment with IFNα was quantified (Fig. 2B). CD4+ T cells from HIV+ patients trended toward elevated background levels of pSTAT1 compared with CD4+ T cells from healthy donors (Fig. 2, C–E), and this difference was statistically significant in the CD45RO− (nonmemory) CD4+ T cells (Fig. 2E). Upon the addition of IFNα, similar induction of pSTAT1 was observed in CD4+ T cells from HIV+ donors and healthy donors (Fig. 2, C–E). Conversely, CD8+ cells from HIV+ donors had diminished levels of IFNα-induced pSTAT1 compared with healthy donors (Fig. 2E). Furthermore, treatment with THC significantly suppressed IFNα-induced pSTAT1 expression in CD4+ and CD8+ T cells from both HIV+ and healthy donors (Fig. 2, C–H), but CD45RO− (nonmemory) CD4+ T cells from HIV+ donors were less sensitive to THC-mediated suppression compared with CD45RO− (nonmemory) CD4+ T cells from healthy donors (Fig. 2E).
IFNα Upregulates IL-7Rα Expression in T Cells from Healthy and HIV+ Donors, and T Cells from Healthy Donors Were More Sensitive to THC-Mediated Suppression than T Cells from HIV+ Donors.
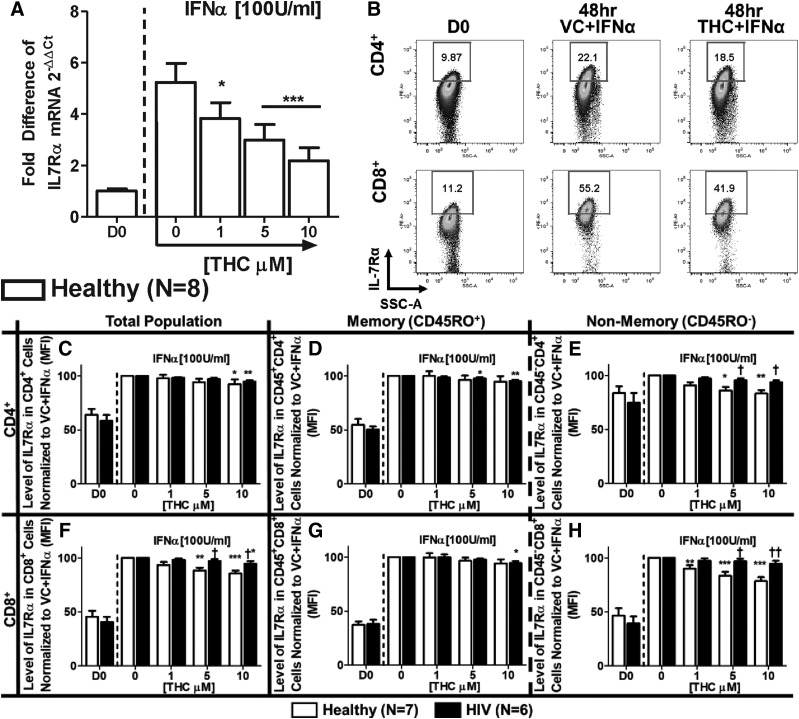
Because the IL-7Rα gene promoter region contains an IFN-sensitive responsible element (Mazzucchelli and Durum, 2007), studies were conducted in purified T cells from healthy donors to determine the effects of THC on IFNα-induced IL-7Rα mRNA expression. IFNα treatment induced mRNA expression of IL-7Rα, which was significantly suppressed by THC (Fig. 3A). Interestingly, we found that the effects of IFNα on IFNAR2 expression were insensitive to THC treatment in T cells from healthy donors (Supplemental Fig. 1).
Fig. 3.
THC suppresses IFNα-induced expression of IL-7Rα mRNA in T cells from healthy donors but differentially affects IFNα-induced surface expression of IL-7Rα in T cells from healthy vs. HIV+ T donors. (A) To determine the effects of IFNα and THC on IL-7Rα mRNA expression, T cells were purified from healthy donors, and treated with either vehicle (0.03% EtOH) or various concentrations of THC (1, 5, or 10 µM) for 30 minutes. After treatment, cells were stimulated with IFNα (100 U/ml), incubated for 48 hours, and harvested for quantification of IL-7Rα mRNA levels by real-time quantitative PCR. For the determination of IL-7Rα surface expression, PBMCs from healthy and HIV+ donors were isolated through Ficoll Paque density gradient centrifugation and either immediately stained for CD3, CD4, CD8, CD45RO, and IL-7Rα (D0) or treated with THC and IFNα, as described above, and measured for IL-7Rα expression after 48 hours. (B) Example of IFNα-mediated IL-7Rα expression and THC (10 µM)-mediated suppression in a healthy donor. The effects of THC on the expression level [mean fluorescence intensity (MFI)] of IL-7Rα in T cells from healthy and HIV+ donors in total (C), memory (D), and nonmemory (E) CD4+ cells; and total (F), memory (G), and nonmemory (H) CD8+ cells. Asterisks indicate statistically significant differences of the treatment with the HIV status–matched VC (0 THC) (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). Daggers indicate statistically significant differences of treatment-matched groups between Healthy and HIV+ T cells (†P ≤ 0.05; ††P ≤ 0.01) (two-way analysis of variance with Bonferroni multiple-comparisons post-test). D0, day 0; SSC-A, side scatter area.
Studies were also performed to determine the direct effects of THC on IFNα-induced IL-7Rα protein expression (Fig. 3B). IFNα augmented the levels of cell surface IL-7Rα expression on memory and nonmemory CD4+ and CD8+ T cells from healthy and HIV+ donors (Fig. 3, C–H). In addition, THC produced differential effects between donor groups and T-cell populations. Specifically, CD45RO− (nonmemory) CD4+ and CD8+ T cells from healthy donors exhibited greater sensitivity to THC-mediated suppression compared with matched T cells from HIV+ donors and memory (CD45RO+) cells (Fig. 3, E and H).
IFNα Augments IL-7–Induced Phosphorylation of STAT5 in CD4+ and CD8+ T Cells from Healthy and HIV+ Donors, and T Cells from Healthy Donors Were More Sensitive to THC-Mediated Suppression than T Cells from HIV+ Donors.
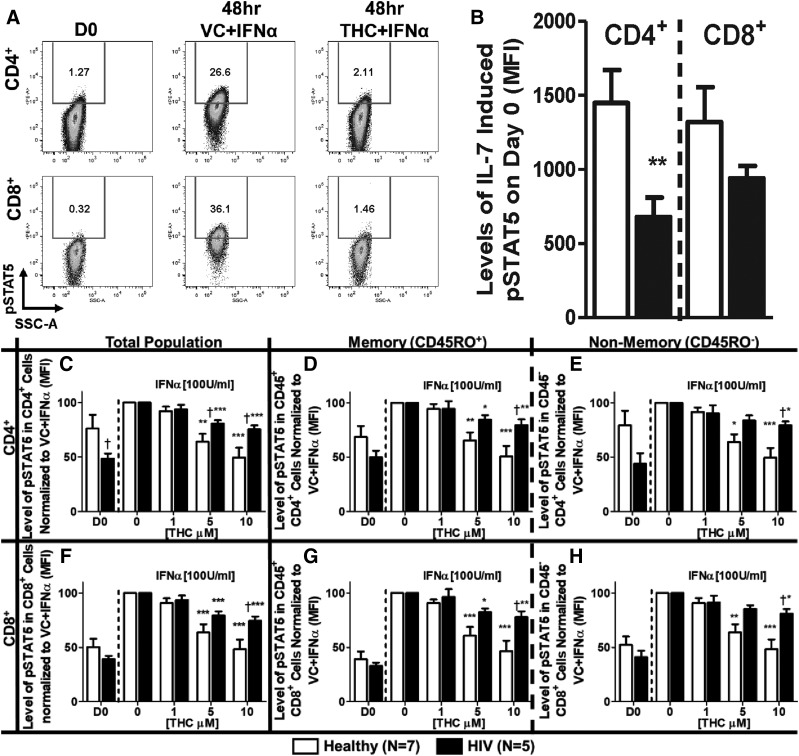
Cell surface receptor expression does not necessarily correlate with biologic activity described by the “spare receptor theory.” By extension, the magnitude of IL-7Rα expression is not necessarily indicative of receptor function, nor does it delineate differences between T cells from healthy and HIV+ donors. Therefore, studies were performed to evaluate the effect of IFNα and THC on IL-7–induced signaling by quantifying the magnitude of STAT5 phosphorylation (Fig. 4A). These studies showed that CD4+ T cells from HIV+ donors had diminished IL-7–induced pSTAT5 levels before IFNα stimulation compared with CD4+ T cells from healthy donors (Fig. 4B). Treatment with IFNα augmented IL-7–induced pSTAT5 in both CD4+ and CD8+ T cells from healthy and HIV+ donors, which was suppressed by THC (Fig. 4, C–H). Moreover, both CD4+ and CD8+ T cells from HIV+ donors were less sensitive to THC-mediated suppression than cells from healthy donors, and the difference was significant when comparing both total and nonmemory (CD45RO−) CD4+ (Fig. 4, C and H) and CD8+ (Fig. 4, F and H) T cells.
Fig. 4.
THC suppresses IFNα-mediated augmentation of IL-7–induced STAT5 phosphorylation. PBMCs from healthy and HIV+ donors were isolated through Ficoll Paque density gradient centrifugation. Cells were either: 1) immediately used for the detection of IL-7–induced pSTAT5 (D0) by treating with IL-7 (10 ng/ml) for 15 minutes then rapidly fixed; or 2) treated with either vehicle (0.03% EtOH) or various concentrations of THC (1, 5, or 10 µM) for 30 minutes, stimulated with IFNα (100 U/ml), incubated for 48, and then used for the detection of IL-7–induced pSTAT5, as described above. (A) Representative experiment of IL-7Rα–induced pSTAT5- and THC (10 µM)-mediated suppression in a healthy donor. (B) Levels [mean fluorescence intensity (MFI)] of pSTAT5 in T cells was determined by flow cytometry on day 0. The effects of THC on the IL-7–induced pSTAT5 level after IFNα stimulation of T cells from healthy and HIV+ donors in: total (C), memory (D), and nonmemory (E) CD4+ cells; and total (F), memory (G), and nonmemory (H) CD8+ cells. Asterisks indicate statistically significant differences between treatments with the HIV status–matched VC (0 THC) (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). Daggers indicate statistically significant differences of treatment-matched groups between healthy and HIV+ T cells (†P ≤ 0.05) (two-way analysis of variance with Bonferroni multiple-comparisons post-test). D0, day 0; SSC-A, side scatter area.
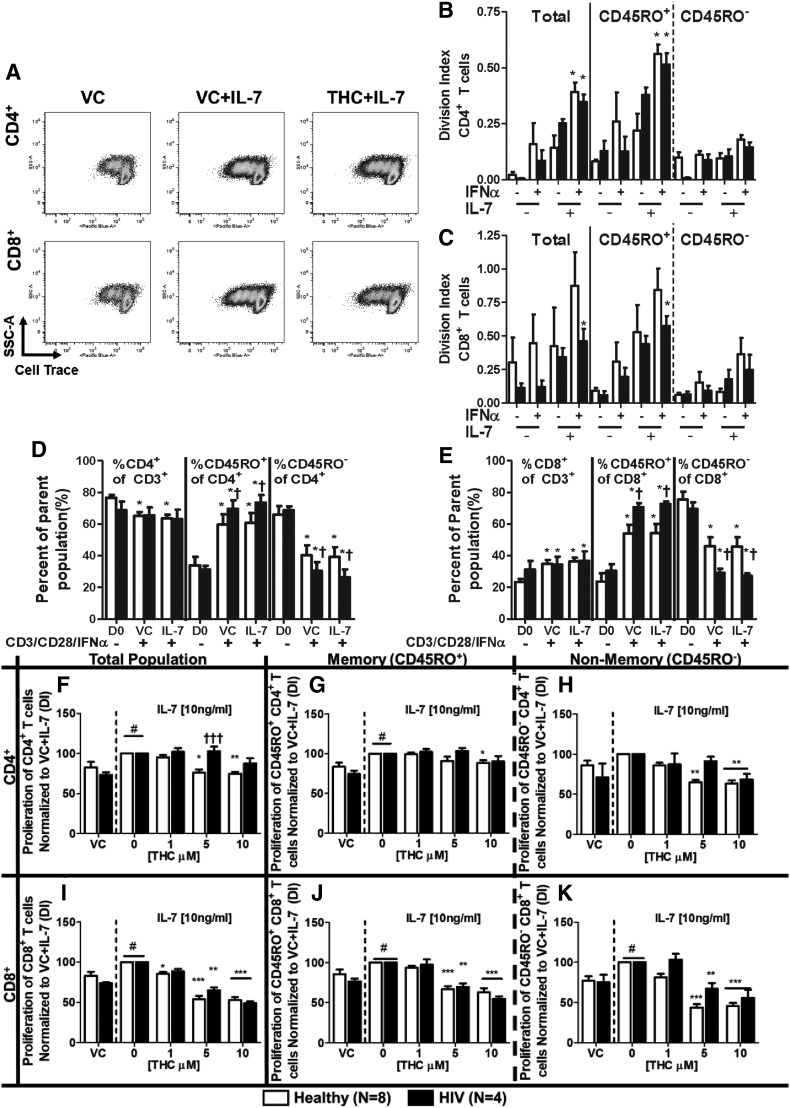
CD3/CD28/IFNα-Induced Proliferation Was Augmented by IL-7 and Suppressed by THC in CD8+ T Cells Regardless of HIV Status, whereas CD4+ T Cells from HIV+ Donors Were Less Sensitive to THC.
The relationship between IFNα and IL-7 stimulation of T cells is poorly characterized (Catalfamo et al., 2011). To better understand how IFNα may affect the homeostatic role of IL-7, studies were performed to address whether IFNα-induced augmentation of IL-7R expression and cognate signaling resulted in an enhanced T-cell proliferative response to IL-7. To mimic in vivo conditions using an in vitro system, T cells were stimulated using anti-CD3/CD28 antibodies and IFNα concurrently (i.e., the “three signal hypothesis”) then stimulated with IL-7 at the peak time of IL-7R expression (48 hours). T-cell proliferation was quantified using a DI (Fig. 5A). Stimulation with IFNα had minimal augmentation of CD3/CD28-induced proliferation in isolation (Fig. 5, B and C). However, stimulation with IFNα before the addition of IL-7 resulted in a significantly stronger proliferative response compared with anti-CD3/CD28 stimulation alone in CD4+ T cells from both healthy and HIV+ donors (Fig. 5B). This phenomenon was also observed in CD8+ T cells from HIV+ donors (Fig. 5C). Stimulation with CD3/CD28/IFNα also increased the proportion of CD45RO+ (memory) cells in CD4+ and CD8+ T cells and was more pronounced in HIV+ donors, but was not significantly affected by treatment with IL-7 (Fig. 5, D and E). In the presence of THC, CD4+ and CD8+ T cells from healthy donors showed a diminished proliferative response to control-treated cells (Fig. 5, F–K). Interestingly, THC-mediated suppression of the proliferative response was diminished in CD4+ T cells from HIV+ donors compared with healthy donors (Fig. 5, F–H). By contrast, CD8+ T cells from HIV+ donors showed suppression in the presence of THC that was comparable to the degree of THC-mediated suppression in CD8+ T cells from healthy donors (Fig. 5, I–K).
Fig. 5.
IL-7 augmented CD3/CD28/IFNα-induced T-cell proliferation is suppressed by THC. PBMCs from healthy and HIV+ donors were isolated through Ficoll Paque density gradient centrifugation. Cells were stained with violet CellTrace dye and then treated with either vehicle (0.03% EtOH) or various concentrations of THC (1, 5, or 10 µM) for 30 minutes. After treatment, cells were stimulated with IFNα (100 U/ml) and anti-CD3 and anti-CD28 antibodies (2.5 μg/ml each) for 48 hours, treated with IL-7 (10 ng/ml) or vehicle (endotoxin-free H2O), and incubated for another 48 hours before harvesting. T-cell proliferation is represented as the DI, as determined by the FlowJo proliferation tool. (A) Representative experiment of IL-7–mediated augmentation of T-cell proliferation in CD3/CD28/IFNα-stimulated T cells and THC (10 µM)-mediated suppression in a healthy donor. The effects of treatment with IFNα and IL-7 on anti-CD3/CD28-mediated T-cell proliferation in CD4+ (B) and CD8+ (C) T cells from healthy and HIV+ donors. The effect of IL-7 stimulation on total CD3+ T-cell composition and between memory (CD45RO+) and nonmemory (CD45RO−) cells in CD4+ (D) and CD8+ (E) T cells. The effects of THC on the IL-7–induced augmentation of CD3/CD28/IFNα-induced proliferation of T cells from healthy and HIV+ donors in total (F), memory (G), and nonmemory (H) CD4+ cells; and total (I), memory (J), and nonmemory (K) CD8+ cells. Pound signs indicate statistically significant differences in the proliferation of IL-7 treated T cells compared to T cells without IL-7 treatment (#P ≤ 0.05). Asterisks indicate statistically significant differences of the treatment with the HIV status–matched VC (0 THC or D0) (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). Daggers indicate statistically significant differences of treatment-matched groups between healthy and HIV+ T cells (†P ≤ 0.05; ††P ≤ 0.01) (two-way analysis of variance with Bonferroni multiple-comparisons post-test). D0, day 0; SSC-A, side scatter area.
Discussion
Presented here is the first report of differential THC-mediated suppression in response to IFNα by T cells from healthy and HIV-infected donors. Our goals were to investigate whether HIV infection affects the role of IFNα in maintaining peripheral T-cell populations and to determine whether cannabinoids can influence these processes. To address these goals, donors included in this study had no detectable HIV viral load, were not coinfected with any screened pathogen, did not use cannabinoids, and had comparable CD4+ as well as CD8+ T-cell counts.
Although the similarity of CD4+ and CD8+ T-cell composition was critical for making comparisons between healthy and HIV+ donors, HIV infection is known to alter the number and function of certain immune cells (Donaghy et al., 2001; Chehimi et al., 2002; Gulzar and Copeland, 2004; Benlahrech and Patterson, 2011; Catalfamo et al., 2011; Février et al., 2011; Rizzo et al., 2017). Therefore, we investigated the responsiveness of T cells to IFNα, which is crucial to maintaining T-cell homeostasis and is a critical mediator of antiviral responses. We found that IFNα-induced phosphorylation of STAT1, one of the most proximal biologic events in response to ligation of the IFNAR2, differed between healthy and HIV+ CD8+ T cells. Specifically, CD8+ T cells from HIV+ donors were less responsive to IFNα, as evidenced by reduced pSTAT1. Moreover, this difference was not observed in CD4+ T cells even though HIV-derived CD4+ T cells possessed lower IFNAR2 expression than those from healthy donors. CD8+ T cells from HIV+ donors also had lower pSTAT1 induction compared with CD8+ cells from healthy donors despite having comparable IFNAR2 expression. These observations agree with previous findings (Rodriguez et al., 2006) that demonstrated that CD4+ and CD8+ T cells from HIV+ patients had differential responses to IFNα-mediated stimulation. These data also indicate that CD8+ T cells in HIV+ donors have a diminished response to IFNα-mediated activation while strengthening the link between the role of IFNα in directing CD4+ T cells in viral infection (Brinkmann et al., 1993).
The differential effects of IFNα in stimulating T-cell subtypes is significant in HIV infection because IFNα plays a key role in maintaining activated T-cell populations (Marrack et al., 1999; Kolumam et al., 2005; Huber and Farrar, 2011) and potentially synergizes with IL-7 to stimulate HIV+ donor T cells (Catalfamo et al., 2011). We show here that IFNα drives IL-7R expression and potentiates IL-7 signaling, as evidenced by augmented IL-7–induced pSTAT5, in cells treated with IFNα. We also show that IL-7 drove robust proliferation of T cells treated with IFNα. These results partially agree with previous findings (Cha et al., 2014) and strengthen the link among IFNα, pDC number, and CD4+ T-cell number in HIV+ patients (Lapenta et al., 1999; Rissoan et al., 1999; Donaghy et al., 2001; Rodriguez et al., 2006). Specifically, circulating pDCs secrete IFNα, which may play a role in sensitizing T cells in their response to IL-7.
HIV+ patients routinely use medicinal cannabinoids (Abrams, 2000; Prentiss et al., 2004; Haney et al., 2007; Ellis et al., 2009). Cannabis use reduces the efficacy of IFNα as a therapeutic agent (Gross et al., 1991). Among healthy donors, the observed suppressive effect of THC on T-cell activation by IFNα is mediated, at least in part, by decreased STAT1 phosphorylation. This observation agrees with previous work on IFNβ, which also binds IFNAR (Kozela et al., 2010). Likewise, THC also suppresses the induction of IL-7Rα mRNA and protein expression, which putatively is mediated through the loss of both homo- and hetero-STAT-dimer formation and subsequent gene transcription. Additionally, THC significantly suppresses the effects of IL-7 on proliferation, likely through the suppression of IL-7–induced STAT5 phosphorylation. Interestingly, THC had no effect on the IFNα-induced expression of IFNAR2, indicating that THC has a specific effect on the IFNα-IL-7 axis.
The most surprising finding of these studies was the reduced sensitivity of T cells in patients with HIV to THC-mediated suppression. Although the initial suppression of IFNα-induced pSTAT1 showed similar trends in both healthy and HIV-infected donors, endpoints temporally distal to STAT1 phosphorylation demonstrated reduced sensitivity to THC-mediated suppression in T cells from HIV-infected donors. This trend was most pronounced in CD4+ T cells from HIV donors, especially with respect to proliferation. This finding, although unexpected, agrees with those of previous studies showing that CD4+ T-cell number was not affected in HIV+ patients using medicinal marijuana (Abrams et al., 2003). Conversely, CD8+ T cells from HIV+ patients showed marked suppression of proliferation by THC despite being less sensitive to THC-mediated impairment of other endpoints.
The limitations of these studies underlie possible reasons for the observed differences. First, the composition of the memory and nonmemory cells could produce some of the differences in IFNα-mediated activation and sensitivity to THC. Memory T cells can be divided into central and effector cells, and nonmemory cells can be divided into naive and effector cells by using surface expression of CD62L (Ammirati et al., 2012). Furthermore, we did not distinguish the T regulatory (Treg) CD4+ T cells from non–Treg-CD4+ T cells. It is noteworthy that IFNα can suppress Treg function (Becker et al., 2013). Last, proliferation was induced by simulating a T-cell receptor–like response using antibodies directed against CD3 and CD28, which differs from antigen-specific stimulation (Lo et al., 2013).
Most significantly, our studies were designed to limit the number of confounding factors by using only male HIV+ patients with the following: 1) CD4+ T-cell counts comparable to those of healthy donors; 2) CD4/CD8 T-cell ratios within the normal range (>1); 3) no coinfection with any strain of hepatitis; and 4) no medicinal or current recreational cannabinoid use. Although these parameters enabled a direct comparison with healthy donors, the profiles for T-cell activation presented in this article may vary significantly from other HIV+ patient populations. Specifically, our data do not address 1) the effects of HIV infection in female HIV+ patients, who have different immunologic responses to HIV infection compared with men (Berghöfer et al., 2006; Meier et al., 2009; Addo and Altfeld, 2014); 2) patients with HIV treated successfully with ART without restoration of CD4+ T-cell counts, which have elevated CD8+ T-cell activity and higher HIV-related mortality (Kelley et al., 2009; Serrano-Villar et al., 2014); 3) patients coinfected with a virus, since infections with hepatitis C virus can alter IFN responses and T-cell activation (Lincoln et al., 2003; Dolganiuc et al., 2006); 4) patients with HIV experiencing early versus established T-cell exhaustion, since the response to IL-7 likely changes as IL-7R expression is lost and as T-cell exhaustion progresses (Yi et al., 2010); and 5) patients using medicinal cannabinoids, since chronic THC exposure can lead to tolerance through various pharmacodynamic mechanisms (González et al., 2005), but it is unknown whether chronic cannabis use can lead to THC tolerance in leukocytes. Finally, these studies were designed to address the effects of THC treatment on early signaling events by IFNα, CD3/CD28, and IL-7–mediated stimulation by single-dose pretreatment with THC, and do not address the effects of repeat treatment of THC or of THC treatment on established effector cell functions. Further studies will be required to characterize the effects of THC in these various patient and cell populations to understand the consequences of cannabinoid use by HIV+ patients.
The findings presented in this article are the first to show a direct link between IFNα- and IL-7–mediated augmentation of CD3/CD28-induced T-cell proliferation. This work is also the first to show differences in the sensitivity to THC-mediated modulation of T-cell stimulation from healthy and HIV-infected donors. The implications of this work are complex and multifaceted. Specifically, IFNα secretion by pDCs from HIV+ donors is acutely sensitive to THC-mediated suppression (Henriquez et al., 2017), and elevated activation of pDCs in women with HIV is linked to faster T-cell depletion (Berghöfer et al., 2006), which is associated with more severe neurocognitive deficiency (Burlacu et al., 2018). Additionally, peripheral immune activation of CD8+ T cells (Kessing et al., 2017) and monocytes is tied to the development of HIV-associated neural inflammation, which could explain why cannabis users have reduced inflammatory monocyte numbers (Rizzo et al., 2017). Collectively, our findings imply that the use of cannabinoids by HIV+ patients undergoing ART may be beneficial within the context of suppressing the activation of cell association with neural inflammation while maintaining CD4+ T cells largely unaffected.
Acknowledgments
We thank Linda Dale, for coordinating blood collection from HIV+ donors; Yulia Pepelyayeva and Patrick O’Connell, for assisting with the isolation of PBMCs from healthy and HIV+ donors; Jiajun Zhou, for formatting figures in preparation for article submission; and Kimberly Hambleton for assistance in the submission of this article.
Abbreviations
- ART
antiretroviral therapy
- C-RPMI
complete RPMI
- DI
division index
- HIV
human immunodeficiency virus
- IFN
interferon
- IFNAR
interferon-α receptor
- IL-7
interleukin-7
- IL-7R
interleukin-7 receptor
- MACS
magnetic activated cell sorting
- MMHC
Mid-Michigan HIV Consortium
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- pDC
plasmacytoid dendritic cell
- pSTAT
phosphorylated signal transducer and activator of transcription
- THC
Δ9-tetrahydrocannabinol
- Treg
T regulatory
- VC
vehicle control
Authorship Contributions
Participated in research design: Henriquez, Rizzo, Crawford, Gulick, and Kaminski.
Conducted experiments: Henriquez and Rizzo.
Performed data analysis: Henriquez.
Wrote or contributed to the writing of the manuscript: Henriquez and Kaminski.
Footnotes
All funds for these studies were supplied through the National Institute on Drug Abuse Grants DA007908 and DA047180; and a National Institute of Environmental Health Sciences Training Grant T32-ES007255. No potential conflicts of interest relevant to this article are reported.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Abrams DI. (2000) Potential interventions for HIV/AIDS wasting: an overview. J Acquir Immune Defic Syndr 25 (Suppl 1):S74–S80. [DOI] [PubMed] [Google Scholar]
- Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, et al. (2003) Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med 139:258–266. [DOI] [PubMed] [Google Scholar]
- Addo MM, Altfeld M. (2014) Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 209 (Suppl 3):S86–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M, Grigore L, Pellegatta F, Pirillo A, Garlaschelli K, et al. (2012) Effector memory T cells are associated with atherosclerosis in humans and animal models. J Am Heart Assoc 1:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J. (1997) Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112–116. [DOI] [PubMed] [Google Scholar]
- Bazdar DA, Sieg SF. (2007) Interleukin-7 enhances proliferation responses to T-cell receptor stimulation in naïve CD4+ T cells from human immunodeficiency virus-infected persons. J Virol 81:12670–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Bopp T, Steinbrink K. (2013) Interferon α interferes with immunological tolerance. OncoImmunology 2:e27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlahrech A, Patterson S. (2011) HIV-1 infection and induction of interferon alpha in plasmacytoid dendritic cells. Curr Opin HIV AIDS 6:373–378. [DOI] [PubMed] [Google Scholar]
- Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. (2006) TLR7 ligands induce higher IFN-α production in females. J Immunol 177:2088–2096. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Geiger T, Alkan S, Heusser CH. (1993) Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med 178:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlacu R, Umlauf A, Luca A, Gianella S, Radoi R, Ruta SM, Marcotte TD, Ene L, Achim CL. (2018) Sex-based differences in neurocognitive functioning in HIV infected young adults. AIDS 32:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park J-H, Adelsberger J, Baseler M, Maldarelli F, Davey R, et al. (2011) CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol 186:2106–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha L, de Jong E, French MA, Fernandez S. (2014) IFN-α exerts opposing effects on activation-induced and IL-7-induced proliferation of T cells that may impair homeostatic maintenance of CD4+ T cell numbers in treated HIV infection. J Immunol 193:2178–2186. [DOI] [PubMed] [Google Scholar]
- Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ. (2002) Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol 168:4796–4801. [DOI] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu Y-J. (2004) Plasmacytoid dendritic cells in immunity. Nat Immunol 5:1219–1226. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. (2006) Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-α and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol 177:6758–6768. [DOI] [PubMed] [Google Scholar]
- Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. (2001) Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574–2576. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, et al. (1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Meissler JJ. (2015) Effects of cannabinoids on T-cell function and resistance to infection. J Neuroimmune Pharmacol 10:204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH. (2009) Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 34:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Février M, Dorgham K, Rebollo A. (2011) CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses 3:586–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sastre A, Biron CA. (2006) Type 1 interferons and the virus-host relationship: a lesson in détente. Science 312:879–882. [DOI] [PubMed] [Google Scholar]
- González S, Cebeira M, Fernández-Ruiz J. (2005) Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 81:300–318. [DOI] [PubMed] [Google Scholar]
- Gross G, Roussaki A, Ikenberg H, Drees N. (1991) Genital warts do not respond to systemic recombinant interferon alfa-2a treatment during cannabis consumption. Dermatologica 183:203–207. [DOI] [PubMed] [Google Scholar]
- Gulzar N, Copeland KF. (2004) CD8+ T-cells: function and response to HIV infection. Curr HIV Res 2:23–37. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. (2007) Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr 45:545–554. [DOI] [PubMed] [Google Scholar]
- Hardy GA, Sieg SF, Rodriguez B, Jiang W, Asaad R, Lederman MM, Harding CV. (2009) Desensitization to type I interferon in HIV-1 infection correlates with markers of immune activation and disease progression. Blood 113:5497–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartling HJ, Jespersen S, Gaardbo JC, Sambleben C, Thorsteinsson K, Gerstoft J, Ullum H, Nielsen SD. (2017) Reduced IL-7R T cell expression and increased plasma sCD127 in late presenting HIV-infected individuals. J Acquir Immune Defic Syndr 74:81–90. [DOI] [PubMed] [Google Scholar]
- Henriquez JE, Rizzo MD, Schulz MA, Crawford RB, Gulick P, Kaminski NE. (2017) Δ9-Tetrahydrocannabinol suppresses secretion of IFNα by plasmacytoid dendritic cells from healthy and HIV-infected individuals. J Acquir Immune Defic Syndr 75:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KL, Hu X, Lansky A, Mermin J, Hall HI. (2017) Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol 27:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JP, Farrar JD. (2011) Regulation of effector and memory T-cell functions by type I interferon. Immunology 132:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob SA, Iacob DG, Jugulete G. (2017) Improving the adherence to antiretroviral therapy, a difficult but essential task for HIV treatment success. Front Pharmacol 8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MC, Park HW, Choi D-H, Choi YW, Park Y, Sung YC, Lee S-W. (2017) Plasmacytoid dendritic cells contribute to the protective immunity induced by intranasal treatment with Fc-fused interleukin-7 against lethal influenza virus infection. Immune Netw 17:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim QA. (2017) Current status of the HIV epidemic & challenges in prevention. Indian J Med Res 146:673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, Crane HM, Willig J, Mugavero M, Saag M, et al. (2009) Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 48:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing CF, Spudich S, Valcour V, Cartwright P, Chalermchai T, Fletcher JL, Takata H, Nichols C, Josey BJ, Slike B, et al. (2017) High number of activated CD8+ T cells targeting HIV antigens are present in cerebrospinal fluid in acute HIV infection. J Acquir Immune Defic Syndr 75:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, Newton C, Friedman H. (1998) Cannabinoid receptors and immunity. Immunol Today 19:373–381. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. (2005) Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 202:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot M, Keet IP, Vos AH, de Goede RE, Roos MTL, Coutinho RA, Miedema F, Schellekens PTA, Tersmette M. (1993) Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med 118:681–688. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. (2010) Cannabinoids Δ(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem 285:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang KS, Recher M, Navarini AA, Harris NL, Löhning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. (2005) Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol 35:738–745. [DOI] [PubMed] [Google Scholar]
- Lapenta C, Santini SM, Proietti E, Rizza P, Logozzi M, Spada M, Parlato S, Fais S, Pitha PM, Belardelli F. (1999) Type I interferon is a powerful inhibitor of in vivo HIV-1 infection and preserves human CD4(+) T cells from virus-induced depletion in SCID mice transplanted with human cells. Virology 263:78–88. [DOI] [PubMed] [Google Scholar]
- Levy Y, Lacabaratz C, Weiss L, Viard J-P, Goujard C, Lelièvre J-D, Boué F, Molina J-M, Rouzioux C, Avettand-Fénoêl V, et al. (2009) Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest 119:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln D, Petoumenos K, Dore GJ, Australian HIV Observational Database (2003) HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med 4:241–249. [DOI] [PubMed] [Google Scholar]
- Lo Y-C, Edidin MA, Powell JD. (2013) Selective activation of antigen-experienced T cells by anti-CD3 constrained on nanoparticles. J Immunol 191:5107–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. (2001) Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J Acquir Immune Defic Syndr 28:454–457. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. (1999) Type I interferons keep activated T cells alive. J Exp Med 189:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli R, Durum SK. (2007) Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol 7:144–154. [DOI] [PubMed] [Google Scholar]
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, et al. (2009) Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15:955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TP, Shukla S, Asaad R, Freeman ML, Lederman MM, Harding CV, Sieg SF. (2016) Responsiveness to IL-7 but not to IFN-α is diminished in CD4+ T cells from treated HIV infected patients who experience poor CD4+ T-cell recovery. AIDS 30:2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A, Price DA, Easterbrook PJ, O’Callaghan CA, Kelleher AD, Whelan JA, Sontag G, Sewell AK, Phillips RE. (2000) Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA 97:3382–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Orenstein JM, Kinter A, Folks TM, Fauci AS. (1989) Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science 244:575–577. [DOI] [PubMed] [Google Scholar]
- Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM. (2004) Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J Acquir Immune Defic Syndr 35:38–45. [DOI] [PubMed] [Google Scholar]
- Reiss CS. (2010) Cannabinoids and viral infections. Pharmaceuticals (Basel) 3:1873–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan M-C, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu Y-J. (1999) Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183–1186. [DOI] [PubMed] [Google Scholar]
- Rizzo MD, Crawford RB, Henriquez JE, Aldhamen Y, Gulick P, Amalfitano A, Kaminski NE. (2017) HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-γ-inducible protein 10 levels compared to non-using HIV patients. AIDS 32:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez B, Lederman MM, Jiang W, Bazdar DA, Gàrate K, Harding CV, Sieg SF. (2006) Interferon-α differentially rescues CD4 and CD8 T cells from apoptosis in HIV infection. AIDS 20:1379–1389. [DOI] [PubMed] [Google Scholar]
- Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. (1995) CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest 95:2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC. (2005) Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci 77:1711–1722. [DOI] [PubMed] [Google Scholar]
- Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, et al. ACTG 5214 Study Team (2009) IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113:6304–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, Beq S, Lafaye de Micheaux S, Yao MD, Ober A, et al. (2014) Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog 10:e1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY, et al. (2014) HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. (2002) CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol 169:3400–3406. [DOI] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. (2001) IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA 98:8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R, Constantinescu CS. (2010) Cannabinoids and the immune system: an overview. Immunobiology 215:588–597. [DOI] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. (2010) T-cell exhaustion: characteristics, causes and conversion. Immunology 129:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]