Summary
Background:
The development of neutralizing anti-factor VIII (fVIII) antibodies remains a challenging complication of modern hemophilia A care. In vitro assays are the primary method used for quantifying inhibitor titers, predicting bleeding risk, and determining bleeding management. However, other mechanisms of inhibition are not accounted for in these assays, which may result in discrepancies between the inhibitor titer and clinical bleeding symptoms.
Objectives:
We evaluated fVIII clearance in vivo as a potential mechanism for antibody pathogenicity and whether increased fVIII dosing regimens corrected the associated bleeding phenotype.
Methods:
fVIII−/− or fVIII−/−/von Willebrand factor (VWF)−/− mice were infused with anti-fVIII monoclonal antibodies (MAbs) directed against fVIII C1, C2, or A2 domains followed by infusion of fVIII. Blood loss via the tail snip bleeding model, fVIII activity, and fVIII antigen levels were subsequently measured.
Results
Pathogenic anti-C1 MAbs that compete with VWF for fVIII binding increased clearance of fVIII/MAb complexes in fVIII−/− mice but not fVIII−/−/VWF−/− mice. Additionally, pathogenic anti-C2 MAbs that inhibit fVIII binding to VWF increased fVIII clearance in fVIII−/− mice. Anti-C1, C2, and A2 MAbs that do not inhibit VWF binding did not accelerate fVIII clearance. Infusion of increased doses of fVIII in the presence of anti-C1 MAbs partially corrected blood loss in fVIII−/− mice.
Conclusions
A subset of antibodies that inhibit VWF binding to fVIII increase clearance of fVIII/MAb complexes, which contributes to antibody pathogenicity. This may explain differences in the bleeding phenotype observed despite factor replacement in some patients with hemophilia A and low titer inhibitors.
Keywords: antibody, factor VIII, hemophilia A, inhibitors, von Willebrand factor
Introduction
Hemophilia A is an X-linked congenital bleeding disorder defined by a deficiency or absence of coagulation protein factor VIII (fVIII). Individuals with this disorder may present with spontaneous or trauma induced hemarthrosis, muscle bleeds, or intracranial hemorrhage [1, 2]. A complication of treatment is the development of neutralizing allo-antibodies (i.e. inhibitors) that occur in approximately 30% of patients with severe hemophilia A [3–5]. The most widely used method for detecting an inhibitor and predicting bleeding risk is the Bethesda assay. The Bethesda assay is an in vitro one-stage clot-based assay that quantifies the inhibition of fVIII procoagulant activity by antibodies in inhibitor plasma [6–8]. However, the Bethesda assay has limitations [9–11]. The Bethesda assay does not account for a diverse spectrum of multi-domain inhibitory and non-inhibitory anti-fVIII antibodies [7, 12, 13] or the effect of these antibodies on fVIII binding to von Willebrand factor (VWF), activation of fVIII by thrombin, or fVIII clearance. For instance, we have described that the Bethesda assay did not predict pathogenicity or response to fVIII in a subset of high titer anti-A2 and C2 antibodies [14, 15]. Additionally, antibodies that are characterized as low titer inhibitors in the Bethesda assay may accelerate the clearance of fVIII. This can result in discrepancies between the inhibitor titer, the fVIII pharmacokinetic profile in the presence of an inhibitor, and the clinical bleeding phenotype.
In patients with hemophilia A and low titer inhibitors, defined as a titer <5 Bethesda Units (BU)/mL, infusion of high doses of fVIII ranging from 50–200 units/kg is a strategy often utilized to prevent and treat bleeding symptoms [16, 17]. However, there are anecdotal reports of poor responses to fVIII in patients with low titer inhibitors despite high dose or frequently dosed factor regimens. In some cases, these patients have required immune tolerance induction (ITI) for inhibitor eradication or a bypassing agent to treat bleeding symptoms. In our previous work characterizing antibodies directed against the C1 domain of fVIII using a hemophilia A murine model, we found that weakly inhibitory antibodies induced bleeding in the tail snip bleeding model after fVIII replacement [18]. Low titer anti-C1 monoclonal antibodies (MAbs), specifically MAbs 2A9 and M6143, not only showed weak inhibition of fVIII but also incomplete (type 2) inhibition at saturating concentrations in the Bethesda assay. The pathogenicity of weakly inhibitory anti-C1 MAbs was hypothesized to result from increased clearance of the fVIII/MAb complex due to inhibition of fVIII binding to VWF. The characteristics of these antibodies may explain the discordant inhibitor titers obtained by the Bethesda assay and tail snip bleeding phenotype in hemophilia A mice.
In this study, we evaluated the contribution of fVIII clearance on bleeding phenotype in fVIII and fVIII/VWF null mice. We postulate that increased clearance of the fVIII/MAb complex due to antibody disruption of the fVIII and VWF binding interaction is an important determinant of the pathogenicity of anti-fVIII antibodies.
Materials and Methods
Materials
Murine anti-human anti-C1, C2 and A2 MAbs were purified from anti-fVIII hybridomas as previously described [19, 20]. The human-derived anti-C1 MAb KM33 was previously received as a gift from Dr. Jan Voorberg. The characteristics of MAbs used in this study are summarized in Table 1. Citrated pooled normal plasma (FACT) and fVIII deficient plasma were purchased from George King Biomedical (Overland Park, KS). B-domain deleted fVIII was expressed and purified as previously described [21–23]. All other materials were reagent grade or are described in the cited literature.
Table 1.
Summary of anti-fVIII MAbs characteristics
| MAb | Domain | Bethesda Titer (BU/mg IgG) |
VWF IC50 (µg/mL) |
PL IC50 (µg/mL) |
|---|---|---|---|---|
| 4A4 | A2 | 40000 | – | – |
| 2A9 | C1 | 23 | 1.1 | 0.9 |
| F156 | C1 | 7 | >10 | >10 |
| M6143 | C1 | 180 | 0.6 | >10 |
| KM33 | C1 | 3700 | 0.03 | 0.03 |
| B136 | C1 | 700 | 0.4 | 0.04 |
| 3D12 | C2 | 2600 | 0.04 | 0.05 |
| I109 | C2 | 1500 | 0.04 | 0.07 |
| 2–117 | C2 | <0.4 | >10 | >10 |
IC50: Concentration producing 50% inhibition of binding
PL: Phospholipid
Mice
Exon 16 disrupted hemophilia A mice (fVIII−/− mice) on a mixed C57BL/6 and 129S4 background were originally obtained from Leon Hoyer (American Red Cross, Holland Laboratory). FVIII/VWF double knockout mice (fVIII−/−/VWF−/− mice) were generated by crossing fVIII−/− mice with VWF−/− mice on a C57BL/6 background originally obtained as a generous gift from Denisa Wagner [24]. Approval for the use of animals and study methods was granted by the Emory University Institutional Animal Care and Use Committee (IACUC). The Emory University School of Medicine Division of Animal Resources (DAR) provided training for the proper handling and euthanasia of animals.
Mouse tail snip bleeding model
The in vivo tail snip bleeding model was utilized to determine bleeding phenotype in mice as previously described with minor modifications [14, 15]. Briefly, 8–12 week old mice received 100 µl injections of anti-C1 MAbs at a concentration of 0.5 mg/kg (65 nM estimated peak plasma concentration) or normal saline, followed by 180 or 360 units/kg fVIII (2.5 or 5 nM estimated peak plasma concentration, respectively) 15 minutes later. The 180 units/kg dose was chosen as “low dose” fVIII as this is the minimum dose necessary to correct the bleeding phenotype in the majority of fVIII−/− mice without inhibitors. Tails were warmed then transected at 4 mm distally two hours after fVIII injection. Blood loss per mouse body weight (mg/g) following tail snips was measured using a pre-weighed 15-mL conical tube containing normal saline. Predicted peak inhibitor titers of antibodies used in this model were calculated using the Bethesda titer, MAb concentration, and estimated mouse plasma volume per average mouse weight.
FVIII activity and antigen levels
FVIII activity and antigen levels were measured to evaluate fVIII clearance as previously described [18]. Briefly, mice were euthanized 15 minutes following fVIII injections and plasma samples collected by cardiac puncture. FVIII antigen levels were determined by ELISA as follows: anti-A1 MAb 2–116 (6 µg/mL) in phosphate buffered saline (PBS)/0.05% sodium azide was coated on ELISA plates, incubated overnight at 4°C, then blocked. Serial dilutions of mice plasma samples in 2-mercaptoethanol (90 mM) were prepared on a polypropylene plate and transferred to ELISA plates. FVIII, diluted to 1 µg/ml in fVIII deficient plasma, was serially diluted in 2-mercaptoethanol on a separate polypropylene plate and transferred to the ELISA plates to construct a standard curve. FVIII was detected by biotinylated anti-A2 MAb 2–76 (non-competing for anti-C1 MAbs) for anti-C1 MAbs or anti-C1 MAb B136 (non-competing for anti-C2 or anti-A2 MAbs) for anti-C2 or anti-A2 MAbs and alkaline phosphate-conjugated streptavidin. No significant differences were noted for standard curves using different MAbs for detection. FVIII antigen levels in mice plasmas were quantitated using the standard curve. FVIII procoagulant activity was measured using the activated partial thromboplastin reagent-based one-stage coagulation assay as previously described [25] and pooled fVIII−/− mouse plasma spiked with fVIII to 1 U/mL for the standard curve.
Statistical Analysis
Data are presented as medians with interquartile ranges (IQR) for blood loss in the tail snip bleeding model or as means with standard deviations (SD) for fVIII activity and antigen levels. Significant differences in blood loss in the tail snip bleeding model were determined by the Mann-Whitney U-test and in fVIII activity and antigen levels by the Kruskal-Wallis test with Dunn’s correction for multiple comparisons. The relationship between median blood loss and predicted peak inhibitor titer was determined by Pearson’s correlation coefficient. Statistical analyses were performed using Prism 6.0 (GraphPad Software Inc., La Jolla, CA). Differences in fVIII antigen levels in fVIII−/− mice that received anti-A2 or anti-C2 MAbs compared to no MAb control starting at 30 minutes and concluding at 8 hours was determined using non-linear regression to fit exponential decay models and the Sum of Squares Reduction test with Bonferroni method for multiple comparisons (significance level α = 0.007). A p value < 0.05 was considered statistically significant and is denoted as * p < 0.05, ** p < 0.01, or *** p < 0.001 unless stated otherwise.
Results
High-dose fVIII replacement partially reduces bleeding in hemophilia A mice infused with anti-C1 MAbs
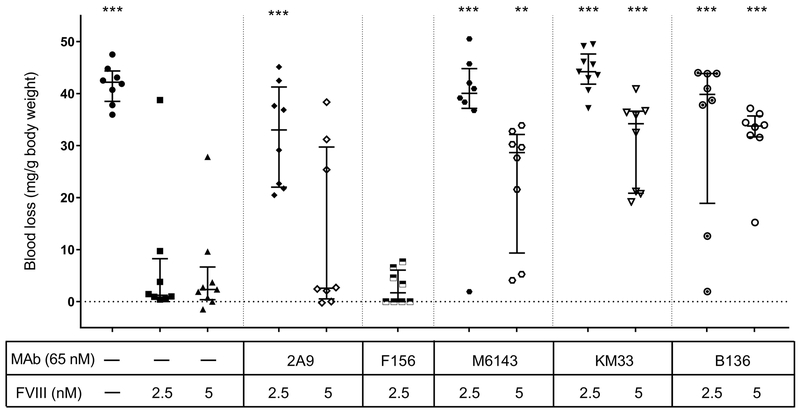
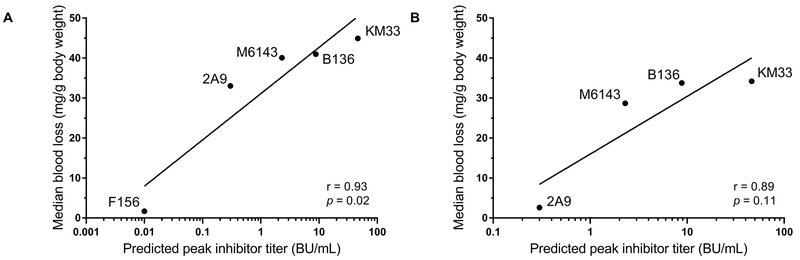
We have previously reported that anti-C1 MAbs 2A9, M6143, B136, and KM33 are pathogenic in a murine model [18]. In this model, the ability of fVIII to correct excessive bleeding following tail snip in fVIII−/− mice is tested in mice that have been injected with anti-fVIII MAbs. The inhibitory activity in the Bethesda assay of these MAbs ranged from weak to strong (23 to 3700 BU/mg) (Table 1). All four antibodies inhibit the binding of fVIII to VWF (Table 1). Previous studies have reported that abnormal bleeding produced by some inhibitory anti-A2 and anti-C2 MAbs could be overcome by increasing the fVIII dose [14, 15]. To determine whether abnormal bleeding could be corrected in mice injected with anti-C1 MAbs, blood loss was measured in the tail snip bleeding model in fVIII−/− mice following injection of “high dose” fVIII at 360 units/kg (estimated peak plasma concentration 5 nM) or “low dose” fVIII at 180 units/kg (estimated peak plasma concentration 2.5 nM) (Fig. 1). Injection of high dose fVIII did not alter the bleeding phenotype in mice injected with strongly inhibitory anti-C1 MAbs KM33 and B136 (median blood loss 34.2 and 33.8 mg/g and predicted peak plasma inhibitor titers of 46.3 and 8.8 BU/mL, respectively). Compared to fVIII−/− mice that received 180 units/kg fVIII alone, high dose 360 units/kg fVIII corrected the bleeding phenotype in mice that received weakly inhibitory MAb 2A9 (median blood loss 2.6 mg/g, estimated plasma inhibitor titer <1 BU/mL) but not MAb M6143 (median blood loss 28.7 mg/g, estimated plasma inhibitor titer 2.3 BU/mL). Notably, mice that received 180 units/kg fVIII bled in response to infusions of MAb 2A9 that produced undetectable inhibitor levels. MAb F156, a weakly inhibitory anti-C1 MAb (Table 1) was not pathogenic at low dose fVIII and thus not tested at high dose fVIII. Incomplete correction of blood loss in fVIII−/− mice was positively correlated with the predicted peak inhibitor titer for anti-C1 MAbs at low dose and high dose fVIII (Fig. 2A, B) although the trend with high dose fVIII did not reach statistical significance. These results may be due to the combined presence of antibodies that inhibit fVIII function and weakly inhibitory antibodies that accelerate fVIII clearance.
Figure 1. High dose fVIII infusion after injection of anti-C1 MAbs partially corrects the bleeding phenotype in fVIII−/− mice.
Blood loss per gram body weight was measured using the tail snip bleeding model in fVIII−/− mice. Mice were injected with 0.5 mg/kg (estimated peak plasma concentration of 65 nM) of anti-C1 MAbs 2A9, M6143, KM33, or B136, followed 15 minutes later by “low dose” 180 units/kg or “high dose” 360 units/kg fVIII (estimated peak plasma concentrations of 2.5 nM or 5 nM, respectively). Tails snips were performed at two hours after injection of fVIII and blood loss was measured for 40 minutes or until mouse death. Data are presented as medians with IQRs (n = 8–9 mice per group). Blood loss for each group was compared to fVIII−/− mice that received 2.5 nM fVIII in the absence of antibody using the Mann-Whitney U test, * p < 0.05, ** p < 0.01, or *** p < 0.001.
Figure 2. Incomplete correction of blood loss in fVIII−/− mice and correlation with anti-C1 MAbs predicted inhibitor titer.
Correlation of predicted peak anti-C1 MAb inhibitor titers with median blood loss in fVIII−/− mice receiving “low dose” 180 units/kg fVIII (A) or “high dose” 360 units/kg fVIII (B) in the tail snip bleeding model is shown. Blood loss in mice injected with anti-C1 MAb F156 was not tested at high dose fVIII due to lack of bleeding at low dose fVIII. The relationship between median blood loss and predicted peak inhibitor titer was determined by Pearson’s correlation coefficient.
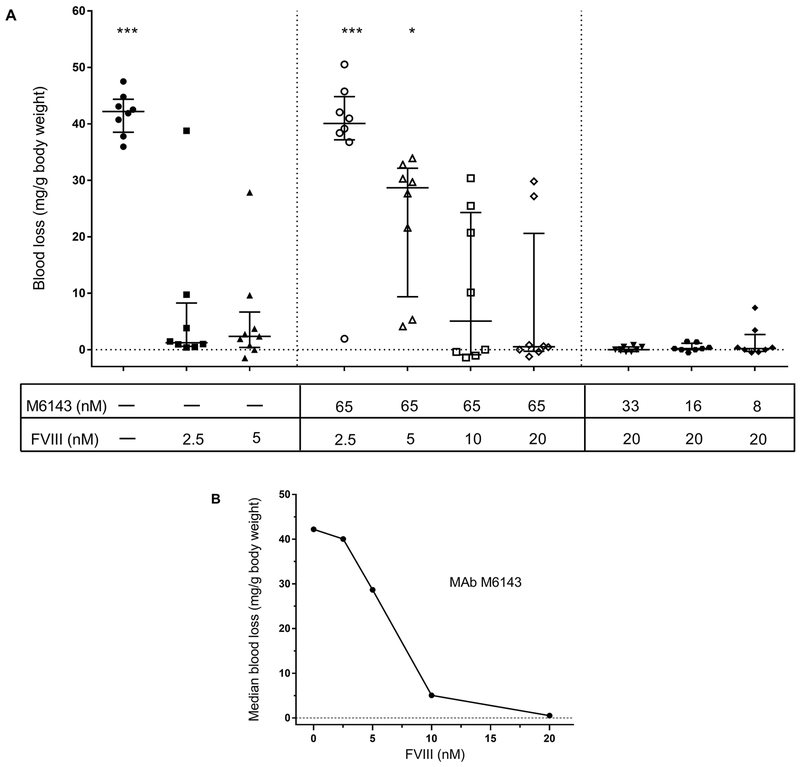
Very high doses of fVIII are required to reduce bleeding in fVIII−/− mice given the weakly inhibitory anti-C1 MAb M6143
High dose fVIII did not correct the blood loss in fVIII−/− mice that received the weakly inhibitory MAb M6143 compared to mice that received low dose fVIII in the absence of antibody, although there was a trend towards reduction (Fig. 1). To determine the fVIII concentration necessary for complete correction of bleeding, fVIII−/−mice receiving MAb M6143 were injected with increasing doses of fVIII. Less bleeding was observed in mice that received 720 units/kg or 1440 units/kg fVIII, producing estimated peak plasma concentrations of 10 and 20 nM, respectively (Fig. 3A). Despite an estimated peak MAb plasma concentration of 65 nM, there was a dose-dependent decrease in blood loss with increasing fVIII dosing (Fig. 3B). MAb M6143 is a high-affinity antibody with a dissociation constant for binding to fVIII of 0.2 nM [18]. Thus, the in vivo molar excess of MAb M6143 over fVIII would not predict the reduction in bleeding that was observed at sub-stoichiometric concentrations of fVIII.
Figure 3. Supraphysiologic fVIII replacement is required to reduce bleeding in fVIII−/− mice infused with weakly inhibitory anti-C1 MAb M6143.
(A) Blood loss was measured in fVIII−/− mice infused with increasing doses of fVIII (180, 360, 720, or 1440 units/kg, corresponding to estimated peak plasma concentrations of 2.5, 5, 10, or 20 nM, respectively) or decreasing doses of MAb M6143 (0.5, 0.25, 0.13, or 0.06 mg/kg; corresponding to estimated peak plasma concentration of 65, 33, 16, or 8 nM, respectively). Data are presented as medians with IQR (n = 8 mice per group). Blood loss for each group was compared to fVIII−/− mice that received 2.5 nM fVIII alone using the Mann-Whitney U test, * p < 0.05, ** p < 0.01, or *** p < 0.001. (B) Concentration-dependent decrease in median blood loss in the tail snip bleeding model in fVIII−/− mice injected with 0.5 mg/kg (estimated peak plasma concentration 65 nM) MAb M6143 and increasing doses of fVIII.
Anti-C1 MAbs do not accelerate fVIII clearance in fVIII−/−/VWF−/− mice
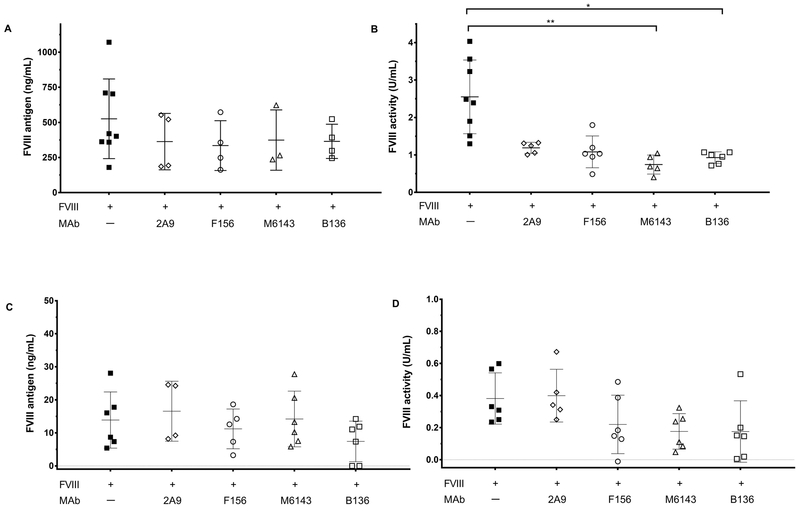
The efficacy of sub-stoichiometric concentrations of fVIII observed in MAb M6143-treated mice suggest that VWF may compete with some anti-fVIII MAbs for binding to fVIII in vivo and limit their pathogenicity. VWF is critical in limiting rapid fVIII clearance by acting as a carrier protein for fVIII. The inhibition of fVIII binding to VWF by anti-C1 MAbs observed in vitro predicts that the increased clearance they produce in fVIII−/− mice [18] would not be observed in fVIII−/−/VWF−/− mice. Thus, we proposed that the pathogenicity of anti-C1 antibodies may be partially or entirely due to inhibition of fVIII binding to VWF. Accordingly, fVIII activity and antigen levels were measured in fVIII−/−/VWF−/− mice following injection of anti-C1 MAbs 2A9, M6143, and B136. Additionally, we evaluated MAb F156, which weakly inhibits fVIII in the Bethesda assay, but does not inhibit fVIII binding to VWF in vitro, increase clearance of fVIII, or produce increased bleeding in the tail snip bleeding model [18].
A notable difference between fVIII−/−/VWF−/− and fVIII−/− mice is the increased dose of fVIII required for hemostatic efficacy in fVIII−/−/VWF−/− mice. Additionally, fVIII antigen levels 15 minutes after injection are reduced in fVIII−/−/VWF−/− mice compared to fVIII−/− mice (data not shown). A fVIII dose of 540 units/kg was used in fVIII−/−/VWF−/− mice instead of 180 units/kg used in our previous study to measure fVIII activity and antigen levels based on the minimum fVIII dose necessary to prevent bleeding in fVIII−/−/VWF−/− mice in the tail snip bleeding model. To account for an increased clearance rate of fVIII in fVIII−/−/VWF−/− mice, we measured fVIII activity and antigen levels at 15 minutes instead of the previously used 40 minutes [18]. Under these conditions there were no significant differences in fVIII antigen levels between groups. However fVIII activity was significantly reduced for MAb groups M6143 and B136 compared to mice that received fVIII alone (Fig. 4A, B). There was also a trend towards a reduction in fVIII activity in the MAb 2A9 group (Fig. 4B) and in the M6143 and B136 groups given 180 units/kg fVIII (Fig. 4D). Carryover of the inhibitory activity of these antibodies in the fVIII activity assay may account for this discrepancy. However because of the relatively large intra-group variation in the fVIII antigen assays (Fig. 4A), the possibility that M6143 and B136 increase the clearance of high dose fVIII in fVIII−/−/VWF−/− mice cannot be excluded.
Figure 4. Anti-C1 MAbs do not accelerate fVIII clearance in fVIII−/−/VWF−/− mice.
(A) FVIII antigen levels and (B) activity in fVIII−/−/VWF−/− mice after infusion of 0.5 mg/kg anti-C1 MAb 2A9, F156, M6143, or B136 and 540 units/kg fVIII (estimated peak plasma concentration 7.5 nM) are shown. (C) FVIII antigen levels and (D) activity in fVIII−/−/VWF−/− mice after 0.5 mg/kg anti-C1 MAb and 180 units/kg of fVIII (estimated peak plasma concentration 2.5 nM) are also shown. Plasma samples were sampled 15 minutes after fVIII injection. FVIII activity data are normalized to normal saline only control group due to background fVIII activity of 0.12 U/mL in mouse plasmas detected in the one-stage clotting assay, which is attributed to increased thrombin generation secondary to compensatory elevated factor VII levels in fVIII−/− and fVIII−/−/VWF−/− mice (unpublished data). Data are presented as mean with SD for fVIII antigen levels and activity (n = 3–8 mice per group). FVIII antigen levels and activities for each MAb group was compared to the fVIII only group using the Kruskal-Wallis tests with Dunn’s correction for multiple comparisons, * p < 0.05, ** p < 0.01, or *** p < 0.001.
One of the challenges encountered in measuring fVIII levels following infusion of 540 units/kg fVIII in fVIII−/−/VWF−/− mice was clotting of samples despite addition of sodium citrate in the collection tubes. To reduce excess sample clotting and compare fVIII levels in fVIII−/−/VWF−/− mice to fVIII−/− mice, the fVIII antigen levels and activities were measured in fVIII−/−/VWF−/− mice following injection of our standard 180 units/kg fVIII. There were no significant differences in the concomitant reduction in fVIII antigen levels and activity in fVIII−/−/VWF−/− mice (Fig. 4C, D). The finding that anti-C1 MAbs 2A9, M6143, and B136 accelerate clearance of fVIII antigen in fVIII−/− mice [18], but not fVIII−/−/VWF−/− mice (Figs. 4A, C) indicate that there are differences in fVIII clearance mechanisms dependent on VWF that could affect the bleeding phenotype induced by anti-fVIII antibodies.
Anti-fVIII MAbs directed against non-C1 domain VWF epitopes increase fVIII clearance in fVIII−/− mice
Previously we investigated the in vivo pathogenicity of antibodies to the A2 and C2 domains of fVIII [14, 15]. A subset of anti-A2 and C2 MAbs produced bleeding in fVIII−/− mice at low and high doses of fVIII (180 units/kg and 360 units/kg, respectively) in the tail snip bleeding model. The in vivo pathogenicity of these MAbs was attributed to their inhibition of fVIII activity. Antibodies that inhibit the binding of fVIII to phospholipid inhibit fVIII procoagulant activity in vitro and produce a positive Bethesda titer. In contrast, antibodies that inhibit the binding of fVIII to VWF but not phospholipid do not necessarily inhibit fVIII procoagulant activity in vitro. The in vitro properties of these antibodies on anti-fVIII activity and inhibition of fVIII binding to VWF and phospholipid are summarized in Table 1. Given the role of increased fVIII clearance produced by anti-C1 MAbs [18], we investigated the effect of a subset of anti-A2 and C2 antibodies directed against immunodominant epitopes in human fVIII.
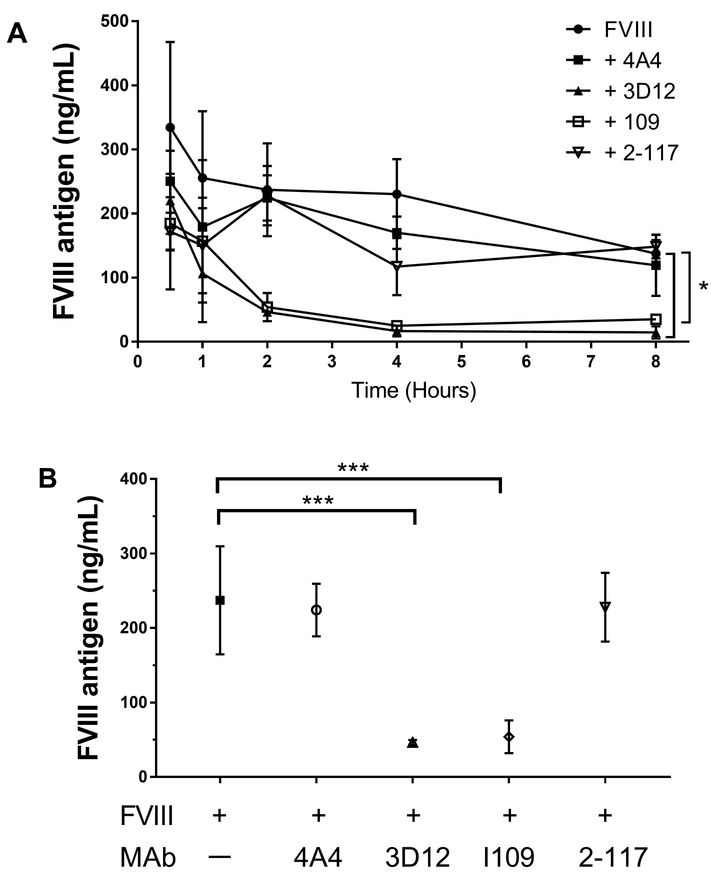
We measured plasma fVIII antigen levels at 0.5, 1, 2, 4 and 8 hours in fVIII−/− mice following injection of anti-A2 MAb 4A4 or anti-C2 MAbs 3D12, I109, or 2–117. Anti-C2 MAbs 3D12 and I109, which inhibit the binding of fVIII to VWF and phospholipid, significantly increased fVIII clearance over 8 hours compared to mice that received fVIII alone (Fig. 5A, B). However, strongly inhibitory anti-A2 MAb 4A4 and non-inhibitory anti-C2 domain MAb 2–117, neither of which inhibit fVIII binding to VWF or phospholipid, did not affect fVIII clearance. Additionally, 2 non-inhibitory anti-B MAbs that do not affect fVIII binding to VWF or phospholipid had no effect on fVIII clearance (data not shown). This suggests that the pathogenicity of both low and high titer anti-fVIII antibodies that compete with VWF for fVIII binding may be due, in part, to increased fVIII clearance.
Figure 5. Pathogenic anti-C2 antibodies, but not anti-A2 antibodies, increase fVIII clearance in fVIII−/− mice.
(A) FVIII antigen levels were measured at 0.5, 1, 2, 4, and 8 hours after infusion of ~0.5 mg/kg anti-fVIII MAbs 4A4 (anti-A2 MAb), 3D12 (anti-C2 MAb), I109 (anti-C2 MAb), and 2–117 (anti-C2 MAb) followed by ~180 units/kg fVIII in fVIII−/− mice. Differences in fVIII antigen levels in MAb groups 4A4 (p = 0.62), 3D12 (p <0.001), I109 (p <0.001), or 2–117 (p = 0.26) compared to no MAb controls starting at 30 minutes and concluding at 8 hours was determined using non-linear regression to fit exponential decay models and the Sum of Squares Reduction test with Bonferroni method for multiple comparisons (significance level α= 0.007). (B) FVIII antigen levels two hours after fVIII infusion is compared to mice that received fVIII alone using Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Data are presented as means with SD (n = 3–7 mice per group), * p < 0.05, ** p < 0.01, or *** p < 0.001
Discussion
In this study, we hypothesized that antibodies that inhibit fVIII binding to VWF increase clearance of fVIII and contribute to antibody pathogenicity. Our prior work showed that weakly inhibitory anti-C1 MAbs 2A9 and M6143 and strongly inhibitory MAbs B136 and KM33, which inhibit fVIII binding to VWF, increased fVIII clearance and produced bleeding in fVIII−/− mice [18]. Additional studies have reported that the pathogenic bleeding phenotype produced by a subset of anti-A2 and anti-C2 MAbs that do not compete with VWF for fVIII binding could be reversed with increased fVIII dosing [14, 15]. To evaluate whether increasing the fVIII dose would correct bleeding in fVIII−/− mice that received anti-C1 MAbs, blood loss was measured following infusion of “high dose” 360 units/kg fVIII. Compared to mice that received fVIII in the absence of antibodies, high dose fVIII replacement did not abrogate blood loss produced by anti-C1 MAbs M6143, B136, and KM33, but did reduce blood loss in mice that received MAb 2A9 (Fig. 1). Anti-C1 MAb F156, a low titer anti-C1 MAb that does not inhibit fVIII binding to VWF or phospholipid, does not produce blood loss at low dose 180 units/kg fVIII. Incomplete correction of median blood loss in fVIII−/− mice treated with anti-C1 MAbs showed a significantly positive correlation with the predicted peak inhibitor titers at low dose but not high dose fVIII (Fig. 2). This suggests that some patients with a low titer inhibitor may have a lower than expected hemostatic response due to the presence of anti-fVIII antibodies that increase fVIII clearance.
The partial correction of bleeding in fVIII−/− mice treated with anti-C1 MAbs was further explored by measuring dose-dependent blood loss in mice injected with increasing doses of fVIII and MAb M6143 at a peak concentration of 65 nM. Unexpectedly, mice required doses of fVIII up to 720 units/kg and 1440 units/kg (peak concentrations of 10 nM and 20 nM, respectively) for bleeding reduction following M6143 injection (Fig. 3). MAb M6143 is a weakly inhibitory MAb that has an inhibitor titer of 180 BU/mg IgG, an estimated peak plasma inhibitor titer of 2.3 BU/mL at the dose used in this study, and a high affinity dissociation constant for fVIII binding of 0.2 nM [18]. High doses of fVIII have been used clinically to achieve hemostasis in patients with non-amnestic low titer inhibitors (defined as <5 BU/mL) by saturating the inhibitor [16, 17, 26, 27]. Multiplying the plasma volume by the inhibitor titer has been reported as a formula for determining the amount of fVIII units required to saturate the inhibitor and achieve hemostasis [26]. For instance, in the murine tail snip bleeding model 180 units/kg fVIII, corresponding to a peak plasma concentration of 2.5 nM, is the estimated dose required to saturate the inhibitor in a mouse injected with 0.5 mg/kg MAb M6143. However, fVIII−/− mice required greater than twice this dose to reverse the bleeding phenotype (Fig. 3). Though it remains unclear whether the inhibitory effect of MAbs in mice plasmas correlate to the inhibitory effect in humans at the same Bethesda titer or whether the definitions of low and high titer inhibitors used in patients are applicable in the mouse model.
Although supraphysiologic doses of fVIII were required to reverse bleeding in the tail snip bleeding model, the predicted plasma concentration of MAb M6143 was still in molar excess of fVIII. This correction of bleeding at sub-stoichiometric concentrations of fVIII could result from VWF competition with the MAb for fVIII binding. This would favor formation of the fVIII/VWF complex at a hemostatically active fVIII dose that limits antibody pathogenicity. In contrast, at lower doses fVIII is partitioned into rapidly cleared, hemostatically ineffective fVIII/MAb immune complexes. Additionally, fVIII clearance was not accelerated in fVIII−/−/VWF−/− mice in the presence of anti-fVIII MAbs (Fig. 4). These results are consistent with competitive partitioning of fVIII between fVIII/VWF and fVIII/MAb complexes. Given the high binding affinity of fVIII for VWF (Kd 0.2 nM) [28], the use of fVIII products pre-bound to VWF may be beneficial in some patients for treatment of bleeding due to competition between VWF and anti-fVIII antibodies directed against “clearance” epitopes.
Although increased fVIII dosing reduced bleeding in a group of anti-A2 and C2 MAbs in the tail snip bleeding model, a subset of these MAbs remained pathogenic at low and high doses of fVIII [14, 15, 29]. Their pathogenicity was attributed to inhibition of fVIII procoagulant function. However, classical anti-C2 MAbs also inhibit fVIII binding to VWF and do not respond to increased fVIII doses, suggesting that their pathogenicity is at least partly due to increased clearance of fVIII. Consistent with this hypothesis, anti-C2 MAbs 3D12 and I109 that inhibit VWF and phospholipid binding accelerated fVIII clearance, in contrast to anti-A2 MAb 4A4 and anti-C2 MAb 2–117 that do not interfere with VWF or phospholipid binding (Fig. 5). Thus, the inhibition of fVIII procoagulant activity may be sufficient to explain the pathogenicity of high titer inhibitors. However for some low titer antibodies, fVIII clearance may result in greater pathogenicity than predicted by the Bethesda assay alone. This may explain why some patients with low titer inhibitors have significant bleeding despite aggressive fVIII replacement. Some of these non-responding patients may require treatment with a bypassing agent or ITI for inhibitor eradication.
While the A2 and C2 domains of fVIII are considered the predominant immunogenic epitopes, neutralizing and non-neutralizing antibodies against all the domains within fVIII have been described in humans and mice as detected by ELISA and chromogenic based assays in addition to the one-stage clot based Bethesda assay [20, 30–33]. One group postulated that antibodies against non-neutralizing antibodies (defined by a negative Bethesda titer but high anti-fVIII ELISA titer) in hemophilia patients may accelerate clearance of infused fVIII [34]. A separate study examining different methods of inhibitor detection described a group of seven patients with congenital hemophilia A and low titer inhibitors defined by a Nijmegen Bethesda titer <2.0 NBU and a positive chromogenic Bethesda assay [35]. Three of these patients with low titer inhibitors maintained response to fVIII replacement therapy, while 4 patients required treatment with a bypassing agent and/or ITI. Another study reported a linear relationship between the concentration of porcine fVIII required to produce in vitro recovery to 50% of normal and the anti-porcine fVIII inhibitor titer, suggesting an inhibitor neutralization assay may be more sensitive and quantitative than the Bethesda [7]. Laboratory assays that account for antibody characteristics such as antibody binding affinity, epitope specificity, the effect on fVIII biochemical properties and clearance not addressed by the Bethesda assay could help optimize management of patients with inhibitors.
This work underlies the importance of understanding the underlying mechanisms of fVIII clearance, the role of VWF in fVIII procoagulant function and clearance in the setting of antigen/antibody immune complexes, and the epitope specific pathogenicity of anti-fVIII antibodies. It suggests that there are distinct clearance pathways of fVIII versus the fVIII/VWF complex in the presence and absence of anti-fVIII inhibitors. Several receptors have been shown to bind fVIII, VWF, or both and contribute to fVIII clearance or endocytosis in circulation including receptors that bind to the low density lipoprotein (LDL), lecithin, and scavenger receptor families [36]. For instance, the clearance receptor LDL-related protein receptor (LRP), a member of the LDL receptor family, has demonstrated binding to fVIII as a ligand via the C2 domain and is downregulated by VWF [37]. Whether different classes of anti-fVIII inhibitors differentially modulate clearance receptors is not known. Additionally, the clearance of fVIII immune complexes by Fc receptors or other mechanisms is not understood.
In this study we show that anti-fVIII MAbs directed against various fVIII epitopes that compete with VWF for fVIII binding increase clearance of fVIII/MAb immune complexes and contribute to antibody pathogenicity. In-depth investigation of the critical fVIII clearance pathways involved in the presence and absence of inhibitors and the contribution of VWF could provide greater insight into mechanisms that determine pathogenicity of anti-fVIII antibodies in patients. Development of laboratory methods that account for the effect of anti-fVIII antibodies on fVIII biology may complement current methods used to quantify anti-fVIII antibodies and help guide determination of optimal treatment regimens.
Essentials.
Inhibitor formation remains a challenging complication of hemophilia A care.
The Bethesda assay is the primary method used for determining bleeding risk and management.
Antibodies that block factor VIII binding to von Willebrand factor can increase fVIII clearance.
Antibodies that increase clearance contribute to antibody pathogenicity.
Acknowledgments
This research was supported by Atlanta Pediatric Scholars Program K12 HD072245 (G. Batsuli), 2016 HTRS/Novo Nordisk Mentored Research Award in Hemophilia and Rare Bleeding Disorders from the Hemostasis and Thrombosis Research Society (HTRS) supported by an educational grant from Novo Nordisk Inc (G. Batsuli), NIH grant U54 HL112309 (P. Lollar, S.L. Meeks), and Hemophilia of Georgia, Inc (P. Lollar, S.L. Meeks). We thank Dr. Jan Voorberg for providing the human-derived anti-fVIII C1 antibody KM33 and Dr. Denisa Wagner for the fVIII−/−/VWF−/− mice.
Footnotes
Addendum
G. Batsuli designed and performed research, analyzed data, and co-wrote the paper. J. Ito, R. Mercer, W.H. Baldwin, C. Cox, E.T. Parker, and J.F. Healey performed research. S.L. Meeks and P. Lollar analyzed data and co-wrote the paper.
Conflict of Interest
The authors declare no interests that might be perceived as posing a conflict or bias. S.L. Meeks reports personal fees from Bioverativ, Bayer, CSL Behring, Catalyst Biosciences, Genentech, HEMA Biologics, Shire and grants from Pfizer outside the submitted work. The other authors state that they have no conflict of interest.
References
- 1.Franchini M, Mannucci PM. Hemophilia A in the third millennium. Blood reviews. 2013; 27: 179–84. 10.1016/j.blre.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Carcao MD. The diagnosis and management of congenital hemophilia. Semin Thromb Hemost. 2012; 38: 727–34. 10.1055/s-0032-1326786. [DOI] [PubMed] [Google Scholar]
- 3.Dimichele D Inhibitors: resolving diagnostic and therapeutic dilemmas. Haemophilia. 2002; 8: 280–7. [DOI] [PubMed] [Google Scholar]
- 4.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003; 9: 418–35. [DOI] [PubMed] [Google Scholar]
- 5.Gouw SC, van den Berg HM. The multifactorial etiology of inhibitor development in hemophilia: genetics and environment. Semin Thromb Hemost. 2009; 35: 723–34. 10.1055/s-0029-1245105. [DOI] [PubMed] [Google Scholar]
- 6.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995; 73: 247–51. [PubMed] [Google Scholar]
- 7.Barrow RT, Lollar P. Neutralization of antifactor VIII inhibitors by recombinant porcine factor VIII. Journal of thrombosis and haemostasis : JTH. 2006; 4: 2223–9. 10.1111/j.1538-7836.2006.02135.x. [DOI] [PubMed] [Google Scholar]
- 8.Kasper CK, Aledort L, Aronson D, Counts R, Edson JR, van Eys J, Fratantoni J, Green D, Hampton J, Hilgartner M, Levine P, Lazerson J, McMillan C, Penner J, Shapiro S, Shulman NR. Proceedings: A more uniform measurement of factor VIII inhibitors. Thrombosis et diathesis haemorrhagica. 1975; 34: 612. [PubMed] [Google Scholar]
- 9.Miller CH, Boylan B, Shapiro AD, Lentz SR, Wicklund BM, Hemophilia Inhibitor Research Study I. Limit of detection and threshold for positivity of the Centers for Disease Control and Prevention assay for factor VIII inhibitors. Journal of thrombosis and haemostasis : JTH. 2017; 15: 1971–6. 10.1111/jth.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verbruggen B, van Heerde WL, Laros-van Gorkom BA. Improvements in factor VIII inhibitor detection: From Bethesda to Nijmegen. Semin Thromb Hemost. 2009; 35: 752–9. 10.1055/s-0029-1245107. [DOI] [PubMed] [Google Scholar]
- 11.Yada K, Nogami K, Shima M. Different factor VIII neutralizing effects on anti-factor VIII inhibitor antibodies associated with epitope specificity and von Willebrand factor. Br J Haematol. 2013; 163: 104–11. 10.1111/bjh.12473. [DOI] [PubMed] [Google Scholar]
- 12.Hofbauer CJ, Whelan SF, Hirschler M, Allacher P, Horling FM, Lawo JP, Oldenburg J, Tiede A, Male C, Windyga J, Greinacher A, Knobl PN, Schrenk G, Koehn J, Scheiflinger F, Reipert BM. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood. 2015; 125: 1180–8. 10.1182/blood-2014-09-598268. [DOI] [PubMed] [Google Scholar]
- 13.Cannavo A, Valsecchi C, Garagiola I, Palla R, Mannucci PM, Rosendaal FR, Peyvandi F. Nonneutralizing antibodies against factor VIII and risk of inhibitor development in severe hemophilia A. Blood. 2017; 129: 1245–50. 10.1182/blood-2016-06-720086. [DOI] [PubMed] [Google Scholar]
- 14.Eubanks J, Baldwin WH, Markovitz R, Parker ET, Cox C, Kempton CL, Meeks SL. A subset of high titer anti-factor VIII A2 domain antibodies are responsive to treatment with factor VIII. Blood. 2016; 127: 2028–34. 10.1182/blood-2015-09-670034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Non-classical anti-factor VIII C2 domain antibodies are pathogenic in a murine in vivo bleeding model. Journal of thrombosis and haemostasis : JTH. 2009; 7: 658–64. 10.1111/j.1538-7836.2009.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins PW, Chalmers E, Hart DP, Liesner R, Rangarajan S, Talks K, Williams M, Hay CR. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). UK Haemophilia Centre Doctors Organization. Br J Haematol. 2013; 160: 153–70. 10.1111/bjh.12091. [DOI] [PubMed] [Google Scholar]
- 17.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014; 124: 3365–72. 10.1182/blood-2014-05-577643. [DOI] [PubMed] [Google Scholar]
- 18.Batsuli G, Deng W, Healey JF, Parker ET, Baldwin WH, Cox C, Nguyen B, Kahle J, Konigs C, Li R, Lollar P, Meeks SL. High-affinity, non-inhibitory pathogenic C1 domain antibodies are present in patients with hemophilia A and inhibitors. Blood. 2016; 128: 2055–67. 10.1182/blood-2016-02-701805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohler G, Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. European journal of immunology. 1976; 6: 511–9. 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- 20.Healey JF, Parker ET, Barrow RT, Langley TJ, Church WR, Lollar P. The humoral response to human factor VIII in hemophilia A mice. Journal of thrombosis and haemostasis : JTH. 2007; 5: 512–9. 10.1111/j.1538-7836.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 21.Barrow RT, Healey JF, Jacquemin MG, Saint-Remy JM, Lollar P. Antigenicity of putative phospholipid membrane-binding residues in factor VIII. Blood. 2001; 97: 169–74. [DOI] [PubMed] [Google Scholar]
- 22.Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods in enzymology. 1993; 217: 270–9. [DOI] [PubMed] [Google Scholar]
- 23.Lind P, Larsson K, Spira J, Sydow-Backman M, Almstedt A, Gray E, Sandberg H. Novel forms of B-domain-deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur J Biochem. 1995; 232: 19–27. [DOI] [PubMed] [Google Scholar]
- 24.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 1998; 95: 9524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeks SL, Cox CL, Healey JF, Parker ET, Doshi BS, Gangadharan B, Barrow RT, Lollar P. A major determinant of the immunogenicity of factor VIII in a murine model is independent of its procoagulant function. Blood. 2012; 120: 2512–20. 10.1182/blood-2012-02-412361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CA, Berntorp EE, Hoots WK. Textbook of Hemophilia. Hoboken, UNITED KINGDOM: John Wiley & Sons, Incorporated, 2014. [Google Scholar]
- 27.Valentino LA, Kempton CL, Kruse-Jarres R, Mathew P, Meeks SL, Reiss UM, International Immune Tolerance Induction Study I. US Guidelines for immune tolerance induction in patients with haemophilia a and inhibitors. Haemophilia. 2015; 21: 559–67. 10.1111/hae.12730. [DOI] [PubMed] [Google Scholar]
- 28.Vlot AJ, Koppelman SJ, van den Berg MH, Bouma BN, Sixma JJ. The affinity and stoichiometry of binding of human factor VIII to von Willebrand factor. Blood. 1995; 85: 3150–7. [PubMed] [Google Scholar]
- 29.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. 2007; 110: 4234–42. 10.1182/blood-2007-06-096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astermark J Basic aspects of inhibitors to factors VIII and IX and the influence of non-genetic risk factors. Haemophilia. 2006; 12 Suppl 6: 8–13; discussion-4 − 10.1111/j.1365-2516.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 31.Prescott R, Nakai H, Saenko EL, Scharrer I, Nilsson IM, Humphries JE, Hurst D, Bray G, Scandella D. The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Recombinate and Kogenate Study Groups. Blood. 1997; 89: 3663–71. [PubMed] [Google Scholar]
- 32.Scandella DH, Nakai H, Felch M, Mondorf W, Scharrer I, Hoyer LW, Saenko EL. In hemophilia A and autoantibody inhibitor patients: the factor VIII A2 domain and light chain are most immunogenic. Thrombosis research. 2001; 101: 377–85. [DOI] [PubMed] [Google Scholar]
- 33.Lavigne-Lissalde G, Lacroix-Desmazes S, Wootla B, Tarrade C, Schved JF, Kaveri SV, Granier C, Villard-Saussine S. Molecular characterization of human B domain-specific anti-factor VIII monoclonal antibodies generated in transgenic mice. Thromb Haemost. 2007; 98: 138–47. [PubMed] [Google Scholar]
- 34.Dazzi F, Tison T, Vianello F, Radossi P, Zerbinati P, Carraro P, Poletti A, Girolami A. High incidence of anti-FVIII antibodies against non-coagulant epitopes in haemophilia A patients: a possible role for the half-life of transfused FVIII. Br J Haematol. 1996; 93: 688–93. [DOI] [PubMed] [Google Scholar]
- 35.Miller CH, Rice AS, Boylan B, Shapiro AD, Lentz SR, Wicklund BM, Kelly FM, Soucie JM, Hemophilia Inhibitor Research Study I. Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study. Journal of thrombosis and haemostasis : JTH. 2013; 11: 1300–9. 10.1111/jth.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartholt RB, van Velzen AS, Peyron I, Ten Brinke A, Fijnvandraat K, Voorberg J. To serve and protect: The modulatory role of von Willebrand factor on factor VIII immunogenicity. Blood reviews. 2017; 31: 339–47. 10.1016/j.blre.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Lenting PJ, Neels JG, van den Berg BM, Clijsters PP, Meijerman DW, Pannekoek H, van Mourik JA, Mertens K, van Zonneveld AJ. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. The Journal of biological chemistry. 1999; 274: 23734–9. [DOI] [PubMed] [Google Scholar]