Abstract
Understanding cellular response to mechanical forces is immensely important for a plethora of biological processes. Focal adhesions are multimolecular protein assemblies that connect the cell to the extracellular matrix and play a pivotal role in cell mechanosensing. Under time-varying stretches, focal adhesions dynamically reorganize and reorient and as a result, regulate the response of cells in tissues. Here I present a simple theoretical model based on, to my knowledge, a novel approach in the understanding of stretch-sensitive bond association and dissociation processes together with the elasticity of the cell-substrate system to predict the growth, stability, and the orientation of focal adhesions in the presence of static as well as cyclically varying stretches. The model agrees well with several experimental observations; most importantly, it explains the puzzling observations of parallel orientation of focal adhesions under static stretch and nearly perpendicular orientation in response to fast varying cyclic stretch.
Rumi De presents a model for focal adhesion dynamics under static stretch and cyclic stretch conditions. The predictions agree with prior observations and may explain the puzzling observation that focal adhesions orient toward the parallel direction in the presence of static or quasi-static stretch, but toward the perpendicular direction under fast varying cyclic stretch.
Introduction
Mechanical forces have long been known to play an important role in determining cellular functions and behaviors1. Living cells actively respond to the mechanical properties of the extracellular matrix, its rigidity, also to the presence of external forces by regulating various processes such as cell adhesion, orientation, migration, differentiation, alteration in morphology, and even apoptosis1–4. Moreover, the effect of time-varying cyclic stretch is particularly striking where each cycle of contraction and relaxation leads to dynamic changes that affect a wide range of activities involving cardiovascular cells, muscle cells, stem cells, and connective tissue cells to name a few1, 5–7. It is not yet well understood how the stretch induces reorganization of the cellular cytoskeleton, assembly and disassembly of focal adhesions (FAs), or how it alters the gene expression, and affects the whole tissue adaptation in general. A deeper insight into the mechanical stretch-induced responses is thus envisaged to have wide impact in many cellular processes, wound healing, tissue engineering, and also regenerative medicine1.
Recent researches have established that FAs play a crucial role in cell mechanotransduction1–4. FAs are micron-sized complex multimolecular protein assemblies linked on one side to the extracellular matrix via membrane-bound receptors and on other side to the actin stress fibers in the cell cytoskeleton. Experiments show that in response to substrate stretch, FAs reorganize and reorient and as a result, regulate the response of the cell2, 7–12. It is also found that external force strongly affects the growth and the stability of FA contacts1–4. FAs grow in the direction of tensile stretch13 and the stability increases with the increase in applied stretch magnitude upto an optimal value, however, eventually become unstable at higher stretches14, 15. Moreover, another puzzling experimental observation is that FAs respond differently to static stretch compared to rapidly varying cyclic stretch. Subjected to static or quasi-static stretch, FAs tend to orient along the stretch direction7, 16–18, whereas under fast varying stretch, FAs opt to orient away from the stretch direction; for high-frequency cyclic stretch, FAs align nearly perpendicular to the applied stretch direction8–12, 19–21.
There are many theoretical studies that have contributed significantly to the understanding of how cells actively respond to the mechanical forces and regulate force transmission3, 4. Quite a few studies have also been carried out to understand the stability and growth of FAs in response to forces. In a seminal work by Bell, the stability of adhesion cluster, modeled as a collection of molecular bonds, was first addressed using kinetic theory of chemical reactions. In Bell’s model the rupture rate of ligand-receptor bonds was proposed to increase exponentially with the mechanical force22. Later, the bond dissociation pathways have been studied in the framework of Kramers theory as thermally assisted escape over an energy barrier under applied forces23, 24. Also, the stochasticity in bond breaking and binding processes has been incorporated through the one-step master equation to investigate the stability of FAs under constant loading25. However, relatively few studies have been performed on the orientational response of FAs to substrate stretching. Moreover, existing theories, which have provided many insights into the cellular orientation problem, including our earlier works, mostly dealt with the orientation of the whole cell modeled as a contractile force dipole in a coarse-grained picture26–28; or studied the reorientation of two-dimensional cells emphasizing on passively stored elastic energy29; else described the formation and realignment of stress fibers in response to cyclic stretch21, 30. Recently, Qian et al.31 constructed rate equations of the density of stress fibers and adhesive receptor-ligand bonds to describe the dynamics of cell realignment in response to cyclic stretch. It has been hypothesized that cells tend to orient in the direction where the formation of stress fibers is energetically most favorable. Moreover, the frequency-dependent force generated within the stress fibers solely obtained from the structural considerations of the filament and does not depend on the description of assembly or disassembly of FA bonds. However, recent experiments have shown that orientation-specific activation of stretch-sensitive proteins in FAs controls the orientation-specific responses of FA growth and disassembly7. Also, other studies on FAs attempted till now have ignored the parallel orientation of FAs and focused only on the perpendicular orientation under cyclic stretching32–34. Moreover, these studies predict that the orientational response of FAs remains unaffected under low frequency or quasi-static stretch32–34. Therefore, though the previous studies have provided many insights into the orientation-specific response, however, so far the focus mainly remained on the dynamics of the cell, or the stress fibers, or the perpendicular orientation of FAs. Thus, a single theory, which explains the parallel orientation of FAs toward the static stretch direction as well as the perpendicular orientation under fast varying cyclic stretch, remains elusive.
In this paper, a theoretical model is presented to study the orientation-specific response of FAs in the presence of static as well as time-varying stretches within an unified framework. The crux of this model lies on a novel approach in the understanding of stretch-sensitive bond association and dissociation processes of FA assembly, which play a crucial role in determining the orientational response of FAs. It also takes into account the elasticity of the cell-matrix system and the stochastic behavior of ligand-receptor bond breaking and binding processes of FA assembly. In particular, the force-sensitive catch-bond behavior and also the time-dependent binding rates under substrate stretching have been incorporated, which allow to capture the experimentally observed puzzle of the parallel alignment of FAs in response to static and quasi-static applied stretch, whereas nearly perpendicular alignment under fast varying cyclic stretch7–12, 16–21. In addition, this theory predicts several other experimental observations such as the growth and the stability of FAs in the direction of tensile stretch13–15 and also the existence of threshold frequency and magnitude of the applied stretch that triggers reorganization of FAs19, 21, 35. Moreover, it also elucidates the experimental observations where orientational responses have been found to vary across cell types as a function of frequency of the substrate stretch19–21.
Results
Theoretical model of focal adhesions
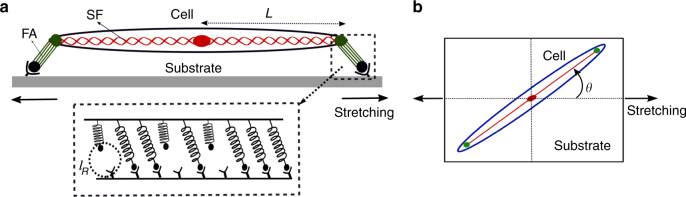
The model takes the cue from the fact that FAs are clusters of ligand-receptor molecular bonds that provide the physical connection between the cell and the extracellular matrix. Figure 1a shows a schematic representation of a cell adhering to a substrate through two FAs. In a minimal model, it could be thought of an actin stress fiber (SF) adhered via two FAs. Figure 1b illustrates the instantaneous position of the cell, and hence of the FA, oriented at an angle θ relative to the applied stretch direction. Each FA site consists of uniformly distributed ligand-receptor bonds connected in parallel to the substrate. These bonds are considered as Hookian elastic springs of stiffness, kb. Moreover, in this model, for simplicity, the elasticity of the cell or the SF is represented by a spring of rigidity kc (is referred as cellular spring). The ligand-receptor bonds are considered to be either in closed or in open state. Due to substrate stretching, the closed bonds, attached at one side to the substrate, get elongated and thus, experience an additional tension (as illustrated in Fig. 1a); since the other side of the bonds is connected to the SF, the cellular spring also gets stretched. Thus, if the bond displacement along the stretch direction is denoted by ub and the cell spring displacement as uc, then the geometric constraint relation of elastic displacements is given by ub + uc = Lε. Here ε is the strain magnitude and L is the distance of the FA site from the cell center as shown in Fig. 1a (also, see Supplementary Fig. 1). Moreover, considering the force balance condition, , the total force summing over all closed bonds must be balanced by the tension in the stretched cellular spring. The above elasticity modeling enables us to determine the single bond force fb calculated as fb = kbub = Lεkbkc/(kc + nkb); n denotes the number of closed bonds at any instant.
Fig. 1.
a Schematic view of the cell and the stress fiber (SF) adhering through two focal adhesions (FAs) under substrate stretching. The inset (dotted box) illustrates the ligand-receptor bonds modeled as Hookian springs. b Illustration of instantaneous orientation of the cell at an angle θ relative to applied stretching direction
Moreover, the dynamics of FAs is subjected to fluctuations in the surrounding microenvironment, thus, bonds can also undergo stochastic breaking or rebinding. To study the time evolution of the FA cluster, a master equation has been written by coupling the elasticity of the cell-matrix system with the statistical behavior of bond association and dissociation processes3, 25, 36,
| 1 |
where Pn(t) is the probability that n bonds are formed at time t. The first two terms in the right-hand side represent the gain term, i.e., the tendency for the number of bonds in state n to increase due to the formation of new bond in state (n − 1) and the dissociation of bond in state (n + 1), respectively. The last term represents the loss of bonds in state n, whereas Kon and Koff denote the total association and total dissociation rate of bonds at the respective state, n, at any instant of time t. This is further to note here that for mathematical simplicity, it has been considered that all bonds in the adhesion cluster experience the same elastic force or deformation. However, since the rates are subjected to fluctuations during stochastic simulation of the model, thus, it also takes care of the nonuniformity that may arise in bond forces across the adhesion cluster.
Moreover, during the time evolution, the bond reaction rate strongly depends on the instantaneous bond configuration. Recent single-molecule experiments have revealed that the external force increases the lifetime of many FA molecules37, 38. Tensile force, upto an optimal value, is found to strengthen and reinforce the molecular bonds; and bonds’ lifetime decreases with further increase in force14. These type of force-strengthening bonds are called catch bonds and are believed to play a crucial role in stabilizing the FA cluster15. Motivated by the experimental findings, in this model, it is assumed that the FA cluster consists of catch bonds and thus the dissociation rate koff of the closed bond is proposed to demonstrate the catch behavior as15, 39,
| 2 |
where kslip and kcatch denote the rate constants for dissociation of the ligand-receptor pair via a slip pathway promoted by the force and a catch pathway opposed by the force, respectively39; these rate constants depend on the type of the adhesion molecules. The total dissociation rate Koff is thus , where n is the number of closed bonds at any given instant and f0 denotes a molecular force scale, typically of the order of piconewton.
On the other hand, it has generally been considered that the association or rebinding rate, kon, increases with the number of available unbound bonds at any instant; thus, kon = γ(N − n), where N is the total number of binding sites, n is the number of closed bond, and γ is the binding rate constant25. However, in this model, motivated by the experiments40, 41, it is considered that during the rebinding process, to facilitate the ligand-receptor bond formation, the pair needs to be in close proximity within a reaction radius lR, and for a characteristic contact time tR. Thus, the ligand-receptor bond has an intrinsic reaction rate v0 = lR/tR for binding to occur. Therefore, in the presence of a cyclically varying substrate stretch, as the substrate moves back and forth, the ligand attached to the substrate also moves back and forth from the cell receptor and hence the association or rebinding rate strongly depends on the time variation of the applied stretch. Thus, under a cyclic strain ε(t) = ε0(1 − cosωt), where ε0 is the average magnitude and ω is the frequency of the applied stretch; the ligand, which was initially at a distance lR from the cell receptor would now undergo a cyclically varying displacement u(t) = lRε(t); and hence, continuously moves away back and forth from the cell receptor. Therefore, the displacement rate has to be much smaller compared to the intrinsic binding rate v0, so that the ligand-receptor pair gets sufficient time to rebind. If the absolute magnitude of the displacement rate , the ligand-receptor pair is in contact for long enough time so that the reaction can take place and binding can easily occur. However, as the displacement rate increases with increase in stretching magnitude or frequency of the external stretch, the ligand-receptor pair gets to spend less and less contact time as the ligand moves away rapidly from the cell receptor and therefore the probability of bond formation decreases. To incorporate these effects, in this model, the association rate is proposed to be rate-dependent and described by
| 3 |
Also, this is to note here that even though lR does not explicitly appeared in the expression of kon, however, the effect of the reaction radius could be seen through its dependence on the strain magnitude ε0. This dependence solely appears due to the consideration of the reaction radius. Therefore, in this model, the effective timescale turns out to be tω = 1/(ε0ω). Thus, the competition between the two timescales, the time variation of the substrate displacement tω and the intrinsic binding timescale tR, determines the probability of bond formation.
Now, in the presence of a static stretch, the association rate turns out to be kon = γ(N − n), as ω = 0. Under a static stretch, the distance between the ligand-receptor pair increases with the external stretch; however, since the pair spends sufficiently long time in close contact, thus, there is always chances of ligand-receptor rebinding to occur and hence the adhesion bonds could be formed. In case of a static stretch, the dissociation rate koff plays a major role in determining the stability of the adhesion cluster. Due to the catch-bond behavior of the dissociation rate, FAs get strengthen with tensile stretch, however, above a threshold stretch value, the dissociation rate start increasing and eventually wins over the association rate and hence the cluster becomes unstable and thus, disassemble.
Numerical simulation method
All parameters of the model have been rewritten in dimensionless units. The scaled time is defined as τ = k0t, where k0 is the spontaneous dissociation rate in the absence of any external force. Similarly, all other rates have been scaled, such as, Ks = kslip/k0, Kc = kcatch/k0, Γ = γ/k0, TR = k0tR, and the scaled frequency ω = ω0/k0. The normalized bond force is defined as Fb = fb/f0. Moreover, all displacements have been scaled by the characteristic length lR, such that Ub = ub/lR, Uc = uc/lR, and Ls = L/lR.
The master equation has been numerically solved to investigate the time evolution, growth, stability, and the orientation of adhesion clusters in response to static as well as time-varying cyclic stretch. Monte-Carlo method has been used based on Gillespie’s algorithm42. In simulations, the FA cluster consisting of N binding sites, starts from an initial state with all bonds at closed state and proceeds until all bonds become open. Thus, the stochastic trajectories are simulated, which share many similarities with experimental realizations. Averaging has been done over many such trajectories to extract useful statistical information. In case of stable clusters, each simulation has run for at least one million events. The overall statistics have been accumulated from 500 such simulation results. In this model, following the existing literature, stability and growth of the adhesion cluster are represented by the mean number of closed bonds, n, under different conditions such as varying stretch, frequency, etc. Dynamics have been studied for a wide range of parameter values. Here the results presented for a cluster of size N = 200. The length L is taken as 20 μm and lR ~ 1 nm. The ratio of single bond stiffness to cellular spring is taken as kb/kc = 5. The other scaled parameter values are kept at Γ = 2, Ks = 0.10, and Kc = 120 (following ref. 39). The magnitude and frequency of the applied stretch and TR remain variable parameters in the model.
Orientational response in the presence of static stretch
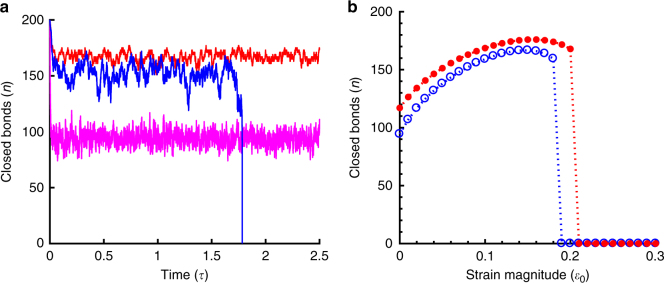
Figure 2a shows the typical simulation trajectories of instantaneous number of closed bonds for three different magnitudes of static strain. It is observed that the number of closed bonds stochastically varies around a mean value depending on the applied stretch magnitude and the cluster eventually becomes unstable above a threshold stretch. The effective strain magnitude along the FA cluster oriented at an angle θ is (as in Fig. 1b). The stability and the growth of the adhesion cluster (represented by the mean number of closed bonds, n) along the direction of the applied static stretch (i.e., θ = 0) have been studied as a function of strain magnitude as shown in Fig. 2b. It is found, as observed in experiments, the adhesion cluster grows with increasing strain, reaches a maximum under an optimal strain value, however, a further increase in stretch eventually results in disassembly of the cluster. This could be attributed to the catch behavior of FA molecules. Since the catch bonds in FAs get strengthen with tensile stretch, the dissociation rate decreases and that, in turn, promotes the growth and stability of FAs. The cluster becomes most stable for an optimal stretch at which the bond dissociation rate is minimum. Thus, in the presence of a static stretch, below the threshold magnitude, parallel to the stretch direction becomes the most stable direction for FA growth; whereas, perpendicular direction is the least stable (as εa = 0) associated with high dissociation rate of bonds. Therefore, FAs tend to align along the parallel direction of the applied stretch. The findings, thus, explain the recent experimental observation where FAs initially oriented at perpendicular direction, disassemble under a static stretch, and reorient toward the parallel direction of higher stability; whereas, FAs initially oriented parallel to the applied stretch direction remain stable7.
Fig. 2.
Simulation results for static stretch. a Time evolution of number of closed bonds for three different strain magnitudes: 1% (pink: stable cluster); 10% (red: stable cluster); and 20% (blue: unstable cluster). b Mean number of closed bonds as a function of strain magnitude for Γ = 1 (open blue circles) and Γ = 2 (solid red dots). Cluster size increases with increase in Γ value
Orientational response in the presence of dynamic stretch
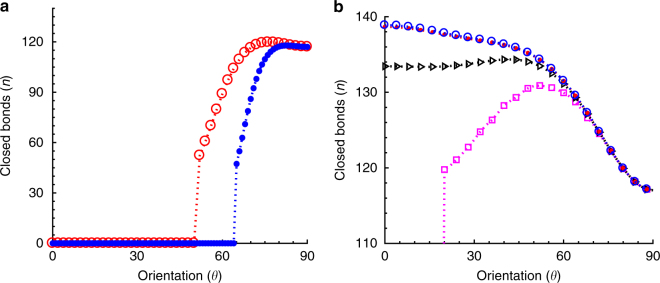
However, in the presence of a high-frequency cyclic stretch, orientational response of FAs is found to be quite different8–12, 19–21. Under a cyclically varying stretch ε(τ) = ε0(1 − cosωτ), the competition between the two timescales, Tω = 1/ε0ω, the time variation of the substrate displacement compared to TR, the intrinsic binding timescale of the ligand-receptor pair determines the formation and stability of FA cluster. For high-frequency stretch, if , the ligand-receptor pairs do not get sufficient contact time to rebind as the ligand on the substrate moves away rapidly from the cell receptor. Therefore, the chances of new bond formation decreases with increasing frequency and the FA cluster becomes unstable along the stretch direction. However, if FA orients, away from the stretch direction, at an angle θ, then the effective strain magnitude acting along the FA decreases and hence the contact time between the ligand-receptor pairs Tω increases with Tω ∝ 1/εa. Therefore, as the cluster orients away from the stretch direction, it becomes more and more stable with increase in probability of bond formation. At perpendicular direction, which is the zero strain direction, the binding of ligand-receptor pair is no longer affected by the fast varying stretch and thus, FAs become stable. Figure 3a shows the stability of the adhesion cluster as a function of orientation angle θ under high-frequency (for ) cyclic stretch. As seen from the figure, cluster grows in size as it orients near perpendicular direction. There exists a distribution of orientation angles toward the perpendicular direction as found in experiments. However, as the stretching frequency decreases, ligand-receptor pairs get longer contact time and the ratio of the two timescales, Tω and TR, determines the most stable orientation angle. When , as the substrate moves slowly, ligand-receptor pairs get sufficient time to form new bonds and therefore, FAs, due to strengthening of the catch bonds, tend to align along the maximal stretch direction (εa = ε0 for θ = 0), i.e., parallel to the applied stretch, as shown in Fig. 3b.
Fig. 3.
Simulation results for time-varying stretch. a Stability of the adhesion cluster as a function of orientation angle θ (in degree) under fast varying cyclic stretch (when Tω < TR) with ω = 10 (solid blue dots) and ω = 5 (open red circles). b The plot shows the cluster stability with decrease in stretching frequency for ω = 1 (open magenta squares), ω = 0.5 (open black triangles), ω = 0.1 (solid red dots), and ω = 0.01 (open blue circles), keeping TR = 10 and for 10% stretch
Effect of intrinsic binding timescale on FA orientation
Moreover, this theory also elucidates the experimental observations where orientational responses have been found to vary from cell type to cell type under time-dependent cyclic stretches. It is observed that some cell types prefer near perpendicular alignment, whereas some orient at different angles, or some do not exhibit considerable reorientation below a certain frequency19–21. In this model, it could be attributed to different intrinsic timescales, TR, characteristic to different cell types. Figure 4 shows the orientational stability of the adhesion cluster for different TR values while keeping the magnitude and the frequency of the cyclic stretch constant. As seen from the figure, with decrease in the characteristic rebinding time TR, the cluster becomes stable at a wide range of orientation angles. As TR decreases, since the binding occurs at a faster rate, ligand-receptor pairs could increasingly cope up with the varying substrate stretch; and thus, the cluster becomes stable. Therefore, FAs and thus cells with fast binding rates do not show significant reorientation.
Fig. 4.
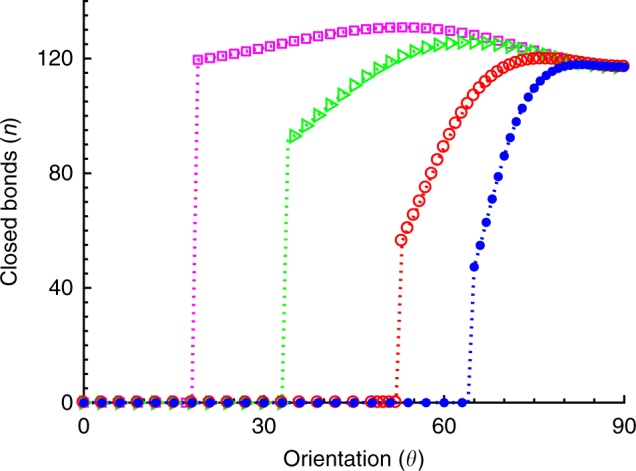
Effect of intrinsic binding timescale, TR, on the cluster orientation for different values of TR = 10 (solid blue dots), 5 (open red circles), 2 (open green triangles), and 1 (open magenta squares); for 10% stretch and frequency, ω = 10
Existence of threshold stretch magnitude
Moreover, as found in experiments, this theory also captures the existence of a threshold stretch magnitude above which the orientational response becomes prominent19, 21, 35. Figure 5 shows the stability of the adhesion cluster, oriented parallel to the applied stretch direction (θ = 0), as a function of strain magnitude and for different stretching frequencies. While keeping the frequency constant, if the magnitude of the applied stretch decreases, since Tω ∝ 1/εa, this leads to increase in the contact time between the ligand-receptor pair. As a result, for smaller stretch magnitudes, below a threshold value, when Tω > TR, since the probability of bond formation increases with increasing contact time, hence, the adhesion cluster becomes stable. However, above the threshold stretch, as Tω < TR, ligand-receptor pairs do not get sufficient contact time to rebind with the fast varying substrate; thus, the cluster becomes unstable and so orients away from the stretch direction. Therefore, orientational response of FAs becomes prominent above a threshold stretch value. Moreover, with increase in stretching frequency, as shown in Fig. 5, since Tω decreases with increasing ω (as Tω ∝ 1/ω), the magnitude of the threshold stretch shifts to a lower value.
Fig. 5.
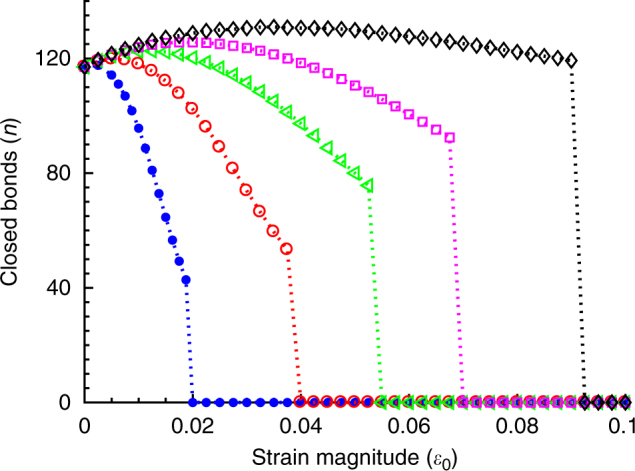
The stability of the adhesion cluster as a function of strain magnitude, ε0, with varying frequency as ω = 10 (solid blue dots), 5 (open red circles), 3 (open green triangles), 2 (open magenta squares), and 1 (open black diamonds); keeping TR = 10
Discussion
In summary, the theoretical model presented, by incorporating the catch-bond behavior of FA assembly and the time-dependent binding rates under substrate stretching, agrees well with several experimental observations. Apart from capturing the force-sensitive stability of FA clusters under tensile stretch, this model in an unified framework also unravels the puzzling observation of orientation of FAs along the parallel direction in response to static and quasi-static stretch, as well as the perpendicular orientation under fast varying cyclic stretch. Moreover, it explains the variation in alignment angles in different cell types and also predicts the existence of threshold stretch as observed in experiments. As discussed, the competition between the timescales involved in the process, namely, the time variation of the substrate displacement and the intrinsic binding time of the ligand-receptor pairs determines the stability of FAs under time-varying stretches. Importantly, it is shown that the sole consideration of the stretch-dependent association and dissociation processes of adhesion clusters could successfully predict the orientational response of FAs. Indeed, it has been shown in recent experiments that the orientation-specific activation of stretch-sensitive proteins in FAs play a crucial role in determining the orientation-specific FA growth and disassembly7. This is also to note that the complex mechanisms of viscoelasticity of stress fibers and actin-myosin contraction may affect the overall cellular mechanosensing processes43, 44. Thus, adding these effects one would get an inclusive picture, however, it is outside the present theory. While this model correctly predicts the major experimental features on the orientation of FAs reported so far, the finer predictions could be further tested by suitable experiments.
Data availability
The data supporting the findings of this study are available within the paper and its supplementary information file.
Electronic supplementary material
Acknowledgements
The author thanks Dr. Rangeet Bhattacharyya for insightful discussions and also acknowledges the financial support from Science and Engineering Research Board (SERB), Grant No. SR/FTP/PS-105/2013, Department of Science & Technology (DST), India.
Author contributions
R.D. developed the model, carried out the entire work, and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s42003-018-0084-9.
References
- 1.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014;12:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz US, Safran SA. Physics of adherent cells. Rev. Mod. Phys. 2013;85:1327–1381. doi: 10.1103/RevModPhys.85.1327. [DOI] [Google Scholar]
- 4.Ladoux B, Nicolas A. Physically based principles of cell adhesion mechanosensitivity in tissues. Rep. Prog. Phys. 2012;72:116601–116625. doi: 10.1088/0034-4885/75/11/116601. [DOI] [PubMed] [Google Scholar]
- 5.De R, Zemel A, Safran SA. Theoretical concepts and models of cellular mechanosensing. Methods Cell Biol. 2010;98:143–175. doi: 10.1016/S0091-679X(10)98007-2. [DOI] [PubMed] [Google Scholar]
- 6.Tamada M, Sheetz MP, Sawada Y. Activation of a signalling cascade by cytoskeleton stretch. Dev. Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Pasapera AM, Koretsky AP, Waterman CM. Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc. Natl Acad. Sci. USA. 2013;110:E2352–E2361. doi: 10.1073/pnas.1221637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarization is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J. Cell Sci. 2009;122:3644–3651. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carisey A, et al. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr. Biol. 2013;23:271–281. doi: 10.1016/j.cub.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greiner AM, Chen H, Spatz JP, Kemkemer R. Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS ONE. 2013;8:e77328–e77336. doi: 10.1371/journal.pone.0077328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W, Sakamoto N, Miyazawa R, Sato M. Role of paxillin in the early phase of orientation of the vascular endothelial cells exposed to cyclic stretching. Biochem. Biophys. Res. Commun. 2012;418:708–713. doi: 10.1016/j.bbrc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 12.Yoshigi. M, Hoffman LM, Jensen C, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 14.Marshall BT, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 15.Thomas WE, Vogel V, Sokurenko E. Biophysics of catch bonds. Annu. Rev. Biophys. 2008;37:399–416. doi: 10.1146/annurev.biophys.37.032807.125804. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, et al. Effect of static pre-stretch induced surface anisotropy on orientation of mesenchymal stem cells. Cell. Mol. Bioeng. 2014;7:106–121. doi: 10.1007/s12195-013-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eastwood M, Mudera VC, McGrouther DA, Brown RA. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil. Cytoskelet. 1998;40:13–21. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Collinsworth AM, et al. Orientation and length of mammalian skeletal myocytes in response to a unidirectional stretch. Cell Tissue Res. 2000;302:243–251. doi: 10.1007/s004410000224. [DOI] [PubMed] [Google Scholar]
- 19.Jungbauer S, Gao HJ, Spatz JP, Kemkemer R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J. 2008;95:3470–3478. doi: 10.1529/biophysj.107.128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JHC, Goldschmidt-Clermont P, Wille J, Yin FCP. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001;34:1563–1572. doi: 10.1016/S0021-9290(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 21.Hsu HJ, Lee CF, Kaunas R. A dynamic stochastic model of frequencydependent stress fiber alignment induced by cyclic stretch. PLoS ONE. 2009;4:e4853–e4860. doi: 10.1371/journal.pone.0004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 23.Evans E, Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys. J. 1997;72:1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion. Science. 2007;316:1148–1153. doi: 10.1126/science.1137592. [DOI] [PubMed] [Google Scholar]
- 25.Erdmann T, Schwarz US. Stability of adhesion clusters under constant force. Phys. Rev. Lett. 2004;92:108102–108105. doi: 10.1103/PhysRevLett.92.108102. [DOI] [PubMed] [Google Scholar]
- 26.De R, Zemel A, Safran SA. Dynamics of cell orientation. Nat. Phys. 2007;3:655–659. doi: 10.1038/nphys680. [DOI] [Google Scholar]
- 27.De R, Safran SA. Dynamical theory of active cellular response to external stress. Phys. Rev. E. 2008;78:031923–031940. doi: 10.1103/PhysRevE.78.031923. [DOI] [PubMed] [Google Scholar]
- 28.De R, Zemel A, Safran SA. Do cells sense stress or strain? Measurement of cellular orientation can provide a clue. Biophys. J. 2008;94:L29–L31. doi: 10.1529/biophysj.107.126060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livne A, Bouchbinder E, Geiger B. Cell orientation under cyclic stretching. Nat. Commun. 2014;5:3938–3945. doi: 10.1038/ncomms4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Z, Deshpande VS, McMeeking RM, Evans AG. Analysis and interpretation of stress fiber organization in cells subject to cyclic stretch. J. Biomech. Eng. 2008;130:031009–031017. doi: 10.1115/1.2907745. [DOI] [PubMed] [Google Scholar]
- 31.Qian J, Liu H, Lin Y, Chen W, Gao H. A mechanochemical model of cell reorientation on substrates under cyclic stretch. PLoS ONE. 2013;8:e65864–e65876. doi: 10.1371/journal.pone.0065864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Kemkemer R, Deibler M, Spatz J, Gao H. Cyclic stretch induces cell reorientation on substrates by destabilizing catch bonds in focal adhesions. PLoS ONE. 2012;7:e48346–e48356. doi: 10.1371/journal.pone.0048346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong Y, Kong D, Dai L, Ji B. Frequency-dependent focal adhesion instability and cell reorientation under cyclic substrate stretching. Cell. Mol. Bioeng. 2011;4:442–456. doi: 10.1007/s12195-011-0187-6. [DOI] [Google Scholar]
- 34.Kong D, Ji B, Dai L. Stability of adhesion clusters and cell reorientation under lateral cyclic tension. Biophys. J. 2008;95:4034–4044. doi: 10.1529/biophysj.108.131342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dartsch PC, Hammerle H. Orientation response of arterial smoothmuscle cells to mechanical stimulation. Eur. J. Cell Biol. 1986;41:339–346. [PubMed] [Google Scholar]
- 36.Van Kampen, N. G. Stochastic Processes in Physics and Chemistry (Elsevier, North Holland, Amsterdam, 2011).
- 37.Kong F, García AJ, Paul Mould A, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roca-Cusachs P, Gauthier NC, Rio A, Sheetz MP. Clustering of α5β1 integrins determines adhesion strength whereas αvβ3 and talin enable mechanotransduction. Proc. Natl Acad. Sci. USA. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereverzev YV, Prezhdo OV, Forero M, Sokurenko EV, Thomas WE. The two-pathway model for the catch-slip transition in biological adhesion. Biophys. J. 2005;89:1446–1454. doi: 10.1529/biophysj.105.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert P, Limozin L, Pierres A, Bongrand P. Biomolecule association rates do not provide a complete description of bond formation. Biophys. J. 2009;96:4642–4650. doi: 10.1016/j.bpj.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robert P, Nicolas A, Aranda-Espinoza S, Bongrand P, Limozin L. Minimal encounter time and separation determine ligand-receptor binding in cell adhesion. Biophys. J. 2011;100:2642–2651. doi: 10.1016/j.bpj.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillespie DT. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 1977;81:2340–2361. doi: 10.1021/j100540a008. [DOI] [Google Scholar]
- 43.Deguchi S, Ohashi T, Sato M. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech. 2006;39:2603–2610. doi: 10.1016/j.jbiomech.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the paper and its supplementary information file.