Abstract
The Marine Mesozoic Revolution (MMR, starting ~200 million years ago) changed the ecological structure of sea floor communities due to increased predation pressure. It was thought to have caused the migration of less mobile invertebrates, such as stalked isocrinid crinoids, into deeper marine environments by the end of the Mesozoic. Recent studies questioned this hypothesis, suggesting the MMR was globally asynchronous. Alternatively, Cenozoic occurrences from Antarctica and South America were described as retrograde reversions to Palaeozoic type communities in cool water. Our results provide conclusive evidence that isocrinid migration from shallow to deep water did not occur at the same time all over the world. The description of a substantial new fauna from Antarctica and Australia, from often-overlooked isolated columnals and articulated crinoids, in addition to the first compilation to our knowledge of Cenozoic Southern Hemisphere isocrinid data, demonstrates a continuous record of shallow marine isocrinids from the Cretaceous-Paleogene to the Eocene/Oligocene boundary.
Rowan Whittle et al. present fossil evidence of new isocrinid (sea lily) species from Antarctica and Australia. They show that isocrinid migration from shallow to deep water occurred at different times across the globe, spanning the Cretaceous-Paleogene to the Eocene/Oligocene.
Introduction
Interactions between predators and prey have shaped the evolution of life and predation is thought to have been responsible for many major trends in the fossil record1–3. During the Marine Mesozoic Revolution (MMR, starting ~200 million years ago2), the evolution of shell-crushing (durophagous) and boring predation in marine organisms caused a change from the dominance of sedentary, epifaunal suspension feeders to more mobile organisms including infauna and predators2–5. It is thought that the MMR heavily affected the stalked crinoids (sea lilies), making the majority of forms extinct as their sessile nature made them easy prey for durophagous predators in shallow waters. Stalked isocrinid crinoids (Order Isocrinida) were displaced into deeper water4,6–8, potentially by the more mobile comatulid crinoids (featherstars, Order Comatulida), which were better able to evade predation and which underwent a series of radiations during the MMR9,10.
Today stalked isocrinids are almost entirely restricted to deeper water environments, their shallowest occurrences being 100–170 m in the western Pacific11,12 and western Atlantic6,13. They occur to depths of 200–300 m and, rarely, they occur at >400 m14. Isocrinids are more mobile than other stalked forms and capable of local relocation15–18. Despite this, it was thought that isocrinids were restricted to middle-shelf and deeper environments during the Late Cretaceous and to outer-shelf and deeper by the Eocene6,13.
There is fossil evidence for an increase in predation on shallow water crinoids in the Mesozoic1,10, including an increased frequency of bite marks and rate of regenerated arms as a result of autotomy (arm shedding)12,19. In modern populations, elevated rates of predation in shallower waters compared with deep waters has also been cited as evidence of increased predation during the MMR12,19. However, the main lines of evidence for changes in predation intensity on isocrinids bought about by the MMR are the apparent lack of isocrinids from shallow water fossil sites in the Cenozoic and their absence from shallow waters at the present day.
Globally, the fossil record of stalked crinoids is extremely good for the Middle to Late Cretaceous20–22. Deep water isocrinid occurrences are found from the early Eocene (Rösnäs Formation, Denmark20, the Eocene London Clay, England23), the early Oligocene (Keasey Formation, Oregon, USA24–27), the late Oligocene (West Indies28), the Miocene (Japan29,30) and the Pliocene (Philippines31), and these are consistent with the argument that stalked crinoids migrated from shallower to deeper water in the early Cenozoic4,6–8. However, in the Northern Hemisphere some shallow water isocrinids persisted until the end of the Danian20,24, and there are a few isolated occurrences from the late Paleocene6 and the late Oligocene6. Recently stalked crinoids have been described from the early Paleogene of Central Europe21, indicating that stalked forms remained in shallow water settings for some time after the initiation of the MMR, until the late Mesozoic and into the early Cenozoic21. This led to the suggestion that predation intensity during the Mesozoic was not the only factor controlling the presence or absence of stalked forms in shallow and deep water environments22 and the off-shore displacement of isocrinids was a gradual process that occurred later than previously supposed9. Isolated occurrences of Cenozoic stalked isocrinids from Antarctica32–36, New Zealand37–46, South America47, and Australia48, have also been described from shallow water deposits. Explanations for the South American and Antarctic occurrences have focused on a hypothetical reversion to Palaeozoic type communities in response to environmental perturbations35,47,49. However, isolated occurrences of isocrinids in the Cenozoic have led to suggestions that the MMR was not globally synchronous9,22,34 or that there was a possible delayed onset of MMR38 in Southern Hemisphere regions.
We describe 37 new Antarctic and Australian isocrinid occurrences of isolated columnals (often ignored in evolutionary studies) and articulated crowns, assigned to nine different species in three genera. Crinoids from the Cenozoic basins of Australia, one of the largest packages of shallow water sediment of this age, have not been studied in detail and, until now, have only yielded one crinoid occurrence48. Exhaustive studies of museum collections and detailed provenance information were applied together with field sampling. Antarctic isocrinids were collected with detailed sedimentological information, enabling accurate environmental and temporal placement. In addition to previously described fossil occurrences32–48, this substantial new body of data indicates that the Southern Hemisphere was an important shallow water isocrinid province during the Paleogene. The data presented herein provides conclusive evidence that the migration of stalked isocrinids from shallow to deep water did not occur at the same time all over the world.
Results
Identification of new isocrinid species
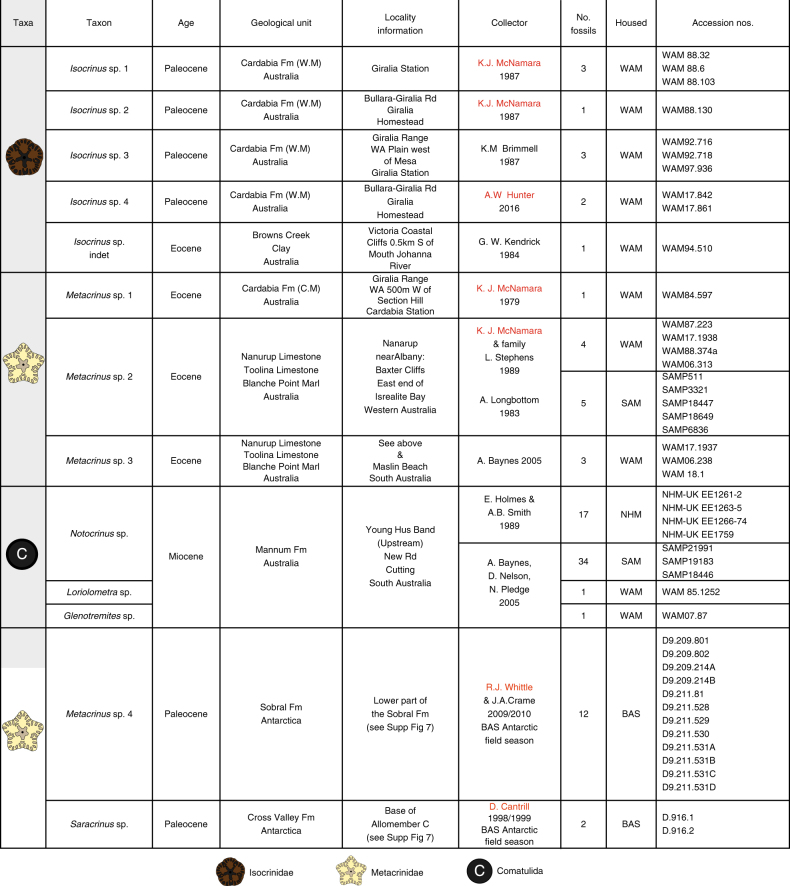
Nine new Cenozoic species (and one indeterminate species) of the Order Isocrinida are identified from shallow water deposits in Antarctica and Australia (Figs. 1and 2) using traditional crown characters as well as columnals or sets of columnals (pluricolumnals) (Supplementary Note 1, Supplementary Figs. 1–5). Three genera of the Order Comatulida are identified from Australia (Fig. 2, Supplementary Note 1, Supplementary Fig. 5c, g-i). Two different isocrinid families are identified: the Metacrinidae (Metacrinus and Saracrinus) and Isocrindae (Isocrinus). New occurrences of Metacrinidae are identified from Antarctica (Figs. 1–4, Supplementary Note 1, Supplementary Figs. 4 and 6); and Metacrinidae plus Isocrinidae from Australia (Figs. 1–4, Supplementary Note 1, Supplementary Figs. 1–3, 5 and 7). A taxonomic monograph describing all of these new species is in production.
Fig. 1.
Examples of newly discovered and described Southern Hemisphere stalked crinoids. a, b Isocrinus sp. 1 lateral surface views (a WAM 88.32; b WAM 88.6) Cardabia Formation (Wadera Calcarenite Member), Paleocene, Western Australia. c Saracrinus sp. lateral side of the crown (D.916.1) from the Cross Valley Formation, Seymour Island, Antarctica. d, e Metacrinus sp. 2 articular surface views (‘Katie’s Stars’ WAM 17.1938) from Nanarup Limestone, middle Eocene, Western Australia. f Metacrinus sp. 2 lateral surface views (WAM 88.374a) Wilson Bluff Limestone (Toolina Limestone) middle Eocene, Western Australia. g Metacrinus sp. 3 articular surface views (WAM 17.1937) Wilson Bluff Limestone (Toolina Limestone) middle Eocene, Western Australia. Scale bars = 5 mm
Fig. 2.
Information for newly identified fossils included in this study. Descriptions and images of these specimens can be found in the Supplementary Note 1 and Supplementary Figures 1–5. Names in red indicate authors on this paper who originally collected a large proportion of the material in the field. Materials collected by other people, undescribed before this study, were accessed through the institutions in which they are housed. WM Wadera Member, CM Cashin Member, WAM Western Australian Museum, SAM South Australian Museum, BAS British Antarctic Survey, NHM Natural History Museum, UK
Fig. 4.
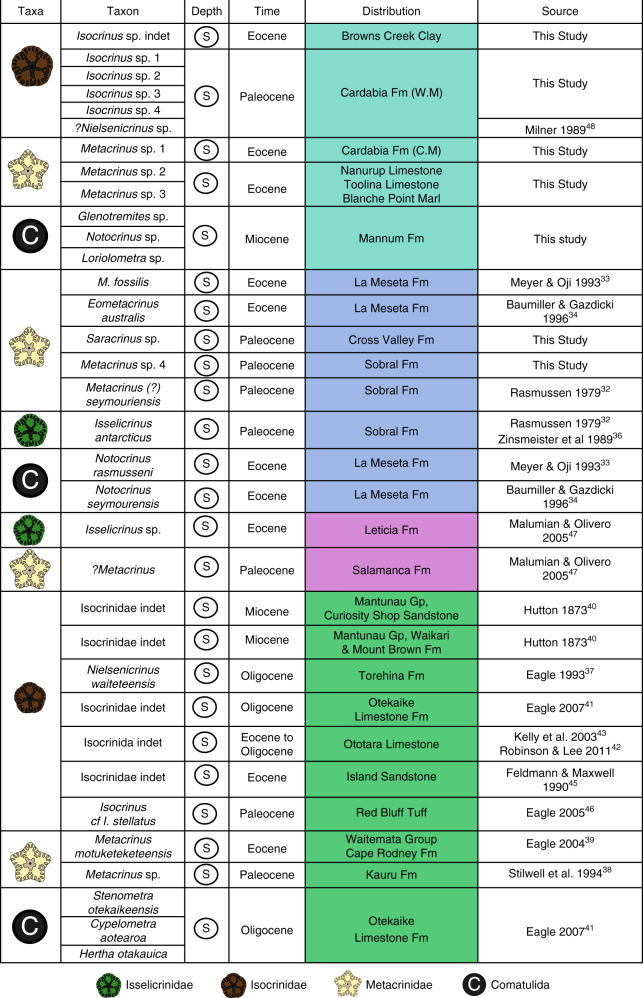
Distribution data for taxa mentioned in Fig. 3, with data sources for this information. All samples were collected in shallow water. In the Distribution column Australian localities are presented in light blue, Antarctic localities are displayed in dark blue, South American localities are shown in pink, New Zealand localities are presented in green
Western Australian isocrinids
In Western Australia four Paleocene species of Isocrinus are identified from shallow marine shelf strata in the Carnarvon Basin (Figs. 2–5, Supplementary Notes 1 and 2, Supplementary Figs. 1, 2 and 7), and isocrinids persisted in this region until the Eocene (Metacrinus sp. 1). Metacrinus species are identified from shallow water deposits in Western and Southern Australia, from the middle and late Eocene (Metacrinus sp.1, Carnarvon Basin, Metacrinus sp. 2; Eucla and St Vincent Basin, Metacrinus sp. 3 Eucla and St Vincent Basin) (Fig. 2, Supplementary Figs. 3, 5d – f and 7 and Supplementary Notes 1 and 2). An indeterminate species of Isocrinus is identified from Eocene shallow water sediments of the Otway Basin, Victoria (Figs. 2–4, Supplementary Fig. 5a, b and Supplementary Notes 1 and 2). In Australia comatulids (the following genera are identified: Glenotremites sp., Notocrinus sp., and Loriolometra sp., Figs. 2–5, Supplementary Note 1, Supplementary Fig. 5c, g–i) first appear in the fossil record in the early Miocene shallow water Mannum Formation50 (Supplementary Note 2). Our descriptions (Supplementary Note 1) of previously collected specimens represent the richest accumulation of fossil comatulids in the Southern Hemisphere.
Fig. 5.
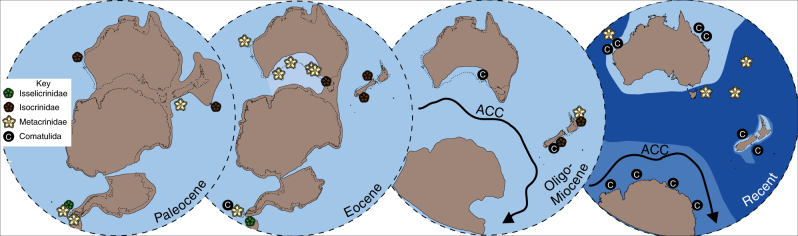
Cenozoic Southern Hemisphere crinoid distribution. The distribution of the Order Isocrinida (Families Isselicrinidae, Isocrinidae and Metacrindae) and Order Comatulida in the Cenozoic of the Southern Hemisphere, from the Paleocene to the Recent. Map outlines modified from Seton et al.88. Darker blues indicate deeper water
Antarctic isocrinids
New specimens of Metacrinus are identified from Antarctic Paleocene deltaic sediments on Seymour Island (Metacrinus sp. 4, Sobral Formation, Supplementary Figs. 4 and 6, Supplementary Notes 1 and 2). These are the oldest confirmed specimens of Metacrinus in the fossil record. Previously described Maastrichtian specimens32 have been cited as being identified from the Sobral Fm and are thus probably also Paleocene in age (Figs. 3 and 4, Supplementary Note 2). Saracrinus sp., also identified from Seymour Island, inhabited a very shallow marine environment (Cross Valley Formation, Supplementary Note 2). This is the oldest confirmed occurrence of the extant genus Saracrinus in the fossil record (Supplementary Note 1). Several Cretaceous and Eocene occurrences of isocrinids have already been described from Seymour Island32–36, and fossil comatulids have previously been described from Antarctica from the early34 and late Eocene33 (Figs. 3, 4).
Fig. 3.
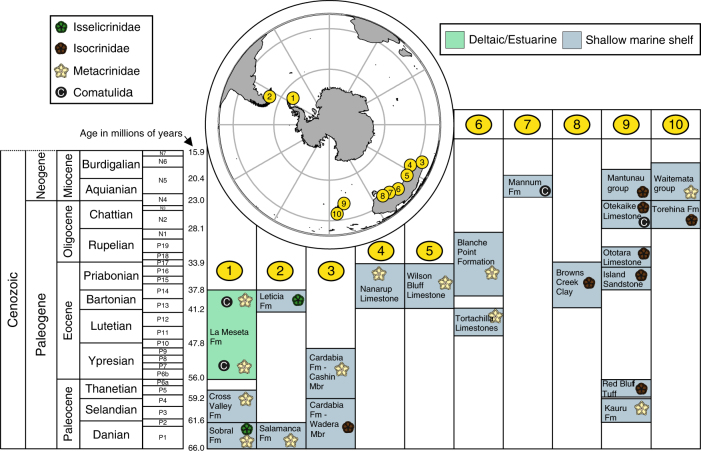
Distribution of shallow marine stalked crinoids in the Cenozoic of the Southern Hemisphere. Newly discovered and described fossils along with those previously described from Antarctica32–36, New Zealand37–46, South America47, and Australia48 are shown in the geological units they were found in (foram zones from McGowran87 are given next to geological stages). Numbered localities are: 1. Antarctic Peninsula – Seymour Island, 2. South America – Patagonia, 3. Western Australia–Carnarvon Basin, 4. Western Australia, western Eucla Basin, 5. Western Australia–eastern Eucla Basin, 6. South Australia Murray Basin. 8. South Australia–Otway Basin, 9. New Zealand South Island, 10. New Zealand, North Island. Map outline modified from Seton et al.88 Geological settings were identified as being shallow water (inner shelf or shallower) based on field studies and published literature (Supplementary Note 2), taxonomic descriptions of the new taxa are detailed in Supplementary Note 1
In addition to previously published shallow water Cenozoic Australian48, New Zealand37–46, Patagonian47 and Antarctic specimens32–36 (Fig. 4), our new occurrences (Fig. 2) provide evidence for a Southern Hemisphere Paleocene to Eocene faunal province inhabited by shallow water isocrinids (Figs. 3 and 5). Isocrinids were not present above the Eocene/Oligocene boundary in Australia or Antarctica; but remained in New Zealand shallow waters until the early Miocene39 (Figs. 3 and 5). The more motile comatulids first occur in the fossil record of Antarctica in the early Eocene and appeared in abundance in Australia in the early Miocene (Figs. 3 and 5).
Discussion
The nine newly identified Cenozoic Southern Hemisphere isocrinid species (Fig. 2), and previously identified occurrences32–48 which have been compiled together for the first time to our knowledge (Fig. 4), confirm that the response of stalked crinoids to increased predation pressure as part of the MMR was asynchronous34,38. Our data refute the hypothesis that the Antarctic and South American benthic communities experienced periodic reversions to a Palaeozoic type community structure as a response to environmental perturbations35,47. The new data provided herein, in addition to previously published occurrences32–48, demonstrate that a shallow water Southern Hemisphere fauna of isocrinid crinoids persisted over the Cretaceous-Paleogene boundary, continued into the early Paleocene and to at least the Eocene/Oligocene boundary (Figs. 3 and 5). The shift in distribution of isocrinids out of shallow water may have occurred at the end of the Eocene around Antarctica and Australia, and later in the Miocene in New Zealand. The modern deep water Isocrinida Metacrinus and Saracrinus may have evolved from shallow water Antarctic habitats in the Paleocene, spreading to the southern margin of Australia in the Eocene, and to their present distribution in deeper waters around Australia, New Zealand, New Caledonia, Indonesia, the Philippines and Japan14,51–53.
The late persistence of isocrinid crinoids in Antarctica, Australia, New Zealand and South America could be explained either as a result of an absence of, or reduced durophagous predation during the MMR in the Southern Hemisphere. Alternatively, it could be as a result of a delayed distribution and/or radiation of motile and more competitive comatulid crinoids which had greater success in shallow waters than the less mobile isocrinids13. These two options are considered below.
The role of durophagous predation in relation to the distribution of isocrinid crinoids is difficult to assess because, until recently, there was little information about predation on crinoids1,10,54–56. Diving investigations have shown predation on recent comatulid crinoids by fishes of several families, consisting of sublethal damage to the crinoid visceral mass and arms56. Crinoid ossicles from the Order Millericrinida were found in bromalites from the Triassic; durophagous sharks, colobodontid fish, placodonts, and some pachypleurosaurs or sauropterygian reptiles were suggested as possible predators57. Predation on comatulid crinoids by cidaroid echinoids has been indicated by studying bite marks on crinoid columnals as well as through direct observation1,10. However, thus far, the only confirmed evidence of predation on isocrinid crinoids has come from laboratory observations and in situ observations using submersibles of predation by cidaroid echinoids10. Therefore, echinoid predation was suggested as a major driver of crinoid radiation and diversity in the Mesozoic1,10. Predation has also been inferred by looking at arm loss and regeneration, suggested to be a response to predation, in fossil isocrinids like Metacrinus from the La Meseta Formation33.
Latitudinal differences in predation may explain the patterns of Cenozoic isocrinid depth distribution seen in the Southern Hemisphere, if predation pressure decreased with increasing latitude3. In modern brachiopods, lower frequencies of repaired predator attacks were observed at high latitudes, possibly due to a lower diversity of crushing predators58. However, it is only today that durophagous predators are rare or absent from Antarctica59. The presence of isocrinids in the La Meseta Formation was attributed to the population being subjected to lower predation pressure than generally prevailed in post-Mesozoic shallow water environments33 as the isocrinids had a lower rate of regenerated arms than in modern settings33. However, taxa thought to predate upon crinoids are found along with isocrinids in Antarctic deposits so a lack of predators cannot be invoked to explain the presence of the isocrinids in the region at the time. Teleost fish, crustaceans and sharks are found in Cretaceous, Paleocene and Eocene deposits of Antarctica60–64 in the same formations as isocrinids. The same is true for Western Australian Eocene deposits (K. McNamara pers. obser.). Isocrinids also co-occur with spines of cidaroid echinoids (known to predate on isocrinids10) in the Sobral Formation, and cidaroid echinoids have also been described from the La Meseta Formation65. Similarly cidaroids and isocrinids are both common in the middle Eocene Nanarup Formation in south-western Australia (McNamara pers. obser.).
Isocrinids are capable, as are comatulids, of autotomy to avoid predatory attacks15. Autotomy planes in stalks and arms and muscular articulations allowing rapid crawling originated in the Middle Triassic57. This, along with recent evidence that isocrinids are motile15, indicates that isocrinids evolved adaptations that enabled them to evade predators during the Mesozoic. Recent specimens of the isocrinids Metacrinus, Saracrinus and Endoxocrinus have been shown to exhibit arm regeneration12,19. Endoxocrinus shows a greater frequency of arm regeneration in shallower (~150 m deep) water than in deeper water (~750 m), leading to the suggestion that predation in shallow water caused isocrinids to move to deeper water12. However, this also shows that today isocrinids are able to inhabit areas which are subject to predation. Isocrinids have been subject to predation throughout their evolutionary history, and have evolved strategies to deal with predatory attacks. Salamon and Gorzelak22 suggested that predation intensity during the Mesozoic was not the only factor controlling the presence or absence of stalked forms in shallow and deep water environments and our data seem to be consistent with this.
Comatulids (feather stars) are thought to have had a higher survival capacity in shallow water than stalked isocrinids13 due to their greater adaptability13. This resulted in comatulids becoming dominant in shallow waters at the present day66. The timing of the onset of comatulid radiation may have not been globally consistent, accounting for longer survival for isocrinids in shallow waters in the Southern Hemisphere. The first true comatulids date from the Early Jurassic66, but overall their fossil record is poor due to a lack of articulated fossils. Using disarticulated elements relies heavily on finding a single centrodorsal ossicle, as arm ossicles are largely taxonomically indeterminate. The oldest known Antarctic comatulid (Notocrinus) was described from the early Eocene and co-occurred with isocrinids34. In South Australia, specimens of comatulids (Glenotremites, Notocrinus, and Loriolometra–Notocrinidae) have been collected in abundance50 from the shallow water early Miocene Mannum Formation, with no co-occurring Isocrinida. This may indicate comatulid dominance in the marine community.
Here we show that Australia has a shallow water fossil record of Isocrinida from the Paleocene to the end of the Eocene (Fig. 3). The oldest (Paleocene) Australian Isocrinida are from Western Australia (Fig. 3). At this time the southern margin of Australia was still connected to Antarctica67 (Fig. 5), but a transgression in the north led to the formation of a shallow water basin68, which the Isocrinida inhabited until the early Eocene. Australia finally separated from Antarctica later in the Eocene, forming an embayment with a complex of shallow water basins from west to east across the southern margin of the Australian continent (Fig. 5). Like echinoids69, foraminifera70, and brachiopods71, the Isocrinida show a pattern of dispersal in a southerly direction along the western Australia coast during the early Paleogene, then an easterly spread across the southern margin of the Australian continent (Fig. 5). Isocrinids do not occur in post- Eocene strata in Australia (Figs. 3 and 5), having seemingly been replaced by comatulids in shallow water habitats. New Zealand was left as an apparent shallow water refugium for isocrinids until the early Miocene (Fig. 3), isocrinids having persisted here from the Paleocene (Figs. 3 and 5)37–46. Following this, isocrinids were displaced to deeper water environments, which they still inhabit today14.
Isocrinids inhabited Antarctic shallow water communities until the end of the Eocene33 (Fig. 3). There is no evidence for fossil isocrinids in Antarctica, Australia or South America after the Eocene (Figs. 2 and 4). This was a time of speciation and radiation in the Southern Hemisphere for many taxa, including comatulids72,73 when changes in continental configuration and ocean circulation brought in different water masses and isolated Antarctic marine faunas74. The Antarctic Circumpolar Current (ACC) started around the Eocene⁄Oligocene boundary to early Oligocene75 physically isolating Antarctica and preventing warmer water masses from reaching the continent. Full development of the ACC resulted in faunal turnover in the Southern Hemisphere, and an increase in cool water cosmopolitan and true Antarctic endemic forms76,77. This is supported by molecular clock data, which shows that modern species of the comatulid Promachocrinus evolved in the Antarctic region after the onset of the ACC73. Similar radiation events after the onset of the ACC are seen in other taxa such as amphipods, isopods and octopods72. The radiation of apparently more successful modern comatulid taxa in the Southern Hemisphere is co-incident with the demise of isocrinids in the region. The onset of the ACC may have caused a local extinction of isocrinids in the Southern Ocean. The repeated extension of ice sheets across the Antarctic continental shelf may also have discouraged the less mobile isocrinids from living at the depths at which they are found elsewhere today.
Overall, based on the evidence presented herein, it is clear that isocrinids inhabited shallow waters in the Southern Hemisphere region in the early Cenozoic, with the oldest metacrinid specimens found in Antarctica. Opening seaways resulted in isocrinids dispersing along newly formed shallow Australian basins around the southern margin of Australia to New Zealand.
Methods
Taxonomic study of isocrinids
The taxonomy of Cenozoic crinoids is virtually unstudied24 other than the notable exceptional occurrences where the crowns have been preserved such as the Rösnäs Formation (Eocene), Denmark, the London Clay (Eocene), England, the Keasey Formation (Oligocene) Mist, Columbia County, Oregon and the La Meseta Formation (Eocene), Seymour Island, Antarctica. The vast majority of material consists of single columnals or sets of columnals, much of which is in need of revision24. We used a new systematic framework based on recent taxonomic work on Jurassic and Cretaceous65 taxa and applied this to the new taxa collected from Australia and Antarctica (Supplementary Note 1). We also compared specimens to recent isocrinids from the Natural History Museum (NHM) UK and the University of Tokyo Museum. Articulated isocrinid crinoids are typically identified based on the number of brachials in the arms and their surface ornamentation. The systematics of isocrinid crinoids has been previously restricted to characters within the crown. In contrast, taxonomy using stem columnals or sets of columnals (pluricolumnals) is considered problematic78. However, there are studies which have extensively utilised columnals in the absence of preserved cup material79–81. We use the methodology detailed in these studies and summarised in Supplementary Fig. 8 for the material described herein. Taxonomic features include the outer surface of the stem (latera), the shape and articular face of the columnals, and its articulations (Supplementary Fig. 8). Sets of columnals called pluricolumnals typically represent stem segments shed in life. These can be quickly incorporated into the sediment or can remain in the substrate where they are subject to abrasion or local transport. The majority of the columnals have not been abraded, suggesting little transport81,82; the high number of articulated sets of columnals in the dataset also suggests rapid burial of columnal segments. However, it should be noted that articulated stalks and headless erect stalks have been observed to survive in the deep-sea and in lab-held Metacrinus from Japan83. Therefore, some caution is needed in claiming that articulated lengths of stalk found widely in the fossil record indicate rapid burial.
Sample collection
Information about the collecting localities of the newly identified specimens in this study can be found in Fig. 2, Supplementary Figs. 6 and 7, and Supplementary Notes 1 and 2. Twelve specimens of Metacrinus sp. 4 from the Paleocene Sobral Formation Seymour Island, Antarctica, were collected in the 2009/2010 British Antarctic Survey (BAS) field season. These fossils were collected by R.J. Whittle and J.A. Crame in conjunction with section lines measured using an Abney level and Jacobs staff, along with detailed field studies and sedimentological logging by J. Francis and J. Ineson (Fig. 2, Supplementary Figs. 4c, d and 6). They are preserved as pluricolumnals only. Two specimens of Saracrinus from the Cross Valley Formation, Seymour Island, Antarctica, were collected by David Cantrill in the 1998/1999 BAS field season (Fig. 2, Supplementary Figs. 4a, b and 6). They are very well preserved with arms attached to the calyx, but with no stalk. To aid identification of Antarctic material, taxonomic comparisons were made with Seymour Island fossil specimens in collections at the Springer Room, National Museum of Natural History, Smithsonian Institution, Washington DC and with modern taxa at the Natural History Museum, London. The ages for the sections and the specimens collected were based on Bowman et al.84. Data for water depth for Antarctic localities was based on the field studies of Dr J. Ineson (Geological Survey of Denmark and Greenland) and have also been the focus of geological study from other authors85,86.
Geological settings and environment of deposition including water depths for rock units mentioned herein are given in Supplementary Note 2 along with the supporting literature references for their interpretation. Herein shallow water is defined as occurring on the inner shelf or shallower.
The 23 Australian crinoid specimens came from spot sampling in the field and museum collections; previously overlooked data from disarticulated columnals were also included. The Paleocene Australian specimens were sampled by A.W. Hunter from the Cardabia Formation (Giralia Anticline, north part of the Southern Carnarvon Basin, Supplementary Fig. 7). Paleocene to Oligocene Australian data came from the series of basins that form the Great Bight Basin System (Supplementary Note 2, Supplementary Fig. 7) and the Southern Carnarvon Basin. They were sampled over a 30 year period by K.J. McNamara and team (S.P. Radford, K.A. McNamara, T. McNamara, J. McNamara, A. Baynes, K.M. Brimmell, G.W. Kendrick and A. Longbottom). Comatulid specimens from the Mannum Formation were collected by A. Baynes, D. Nelson, N. Pledge, E. Holmes and A.B. Smith. To aid identification of the Australian specimens, extant material was studied in reference collections in the Muséum National d’Histoire Naturelle, Paris, the Natural History Museum, London (NHM), the Western Australian Museum, Perth (WAM), the Southern Australian Museum, Adelaide (SAM), the Museum of Victoria, the Australian Museum, Sydney, the University of Tokyo Museum, and the National Museum of Natural History, Smithsonian Institution. Monographs of Cenozoic taxa plus specimens and monographs of modern taxa were compared. Australian and Antarctic fossil sample data were combined with published data from Australia, Antarctica, South America and New Zealand.
Data availability
Information regarding the data that support the findings of this study are available within the paper, Supplementary Figures and Supplementary Notes 1 and 2. All Antarctic fossil specimens are deposited at the British Antarctic Survey, Cambridge. Australian specimens are housed in the Western Australian Museum, South Australian Museum and the Natural History Museum (UK). Detailed provenance information for the newly collected specimens is given in Fig. 2, and Supplementary Notes 1 and 2.
Accession Numbers
Western Australian Museum-WAM 88.32, WAM 88.6, WAM 88.103, WAM 88.130, WAM 92.716, WAM 92.718, WAM 97.936, WAM 17.842, WAM 17.861, WAM 94.510, WAM 84.597, WAM 87.223, WAM 17.1938, WAM 88.374a, WAM 06.313, WAM 17.1937, WAM 06.238, WAM 18.1, WAM 85.1252 and WAM 07.87.
South Australian Museum-SAM P511, SAM P3321, SAM P18447, SAM P18649, SAM P6836, SAM P21991, SAM P19183 and SAM P18446.
Natural History Museum (UK)-NHM-UK EE 1261-2, NHM-UK EE 1263-5, NHM-UK EE 1266-74 and NHM-UK EE 1759.
British Antarctic Survey (Cambridge)-D9.209.801, D9.209.802, D9.209.214 A, D9.209.214B, D9.211.81, D9.211.528, D9.211.529, D9.211.530, D9.211.531 A, D9.211.531B, D9.211.531 C, D9.211.531D, D.916.1 and D.916.2.
Electronic supplementary material
Acknowledgements
This study is a part of the BAS Palaeoenvironments, Ice-sheets and Climate Change Programme. This work was funded by NERC (UK) grant NE/I00582X/1. The authors thank the PALEOPOLAR team and Dr J.D. Witts from the American Museum of Natural History. H. Blagbrough assisted with access to BAS collections and technical support, and P. Bucktrout with photography. Dr. D. Hodgson provided critical feedback. BAS provided Antarctic field logistics. We thank A. Cabrinovic and T. Ewin for access to specimens at the Natural History Museum, London. M. Siversson (WAM) and M-A. Binnie (SAM) provided access to Australian collections. The management of Giralia Station allowed access to their land. L.E. Young and R. Nicholls provided Australian field assistance. M.K. Eagle provided discussion. K.J.M. thanks those who helped collect the Australian material now housed in the WAM.
Author contributions
R.J.W. and A.W.H. initiated the study, collected specimens, compiled the data, conducted the analyses and wrote the manuscript. D.J.C. and K.J.M. collected specimens and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rowan J. Whittle, Email: roit@bas.ac.uk
Aaron W. Hunter, Email: awh31@cam.ac.uk
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s42003-018-0048-0.
References
- 1.Gorzelak P, Salamon MA, Baumiller TK. Predator-induced macroevolutionary trends in Mesozoic crinoids. Proc. Natl Acad. Sci. USA. 2012;109:7004–7007. doi: 10.1073/pnas.1201573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeij GJ. The Mesozoic Marine Revolution: evidence from snails, predators and grazers. Paleobiology. 1977;3:245–258. doi: 10.1017/S0094837300005352. [DOI] [Google Scholar]
- 3.Vermeij GJ. Evolution and Escalation: An Ecological History of Life. Princeton, New Jersey: Princeton University Press; 1987. p. 527. [Google Scholar]
- 4.Harper, E. M. in Predator-Prey Interactions in the Fossil Record (eds Kelley, P. H., Kowalewski, M. & Hansen, T. A.) pp. 433–455, (Kluwer Academic/Plenum Publishers, New York, 2003).
- 5.Wagner PJ, Kosnik MA, Lidgard S. Abundance distributions imply elevated complexity of Post-Paleozoic marine ecosystems. Science. 2006;314:1289–1292. doi: 10.1126/science.1133795. [DOI] [PubMed] [Google Scholar]
- 6.Bottjer DJ, Jablonski D. Paleoenvironmental patterns in the evolution of post-Paleozoic benthic marine invertebrates. Palaios. 1988;3:540–560. doi: 10.2307/3514444. [DOI] [Google Scholar]
- 7.Aronson RB. Scale-independent biological interactions in the marine environment. Oceanogr. Mar. Biol. - Annu. Rev. 1994;32:435–460. [Google Scholar]
- 8.Jablonski D, Sepkoski JJ., Jr. Paleobiology, community ecology, and scales of ecological pattern. Ecology. 1996;77:1367–1378. doi: 10.2307/2265534. [DOI] [PubMed] [Google Scholar]
- 9.Gorzelak P, Salamon MA, Trzęsiok D, Lach R, Baumiller TK. Diversity dynamics of post-Palaeozoic crinoids – in quest of the factors affecting crinoid macroevolution. Lethaia. 2016;49:231–244. doi: 10.1111/let.12141. [DOI] [Google Scholar]
- 10.Baumiller TK, et al. Post-Paleozoic crinoid radiation in response to benthic predation preceded the Mesozoic marine revolution. Proc. Natl Acad. Sci. USA. 2010;107:5893–5896. doi: 10.1073/pnas.0914199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oji, T. in Current Aspects of Biogeography in West Pacific and East Asian Regions. Nature and Culture, No. 1., (eds Ohba, H., Hayami, I., Mochizuki, K.) pp. 27–43, (The University Museum, The University of Tokyo, 1989).
- 12.Oji T. Is predation intensity reduced with increasing depth? Evidence from the West Atlantic Stalked Crinoid Endoxocrinus parrae (Gervais) and Implications for the Mesozoic Marine Revolution. Paleobiology. 1996;22:339–351. doi: 10.1017/S0094837300016328. [DOI] [Google Scholar]
- 13.Meyer DL, Macurda DB., Jr. Adaptive radiation of comatulid crinoids. Paleobiology. 1977;3:74–82. doi: 10.1017/S0094837300005121. [DOI] [Google Scholar]
- 14.Ameziane-Cominardi N. Distribution bathymetrique des pentacrines du Pacifique occidental: essai de modelisation et d’application aux faunes du lias: problèmes de tectono-eustatisme au cours du rifting téthysien. Doc. Des. Lab. De. géologie Lyon. 1991;116:1–253. [Google Scholar]
- 15.Baumiller TK. Crinoid Ecological Morphology. Annu Rev. Earth Planet Sci. 2008;36:221–249. doi: 10.1146/annurev.earth.36.031207.124116. [DOI] [Google Scholar]
- 16.Baumiller TK, Messing CG. Stalked crinoid locomotion, and its ecological and evolutionary implications. Palaeontol. Electron. 2007;10:1–10. [Google Scholar]
- 17.Baumiller TK, LaBarbera M, Woodley JD. Ecology and functional morphology of the isocrinid Cenocrinus asterius (Linnaeus) (Echinodermata: Crinoidea): in situ and laboratory experiments and observations. Bull. Mar. Sci. 1991;48:731–748. [Google Scholar]
- 18.Messing CG, RoseSmyth MC, Mailer SR, Miller JE. Relocation movement in a stalked crinoid (Echinodermata) Bull. Mar. Sci. 1988;42:480–487. [Google Scholar]
- 19.Oji T. Skeletal variation related to arm regeneration in Metacrinus and Saracrinus, Recent stalked crinoids. Lethaia. 1986;19:355–360. doi: 10.1111/j.1502-3931.1986.tb00752.x. [DOI] [Google Scholar]
- 20.Rasmussen HW. Lower Tertiary Crinoidea, Asteroidea and Ophiuroidea from Northern Europe and Greenland. Det. K. Dan. Vidensk. Selsk. Biol. Skr. 1972;19:1–83. [Google Scholar]
- 21.Salamon MA, Gorzelak P, Borszcz T, Gajerski A, Kaźmierczak J. A crinoid concentration Lagerstätte in the Turonian (Late Cretaceous) Conulus Bed (Miechów-Wolbrom area, Poland) Geobios. 2009;42:351–357. doi: 10.1016/j.geobios.2008.10.008. [DOI] [Google Scholar]
- 22.Salamon M, Gorzelak P. Late Cretaceous crinoids (Crinoidea) from Eastern Poland. Palaeontogr. Abt. A. 2010;291:1–43. [Google Scholar]
- 23.Wignall PB, Simms MJ. Pseudoplankton. Palaeontology. 1990;33:359–378. [Google Scholar]
- 24.Hess, H. in Fossil Crinoids (eds Hess, H., Ausich, W. I., Brett, C. E. & Simms, M. J.) pp. 233–236 (Cambridge University Press, Cambridge, UK, 1999).
- 25.Moore RC, Vokes HE. Lower Tertiary Crinoids from Northwestern Oregon. Geol. Surv. Prof. Pap. 1953;233-E:113–148. [Google Scholar]
- 26.Burns, C. & Mooi, R. in From Greenhouse to Icehouse: The Marine Eocene-Oligocene Transition (eds Prothero, D. R., Ivany, L. C. & Nesbitt, E. A.) pp. 88–106, (Columbia University Press, New York, 2003).
- 27.Burns C, Campbell KA, Mooi R. Exceptional crinoid occurrences and associated carbonates of the Keasey Formation (Early Oligocene) at Mist, Oregon, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;227:210–231. doi: 10.1016/j.palaeo.2005.04.023. [DOI] [Google Scholar]
- 28.Donovan SK, Harper DAT, Portell RW. In deep water: a crinoid–brachiopod association in the Upper Oligocene of Antigua, West Indies. Lethaia. 2015;48:291–298. doi: 10.1111/let.12105. [DOI] [Google Scholar]
- 29.Oji T. Miocene Isocrinidae (stalked Crinoids) from Japan and their biogeographic implication. Trans. Proc. Palaeontol. Soc., Jpn. N. S. 1990;157:412–429. [Google Scholar]
- 30.Fujiwara SndashI, Oji T, Tanaka Y, Kondo Y. Relay Strategy and adaptation to a muddy environment in Isselicrinus (Isselicrinidae: Crinoidea) Palaios. 2005;20:241–248. doi: 10.2110/palo.2004.p04\|[ndash]\|25. [DOI] [Google Scholar]
- 31.Donovan SK, Helwerda RA. Neogene crinoids of southeast Asia: preservation, systematics and significance. Alcheringa. 2016;41:215–221. doi: 10.1080/03115518.2016.1206322. [DOI] [Google Scholar]
- 32.Rasmussen HW. Crinoideos del Cretacico superior y del Terciario inferior de la Isla Vicecomodoro Marambio (Seymour Island), Antartida. Contrib. Científico Del. Inst. Antártico Argent. 1979;4:79–97. [Google Scholar]
- 33.Meyer DL, Oji T. Crinoids from Seymour Island, Antarctic Peninsula: Paleobiogeographic and Paleoecologic implications. J. Paleontol. 1993;67:250–257. doi: 10.1017/S0022336000032170. [DOI] [Google Scholar]
- 34.Baumiller TK, Gaździcki A. New crinoids from the Eocene La Meseta Formation of Seymour Island, Antarctic Peninsula. Palaeontol. Pol. 1996;55:101–116. [Google Scholar]
- 35.Aronson RB, Blake DB, Oji T. Retrograde community structure in the late Eocene of Antarctica. Geology. 1997;25:903–906. doi: 10.1130/0091-7613(1997)025<0903:RCSITL>2.3.CO;2. [DOI] [Google Scholar]
- 36.Zinsmeister WJ, Feldmann RM, Woodburne MO, Elliot DH. Latest Cretaceous/Earliest Tertiary transition on Seymour Island, Antarctica. J. Paleontol. 1989;63:731–738. doi: 10.1017/S0022336000036453. [DOI] [Google Scholar]
- 37.Eagle MK. A new fossil isocrinid crinoid from the Late Oligocene of Waitete Bay, Northern Coromandel. Rec. Auckl. Inst. Mus. 1993;30:1–12. [Google Scholar]
- 38.Stilwell JD, Fordyce RE, Rolfe PJ. Paleocene Isocrinids (Echinodermata:Crinoidea) from the Kauru Formation, South Island, New Zealand. J. Paleontol. 1994;68:135–141. doi: 10.1017/S0022336000025658. [DOI] [Google Scholar]
- 39.Eagle MK. Saracrinus (Crinoidea: Metacrininae) from the Early Miocene of Motuketekete Island, Hauraki Gulf, Auckland, New Zealand. Rec. Auckl. Mus. 2004;41:5–12. [Google Scholar]
- 40.Hutton, F. W. Catalogue of the Tertiary Mollusca and Echinodermata of New Zealand in The Collection of the Colonial Museum (New Zealand Geological Survey, Wellington, 1873; 48).
- 41.Eagle MK. New Fossil Crinoids (Articulata: Comatulida) from the Late Oligocene of The Pentland Hills and Hurstlea, South Island. Rec. Auckl. Mus. 2007;44:85–110. [Google Scholar]
- 42.Robinson JH, Lee DE. A shallow, warm-water calcitic molluscan fauna from an Early Oligocene seamount, North Otago, New Zealand. N.Z. J. Geology Geophysics. 2011;54:135–147. doi: 10.1080/00288306.2011.537612. [DOI] [Google Scholar]
- 43.Kelly M, Lee D, Kelly S, Buckeridge JS. A recent sponge, Pleroma aotea Kelly (“Order” Lithistida: Family Pleromidae), in the late Eocene Ototara Limestone of Otago, New Zealand. N.Z. J. Mar. Freshw. Res. 2003;37:129–148. doi: 10.1080/00288330.2003.9517152. [DOI] [Google Scholar]
- 44.Campbell HJ, et al. Cretaceous-Cenozoic geology and biostratigraphy of the Chatham Islands, New Zealand. Monogr. Inst. Geol. Nucl. Sci. 1993;2:1–269. [Google Scholar]
- 45.Feldmann RM, Maxwell PA. Late Eocene decapod Crustacea from North Westland, South Island, New Zealand. J. Paleontol. 1990;64:779–797. doi: 10.1017/S0022336000042347. [DOI] [Google Scholar]
- 46.Eagle MK. A new genus of fossil crinoid (Cyrtocrinidia: Sclerocrinidae) from Chatham Island, New Zealand. Rec. Auckl. Mus. 2005;42:35–47. [Google Scholar]
- 47.Malumián N, Olivero EB. Shallow-water late middle Eocene crinoids from Tierra del Fuego: a new southern record of a retrograde community structure. Sci. Mar. 2005;69:349–353. doi: 10.3989/scimar.2005.69s2349. [DOI] [Google Scholar]
- 48.Milner GJ. The first record of an isocrinid crinoid from the Tertiary of Australia. Rec. West. Aust. Mus. 1989;14:385–389. [Google Scholar]
- 49.Aronson RB, Blake DB. Global climate change and the origin of modern Benthic communities in Antarctica. Am. Zool. 2001;41:27–39. [Google Scholar]
- 50.Lukasik JJ, James NP. Lithostratigraphic revision and correlation of the Oligo‐Miocene Murray Supergroup, Western Murray Basin, South Australia. Aust. J. Earth Sci. 1998;45:889–902. doi: 10.1080/08120099808728443. [DOI] [Google Scholar]
- 51.Oji T, Kitazawa K. Discovery of two rare species of stalked crinoids from Okinawa Trough, southwestern Japan, and their systematic and biogeographic implications. Zool. Sci. 2008;25:115–121. doi: 10.2108/zsj.25.115. [DOI] [PubMed] [Google Scholar]
- 52.Oji T, Kitazawa K. Distribution of stalked crinoids (Echinodermata) from waters off the southern coasts of Japan. Mem. Natl. Sci. Mus., Tokyo. 2006;41:217–222. [Google Scholar]
- 53.Messing, C. Metacrinus Carpenter, 1882 in Messing, C. (2015) World List of Crinoidea. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetailsid=411397 on2016-03-01 (2015)
- 54.Meyer, D. L. & Ausich, W. I. in Biotic Interactions in Recent and Fossil Benthic Communities (eds Tevesz, J. S. et al.) pp. 377–427, (Springer Science + Business Media, New York, 1983).
- 55.Baumiller TK, Gahn FJ. Testing predator-driven evolution with Paleozoic Crinoid arm regeneration. Science. 2004;305:1453–1455. doi: 10.1126/science.1101009. [DOI] [PubMed] [Google Scholar]
- 56.Meyer DL. Evolutionary implications of predation on Recent comatulid crinoids from the Great Barrier Reef. Paleobiology. 1985;11:154–164. doi: 10.1017/S0094837300011477. [DOI] [Google Scholar]
- 57.Salamon MA, Niedźwiedzki R, Gorzelak P, Lach R, Surmik D. Bromalites from the Middle Triassic of Poland and the rise of the Mesozoic Marine Revolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012;321–322:142–150. doi: 10.1016/j.palaeo.2012.01.029. [DOI] [Google Scholar]
- 58.Harper EM, Peck LS. Latitudinal and depth gradients in marine predation pressure. Glob. Ecol. Biogeogr. 2016;25:670–678. doi: 10.1111/geb.12444. [DOI] [Google Scholar]
- 59.Aronson RB, et al. Climate Change and the Invasibility of the Antarctic Benthos. Annu Rev. Ecol. Evol. Syst. 2007;38:129–154. doi: 10.1146/annurev.ecolsys.38.091206.095525. [DOI] [Google Scholar]
- 60.Grande L, Chatterjee S. New Cretaceous fish fossils from Seymour Island, Antarctic Peninsula. Palaeontology. 1987;30:829–837. [Google Scholar]
- 61.Cione AL, Mercedes Azpelicueta de las M, Bellwood D. An Oplegnathid fish from the Eocene of Antarctica. Palaeontology. 1994;37:931–940. [Google Scholar]
- 62.Feldmann RM, Schweitzer CE. Paleobiogeography of Southern Hemisphere decapod Crustacea. J. Paleontol. 2006;80:83–103. doi: 10.1666/0022-3360(2006)080[0083:POSHDC]2.0.CO;2. [DOI] [Google Scholar]
- 63.Griffiths HJ, Whittle RJ, Roberts SJ, Belchier M, Linse K. Antarctic crabs: invasion or endurance. PLoS ONE. 2013;8:e66981. doi: 10.1371/journal.pone.0066981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whittle RJ, Quaglio F, Griffiths HJ, Linse K, Crame JA. The Early Miocene Cape Melville Formation fossil assemblage and the evolution of modern Antarctic marine communities. Naturwissenschaften. 2014;101:47–59. doi: 10.1007/s00114-013-1128-0. [DOI] [PubMed] [Google Scholar]
- 65.Radwańska U. A new echinoid from the Eocene La Meseta Formation of Seymour Island, Antarctic Peninsula. Palaeontol. Pol. 1996;55:117–125. [Google Scholar]
- 66.Hess H. Origin and radiation of the comatulids (Crinoidea) in the Jurassic. Swiss. J. Paleontol. 2014;133:23–34. [Google Scholar]
- 67.Clarke JDA, Gammon PR, Hou B, Gallagher SJ. Middle to Upper Eocene stratigraphic nomenclature and deposition in the Eucla Basin. Aust. J. Earth Sci. 2003;50:231–248. doi: 10.1046/j.1440-0952.2003.00995.x. [DOI] [Google Scholar]
- 68.Hocking RM, Moors HT, Van de Graaff JE. Geology of the Carnarvon Basin Western Australia. Geol. Surv. West. Aust. Bull. 1987;133:1–288. [Google Scholar]
- 69.McNamara, K. J. in Echinoderm Research 1998 (eds Candia Carnevali, M. D. & Bonasoro, F.) pp. 333–338, (A.A. Balkema, Rotterdam, 1999).
- 70.McGowran B, et al. Australasian palaeobiogeography: the Palaeogene and Neogene record. Mem. Assoc. Australas. Palaeontol. 2000;23:405–470. [Google Scholar]
- 71.Craig RS. The Cenozoic Brachiopods of the Carnarvon Basin, Western Australia. Palaeontology. 2000;43:111–152. doi: 10.1111/1475-4983.00121. [DOI] [Google Scholar]
- 72.Newman L, Convey P, Gibson JAE, Linse K. Antarctic Paleobiology: Glacial refugia and constraints on past ice-sheet reconstructions. PAGES News. 2009;17:22–24. doi: 10.22498/pages.17.1.22. [DOI] [Google Scholar]
- 73.Wilson NG, Hunter RL, Lockhart SJ, Halanych KM. Multiple lineages and absence of panmixia in the “circumpolar” crinoid Promachocrinus kerguelensis from the Atlantic sector of Antarctica. Mar. Biol. 2007;152:895–904. doi: 10.1007/s00227-007-0742-9. [DOI] [Google Scholar]
- 74.Barnes DKA, Clarke A. Antarctic marine biology. Curr. Biol. 2011;21:R451–R457. doi: 10.1016/j.cub.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 75.Barker PF, Filippelli GM, Florindo F, Martin EE, Scher HD. Onset and role of the Antarctic circumpolar current. Deep Sea Res Part 2 Top. Stud. Oceanogr. 2007;54:2388–2398. doi: 10.1016/j.dsr2.2007.07.028. [DOI] [Google Scholar]
- 76.Lazarus, D. & Caulet, J.–P. in The Antarctic Paleoenvironment: A Perspective on Global Change Antarctic Research Series, 60 (eds Kennet, J. P. & Warnke, D. A.) pp. 145–174. (American Geophysical Union, Washington, DC, 1993).
- 77.Brown B, Gaina G, Müller RD. Circum-Antarctic palaeobathymetry: illustrated examples from Cenozoic to recent times. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006;231:158–168. doi: 10.1016/j.palaeo.2005.07.033. [DOI] [Google Scholar]
- 78.Hess, H, Messing, C. G. & Ausich, W. I. in Treatise on Invertebrate Paleontology, Part T, Echinodermata 2 (ed. Seldon, P.A.) pp. 1–261 (The University of Kansas Paleontological Institute, Lawrence, Kansas, 2011).
- 79.Simms MJ. British Lower Jurassic crinoids. Monogr. Palaeontogr. Soc., Lond. 1989;142(581 for 1988):1–103. [Google Scholar]
- 80.Hunter AW, Barras CG, Thuy B. Online field-guide to fossils: British Middle Jurassic echinoderms. Proc. Geol. Assoc. 2011;122:501–503. doi: 10.1016/j.pgeola.2011.03.001. [DOI] [Google Scholar]
- 81.Hunter AW, Underwood CJ. Lithofacies and taphofacies control on distribution of crinoid habitats in the Bathonian (Middle Jurassic) of England and France. Acta Palaeontol. Pol. 2009;54:77–98. doi: 10.4202/app.2009.0109. [DOI] [Google Scholar]
- 82.Hunter AW, Underwood CJ. Comment and Reply on “Palaeoenvironmental Control on Distribution of Crinoids in the Bathonian (Middle Jurassic) of England and France” by Aaron W. Hunter and Charlie J. Underwood. Acta Palaeontol. Pol. 2010;55:174–176. doi: 10.4202/app.2010.0016. [DOI] [Google Scholar]
- 83.Amemiya S, Oji T. Regeneration in sea lilies. Nature. 1992;357:546–555. doi: 10.1038/357546a0. [DOI] [Google Scholar]
- 84.Bowman V, et al. The Paleocene of Antarctica: biostratigraphy and palaeogeographical implications for the palaeo-Pacific margin of Gondwana. Gondwana Res. 2016;38:132–148. doi: 10.1016/j.gr.2015.10.018. [DOI] [Google Scholar]
- 85.Macellari CE. Stratigraphy, sedimentology, and palaeoecology of Upper Cretaceous/Paleocene shelf-deltaic sediments of Seymour Island. Geol. Soc. Am. Mem. 1988;169:25–53. [Google Scholar]
- 86.Marenssi S, Santillana S, Bauer M. Estratigrafía, petrografía sedimentaria y procedencia de las formaciones Sobral y Cross Valley (Paleoceno), isla Marambio (Seymour), Antártica. Andean Geol. 2012;39:67–91. [Google Scholar]
- 87.McGowran B. The tertiary of Australia: foraminiferal overview. Mar. Micropaleontol. 1979;4:235–264. doi: 10.1016/0377-8398(79)90019-7. [DOI] [Google Scholar]
- 88.Seton M, et al. Global continental and ocean basin reconstructions since 200 Ma. Earth-Sci. Rev. 2012;113:212–270. doi: 10.1016/j.earscirev.2012.03.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Information regarding the data that support the findings of this study are available within the paper, Supplementary Figures and Supplementary Notes 1 and 2. All Antarctic fossil specimens are deposited at the British Antarctic Survey, Cambridge. Australian specimens are housed in the Western Australian Museum, South Australian Museum and the Natural History Museum (UK). Detailed provenance information for the newly collected specimens is given in Fig. 2, and Supplementary Notes 1 and 2.