Abstract
Feeding upon vertebrate blood by mosquitoes permits transmission of diverse pathogens, including viruses, protozoa, and nematodes. Despite over a century of intensive study, no mosquito species is known to specialize on non-vertebrate hosts. Using molecular analyses and field observations, we provide the first evidence, to our knowledge, that a mosquito, Uranotaenia sapphirina, specializes on annelid hosts (earthworms and leeches) while its sympatric congener, Uranotaenia lowii, feeds only on anurans (frogs and toads). Our results demonstrate that Ur. sapphirina feeds on annelid hosts (100% of identified blood meals; n = 72; collected throughout Florida), findings that are supported by field observations of these mosquitoes feeding on Sparganophilus worms and freshwater leeches. These findings indicate that adult mosquitoes utilize a much broader range of host taxa than previously recognized, with implications for epidemiology and the evolution of host use patterns in mosquitoes.
Lawrence Reeves et al. report evidence that adult females of the mosquito species Uranotaenia sapphirina feed primarily on annelid hosts. This is the first known example of a mosquito species that specializes on invertebrate blood and suggests that mosquito host use patterns are more diverse than previously recognized.
Introduction
Mosquitoes (Diptera: Culicidae) are the vectors of disease-causing human and wildlife pathogens1–8, and as a result, they have received greater scientific and public attention than any other insect taxon. The evolutionary innovation of blood feeding in the nematoceran flies preceded our species by hundreds of millions of years9, but has had persistent consequences throughout human history. Because blood feeding enables the transmission of pathogens between vertebrate hosts10, it is the primary reason behind the intense study of mosquitoes and their interactions with vertebrates. Despite this, the origin of blood feeding and the evolution of host use patterns in mosquitoes or other dipteran vectors of pathogens remains poorly understood. Blood feeding presents an evolutionary challenge that requires meticulous adaptations that are specific for various host groups. To effectively utilize blood, a mosquito must not only possess olfactory, visual, or thermal machinery to locate hosts and pierce the epidermis, but overcome cellular and molecular barriers to blood feeding11 that vary between host orders12. Most vertebrate animals have evolved complex cellular mechanisms that rapidly respond to blood vessel injuries with a series of immune and hemostatic reactions. Mosquitoes that feed on endothermic hosts (birds and mammals) must also possess thermoregulatory strategies to avoid overheating13. The evolution of mechanisms around these barriers in mosquitoes, including biochemical salivary cocktails that circumvent immune and hemostatic responses of hosts, and thermoregulation and highly specialized mouthparts and sensory organs, have had tremendous implications for human health, throughout history and today.
Feeding on vertebrate blood is characteristic of mosquitoes of all genera, with few exceptions. All species of Toxorhynchites (89 species) and Malaya (12 species), and possibly others (e.g., Topomyia, Maorigoeldia) do not require a blood meal to complete egg development14–18. Adults of species of these genera feed exclusively on plant-derived sugars, either directly15 or from the carbohydrate-rich solution regurgitated by ants18. Autogenous, or partially autogenous, mosquito species are also found in a number of genera which include species that are otherwise blood-feeders19–23. Many blood-feeding mosquito species feed on plant-derived sugars to support metabolism10, but only females feed on blood. Female mosquitoes of hematophagous species feed on diverse vertebrate lineages, including mammals, birds, reptiles, amphibians, and fishes24. Most specialize to varying degrees on certain ranges of vertebrate classes or orders, and these patterns of host use mediate the transmission dynamics of mosquito-vectored pathogens25. No mosquito species studied to date has been found to specialize on the blood of a non-vertebrate animal.
Mitigating the impact of vector-borne pathogens is one of the greatest challenges in epidemiology and medicine. Because the transmission networks of mosquito-vectored pathogens are structured by mosquito host use patterns, understanding mosquito–host interactions is a critical element in confronting this challenge. Blood meal analysis is a collection of techniques that takes advantage of immunological or genetic specificity of host blood in adult mosquito gut contents in order to identify hosts24. Using PCR-based blood meal analyses, we investigated the host use patterns of the two Uranotaenia species that occur in eastern North America: Ur. sapphirina and Ur. lowii.
Uranotaenia is a taxonomically diverse genus, consisting of 270 currently recognized, primarily tropical species14, 26. Although few species have been extensively studied, those investigated feed primarily on anuran hosts27, 28 and at least one feeds on amphibious fishes10. Uranotaenia lowii feeds predominantly on frogs, and host-seeking females are attracted to their songs29. Until now, the host use patterns of Ur. sapphirina were, as far as we are aware, unknown, and despite deficient evidence, assumed to parallel those of Ur. lowii. Previous research using both serological and DNA-based blood meal analyses have attempted to identify the hosts of Ur. sapphirina, but most blood meal assays failed. For example, Irby and Apperson30 identified only two (1.7%) of 120 Ur. sapphirina blood meals (both as an unknown species of snake) and Cupp et al.31 identified 2 (5.7%) of 35 (both as the ranid frog Lithobates catesbeianus), compared with identification rates of 85.8% and 61.4%, respectively, for all mosquito species screened, excluding Ur. sapphirina.
We determined that Ur. sapphirina is a specialist of invertebrate hosts, worms, and leeches of the phylum Annelida, while the sympatric Ur. lowii specializes on the amphibian order Anura (frogs). We demonstrate that adult Ur. sapphirina feed on diverse annelid hosts, and report the first documentation, to our knowledge, of a mosquito specializing on invertebrate hosts. We collected blood-fed Uranotaenia mosquitoes (n = 132; 88 Ur. sapphirina; 44 Ur. lowii) from multiple locations on the Florida Peninsula. We used two diagnostic PCR assays to screen Uranotaenia blood meals for annelid and vertebrate DNA. Annelid DNA was targeted because field observations made at River Styx, Alachua Co., Florida, USA, suggested that this mosquito was feeding on oligochaete earthworm hosts. For each blood meal, we used extracted DNA as amplification templates in two separate reactions: one that targeted the annelid 28S ribosomal RNA and one that targeted the vertebrate cytochrome c oxidase subunit I gene (COI). Amplification reactions and primers were designed to produce an amplicon only in the presence of their respective template. Products of all successful reactions were sequenced to confirm the presence of annelid or vertebrate DNA.
Results
Mosquito collections
In total, 132 blood-fed adult female Uranotaenia mosquitoes were collected in four counties in Florida, representing 88 Ur. sapphirina and 44 Ur. lowii. Uranotaenia sapphirina was collected in Columbia Co. (n = 18), Alachua Co. (n = 14), and Indian River Co. (n = 56). Uranotaenia lowii was collected in Alachua Co. (n = 26), Indian River Co. (n = 14), and Miami-Dade Co. (n = 4).
Host identification
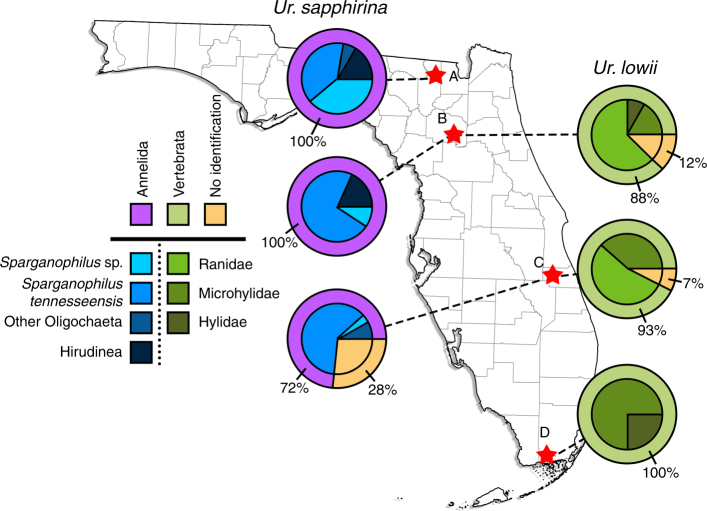
The results of PCR assays indicated that Ur. sapphirina and Ur. lowii had distinct and disparate host specialization patterns (Fig. 1). There was a significant difference in the use of annelid and vertebrate hosts between Ur. sapphirina and Ur. lowii (two-sided Fisher’s exact test; P < 0.001). We found that 100% of identified Ur. sapphirina blood meal DNA was derived from annelid hosts, while 100% of identified Ur. lowii blood meal DNA was derived from anuran hosts. Templates from 80 of 88 Ur. sapphirina blood meals screened positive for annelid DNA, and of these, 72 (81%) were confirmed by Sanger sequencing. All Ur. sapphirina blood meals were negative for vertebrate DNA. Identical screens of Ur. lowii blood meals indicated that 43 of 44 were positive for vertebrate DNA, with 38 (86%) confirmed by Sanger sequencing and attributed to anuran species known to occur in Florida. All Ur. lowii blood meals were negative for annelid DNA. Recovered host DNA sequences were compared against a reference database (GenBank, National Center for Biotechnology Information), or sequences obtained from morphologically identified annelid specimens. The majority (93%) of identified Ur. sapphirina blood meals were attributed to oligochaete earthworms. Sparganophilus tennesseensis, a sparganophilid worm, was the most frequently identified host, detected in 43 of 72 (60%) of Ur. sapphirina blood meals. Two species of freshwater leeches (Macrobdella ditetra, Philobdella floridana) together represented 7% of identified Ur. sapphirina blood meals. In comparison, the hosts of Ur. lowii were exclusively identified as anurans.
Fig. 1.
Host use patterns of Ur. sapphirina and Ur. Lowii. Mosquitoes were collected in four counties (A–D) along the Florida Peninsula, USA, and host use patterns were determined by diagnostic PCR screening of blood meals for annelid and vertebrate DNA. Results of PCR screens for vertebrate and annelid DNA in the blood meals of 88 Ur. sapphirina (left) and 44 Ur. lowii (right), collected from Columbia Co. (A) Alachua Co. (B), Indian River Co. (C), or Miami-Dade Co. (D). Pink shading of outer rings indicates the proportion of blood meals that screened positive for annelid DNA and were confirmed by Sanger sequencing. Green shading on outer rings indicates the proportion of blood meals that screened positive for vertebrate DNA and were confirmed by Sanger sequencing. Orange shading on outer rings and inner circles indicates the proportion of blood meals that either did not produce an amplicon or resulted in ambiguous sequences that could not be attributed to a host taxon. Blue shading of inner circles represents the proportion of blood meals derived from various annelid taxa, as determined by DNA sequences. Green shading of inner circles represents the proportion of blood meals derived from various vertebrate taxa, as determined by DNA sequences. Excluding unidentified blood meals (orange shading), the proportion of annelid and vertebrate hosts overall was significantly different between Ur. sapphirina and Ur. lowii (two-sided Fisher’s Exact Test; P < 0.001). Map created using data obtained from the Florida Geographic Data Library78
Field observations
To further confirm these results, we made field observations of Ur. sapphirina and Ur. lowii in Alachua Co., Florida. We observed and documented female Ur. sapphirina feeding on both oligochaete worms and freshwater leeches (Fig. 2) at the interface between terrestrial and aquatic ecosystems. Female Ur. sapphirina were observed probing the substrate with their proboscises, presumably in attempts to locate hosts (Supplementary Movie 1). While no Ur. lowii were observed feeding on annelids, we documented females feeding on both hylid and ranid frogs (Fig. 2).
Fig. 2.

Interactions between Uranotaenia, Corethrella, and hosts. a, b Female Uranotaenia sapphirina were observed, photographed, and filmed at River Styx, Alachua Co., Florida, USA. a Congregations of female mosquitoes were observed questing among and feeding from partially submerged Sparganophilus earthworms. b Female Uranotaenia sapphirina feeding from the leech Macrobdella ditetra; note reflexed labium and exposed stylets. c Females of Uranotaenia lowii and frog-biting midges (Corethrellidae; indicated by red arrows) feeding from Hyla squirella, Miami-Dade Co., Florida, USA
Discussion
Unlike any previously studied mosquito species, Ur. sapphirina in our sample fed exclusively on annelid hosts. This finding explains the inability of previous investigations to identify Ur. sapphirina blood meals, as these studies were performed under the assumption, and corresponding laboratory methodology, that female mosquitoes take blood meals only from vertebrate animals. Annelids and vertebrates share enclosed circulatory systems and either extracellular (annelids) or intracellular (vertebrates) hemoglobin, which in both groups causes the characteristic red coloration of the blood. The presence of red blood in the guts of Ur. sapphirina females likely contributed to the confusion related to host use of this mosquito by other researchers. Future studies may need to consider the possibility that mosquitoes fed on other types of invertebrates may not display the red gut normally used to classify a mosquito as blood engorged.
The recognition of Ur. sapphirina as a specialist of annelid, not vertebrate, host animals has important implications in mosquito ecology and evolution, and in the epidemiology of mosquito-vectored pathogens. This finding demonstrates that the range of potential mosquito hosts is considerably broader than previously indicated. Uranotaenia sapphirina is a common species throughout eastern North America, where mosquitoes have been under extensive study since their involvement in pathogen transmission was recognized in 1881 (ref. 1). The presumption that adult female mosquitoes blood feed only from vertebrate hosts10 is a source of bias in the methodological framework used to study mosquito ecology. Mosquito blood meals are identified primarily through methods that, by design, selectively react with only vertebrate antigens or DNA. For this reason, estimating the extent to which invertebrate hosts are utilized by female mosquitoes is not possible given previously available methods. In the laboratory, caged mosquitoes, including mammalophilic Aedes and Anopheles species have been documented locating and feeding on lepidopteran larvae in no-choice experiments, and subsequently, in some cases, to produce viable eggs32–35. Anecdotal records of mosquitoes feeding on cicada nymphs, mantids, chironomid midges, and lepidopteran pupae reported in the early 1900s by Howard36, 37 and occasionally referenced in the literature26, 38 have been disputed by Downes10 as mistaken identifications and include few substantive details. Beyond these laboratory experiments and historic records, there is no previous evidence that suggests that such interactions occur in nature, although any instance of invertebrate host use would not be detected by the traditional methods of blood meal analysis. Interestingly, for some mosquito and Corethrellidae (the culicomorph sister taxon to mosquitoes + Chaoboridae39) species, blood meal analyses that are effective in other species have failed, which may indicate that these species also feed upon hosts that cannot be detected using the vertebrate-based methodology40–43. As new sequencing technologies are applied to blood meal analysis, the ability to detect unexpected hosts should improve, particularly with the recognition of the potential for invertebrate feeding in mosquitoes.
Understanding the extent to which mosquitoes, particularly pathogen vectors, interact with invertebrate hosts has epidemiological implications. Uranotaenia sapphirina has been implicated as a potential vector for several arboviruses. Field-collected Ur. sapphirina have tested positive for Eastern equine encephalitis virus2, 4, 44, 45 and West Nile virus3. Our results suggest that Ur. sapphirina is unlikely to become infected with these viruses through feeding on vertebrate hosts. It is possible that by feeding on hematophagous leeches, which themselves often parasitize competent arbovirus hosts46, 47, Ur. sapphirina could act as kleptoparasites, acquiring virus-infected vertebrate blood meals from their leech hosts. Similarly, interactions between Ur. sapphirina and leeches may affect the transmission of pathogens vectored by leeches48. In previous studies30, 31, a small proportion of examined Ur. sapphirina blood meals were derived from snakes and the ranid frog Lithobates catesbeianus. Some snake species (e.g., Agkistrodon piscivorus, Nerodia spp.) and ranid frogs are common at the margins of vegetated waterways at night, a microhabitat where Ur. sapphirina females were observed feeding on annelid hosts. In these microhabitats, snakes and frogs would be available to host-seeking Ur. sapphirina females, and may be fed on incidentally. An alternative possibility for the detection of arboviruses in Ur. sapphirina is that this mosquito occasionally feeds on vertebrate hosts that may be competent for some arboviruses. The identification of two snake-derived Ur. sapphirina blood meals30 is particularly noteworthy, as the role of snakes in arbovirus persistence and transmission has been increasingly supported31, 49–52. Future studies should elucidate how Ur. sapphirina comes into contact with these viruses, and investigate the possibility that Ur. sapphirina could represent a complex of cryptic species with varying host use patterns.
Annelid host specialization by Ur. sapphirina raises important questions about the origin and evolution of blood feeding in mosquitoes. Foremost is whether feeding on invertebrates is an ancestral or derived trait. Divergence and radiation time estimates of Culicomorpha and vertebrate host lineages suggest that mosquitoes, or their culicomorph ancestors, adapted to vertebrate host groups after their diversification. The relationship between host and mosquito/Culicomorpha phylogenies has yet to be assessed, but other hematophagous insects have undergone stepwise transitions, with diversification of hematophagous insect lineages paralleling their host phylogenies53. Understanding how invertebrates factor into the evolution of host use by mosquitoes and other Culicomorpha will ultimately depend on a more complete accounting of mosquito host use patterns and the extent of invertebrate host use, and a well-resolved mosquito phylogeny. Until more information becomes available, understanding the origins and evolution of blood feeding in mosquitoes will remain speculative. However, the evolutionary history of Culicomorpha and host animals, and the host use patterns of basal mosquitoes may provide clues.
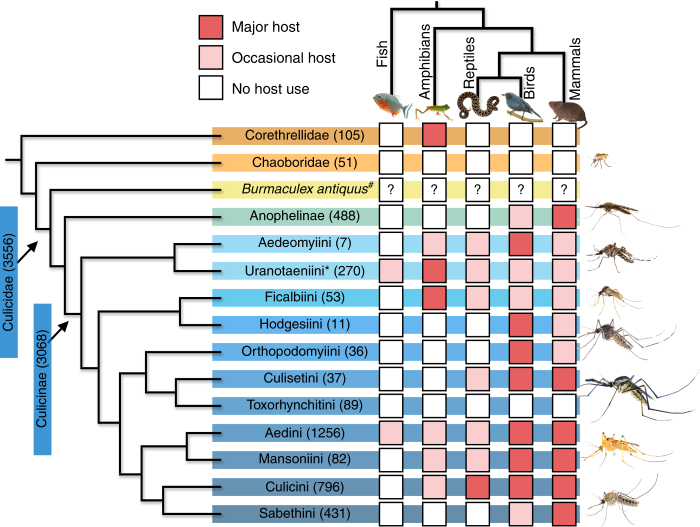
Birds and mammals are the major hosts of many modern mosquitoes, particularly among the more derived lineages (Fig. 3). However, it is unlikely that birds or mammals were the initial hosts of ancestral mosquitoes, as the earliest known fossil mosquito, Burmaculex antiquus54, precedes the diversification of birds55 and mammals56 by 30–40 million years. Modern frog-biting midges (Corethrellidae), sister to the mosquitoes + phantom midges (Chaoboridae)39, are known to feed only on anuran hosts (Fig. 3), and this association dates to the Lower Cretaceous57, pre-dating Burmaculex by 75 million years. The antiquity of anuran host use by Corethrella and the use of endothermic hosts by modern mosquitoes suggests a relationship between the vertebrate and mosquito phylogenies. However, this hypothesis is not supported by the basal placement of Anopheles, the human malaria vectors, that are generally considered specialists of mammalian hosts (Fig. 3). The split between Anophelinae and Culicinae is estimated at 45–126 million years before Burmaculex58, 59, suggesting either that mammal specialization in Anophelinae is not the ancestral trait, or that the basal placement of Anophelinae is incorrect. While phylogenetic analyses based on molecular data have not yet fully resolved deeper (genus-level) divisions within Culicidae26, they have estimated the time of divergence of Uranotaenia from other genera at >150 mya58. This event would have been concurrent with the diversification of major anuran groups60 and the first actual fossils of frog-biting midges57, and 50 million years older than Burmaculex. Uranotaenia, with its sister group Aedeomyia, is placed in a basal position within Culicinae58, 61 (Fig. 3), a diverse clade containing the majority of mosquito genera, implying an ancient origin for Uranotaenia. This might suggest that, if invertebrate feeding is the pleisiomorphic state, host affinities within Uranotaenia are indicative of early patterns of host use within Culicidae that were lost in other basal lineages (e.g., Anophelinae).
Fig. 3.
Patterns of blood-feeding on vertebrate host classes for Culicidae and related lineages (Corethrellidae and Chaoboridae). Composite image adapted using phylogeny from Harbach and Kitching61 and Borkent and Grimaldi54. Numbers in parentheses following mosquito taxa indicate number of species within each taxon14, 57, 79. Shading of squares indicates the degree to which mosquito or related lineages interact with vertebrate classes14, 15, 24, 27, 28, 30, 31, 40, 43, 54, 57, 62, 79–95. Uranotaenia sapphirina is contained within tribe Uranotaeniini*. All mosquito lineages are extant except Burmaculex antiquus#, the oldest known fossil mosquito, from the middle Cretaceous (110 mya). Burmaculex antiquus is presumed to have been a blood feeder, but had a moderately elongate proboscis and lacked the palpal sensillae that function as CO2 receptors for host location in modern mosquitoes54
The limited evidence available indicates that the earliest lineages of Culicomorpha fed on the body fluids (hemolymph) of the open circulatory systems of insects62. This trait is retained in multiple culicomorph families, including some extant chironomids and ceratopogonids, and mammalophilic Anopheles33 and Aedes32, 34 mosquitoes are able to locate insect hosts and utilize hemolymph to mature eggs. The closed circulatory systems of annelids, by contrast, are analogous to those of vertebrates, with phenotypically similar components, including heart(s), blood vessels, and hemoglobin63, 64. Annelid feeding may represent an evolutionary link between by an ancestral culicomorph feeding on free hemolymph in the body cavity and modern mosquitoes feeding on vertebrates with closed circulatory systems. While annelid circulatory systems are analogous to those of vertebrates, annelid immune systems and hemostatic responses are less sophisticated65, 66, suggesting that annelid feeding mosquitoes may encounter fewer defenses. In that context, annelid feeding may have primed the development of physiological, biochemical, behavioral, and morphological adaptations that would enable mosquitoes to eventually circumvent barriers to blood feeding from diverse vertebrate hosts, ultimately leading to the tremendous human health impacts caused by pathogen transmission in modern mosquitoes.
The use of annelid hosts in Ur. sapphirina alternatively could be a trait derived from frog feeding ancestors switching to worms and leeches that were encountered in habitats similar to anuran hosts. The phylogenetic position (Fig. 3) of the frog-biting midges, and their presumed ancient association with frogs57 may suggest that amphibians were the hosts of the common ancestor of Corethrellidae and Culicidae + Chaoboridae. However, a recent study of the hosts of Corethrella found that PCR assays successfully identified only <30% of blood meals, despite the use of primers that amplify a broad range of vertebrate hosts43. The authors of that study43 compared their low success rate with that of Ur. sapphirina from other published works, leading them to conclude that female Corethrella may feed on additional, undetermined hosts.
The function of annelid host use by Ur. sapphirina is not yet established, and the molecular analyses and field observations we report cannot discount the possibility that annelid host use by Ur. sapphirina could be a derived trait that evolved to serve a non-reproductive function, such as preventing dehydration or supplementing energetic reserves. Blood feeding by mosquitoes serves a function that is primarily reproductive: the females of most mosquito species require nutrients, particularly proteins, from host blood to provision developing eggs10, although blood-derived resources can also be diverted to meet metabolic needs67, 68 or in response to dehydration69. Dehydration can alter the behavior of mosquitoes by prompting them to increase host seeking and blood feeding69. Carbohydrates, obtained directly or indirectly from plants, serve primarily as a metabolic resource to both male and female mosquitoes, but can also enhance the reproductive potential of a female mosquito70. Our findings do not exclude the potential that female Ur. sapphirina utilize annelid blood as an energetic resource or a means of maintaining hydration; however, circumstantial evidence supports the idea that annelid feeding plays a reproductive role for this mosquito. For example, despite the collection of numerous male Ur. sapphirina, blood meals were only found in females in our samples. In addition, both males and females have been observed nectaring on flowers at field locations (L.E.R., personal observation), and collected engorged with nectar71. The details of egg production following annelid feeding, and the potential for annelid blood to serve a function other than egg maturation, needs to be explored by subsequent research to better understand the evolutionary context of these findings, as our data are limited to the identification of annelids as the hosts of Ur. sapphirina.
Specialization of Ur. sapphirina on annelid hosts demonstrates that the host breadth of mosquitoes is substantially broader than previously understood. Prior research on the host interactions of mosquitoes has centered around a minor subset of mosquito species, particularly the Aedes, Anopheles, and Culex pathogen vectors. For many genera, particularly those restricted to tropical regions, host use patterns have not been investigated, leaving substantial gaps in the understanding of mosquito–host relations. Combined with the large diversity of mosquitoes, there is potential that invertebrate host specialization extends beyond Ur. sapphirina. The fact that a common North American mosquito specializing on annelid hosts has gone undocumented as far as we are aware for more than a century suggests that invertebrate host use by mosquitoes is easily overlooked. Future work towards a more complete understanding of mosquito host use patterns should consider this possibility and, ideally, make use of novel molecular technologies that are compatible with the detection of invertebrate hosts.
Methods
Mosquito collections
Blood engorged Uranotaenia mosquitoes were collected between 28 September 2015 and 10 May 2017 at sites throughout the Florida Peninsula: Osceola National Forest (Columbia County), River Styx (Alachua County), Newnan’s Lake (Alachua County), the University of Florida Natural Area Teaching Lab (Alachua County), two sites at Blue Cypress Lake Conservation Area (Indian River County), and Everglades National Park (Miami-Dade County; Permit Number EVER-2017-SCI-0011). Mosquitoes were collected from natural resting sites (tree trunks, cypress knees, vegetation, exposed tree roots) using a battery-powered aspirator. Mosquitoes from Osceola National Forest, River Styx, Natural Area Teaching Lab, and Everglades National Park were killed in the field, promptly after collection, by exposure to ethyl-acetate-soaked plaster for approximately 10 min. Host DNA was immediately preserved in the field on Whatman Flinders Technology Associates (FTA) blood cards72, and taken to the Entomology and Nematology Department, University of Florida (Gainesville, Florida) for blood meal analysis. Mosquitoes from Indian River Co. were promptly transported to the laboratory (Florida Medical Entomology Laboratory, Vero Beach, Florida) inside polypropylene collecting cups (BioQuip), chilled on ice inside a cooler. Upon arrival, collecting cups were placed into a −20°C freezer for at least 20 min to kill mosquitoes. Mosquitoes were identified through morphological characters73. Blood-fed individuals were separated from others by visual inspection of the abdomen. DNA was extracted using the hot sodium hydroxide and tris (HotSHOT) method74 or DNEasy blood and tissue kit (Qiagen), following the manufacturer’s protocol.
PCR and DNA amplification
For each blood meal DNA sample, we attempted to amplify template DNA fragments in two independent PCRs targeting vertebrate or annelid DNA, respectively. One PCR was intended to screen for vertebrate DNA and used a vertebrate-specific primer set that amplifies a 664 bp template of the COI barcode region75. The primers RepCOI-F and RepCOI-R (Table 1) were used in 20 µL reactions each consisting 10 µL, 2× Apex Taq RED Mastermix (Genesee Scientific), 0.75 µL each primer (10 μM), 7.5 µL molecular grade water, and 1 µL DNA template. Thermocycler conditions followed a profile of 94 °C for 3 min, followed by 40 cycles of 94 °C for 40 s, 48.5 °C for 30 s, and 72 °C for 1 min, and a final extension step of 72 °C for 7 min. The other PCR was intended to screen for annelid DNA and used an annelid-specific primer set to amplify a 416 bp fragment of the annelid 28S ribosomal RNA. We used forward primer SPARG_C2_416 designed de novo to exclude co-amplification of Uranotaenia templates with reverse primer 28S_C4_R (Table 1)76. PCR reactions consisted of 12.5 μL InvitrogenTM Platinum Green Hot Start PCR 2× Master Mix kit plus 0.5 µL each primer (20 μM), 2.5 µL DNA template, 9 µL molecular grade water, and followed a thermal profile of 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 59.3 °C for 30 s, and 72 °C for 1 min, followed by a final extension step of 72 °C for 1 min. Negative controls were included in each set of amplification reactions and utilized sterile, double-distilled water in place of extracted DNA to monitor for contamination. PCR products were stained with ethidium bromide or SYBR™ Safe DNA Gel Stain (InvitrogenTM) and visualized under ultra-violet or blue light after electrophoresis on a 1.0% or 1.5% agarose gel. DNA ladder, 50 or 100 bp (InvitrogenTM), was used to determine the approximate fragment size of PCR products.
Table 1.
Primer sequences used to screen Uranotaenia blood meals for vertebrate and annelid DNA
| Primer name | Target | Sequence | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| RepCOI-F | Vertebrate COI | 5′-TNT TMT CAA CNA ACC ACA AAG A-3′ | 664 | 75 |
| RepCOI-R | Vertebrate COI | 5′-ACT TCT GGR TGK CCA AAR AAT CA-3′ | 664 | 75 |
| SPARG_C2_416_F | annelid 28S ribosomal RNA | 5′-ATC GGT CGG CAA CCT GTC CG-3′ | 416 | This study |
| 28S_C4_R | annelid 28S ribosomal RNA | 5′-TTC GAT TRG TCT TTC GCC CCT-3′ | 416 | 76 |
DNA sequencing and taxonomic identification
To confirm that the PCRs correctly detected annelid or vertebrate DNA, any PCR product that showed a band at the expected fragment size was submitted to the University of Florida Interdisciplinary Center for Biotechnology Research or Eurofins for Sanger sequencing on an ABI 3130® automated sequencer. Resulting sequence chromatograms were examined and edited for quality in the program Geneious® Version R10 (ref. 77). Unambiguous sequences were searched on the National Center for Biotechnology Information, GenBank database using the Basic Local Alignment Search Tool (BLAST). For COI sequences, species-level taxonomic identities were assigned to blood meals when a host sequence was >97% similar to a sequence referenced in the database. Some unambiguous sequences did not meet this threshold. In these cases, we used the BLAST function to align and compare blood meal sequences with reference sequences obtained from tissue samples of morphologically identified species and used the same >97% homologous criterion to identify host species. For 28S ribosomal RNA sequences, low taxonomic coverage for Annelida in the GenBank database prohibited the application of the 97% similarity criterion used for COI. We attributed 28S ribosomal RNA sequences to Annelida if the most similar reference sequence to an unambiguous high-quality host sequence was derived from an annelid species. We subsequently compared 28S ribosomal RNA blood meal sequences to reference sequences obtained from annelids collected at two Florida sites (River Styx, Alachua Co., Blue Cypress Lake, Indian River Co.). Worm specimens were captured by hand from muddy substrates where Ur. sapphirina mosquitoes had previously been observed. Worms were immediately placed in 95% ethanol and subsequently identified to species by M. Siddall using morphological and molecular markers.
Statistics
The proportion of blood meals derived from vertebrate hosts and annelid hosts (detected by PCR and confirmed by sequencing) for Ur. sapphirina and Ur. lowii was compared using Fisher’s Exact Test. This analysis was performed in the software R® Version 3.2.0 using the stats package. Results were considered significant if P < 0.05.
Data availability
Sequence data generated by this study have been deposited in the National Center for Biotechnology Information GenBank database (Accession Numbers MH384533-MH384601 for annelid host sequences and Accession Numbers MH384497-MH384532 for vertebrate hosts). All other relevant data supporting the findings of this study are within the paper and its Supplementary Files. Any further data or information are available from the corresponding author upon reasonable request.
Electronic supplementary material
Description of Additional Supplementary Uranotaenia sapphirina
Acknowledgements
We thank Mark Siddall for providing advice and assistance in identifying oligochaete worm specimens; Elizabeth Borda for assistance with identifying leeches; Daniel H. Shain and Shirley Lang for suggesting primers for annelid DNA amplification; Carolina Acevedo for assistance in blood meal identification; Lei Xiao for assistance in primer design; P.J. Walker and the National Park Service for assistance with and granting collection permits; and Kristin E. Sloyer for GIS assistance in creating figures. This material is based upon work supported by the National Science Foundation (http://www.nsf.gov) Graduate Research Fellowship under Grant No. 00107251 to L.E.R.
Author Contributions
N.D.B.C, C.J.H., P.E.K., J.L.G.K., A.Y.K., and L.E.R. conceived and designed the study. C.J.H., E.M.B., N.D.B.C., and L.E.R. collected mosquitoes from field sites throughout Florida, N.D.B.C. and L.E.R. obtained permits where necessary. A.Y.K., N.D.B.C., and L.E.R. designed laboratory protocols. P.E.K., and N.D.B.C. provided laboratory space, equipment, and supplies. C.J.H., L.E.R., and N.D.B.C. performed molecular analyses. N.D.B.C. photographed interactions between mosquitoes and frogs. L.E.R. designed primers for amplifying annelid DNA, photographed, and filmed interactions between mosquitoes and annelids, and drafted the initial version of the manuscript. All authors provided input and edits on the manuscript, and approved the final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s42003-018-0096-5.
References
- 1.Finlay C. The mosquito hypothetically considered as an agent in the transmission of yellow fever poison. New Orleans Med. Surg. J. 1881;9:601–616. [PMC free article] [PubMed] [Google Scholar]
- 2.Cupp EW, Klingler K, Hassan HK, Viguers LM, Unnasch TR. Transmission of Eastern equine encephalomyelitis virus in central Alabama. Am. J. Trop. Med. Hyg. 2003;68:495–500. [PMC free article] [PubMed] [Google Scholar]
- 3.Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999-2003. Vector Borne Zoonotic Dis. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong PM, Andreadis TG. Eastern equine encephalitis virus in mosquitoes and their role as bridge vectors. Emerg. Infect. Dis. 2010;16:1869–1874. doi: 10.3201/eid1612.100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkerson RC, et al. Making mosquito taxonomy useful: a stable classification of tribe Aedini that balances utility with current knowledge of evolutionary relationships. PLoS ONE. 2015;10:e0133602. doi: 10.1371/journal.pone.0133602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011;11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements, A. N. The Biology of Mosquitoes. Volume 3, Transmission of Viruses, and Interactions with Bacterial Symbionts (CAB International, Wallingford, 2012).
- 8.Dobson A, Fuofopoulos J. Emerging infectious pathogens of wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mans BJ. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J. Innate Immun. 2011;3:41–55. doi: 10.1159/000321599. [DOI] [PubMed] [Google Scholar]
- 10.Downes JA. The feeding habits of biting flies and their significance in classification. Annu. Rev. Entomol. 1958;3:249–266. doi: 10.1146/annurev.en.03.010158.001341. [DOI] [Google Scholar]
- 11.Ribeiro JMC, Mans BJ, Arcà B. An insight into the sialome of blood-feeding Nematocera. Insect Biochem. Mol. Biol. 2010;40:767–784. doi: 10.1016/j.ibmb.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didisheim P, Hattori K, Lewis JH. Hematologic and coagulation studies in various animal species. J. Lab. Clin. Med. 1959;53:866–875. [PubMed] [Google Scholar]
- 13.Lahondère C, Lazzari CR. Mosquitoes cool down during blood feeding to avoid overheating. Curr. Biol. 2012;22:40–45. doi: 10.1016/j.cub.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Harbach, R. E. Mosquito Taxonomic Inventory. http://mosquito-taxonomic-inventory.info/ (2013).
- 15.Steffan WA, Evenhuis NL. Biology of Toxorhynchites. Ann. Rev. Entomol. 1981;26:159–181. doi: 10.1146/annurev.en.26.010181.001111. [DOI] [Google Scholar]
- 16.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J. Trop. Med. Public Health. 2007;38:1–65. [PubMed] [Google Scholar]
- 17.Snell AE, Derraik JGB, McIntyre M. Maorigoeldia argyropus Walker (Diptera: Culicidae): is this another threatened endemic species? NZ Entomol. 2005;28:95–99. doi: 10.1080/00779962.2005.9722692. [DOI] [Google Scholar]
- 18.Foster, W. A. & Walker, E. D. in Medical and Veterinary Entomology, 2nd edn (eds Mullen, G. R. & Durden, L. A.) 207–259 (Academic Press, San Diego, 2009).
- 19.Tate P, Vincent M. The biology of autogenous and anautogenous races of Culex pipiens L. (Diptera: Culicidae) Parasitology. 1936;28:115–145. doi: 10.1017/S0031182000022319. [DOI] [Google Scholar]
- 20.Speilman A. Bionomics of autogenous mosquitoes. Annu. Rev. Entomol. 1971;16:231–248. doi: 10.1146/annurev.en.16.010171.001311. [DOI] [PubMed] [Google Scholar]
- 21.O’Meara GF, Edman JD. Autogenous egg production in the salt marsh mosquito, Aedes taeniorhynchus. Biol. Bull. 1975;149:384–396. doi: 10.2307/1540534. [DOI] [PubMed] [Google Scholar]
- 22.O’Meara GF. Variable expression of autogeny in three mosquito species. Int. J. Inver. Rep. Dev. 1979;1:253–261. doi: 10.1080/01651269.1979.10553321. [DOI] [Google Scholar]
- 23.Lounibos LP, Van Dover C, O’Meara GF. Fecundity, autogeny, and the larval environment of the pitcher-plant mosquito, Wyeomyia smithii. Oecologia. 1982;55:160–164. doi: 10.1007/BF00384482. [DOI] [PubMed] [Google Scholar]
- 24.Tempelis CH. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J. Med. Entomol. 1975;11:635–653. doi: 10.1093/jmedent/11.6.635. [DOI] [PubMed] [Google Scholar]
- 25.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harbach RE. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa. 2007;1668:591–638. [Google Scholar]
- 27.Christensen HA, de Vasquez AM, Boreham MM. Host-feeding patterns of mosquitoes (Diptera: Culicidae) from central Panama. Am. J. Trop. Med. Hyg. 1996;55:202–208. doi: 10.4269/ajtmh.1996.55.202. [DOI] [PubMed] [Google Scholar]
- 28.Toma T, Miyagi M, Tamashiro M. Blood meal identification and feeding habits of Uranotaenia species collected in the Ryukyu Archipelago. J. Am. Mosq. Control. Assoc. 2014;30:215–218. doi: 10.2987/14-6398R.1. [DOI] [PubMed] [Google Scholar]
- 29.Borkent A, Belton P. Attraction of female Uranotaenia lowii (Diptera: Culicidae) to frog calls in Costa Rica. Can. Entomol. 2006;138:91–94. doi: 10.4039/n04-113. [DOI] [Google Scholar]
- 30.Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J. Med. Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- 31.Cupp EW, et al. Identification of reptilian and amphibian blood meals from mosquitoes in an Eastern equine encephalomyelitis virus focus in central Alabama. Am. J. Trop. Med. Hyg. 2004;71:272–276. [PMC free article] [PubMed] [Google Scholar]
- 32.Harris P, Riordan DF, Cooke D. Mosquitoes feeding on insect larvae. Science. 1969;164:184–185. doi: 10.1126/science.164.3876.184. [DOI] [PubMed] [Google Scholar]
- 33.George J, Blanford S, Thomas MB, Baker TC. Malaria mosquitoes host-locate and feed on caterpillars. PLoS ONE. 2014;9:e108894. doi: 10.1371/journal.pone.0108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hribar LJ. Mosquitoes feeding on caterpillars of the Common Buckeye butterfly, Junonia coenia (Lepidoptera: Nymphalidae) J. Res. Lepid. 2014;47:45–48. [Google Scholar]
- 35.Martel V, Schlyter F, Ignell R, Hansson BS, Anderson P. Mosquito feeding affects larval behavior and development in a moth. PLoS ONE. 2011;6:e25658. doi: 10.1371/journal.pone.0025658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard, L. O. Mosquitoes. How They Live; How They Carry Disease; How They are Classified; How They May Be Destroyed (McClure, Phillips and Co., New York, 1901).
- 37.Howard, L. O., Dyar, H. G. & Knab, F. The Mosquitoes of North and Central America and the West Indies (Carnegie Institution of Washington, The Lord Baltimore Press, Baltimore, 1912).
- 38.Horsfall, W. E. Mosquitoes. Their Bionomics and Relation to Disease (Ronald Press Co., 1955).
- 39.Kutty, S. N., Wong, W. H., Meusemann, K., Meier, R. & Cranston, P. S. A phylogenomic analysis of Culicomorpha (Diptera) resolves the relationships among the eight constituent families. Syst. Entomol. 10.1111/syen.12285 (2018).
- 40.Boreham PFL, Chandler JA, Highton RB. Studies on the feeding patterns of mosquitoes of the genera Ficalbia, Mimomyia and Uranotaenia in the Kisumu area of Kenya. Bull. Entomol. Res. 1975;65:69–74. doi: 10.1017/S0007485300005770. [DOI] [Google Scholar]
- 41.Chandler JA, Boreham PFL, Highton RB, Hill MN. A study of the host selection patterns of the mosquitoes of the Kisumu area of Kenya. Trans. R. Soc. Trop. Med. Hyg. 1975;69:415–425. doi: 10.1016/0035-9203(75)90200-X. [DOI] [PubMed] [Google Scholar]
- 42.Fyodorova MV, et al. Evaluation of potential West Nile virus vectors in Volgograd Region, Russia, 2003 (Diptera: Culicidae): species composition, bloodmeal host utilization, and virus infection rates of mosquitoes. J. Med. Entomol. 2006;43:552–563. doi: 10.1093/jmedent/43.3.552. [DOI] [PubMed] [Google Scholar]
- 43.Camp JV, Irby WS. Molecular confirmation of frogs (Anura) as the hosts of Corethrellidae (Diptera) in the southeastern United States. J. Insect Sci. 2017;17:95. doi: 10.1093/jisesa/iex068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan HK, et al. Avian host preference by vectors of Eastern equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- 45.Molaei G, et al. Dynamics of vector-host interactions in avian communities in four Eastern equine encephalitis virus foci in the northeastern U.S. PLoS Negl. Trop. Dis. 2016;10:e0004347. doi: 10.1371/journal.pntd.0004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trauger DL, Batronek JC. Leech parasitism of waterfowl in North America. Wildfowl. 1977;28:143–152. [Google Scholar]
- 47.Krysko KL, et al. Nerodia fasciata, ectoparasites. Herpetol. Rev. 2012;43:347. [Google Scholar]
- 48.Kang JG, et al. Molecular detection of Bartonella spp. in terrestrial leeches (Haemadipsa rjukjuana) feeding on human and animal blood in Gageo-do, Republic of Korea. Parasit. Vectors. 2016;9:326. doi: 10.1186/s13071-016-1613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White G, Ottendorfer C, Graham S, Unnasch TR. Competency of reptiles and amphibians for Eastern equine encephalitis virus. Am. J. Trop. Med. Hyg. 2011;85:421–425. doi: 10.4269/ajtmh.2011.11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bingham AM, et al. Detection of Eastern equine encephalomyelitis virus RNA in North American snakes. Am. J. Trop. Med. Hyg. 2012;87:1140–1144. doi: 10.4269/ajtmh.2012.12-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham SP, et al. Serosurveillance of Eastern equine encephalitis virus in amphibians and reptiles from Alabama, USA. Am. J. Trop. Med. Hyg. 2012;86:540–544. doi: 10.4269/ajtmh.2012.11-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosco-Lauth AM, Hartwig AE, Bowen RA. Reptiles and amphibians as potential reservoir hosts of Chikungunya virus. Am. J. Trop. Med. Hyg. 2018;98:841–844. doi: 10.4269/ajtmh.17-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page RDM. Parallel phylogenies: reconstructing the history of host-parasite assemblages. Cladistics. 1998;10:155–173. doi: 10.1111/j.1096-0031.1994.tb00170.x. [DOI] [Google Scholar]
- 54.Borkent A, Grimaldi DA. The Cretaceous fossil Burmaculex antiquus confirmed as the earliest known lineage of mosquitoes (Diptera: Culicidae) Zootaxa. 2016;4079:457–466. doi: 10.11646/zootaxa.4079.4.5. [DOI] [PubMed] [Google Scholar]
- 55.Claramunt S, Cracraft J. A new time tree reveals Earth history’s imprint on the evolution of modern birds. Sci. Adv. 2015;1:e1501005. doi: 10.1126/sciadv.1501005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Leary MA, et al. The placental mammal ancestor and the Post-K-Pg radiation of placentals. Science. 2013;339:662–667. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- 57.Borkent A. The frog-biting midges of the world (Corethrellidae: Diptera) Zootaxa. 2008;1804:1–456. [Google Scholar]
- 58.Reidenbach KR, et al. Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol. Biol. 2009;9:298. doi: 10.1186/1471-2148-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krzywinski J, Grushko OG, Besanky NJ. Analysis of the complete mitochondrial DNA from Anopheles funestus: an improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol. Phylogenet. Evol. 2006;39:417–423. doi: 10.1016/j.ympev.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Biju SD, Bossuyt F. New frog family from India reveals an ancient biogeographical link with the Seychelles. Nature. 2003;425:711–714. doi: 10.1038/nature02019. [DOI] [PubMed] [Google Scholar]
- 61.Harbach RE, Kitching IJ. Phylogeny and classification of the Culicidae (Diptera) Syst. Entomol. 1998;23:327–370. doi: 10.1046/j.1365-3113.1998.00072.x. [DOI] [Google Scholar]
- 62.Borkent A. The pupae of Culicomorpha—morphology and a new phylogenetic tree. Zootaxa. 2012;3396:1–90. [Google Scholar]
- 63.Glomski CA, Tamburlin J. The phylogenetic odyssey of the erythrocyte. II. The early or invertebrate prototypes. Histol. Histopath. 1990;5:513–525. [PubMed] [Google Scholar]
- 64.Svoboda O, Bartunek P. Origins of the vertebrate erythro/megakaryocytic system. Biomed. Res. Int. 2015;2015:632171. doi: 10.1155/2015/632171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ratnoff OD. The evolution of hemostatic mechanisms. Perspect. Biol. Med. 1987;31:4–33. doi: 10.1353/pbm.1987.0003. [DOI] [PubMed] [Google Scholar]
- 66.Cooper EL, Kauschke E, Cossarizza A. Digging for innate immunity since Darwin and Metchnikoff. Bioessays. 2002;24:319–333. doi: 10.1002/bies.10077. [DOI] [PubMed] [Google Scholar]
- 67.Clements, A. N. The Biology of Mosquitoes. Volume 1, Development, Nutrition, and Reproduction (CAB International, 1992).
- 68.Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J. Med. Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- 69.Hagan RW, et al. Dehydration prompts increased activity and blood feeding by mosquitoes. Sci. Rep. 2018;8:6804. doi: 10.1038/s41598-018-24893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foster WA. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- 71.Bidlingmayer WL, Hem DG. Sugar feeding by Florida mosquitoes. Mosq. News. 1973;33:535–538. [Google Scholar]
- 72.Reeves LE, Holderman CJ, Gillett-Kaufman JL, Kawahara AY, Kaufman PE. Maintenance of host DNA integrity in field-preserved mosquito (Diptera: Culicidae) blood meals for identification by DNA barcoding. Parasit. Vectors. 2016;9:503. doi: 10.1186/s13071-016-1791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Darsie, R. F. & Morris, C. D. Keys to the Adult Female and Fourth Instar Larvae of the Mosquitoes of Florida (Diptera, Culicidae) (Florida Mosquito Control Association, Ft. Myers, 2000).
- 74.Truett GE, et al. Preparation of PCR quality mouse genomic DNA with sodium hydroxide and Tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 75.Nagy ZT, Sonet G, Glaw F, Vences M. First large-scale DNA barcoding assessment of reptiles in the biodiversity hotspot of Madagascar, based on newly designed COI primers. PLoS ONE. 2012;7:e34506. doi: 10.1371/journal.pone.0034506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lang SA, Saglam N, Kawash J, Shain DH. Punctuated invasion of water, ice, snow and terrestrial ecozones by segmented worms (Oligochaeta: Enchytraeidae: Mesenchytraeus) Proc. R. Soc. B. 2017;284:20171081. doi: 10.1098/rspb.2017.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.FGDL (Florida Geographic Data Library). University of Florida. https://www.fgdl.org/ (accessed 24 May 2018).
- 79.Borkent A. World catalog of extant and fossil Corethrellidae (Diptera) Zootaxa. 2014;3796:453–468. doi: 10.11646/zootaxa.3796.3.3. [DOI] [PubMed] [Google Scholar]
- 80.Borkent, A., World catalog of extant and fossil Chaoboridae (Diptera). Zootaxa 3796, 469–493 (2014). [DOI] [PubMed]
- 81.Logue K, et al. Unbiased characterization of Anopheles mosquito blood meals by targeted high-throughput sequencing. PLoS Negl. Trop. Dis. 2016;10:e0004512. doi: 10.1371/journal.pntd.0004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moreno M, et al. Intensive trapping of blood-fed Anopheles darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. PLoS Negl. Trop. Dis. 2017;11:e0005337. doi: 10.1371/journal.pntd.0005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Navia-Gine WG, Loaiza JR, Miller MJ. Mosquito-host interactions during and after an outbreak of equine viral encephalitis in eastern Panama. PLoS ONE. 2013;8:e81788. doi: 10.1371/journal.pone.0081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aitken TH. The canopy frequenting mosquitoes of Bush Forest, Trinidad, West Indies. Atas Do Simpósio Sobre a Biota Amaz. 1967;6:65–73. [Google Scholar]
- 85.Toma T, et al. Bionomics of the mud lobster-hole mosquito Aedes (Geoskusea) baisasi in the mangrove swamps of the Ryukyu Archipelago, Japan. J. Am. Mosq. Control. Assoc. 2011;27:207–216. doi: 10.2987/11-6123.1. [DOI] [PubMed] [Google Scholar]
- 86.Tamashiro M, Toma T, Mannen K, Higa Y, Miyagi I. Bloodmeal identification and feeding habits of mosquitoes (Diptera: Culicidae) collected at five islands in the Ryukyu Archipelago, Japan. Med. Entomol. Zool. 2011;62:53–70. doi: 10.7601/mez.62.53. [DOI] [Google Scholar]
- 87.Braima KA, et al. Feeding behavior of Mimomyia (Etorleptiomyia) luzonensis (Ludlow, 1905) (Diptera, Culicidae) in Peninsular Malaysia. Acta Trop. 2017;171:138–140. doi: 10.1016/j.actatropica.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 88.Haddow AJ, Ssenkubuge Y. Studies on the biting habits of East African mosquitos in the genera Uranotaenia, Ficalbia and Hodgesia. Bull. Entomol. Res. 1963;53:639–652. doi: 10.1017/S0007485300048380. [DOI] [Google Scholar]
- 89.Williams MC. Studies on mosquitos (Diptera, Culicidae) biting birds, using twenty-four-hour catches, in the Entebbe area, Uganda. Bull. Entomol. Res. 1963;54:407–424. doi: 10.1017/S0007485300048914. [DOI] [Google Scholar]
- 90.Zavortink TJ. Mosquito studies (Diptera, Culicidae) VIII. A prodrome of the genus Orthopodomyia. Contrib. Am. Entomol. Inst. 1968;3:1–221. [Google Scholar]
- 91.Blosser EM, et al. Environmental drivers of seasonal patterns of host utilization by Culiseta melanura (Diptera: Culicidae) in Florida. J. Med. Entomol. 2017;54:1365–1374. doi: 10.1093/jme/tjx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maslov A. V. Blood-Sucking Mosquitoes of the Subtribe Culisetina (Diptera, Culicidae) in World Fauna (Smithsonian Institution Libraries and National Science Foundation, Washington D.C., 1987).
- 93.Okudo H, et al. A crab-hole mosquito, Aedes baisasi, feeding on mudskipper (Gobiidae: Oxudercinae) in the Ryukyu Islands, Japan. J. Am. Mosq. Control Assoc. 2004;20:134–137. [PubMed] [Google Scholar]
- 94.Crans WJ, Rockel EG. The mosquitoes attracted to turtles. Mosq. News. 1968;28:332–337. [Google Scholar]
- 95.De Castro Gomes A, et al. Ecologia de Haemagogus e Sabethes (Diptera: Culicidae) em áreas epizoóticas do vírus da febre amarela, Rio Grande do Sul, Brasil. Epidemiol. Serv. Saúde. 2010;19:101–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Uranotaenia sapphirina
Data Availability Statement
Sequence data generated by this study have been deposited in the National Center for Biotechnology Information GenBank database (Accession Numbers MH384533-MH384601 for annelid host sequences and Accession Numbers MH384497-MH384532 for vertebrate hosts). All other relevant data supporting the findings of this study are within the paper and its Supplementary Files. Any further data or information are available from the corresponding author upon reasonable request.