Abstract
Store-operated calcium (Ca2+) entry (SOCE) in skeletal muscle is rapidly activated across the tubular system during direct activation of Ca2+ release. The tubular system is the invagination of the plasma membrane that forms junctions with the sarcoplasmic reticulum (SR) where STIM1, Orai1 and ryanodine receptors are found. The physiological activation of SOCE in muscle is not defined, thus clouding its physiological role. Here we show that the magnitude of a phasic tubular system Ca2+ influx is dependent on SR Ca2+ depletion magnitude, and define this as SOCE. Consistent with SOCE, the influx was resistant to nifedipine and BayK8644, and silenced by inhibition of SR Ca2+ release during excitation. The SOCE transient was shaped by action potential frequency and SR Ca2+ pump activity. Our results show that SOCE in skeletal muscle acts as an immediate counter-flux to Ca2+ loss across the tubular system during excitation-contraction coupling.
Xaver Koenig et al. demonstrate the physiological activation of store-operated calcium entry in muscle, pointing to a role for this mechanism. The resulting influx of calcium into the cytoplasm is shaped by action potential frequency and calcium pump activity.
Introduction
Store-operated Ca2+ entry (SOCE) is a mechanism that is slowly activating in most cells, where stromal interacting molecule 1 (STIM1) molecules sense Ca2+ depletion in the internal Ca2+ store and migrate to plasma membrane contact sites to trigger Ca2+ influx through Orai1 channels1. In contrast, SOCE has evolved to become a fast mechanism in skeletal muscle, probably in parallel with the evolution of the highly organised membrane structures that support the rapid transients of Ca2+ in the fibre evoked by action potentials2. In muscle, the surface plasma membrane of the fibre has regular invaginations, to form the tubular (t-) system. The dominant element of the t-system, the transverse tubules reach each sarcomere to form junctions with the sarcoplasmic reticulum (SR) terminal cisternae3 where the transverse tubular dihydropyridine receptor (DHPR)/L-type Ca2+ channel directly activates the SR ryanodine receptor (RyR) to rapidly activate Ca2+ release4. This process is known as excitation-contraction coupling (EC coupling) and provides the basis for muscle contraction.
While the presence of a robust and fast SOCE in muscle is clear, the physiological activation and primary roles of SOCE in this tissue remain elusive2,5. The biophysical properties of SOCE in muscle2 suggest its physiological role can be an immediate one, related to compensatory Ca2+ movements during action potential-evoked Ca2+ release. Knowledge of the physiological patterns of store-dependent influx and the conditions associated with its activation are critical in defining physiological role, which will help in our understanding of SOCE-related myopathies, developmental defects and fibre-type shifts in STIM1 and ORAI1 knockout mice6–10.
Ca2+ within the SR ([Ca2+]SR) is strongly buffered by calsequestrin and the high-capacity SR Ca2+ pumps11. This prevents steady state or tonic activation of SOCE in resting fibres5,12. Furthermore, the SR strongly buffers Ca2+ during EC coupling, preventing deep depletion of the [Ca2+]SR13–15, making it difficult to understand how SOCE is activated physiologically in skeletal muscle, if we continue to assume that a bulk depletion of [Ca2+]SR is required for SOCE activation5.
We hypothesized that SOCE could be activated during action potential-evoked Ca2+ release by local depletion of Ca2+ immediately behind the RyRs at the SR terminal cisternae. Local depletions of Ca2+ inside the SR would briefly and uniformly dissociate Ca2+ from the local population of STIM17,16 to rapidly active SOCE. The rapid transduction of such a signal requires the static junction between the transverse tubules and the SR terminal cisternae membranes, allowing RyR activity to intimately link EC coupling and SOCE2. Consistent with this, it has been demonstrated that only the transverse tubules support SOCE; whereas, the longitudinal elements of the t-system that lack proximity to RyRs have become dynamic Ca2+-buffers, devoid of SOCE17. Here we use a microscopy-based technique with the necessary sensitivity and temporal resolution to measure the occurring Ca2+-fluxes across the t-system membrane18,19 under physiological stimulation patterns. This approach allowed us to identify that the physiological activation of SOCE is by every action potential-induced Ca2+ release, presenting as a phasic Ca2+ influx. This finding allows the elucidation of the physiological roles of SOCE in muscle and broadens our understanding of the properties of STIM1 and SOCE more generally.
Results
t-system membrane Ca2+ fluxes following every action potential
To resolve Ca2+ fluxes across the t-system membrane during action potential-evoked Ca2+ release required imaging Ca2+ in the cytoplasm and t-system with high sensitivity and millisecond temporal resolution. To do this we used mechanically skinned fibres (Fig. 1a, Methods section), in which we trapped the Ca2+-sensitive fluorescent dye rhod-5N in the t-system of skinned fast twitch extensor digitorum longus (EDL) fibres from the rat, as previously described19. This preparation preserves intact EC coupling13,20 and respective Ca2+ release and force responses are indistinguishable from those in intact fibres19,21–23. The removal of the sarcolemma during the skinning process grants access to the cytosol and allows for the measurement of the intracellular Ca2+ levels with the use of a spectrally separate Ca2+-sensitive dye from rhod-5N, fluo-4.
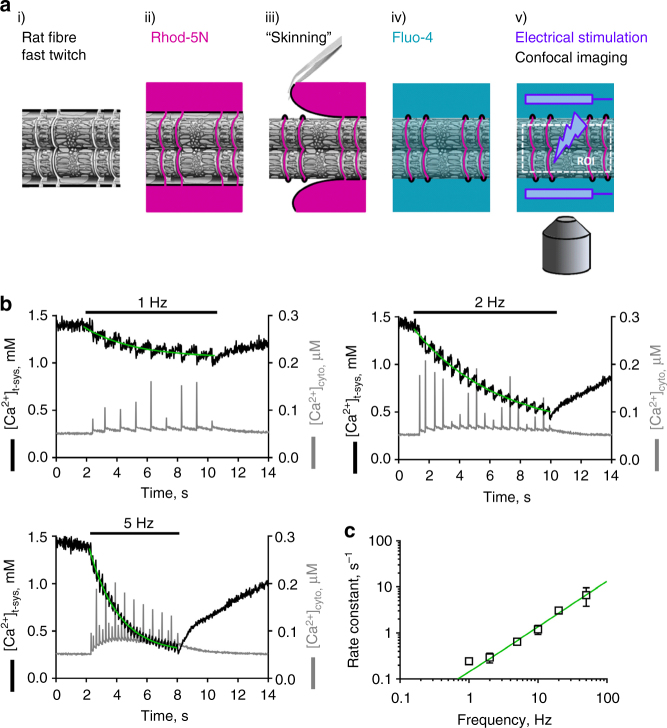
Fig. 1.
Identifying extracellular Ca2+ influx across the t‐system membrane activated by single-action potentials. a Schematic representation of the individual protocol steps of the technique. (i) isolation of a single fibre of rat EDL muscle, (ii) incubation of the fibre with the Ca2+‐sensitive dye Rhod‐5N, (iii) Mechanical removal of the sarcolemma (skinning) and resulting trapping of the dye within the sealed t‐system, (iv) incubation of the fibre in a physiological salt solution containing the Ca2+‐sensitive dye Fluo‐4, (v) electrical field stimulation via platinum electrodes in parallel to the fibre’s long axis, and simultaneous confocal imaging of both dye fluorescence within the region of interest (ROI). b Typical recordings of [Ca2+]t‐sys (left axes) and [Ca2+]cyto (right axes) over time as derived from skinned rat EDL fibres during electrical stimulation. xyt image series of the fluorescent signals of rhod‐5N and fluo‐4 trapped in the t‐system and loaded into the cytosol, respectively, were spatially averaged and calibrated (Methods section). Electrical stimulation at 1, 2 and 5 Hz as indicated was started shortly after beginning of the recording and was continued until a new steady state in the t‐system was reached, unambiguously identifiable by the termination of cytosolic transients. Note that the employed sampling rate of 55 frames s−1 is below the Nyquist criterion, which results in an apparent modulation of cytosolic Ca2+ transient amplitudes. Green lines represent mono‐exponential fits to the decaying phase of [Ca2+]t‐sys upon stimulation. c A summary of the respective rate constants as derived from the exponential fits for all tested frequencies. Data are derived from 10 fibres and given as mean ± SEM. EDL, extensor digitorum longus
Figure 1b is an example of spatially averaged profiles of cytoplasmic fluo-4 and t-system-trapped rhod-5N fluorescence during electrical field stimulation of a skinned fibre at 1, 2 and 5 Hz using the approach described in Fig. 1a. In each of these cases, field stimulation induced a transient rise in cytosolic Ca2+ (Fig. 1b, grey trace). The simultaneous recording of rhod-5N fluorescence showed a decline, indicating a concurrent influx of Ca2+ from the t-system to the cytoplasm with each action potential (Fig. 1b, black trace). Both signals returned to their respective baseline levels following the cessation of stimulation. Note that the cytoplasmic solution contained 10 mM EGTA with free [Ca2+]cyto buffered to 50 nM. The introduction of EGTA as the dominant cytoplasmic Ca2+-buffer allows the calculation of the amount of total Ca2+ released from the SR (Methods).
Close inspection of the [Ca2+]t-sys signals shows a fast decline in t-system Ca2+ associated with each action potential-evoked Ca2+ release (Fig. 1b). Termination of Ca2+ efflux from the t-system allows the signal to recover toward baseline in between individual stimulations pulses, generating a saw-tooth like pattern. As the time for recovery of [Ca2+]t-sys became too short at increasing stimulation frequencies, there was a progressive summation of individual [Ca2+]t-sys depletions to reduce [Ca2+]t-sys in a frequency-dependent manner (Fig. 1b). The overall decline in the [Ca2+]t-sys signal during repetitive electrical stimulation could be fitted by an exponential function. A summary of the rate constants at the stimulation frequencies applied is displayed in Fig. 1c. A positive relationship between stimulation frequency and the rate constant of [Ca2+]t-sys depletion was observed. At stimulation frequencies of 20 and 50 Hz, the temporal resolution of the recordings (18 ms, Methods section) omitted to resolve individual [Ca2+]t-sys depletion events. Still the depletion of [Ca2+]t-sys could be fitted by exponential functions (Supplementary Figure 1). The coherence to the observed relationship is consistent with a successful electrical stimulation at these high frequencies and indicates a coupled Ca2+ influx from the t-system to the cytoplasm with each action potential under these conditions.
When we evaluated the [Ca2+]t-sys depletion, as well as the recovery of the individual steps for the tested frequencies we noticed that individual depletion steps were getting smaller with each applied pulse (Supplementary Figure 2a). We reasoned that this would be due to the decreasing levels of [Ca2+]t-sys, the driving force for Ca2+ entry24. When we quantified the change in [Ca2+]t-sys (Δ[Ca2+]t-sys) with respect to [Ca2+]t-sys we obtained a strong linear relationship (Supplementary Figure 2b). Thus, the depletion steps and with it the underlying calcium flux were directly proportional to the Ca2+ gradient across the t-system (neglecting the much smaller cytosolic Ca2+ concentration) suggesting that the observed Ca2+ flux was carried by a Ca2+-selective ion channel24.
In order to improve the temporal resolution of our signal, we performed linescans, accepting a dramatic loss in signal to noise ratio due to the loss of the spatial averaging. To partly restore for the deteriorated signal we averaged at least 10 consecutive linescans aligned by the peak of the action potential-induced Ca2+ release. This provided an improvement in temporal resolution to 80 µs. With this approach, we determined the activation of the Ca2+ influx during a single-action potential occurred with a time constant of 10 ms (Supplementary Figure 2c and 2d).
Every action potential activates SOCE
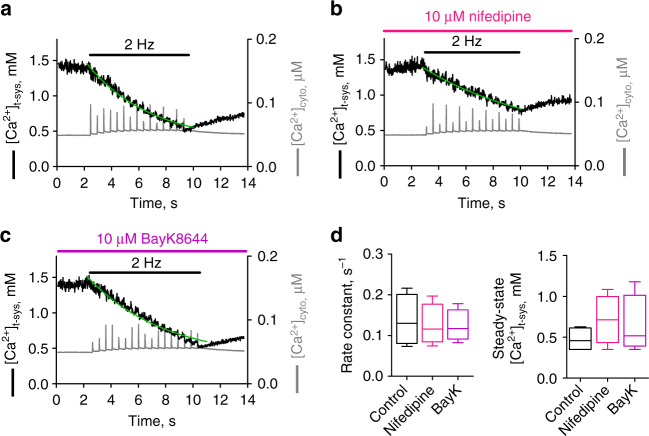
The known t-system Ca2+ fluxes that could be carrying the observed phasic influx during EC coupling are L-type Ca2+ channel or SOCE. To determine whether the phasic Ca2+ flux was carried by the L-type Ca2+ channel or not we applied the known antagonist nifedipine and the agonist BayK8644 and assessed [Ca2+]t-sys depletion during trains of action potentials under these conditions. Neither nifedipine, at a close to saturating concentration (10 µM)25,26, nor BayK8644 had any effect on the observed phasic [Ca2+]t-sys depletion nor its rate of depletion (Fig. 2). These results exclude the possibility that the L-type Ca2+ channel is responsible for carrying the observed phasic t-system Ca2+ flux.
Fig. 2.
The phasic Ca2+ influx is resistant to the DHPR antagonist and agonist, nifedipine and BayK8644, respectively. Depletion of [Ca2+]t-sys during 2 Hz stimulation of a skinned fibre under control conditions (a), and in the presence of either 10 µM nifedipine (b) or 10 µM BayK8644 (c). An exponential curve was fitted to the declining phase of the [Ca2+]t-sys in each case. The mean rate constant and [Ca2+]t-sys steady-state lower plateau levels fitted from four fibres as presented as box and whisker plots in d. One-way ANOVA revealed no statistical significance for comparing the rate constants (p = 0.8128) and the steady-state levels (p = 0.4412), respectively
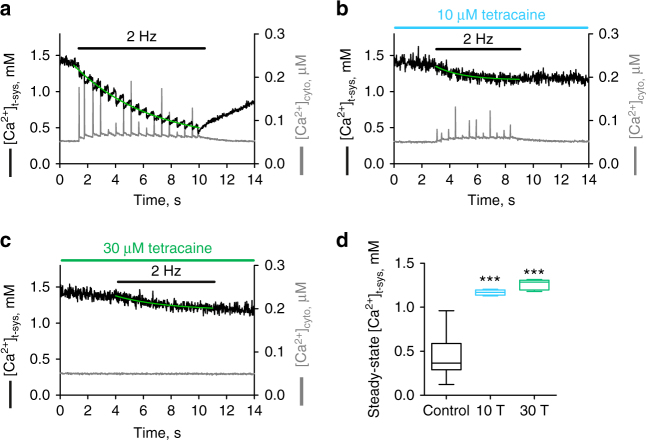
Next, to test whether the phasic influx was store-dependent, we examined whether interfering with store-depletion during excitation of the fibre affected the phasic influx. To do this we blocked the RyRs directly, to prevent Ca2+ release during field stimulation, using three independent RyR antagonists: tetracaine, ryanodine, and raised free [Mg2+]cyto. This approach was expected to separate t-system Ca2+ fluxes dependent directly on excitability or Ca2+ release. First, we tried tetracaine. As expected, 10 µM of the RyR inhibitor tetracaine inhibited the electrically evoked cytosolic Ca2+ transients. This concurrently reduced the amount of Ca2+ depleted from the t-system (Fig. 3). Raising the tetracaine concentration to 30 µM abolished the cytosolic Ca2+ transients and the phasic depletions of [Ca2+]t-sys (Fig. 3). Interestingly, although individual depletion steps could not be resolved anymore under these conditions, it was evident that [Ca2+]t-sys steady-state values were different before and after electrical stimulation. Thus, electrical stimulation was able to deplete some [Ca2+]t-sys even in the absence of any detectable SR Ca2+ release. This result is consistent with maintained excitability of the t-system following action potentials in the presence of µM tetracaine and the activation of an apparently tonic, low-amplitude Ca2+ current activated by the train of action potentials.
Fig. 3.
Action potential-activated extracellular Ca2+ influx is store‐operated. Representative traces of [Ca2+]cyto and [Ca2+]t‐sys under control conditions (a) or in the presence of either 10 µM tetracaine (b) or 30 µM tetracaine (c). Green lines represent mono‐exponential fits to the decay in the [Ca2+]t‐sys signal during 2 Hz stimulation. d The mean [Ca2+]t-sys steady-state lower plateaus at the end of the 2 Hz stimulation are presented as box and whisker plots. Data under control conditions (n = 7), and in the presence of 10 µM (n = 4) and 30 µM tetracaine (n = 5) were analysed with One-way ANOVA and Tukey’s post hoc test. ***statistical significance with p < 0.0001. Abbreviations on figure: 10 µM tetracaine, 10 T; 30 µM tetracaine, 30 T
In the next experiments, raised cytosolic [Mg2+]cyto and ryanodine were used to inhibit the RyR, both of which are known not to affect t-system excitability27. A concentration of 10 µM ryanodine completely abolished SR Ca2+ release and the observed phasic depletions of [Ca2+]t-sys. Consistent with 30 µM tetracaine, a small reduction of the [Ca2+]t-sys steady state with stimulation was observed (Supplementary Figure 3). 3 mM free [Mg2+]cyto caused a reduction in SR Ca2+ release and a reduction in the depletion of [Ca2+]t-sys during excitation (Supplementary Figure 4).
We are aware that it is possible for ryanodine and tetracaine to affect the DHPR and voltage-gated Na+ channel, respectively. However, the concentrations of ryanodine we have used is well-below the Ki for binding to the DHPR (45 µM)28. The presence of 10 µM tetracaine allowed action potential-induced Ca2+ release to remain active, though reduced. Furthermore, the use of raised Mg2+ as a RyR antagonist is known not to affect t-system excitability29,30. Regardless, the presence of the antagonist did not affect the maintenance of excitability as we observed the tonic Ca2+ influx in each case, which likely reflects L-type Ca2+ channel influx31.
Additionally, we examined the possibility that the Ca2+ released from the SR into the junctional space per se rather than SR Ca2+ depletion caused activation of phasic t-system Ca2+ flux. To this end we manipulated the [Ca2+] in the junctional space during SR Ca2+ release by bathing the fibre in a solution containing 10 mM BAPTA instead of EGTA. BAPTA provides a much faster rate of Ca2+ chelation. Under these conditions we observed a suppression of the cytosolic Ca2+ transients during EC coupling, as expected, but also observed the persistence of the phasic Ca2+ influx from the t-system (Supplementary Figure 5). The rate constant from exponential curves fitted to the decline in [Ca2+]t-sys during 2 Hz stimulation and the plateau of [Ca2+]t-sys depletion were not significantly different from that in EGTA (two-tailed t-tests, p = 0.4728 and 0.5833, respectively). This is consistent with depletion of Ca2+ from within the SR as the trigger for the phasic t-system Ca2+ flux and strongly argues against a mechanism of Ca2+-induced Ca2+ influx, that could have potentially been carried by members of the TRP family of ion channels
Together, these experiments (Figs. 2, 3 and Supplementary Figures 3-5) clearly demonstrate that the observed rapid [Ca2+]t-sys depletion is dependent on the Ca2+ depleted from within the SR.
To be consistent with the phasic flux being SOCE, the degree of SR depletion should be represented in the magnitude of the t-system Ca2+ flux2. To determine the relationship between the amount of Ca2+ released from the SR and the amount of Ca2+ depleted from the t-system, we determined the total Ca2+ released from the SR under control conditions and under conditions where Ca2+ release was either suppressed or facilitated (for example, when Ca2+ release was suppressed in tetracaine, Fig. 3, see Methods section for calculations). A summary of this analysis is plotted in Fig. 4. A two component model fitted the data. For very small and non-detectable Ca2+ release from the SR (left branch of the graph) there was a constant amount of about 0.14 mM Ca2+ depleted from the t-system (with respect to t-system volume). When SR Ca2+ release surpassed a threshold of 1.2 mM Ca2+ (with respect to SR volume) depletion of the t-system Ca2+ increased with increasing Ca2+ released from the SR. A maximum plateau was reached at a value of about 0.3 mM (right branch of the graph). The amount of extracellular Ca2+ entering the cytoplasm from the t-system was only a small fraction relative to the contribution of the SR during EC coupling. Ca2+ released from the SR contributed about 99% to the Ca2+ entering the cytoplasm (blue trace, right axis, Fig. 4). Taken together, this data show the presence of a store-independent mechanism (the apparently tonic flux) that can be observed in isolation when no or very little Ca2+ was released from the SR and the emergence of a second, store-dependent mechanism (the phasic flux) when a certain threshold of SR Ca2+ release was exceeded.
Fig. 4.
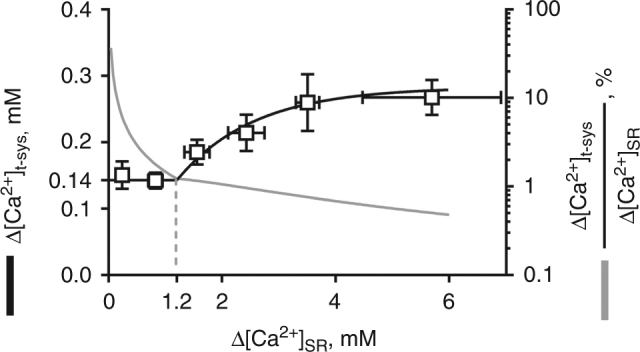
Store dependence of Ca2+ influx during electrical stimulation. A summary of the amount of Ca2+ entering the cytoplasm from the t‐system in response to the total Ca2+ released from the SR following a single-action potential (black line). The ratio of Ca2+ entering the cytoplasm from the t‐system and the SR is shown as grey line. Mean ± SEM from six fibres
The SR Ca2+ pump restricts SOCE
We have proposed that the SR Ca2+ pump strongly restricts the activation of SOCE by re-sequestering virtually all of the released Ca2+, eliminating the possibility that SOCE refills the SR following EC coupling5. To test this we directly assessed SOCE during EC coupling in the presence and absence of a functional SR Ca2+ pump. The SR Ca2+ pump was poisoned with cyclopiazonic acid (CPA) added to the cytoplasmic solution to inhibit the uptake of Ca2+. Figure 5 shows that the addition of 100 µM CPA caused [Ca2+]t-sys to be lowered to a new steady level prior to electrical stimulation. This change in [Ca2+]t-sys occurred in the absence of a change in the t-system electrochemical gradient for Ca2+, indicating that the lowering of [Ca2+]SR to a new steady level upon blocking of the SR Ca2+ pump caused tonic activation of a subpopulation of SOCE channels. Importantly, the commencement of phasic [Ca2+]t-sys depletions with individual electrical stimuli, indicated the additional, rapid recruitment of SOCE channels during the rapid release of Ca2+ from SR. This also indicates that [Ca2+]SR remained high enough in the presence of CPA prior to electrical stimulation to keep a population of SOCE channels inactive. With cessation of electrical stimulation, the lowered [Ca2+]t-sys did not recover in the continued presence of CPA, in contrast to the control (Fig. 5). This indicates that SOCE channels opened phasically during stimulation in the presence of CPA probably remained tonically active as Ca2+ re-uptake into the SR was inhibited. The immediate recovery of [Ca2+]t-sys following cessation of electrical stimulation in the absence of CPA shows that SOCE channels close or become inactive immediately following the termination of action potential-induced opening of the RyRs.
Fig. 5.
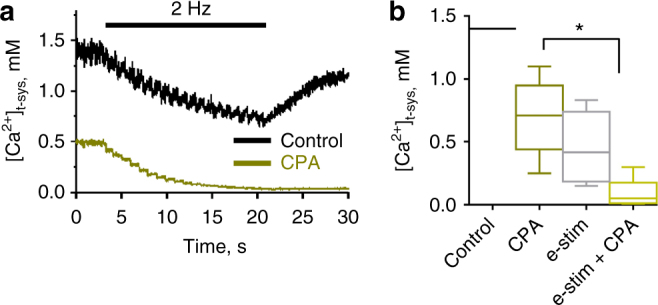
Tonic and phasic SOCE are observed under SR Ca2+ pump inhibition. Representative traces of [Ca2+]cyto and [Ca2+]t‐sys at rest and during 2 Hz field stimulation in the absence (a, top trace) and presence (a, lower trace) of 100 µM CPA. Note that the addition of CPA caused the [Ca2+]t‐sys to decline to a new steady‐state level. This new, lower level did not affect the phasic activation of SOCE upon electrical stimulation in the presence of CPA. b Summary of [Ca2+]t‐sys steady‐state plateau levels before and after 2 Hz electrical stimulation in the absence and presence of CPA. Result from 6 fibres is displayed as a box and whisker plot. One-way ANOVA and Tukey’s post hoc test. *indicates statistical significance with p < 0.001
SOCE is a counter-flux
While the presence of 10 mM EGTA in the cytoplasmic solution allowed the calculation of the total Ca2+ released from the SR, this level of Ca2+-buffering far exceeds endogenous levels. Thus we performed experiments under levels more equivalent to physiological buffering, with [EGTA] lowered from 10 to 0.2 mM22. Under field stimulation, with 0.2 mM EGTA in the cytoplasmic solution, there was a marked increase and broadening of the cytosolic Ca2+ transients. Importantly, the phasic depletions of [Ca2+]t-sys observed in 10 mM EGTA (Fig. 1) could no longer be resolved (Fig. 6). In the same fibre, the re-introduction of 10 mM EGTA allowed these fast [Ca2+]t-sys depletions to be observed again (Supplementary Figure 6). Under 2 Hz field stimulation, the steady-state depletion of [Ca2+]t-sys was only significant in high [EGTA] (Fig. 6b) (two-tailed t-test, p = 0.0005). This suggests that high-EGTA suppresses the rise of cytoplasmic [Ca2+]cyto to critically reduce the amount of Ca2+ able to bind to t-system Ca2+-pump binding sites during Ca2+ release to reduce Ca2+ extrusion. In contrast, under weakly buffered conditions, the higher [Ca2+]cyto allows the t-system Ca2+ fluxes to be bidirectional and effectively nullify each other. Taken together, the presence of high EGTA provides Ca2+-buffering in the junctional space to reduce the ability of the t-system to extrude Ca2+, thereby isolating SOCE and the tonic influx of Ca2+ during a train of action potentials that results in the observed [Ca2+]t-sys depletion (Figs. 1–6)19.
Fig. 6.
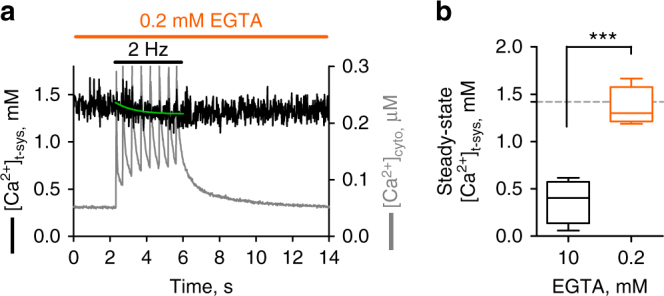
SOCE is an immediate counter‐flux isolated by cytosolic Ca2+ buffering. a [Ca2+]t‐sys and cytoplasmic Ca2+ transients in a skinned fibre in the presence of 0.2 mM EGTA during field stimulation of 2 Hz. Note the absence of the phasic [Ca2+]t‐sys depletion observed under these conditions, in contrast to cytosolic Ca2+‐buffering with 10 mM EGTA (Fig. 1). b Summary of steady‐state [Ca2+]t‐sys in skinned fibres following 2 Hz field stimulation in the presence of 0.2 and 10 mM EGTA. Result from 4 fibres is presented as a box and whisker plot. Paired two-tailed Student’s t-test, ***indicating statistical significance with p = 0.0005.
Discussion
The physiological activation mechanism and a primary physiological role of SOCE in skeletal muscle have not been defined, even though the molecular identities and biophysical properties of SOCE have been described in some detail2,7–9. The main obstacle in determining the physiological activation and role of SOCE in muscle has been the lack of direct measurements of SOCE during EC coupling under physiological conditions, which we have addressed (Fig. 1). Using this approach we were able to observe a phasic influx of Ca2+ from the t-system lumen with every action potential at all stimulation frequencies tested here. The pathway responsible for the Ca2+ influx was established as predominantly store-dependent channels by showing (i) a dependence on the amount of Ca2+ depleted from the SR (Fig. 4), (ii) resistance to the L-type Ca2+ channel antagonist and agonist, nifedipine and BayK8644 (Fig. 2), (iii) an elimination by the blockade of SR Ca2+ depletion with three independent RyR antagonists during excitation (Fig. 3 and Supplementary Figures 3, 4), (iv) fast kinetics consistent with a high off-rate of Ca2+ for STIM1 (and inconsistent with the slow activation of the L-type Ca2+ channel) (Fig. 1), (v) a linear dependence on the t-system Ca2+ gradient (Supplementary Figure 2b), and (vi) persistence in the presence of either cytoplasmic EGTA or BAPTA (Supplementary Figure 5). In the absence of Ca2+ release, repetitive field stimulation did allow the isolation of an additional mode of slowly activating, apparently tonic Ca2+ influx during repetitive action potentials consistent with L-type Ca2+ channel activation31 (Fig. 3). Changing the Ca2+-buffering capacity of the cytoplasm also allowed the manipulation of the ability of the t-system to extrude Ca2+ during SR Ca2+ release19,32. This exposed a role of rapidly activated SOCE during EC coupling was to act as an immediate counter-flux to t-system Ca2+ extrusion to prevent the loss of fibre Ca2+ during muscle stimulation.
It has been implied from indirect evidence that SOCE is active during the declining phase of the Ca2+ transient of a tetanus9,15. However, by directly measuring SOCE during EC coupling we show that this is not the case (Figs. 1–3). The physiological waveform of SOCE influx is phasic, following each action potential. The heavily Ca2+-buffered SR rapidly inactivated SOCE (Figs. 1–3). We could only observe a tonic activation of SOCE under non-physiological conditions of a poisoned SR Ca2+ pump and hence depleted SR Ca2+ stores (Fig. 5). During periods of fibre quiescence the electrochemical gradient for Ca2+ across the t-system is sufficient for the passive leak of extracellular Ca2+ into the fibre to set the required level of Ca2+ inside the SR5,33. This is consistent with STIM1 or ORAI1 null muscle responding normally to field stimulation at 50 Hz, which implies a normal load of SR Ca2+5,7,9,34. Furthermore, during prolonged periods of muscle stimulation where the muscle experiences metabolic fatigue, the total amount of Ca2+ inside the SR is still significant and is not the limiting factor in Ca2+ release35–37.
The rapid activation of SOCE in conjunction with action potential-induced Ca2+ release means only a relatively small quantum of the total Ca2+ held within the SR gets released as the trigger SR Ca2+ depletion for Ca2+ dissociation from STIM1. The steady level of bulk [Ca2+]SR does not drop significantly13–15 and cannot be critical to SOCE activation. It must be the Ca2+ dropping during release in a locally restricted domain behind the RyR channel pore that is critical for SOCE activation. Thus, we suggest that SOCE is rapidly activated by local depletions of Ca2+ at the near membrane inside the SR terminal cisternae following the action potential-coordinated Ca2+ release. This is, of course, consistent with a koff Ca2+ from STIM1 of probably >2000 s−138. Near membrane Ca2+ depletion will uniformly occur at all the terminal cisternae of the fibre, where STIM1 and RyRs are likely in close quarters2,16,24. Under this condition the bulk [Ca2+]SR during twitches and tetani can remain high13–15 while SOCE becomes briefly active (Figs. 1–4). Our findings provide further support to the proposal that Orai1 and STIM1 are in prearranged complexes that can be rapidly activated upon Ca2+ release2,16,24,32. Others have challenged this idea based on the increase of a low-biomolecular fluorescence complementation (BiFC) signal in a resting fibre upon poisoning of the SR Ca2+ pump and thorough depletion of SR Ca2+9. The conclusion was based on the assumption that only the stronger BiFC signal represents prearranged complexes. It is, however, possible that both the full and depleted SR have prearranged Orai1-STIM1 complexes, differing only in absolute number, and therefore BiFC signal.
Our line of argument of pre-coupled Orai1-STIM1 complexes is further supported by the observation that SOCE activated with a time constant of only 10 ms (Supplementary Figure 2c, d). In fact this makes it hard to envision STIM1 and Orai1 physically separated at the junctional membranes, given that respective activation kinetics of uncoupled Orai1-STIM1, as has been demonstrated in multiple non-muscle tissues, lie in the seconds range1.
The simultaneous imaging of Ca2+ in the cytoplasm and SR lumen has shown that the nadir of Ca2+ depletion inside the SR lags behind the closure of RyRs during sparks and action potential-induced Ca2+ release by 50 and 30 ms, respectively13. To explain the anomaly of SR Ca2+ depletion continuing after the cessation of Ca2+ release, the source of the Ca2+ during these events was proposed to originate from a locally restricted domain near the membrane behind the RyR channel pore whose diffusional properties differ from the bulk SR13. As such, the observed delay of the nadir of the measured bulk SR Ca2+ depletion must be associated with the equilibration of these local Ca2+ gradients. Given the length of the delay between closure of the RyRs and the time to [Ca2+]SR nadir, it is reasonable to suggest that there is a barrier to Ca2+ diffusion from the bulk SR to the near membrane as Ca2+ is released. This is likely to be calsequestrin (CSQ), given the positioning of CSQ at the SR terminal cisternae, immediately behind the RyRs and the unique Ca2+-adsorbing and high Ca2+-buffering capacity of CSQ39–41. Thus the spatiotemporal separation of Ca2+ sources inside the SR makes the near membrane a privileged signalling environment where Ca2+ can rapidly and locally deplete, with the opening and closing of RyRs. These transient depletions of Ca2+ near the membrane can happen without major depletions of the bulk [Ca2+]SR be it during a spark, twitch or punctuated throughout a tetanus13–15. This system provides the environment for the rapid dissociation of Ca2+ from STIM1 in the near membrane16 for activation of SOCE following every action potential, as we have demonstrated (Figs. 1–4). Consistent with this, preliminary reports using Ca2+ indicators targeted close to the RyR channel within the SR terminal cisternae lumen have revealed that local Ca2+ release is greater and faster than that occurring in the bulk SR lumen during EC coupling42.
In respect thereof, the abundance and positioning of CSQ, RyRs and STIM1 in this environment are paramount to muscle Ca2+ homoeostasis and, ultimately, muscle physiology and health (e.g., refs. 6,22,43–45). While the normal positioning of CSQ and RyRs has been shown39,46, the exact positioning of STIM1 is unknown because of the lack of a specific STIM1 antibody to use for localization imaging32. Developing a picture of how STIM1 and RyRs position themselves relative to each other at the terminal SR and identifying how this arrangement changes under different conditions will be required to gain a further understanding of signalling at this nano-domain. However, our high-spatiotemporal imaging of Ca2+ transients (Figs. 1–4) during physiological Ca2+ release limits the possible location of the active proteins in the fibre to the junctional membranes, nearby the RyRs.
We propose that a primary physiological role of SOCE is an immediate, rapidly activated counter-flux to t-system Ca2+ extrusion in skeletal muscle fibres that is activated in response to the local depletion of SR terminal cisternae Ca2+ as evoked by action potentials. In the presence of a weakly Ca2+-buffered cytoplasmic environment, there was only a small net change in the [Ca2+]t-sys during a train of action potentials (Fig. 6). This level of Ca2+-buffering is equivalent to the situation in the muscle, so allowing a build-up of Ca2+ at the cytoplasmic side of the t-system membrane during EC coupling to cause activation of Ca2+ transport from the junctional space to the t-system lumen. The simultaneous activation of SOCE with t-system Ca2+ extrusion represents an efficient mechanism to keep Ca2+ in the fibre during any period of stimulation. The advantage of the phasic Ca2+ influx being store-dependent is that the recovery of Ca2+ that enters the t-system during EC coupling is tightly regulated by the amount of Ca2+ exiting the SR, allowing balance of fluxes, or net Ca2+ influx that may assist muscle activity. Tracking the entry of radioactive Ca2+ into intact muscle during continuous, low-frequency stimulation has shown an accumulation of Ca2+ in the muscle47, suggesting that Ca2+ influx is greater than efflux in vivo48. The major component of this Ca2+ influx would be SOCE at the transverse tubules in conjunction with excitation (Figs. 1–4)47. The net movement of Ca2+ across the t-system is probably tuneable by regulation of the relative activity of the Na+–Ca2+ exchanger, plasma membrane CaATPase and SOCE. It remains possible that deviation from the Ca2+ homoeostasis we describe here may alter signalling cascades10.
Our demonstration that the normal activation of SOCE is immediate following every action potential-evoked release of Ca2+ in healthy muscle provides the framework in which to understand SOCE-related myopathies. e.g., ref. 6. It is not currently clear how quickly the absence of SOCE as a counter-flux will compromise fibre Ca2+ levels during bouts of EC coupling, as these fluxes are small (Fig. 4). Certainly, it is likely that changes in Ca2+ levels in the nano-domains of the junctional membranes during muscle activity will be sensitive to SOCE. The in-phase nature of the SOCE influx with the pattern of action potential stimulation (Figs. 1–4) also makes it possible that the SOCE transients entering the junctional space are an essential part of phasic Ca2+ signalling in response to muscle usage patterns8–10,49,50, as local Ca2+ signals activate diffusible messengers51.
Taken together, our results suggest the primary physiological role for SOCE in skeletal muscle to be a counter-flux to t-system Ca2+ extrusion, to maintain Ca2+ homoeostasis. Our observation of the control of t-system Ca2+-handling by action potential-evoked Ca2+ release can explain the classic observation of Armstrong et al.52, where skeletal muscle twitched for long periods in the presence of EGTA and virtual absence of external Ca2+. Under these conditions some of the Ca2+ released from SR is extruded across the t-system membrane to the t-system lumen. Ca2+ entering the t-system lumen requires seconds to diffuse from the fibre53. The simultaneous opening of the store-dependent channels with SR Ca2+ release (Figs. 1–4) turn the t-system into a sieve, causing the Ca2+ that entered the t-system lumen during Ca2+ release to re-enter the cytoplasm. That is, SOCE captures Ca2+ that is extruded to the t-system and returns it to the cytoplasm before it would otherwise be lost to the external environment beyond the t-system lumen.
Materials and methods
Preparation of skinned fibres and solutions for Ca2+ imaging
All experiments on rats were approved by the Animal Ethics Committee at The University of Queensland. Male Wistar and Sprague rats 4–8 months of age (University of Queensland Biological Resources, Brisbane) were killed by asphyxiation in CO2. The extensor digitorum longus (EDL) muscle was rapidly excised and placed in a Petri dish under paraffin oil above a layer of Sylgard. Small bundles of fibres were exposed to a Ringer solution containing 2.5 mM of the Ca2+-sensitive dye rhod-5N for at least 10 min. Thereafter, segments of individual fibres were isolated and the sarcolemma was mechanically removed with forceps (fibre skinning). The skinned fibres were mounted in a custom built chamber on top of a 1.5 coverslip and bathed in an internal solution (in mM): 90 HEPES, 10 EGTA, 40 HDTA (1,6-Diaminohexane-N,N,N′,N′-tetraacetic acid), 8.77 MgO, 1.94 CaCO3, 8 ATP*2Na, 10 creatine phosphate*2Na, with pH adjusted to 7.1 ± 0.1 with KOH and resulting free Ca2+ and Mg2+ concentration of 50 nM and 1 mM, respectively. Fluo-4 was added at a concentration of 10 µM. Electrical field stimulation was delivered by two platinum electrodes running in parallel to the fibre long axis. Individual pulses were applied at various frequencies (0.2–50 Hz) by rectangular voltage steps of up to 100 V in amplitude and 2–4 ms in duration.
The experimental chamber was mounted above a ×20 objective of a Leica TCS SP8 laser scanning confocal microscope system equipped with a 12 kHz resonant scanner. Rhod-5N and Fluo-4 were excited by 561 and 488 nm laser lines, respectively, and emitted light was collected with high-sensitivity GaAsP detectors in bands of 40 nm. xyt image series of the two colours were taken in sequential mode with an individual xy image size of 512 × 128 pixels and a time resolution of 18 ms (55 frames s−1). Pinhole was set to 1.5 AU. Fluorescence intensity across the imaged fibre area was averaged for both colours to derive traces of the mean fluorescence intensity over time that were then converted to [Ca2+]cyto and [Ca2+]t-sys. Calcium concentrations ([Ca2+]), refer to free ion concentrations if not stated otherwise.
Calibration of fluo-4 fluorescence and conversion to [Ca2+]cyto
The dissociation constant (KD) of fluo-4 was determined by measuring the dye fluorescence in the presence of free cytosolic [Ca2+] of 0.05, 0.8, 10 and 2000 µM in 4 fibres. Fluorescence values were normalized to the maximal values and fit with a Hill equation. The so obtained KD-value amounted to 1 µM, which is in good agreement with other reports54. Fluorescence recordings were then transformed to [Ca2+]cyto according to the formula
| 1 |
Fmin was determined at the end of each experiment by exposing the fibre to a calcium free solution. Fmax was calculated from the baseline fluorescence in the standard solution containing 50 nM of free [Ca2+] by rearranging equation (1):
| 2 |
Determination of the amount of total Ca2+ released from the SR during an electrically evoked action potential
This estimation is based on a method originally described by Melzer et al.55. Briefly, the cytosolic Ca2+ signals in 10 mM EGTA show a sharp transient followed by a slow decay upon each action potential (Fig. 1). The sharp transient is caused by the release of Ca2+ from the SR, which is incompletely buffered by EGTA while the slow decline reflects the diffusion of free Ca2+ out of the imaged region of interest, i.e., from within the fibre. Assuming the diffusive loss during the short time of an action potential to be negligible the change in cytosolic free Ca2+ levels before and right after an action potential directly reflects the additional amount of Ca2+ released from the SR. Considering a simple Ca2+-EGTA buffer system (Ca2+ +EGTA<–>Ca2+EGTA) and neglecting the much smaller endogenous buffering capacity56 the total amount of Ca2+ present can be calculated from the free [Ca2+]cyto according to the following equation:
And the amount of total calcium released by the SR is obtained by:
| 3 |
where [Ca2+]before and [Ca2+]after are the free calcium concentrations right before and right after each action potential, KD is the dissociation constant of EGTA (200 nM)56, and [EGTA]T is the total EGTA buffer concentration. The so converted values refer to the cytosolic volume of the fibre and were converted to values referring to the SR volume by assuming a cytosol to fibre volume ratio of 0.7 and a SR to fibre volume ratio of 0.09357.
| 4 |
Calibration of t-system rhod-5N fluorescence
Calibration of rhod-5N fluorescence was performed as previously reported19. Fluorescence values were converted to [Ca2+]t-sys using equation (1) with a KD of 0.872 mM19.
Determination of t-system depletion and recovery values
The change in [Ca2+]t-sys (∆[Ca2+]t-sys depletion) was calculated as the difference between the [Ca2+]t-sys right before and immediately after each electrical stimulus. The recovery of [Ca2+]t-sys (∆[Ca2+]t-sys depletion) of the signal between consecutive stimuli was calculated as the difference between the [Ca2+]t-sys right after and immediately before the following electrical stimulus.
Determination of the relationship between ∆[Ca2+]t-sys and ∆[Ca2+]SR
To derive the relationship between ∆[Ca2+]t-sys and ∆[Ca2+]SR (Fig. 4), [Ca2+]t-sys depletion values and corresponding [Ca2+]SR release values were determined under control conditions, in the presence of 10 µM of tetracaine, by back calculation in the presence of 30 µM of tetracaine, and for so called super events (see below). All of the so obtained values were then binned into the following intervals of ∆[Ca2+]SR: [0–0.5], [0.5–1], [1–2], [2–3], [3–4], [4–oo] mM. Data were fit with a nonlinear least square routine to a segmented equation consisting of an initial constant baseline followed by an exponential rise according to the formula:
| 5 |
with x0 indicating the start of exponential rise.
Back calculation of Ca2+ movements across the t-system membrane
Each action potential was accompanied by a depletion step, as well as a reuptake step. Upon repetitive stimulation [Ca2+]t-sys eventually reached a new steady state, a condition in which the single depletion and reuptake steps must exactly balance each other (Supplementary Figure 2a). This relationship can be used to obtain the amplitude of the first depletion step under conditions in which the individual steps cannot be resolved anymore. The change in t-system steady-state Ca2+ level (∆[Ca2+]SSt-sys) during repetitive stimulation is the sum of all individual depletion and re-uptake steps, i.e.
| 6 |
with Ydi and Yri being the ith amplitude of depletion and re-uptake, respectively. The amplitudes of the individual depletions steps followed an exponential, while the amplitudes of the reuptake remained constant (Supplementary Figure 2a), thus
| 7 |
Summing of the geometric series as obtained by inserting expression (7) into (6) yields an expression for the first depletion step in a train of stimulation as a function of ∆[Ca2+]SSt-sys:
| 8 |
Super events
On a few occasions electrical stimulation did not result in the generation of only a single action potential but was accompanied by a second action potential or even a small burst like discharge. Also, spontaneous action potentials occasionally seen during experiments happened to sometimes coincide with the electrical stimulation. Although these super events released considerably larger amounts of Ca2+ into the cytosol as determined by the fluo-4 signal the respective depletion of the t-system was only moderately increased reaching a limiting level of about 0.3 mM.
Statistical analysis
For comparing two groups of data a two-tailed Student’s t-test was used. For comparing more than two groups ANOVA with Tukey’s post hoc test was used. Paired testing was applied when the data points to compare were sampled from the same fibres. All data are given as mean ± SEM.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
Electronic supplementary material
Acknowledgements
The work was supported by an Australian Research Council (ARC) Discovery Project (DP180100937). B.S.L. was a Future Fellow of the ARC (FT140101309).
Author contributions
X.F. designed, performed and analysed experiments, and interpreted data; R.H.C. interpreted data; B.S.L. designed experiments, interpreted data and wrote the paper. All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s42003-018-0033-7.
References
- 1.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Launikonis BS, Murphy RM, Edwards JN. Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflüg. Arch. 2010;460:813–823. doi: 10.1007/s00424-010-0856-7. [DOI] [PubMed] [Google Scholar]
- 3.Peachey LD. The sarcoplasmic reticulum and transverse tubules of the frog’s sartorius. J. Cell Biol. 1965;25(Supplementary):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- 4.Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- 5.Cully TR, Launikonis BS. Store-operated Ca2+ entry is not required for store refilling in skeletal muscle. Clin. Exp. Pharmacol. Physiol. 2013;40:338–344. doi: 10.1111/1440-1681.12078. [DOI] [PubMed] [Google Scholar]
- 6.McCarl CA, et al. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J. Allergy Clin. Immunol. 2009;124:1311–1318. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stiber J, et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat. Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T. STIM1-Ca2+ signaling is required for the hypertrophic growth of skeletal muscle in mice. Mol. Cell Biol. 2012;32:3009–3017. doi: 10.1128/MCB.06599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei-Lapierre L, Carrell EM, Boncompagni S, Protasi F, Dirksen RT. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 2013;4:2805. doi: 10.1038/ncomms3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrell EM, Coppola AR, McBride HJ, Dirksen RT. Orai1 enhances muscle endurance by promoting fatigue-resistant type I fiber content but not through acute store-operated Ca2+ entry. FASEB J. 2016;30:4109–4119. doi: 10.1096/fj.201600621R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J. Physiol. 2009;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Launikonis BS, et al. Depletion “skraps” and dynamic buffering inside the cellular Ca2+ store. Proc. Natl Acad. Sci. USA. 2006;103:2982–2987. doi: 10.1073/pnas.0511252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolf R, Magalhães PJ, Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J. Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canato M, et al. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl Acad. Sci. USA. 2010;107:22326–22331. doi: 10.1073/pnas.1009168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol. 2011;194:335–346. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cully TR, et al. Human skeletal muscle plasmalemma alters its structure to change its Ca2+-handling following heavy-load resistance exercise. Nat. Commun. 2017;8:14266. doi: 10.1038/ncomms14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. USA. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cully TR, Edwards JN, Murphy RM, Launikonis BS. A quantitative description of tubular system Ca2+-handling properties in fast- and slow-twitch fibres. J. Physiol. 2016;594:2795–2810. doi: 10.1113/JP271658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posterino GS, Lamb GD, Stephenson DG. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J. Physiol. 2000;527:131–137. doi: 10.1111/j.1469-7793.2000.t01-2-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Posterino GS, Lamb GD. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J. Physiol. 2003;551:219–237. doi: 10.1113/jphysiol.2003.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J. Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakker AJ, Cully TR, Wingate C, Barclay CJ, Launikonis BS. Doublet stimulation increases Ca2+ binding to troponin C to ensure rapid force development in skeletal muscle. J. Gen. Physiol. 2017;149:323–334. doi: 10.1085/jgp.201611727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launikonis BS, Ríos E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedetti B, Tuluc P, Mastrolia V, Dlaska C, Flucher BE. Physiological and pharmacological modulation of the embryonic skeletal muscle calcium channel splice variant CaV1.1e. Biophys. J. 2015;108:1072–1080. doi: 10.1016/j.bpj.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posterino GS, Lamb GD. Effect of nifedipine on depolarization-induced force responses in skinned skeletal muscle fibres of rat and toad. J. Muscle Res. Cell Motil. 1998;19:53–65. doi: 10.1007/BF03257390. [DOI] [PubMed] [Google Scholar]
- 27.Lamb GD, Stephenson DG. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J. Physiol. 1990;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdivia HH, Coronado R. Inhibition of dihydropyridine-sensitive calcium channels by plant alkaloid ryanodine. FEBS Lett. 1989;244:333–337. doi: 10.1016/0014-5793(89)80557-5. [DOI] [PubMed] [Google Scholar]
- 29.Lamb GD, Stephenson DG. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J. Physiol. 1991;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi RH, Koenig X, Launikonis BS. Dantrolene requires Mg2+ to arrest malignant hyperthermia. Proc. Natl Acad. Sci. USA. 2017;114:4811–4815. doi: 10.1073/pnas.1619835114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bannister RA, Pessah IN, Beam KG. The skeletal L-type Ca2+ current is a major contributor to excitation-coupled Ca2+ entry. J. Gen. Physiol. 2009;133:79–91. doi: 10.1085/jgp.200810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards JN, et al. Ultra-rapid activation and deactivation of store-operated Ca2+ entry in skeletal muscle. Cell Calcium. 2010;47:458–467. doi: 10.1016/j.ceca.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J. Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutka TL, Cole L, Lamb GD. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces AP-mediated Ca2+ release in mammalian skeletal muscle. Am. J. Physiol. Cell Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- 35.Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J. Gen. Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 37.Cheng, A. J., Place, N. & Westerblad, H. Molecular basis for exercise-induced fatigue: the importance of strictly controlled cellular Ca2+ handling. Cold Spring Harb. Perspect. Med. pii: a029710. 10.1101/cshperspect.a029710, (2017). [DOI] [PMC free article] [PubMed]
- 38.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franzini-Armstrong C, Kenney LJ, Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J. Cell Biol. 1987;105:49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beard NA, Sakowska MM, Dulhunty AF, Laver DR. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 2002;82:310–320. doi: 10.1016/S0006-3495(02)75396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H, et al. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J. Biol. Chem. 2004;279:18026–18033. doi: 10.1074/jbc.M311553200. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, et al. Novel local RYR1 junctional calcium responses and dynamics by improved calcium sensor catcher. Biophys. J. 2017;112:331a. doi: 10.1016/j.bpj.2016.11.1790. [DOI] [Google Scholar]
- 43.Edwards JN, Blackmore DG, Gilbert DF, Murphy RM, Launikonis BS. Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression. Aging Cell. 2011;10:675–685. doi: 10.1111/j.1474-9726.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 44.Lamboley CR, Wyckelsma VL, McKenna MJ, Murphy RM, Lamb GD. Ca2+ leakage out of the sarcoplasmic reticulum is increased in type I skeletal muscle fibres in aged humans. J. Physiol. 2016;594:469–481. doi: 10.1113/JP271382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamboley CR, Wyckelsma VL, Perry BD, McKenna MJ, Lamb GD. Effect of 23-day muscle disuse on sarcoplasmic reticulum Ca2+ properties and contractility in human type I and type II skeletal muscle fibers. J. Appl. Physiol. 2016;121:483–492. doi: 10.1152/japplphysiol.00337.2016. [DOI] [PubMed] [Google Scholar]
- 46.Jayasinghe ID, Munro M, Baddeley D, Launikonis BS, Soeller C. Observation of the molecular organization of calcium release sites in fast- and slow-twitch skeletal muscle with nanoscale imaging. J. R. Soc. Interface. 2014;11:pii: 20140570. doi: 10.1098/rsif.2014.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gissel H, Clausen T. Excitation-induced Ca2+ uptake in rat skeletal muscle. Am. J. Physiol. 1999;276:R331–R339. doi: 10.1152/ajpregu.1999.276.2.R331. [DOI] [PubMed] [Google Scholar]
- 48.Overgaard K, et al. Effects of running distance and training on Ca2+ content and damage in human muscle. Med. Sci. Sports Exerc. 2004;36:821–829. doi: 10.1249/01.MSS.0000126468.65714.60. [DOI] [PubMed] [Google Scholar]
- 49.Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 50.Tavi P, Westerblad H. The role of in vivo Ca²+signals acting on Ca²+-calmodulin-dependent proteins for skeletal muscle plasticity. J. Physiol. 2011;589:5021–5031. doi: 10.1113/jphysiol.2011.212860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saucerman JJ, Bers DM. Calmodulin binding proteins provide domains of local Ca2+ signaling in cardiac myocytes. J. Mol. Cell Cardiol. 2012;52:312–316. doi: 10.1016/j.yjmcc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N’-tetracetic acid. Biochim. Biophys. Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- 53.Friedrich O, Ehmer T, Uttenweiler D, Vogel M, Barry PH, Fink RH. Numerical analysis of Ca2+ depletion in the transverse tubular system of mammalian muscle. Biophys. J. 2001;80:2046–2055. doi: 10.1016/S0006-3495(01)76178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollingworth S, Soeller C, Baylor SM, Cannell MB. Sarcomeric Ca2+ gradients during activation of frog skeletal muscle fibres imaged with confocal and two-photon microscopy. J. Physiol. 2000;526:551–560. doi: 10.1111/j.1469-7793.2000.t01-1-00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melzer W, Rios E, Schneider MF. Time course of calcium release and removal in skeletal muscle fibers. Biophys. J. 1984;45:637–641. doi: 10.1016/S0006-3495(84)84203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J. Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, editor. Handbook of Physiology, section 10, Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1983. pp. 73–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.