Highlights
-
•
Medically unexplained symptoms (MUS) are challenge for electrodiagnostic testing.
-
•
Weakness and sensory deficit can be manifestations of psychogenic disorders.
-
•
This is a review of electrodiagnostic methods used for the assessment of MUS.
Keywords: Functional disorders, Psychogenic paresis, Transcranial magnetic stimulation, Prepulse inhibition
Abstract
Patients with suspected medically unexplained symptoms or psychogenic disorders are frequently requested to undergo an EMG exam. However, the suspected diagnosis is not always told to the electromyography practitioner, who must be able to recognize such a condition to avoid false positive diagnosis without dismissing the possibility to uncover any true dysfunction. There are many clinical manoeuvers to assess the consistency of the patients’ reported weakness or sensory deficit. The electrodiagnostic practitioner should be aware of those clinical tricks and interpret the electrodiagnostic findings in the clinical context. There are many electrodiagnostic tests that the practitioner can use for the assessment of motor and sensory functions but these tests have also important drawbacks and limitations. Only after a good clinical evaluation would the practitioner be able to give his/her opinion on the clinical relevance of the electrodiagnostic findings. Here we review some of the tests that can help the practitioner to define the electrophysiological characteristics of a suspected functional disorder presenting with weakness or sensory deficit.
1. Introduction
Loss of strength or sensation are two of the most common symptoms for referral of patients to electrodiagnostic testing. The examiner can use a variety of techniques in search of a pathophysiological explanation for those symptoms compatible with the clinical context. In theory, the examiner carrying out electrodiagnostic tests should report on objective parametric data, with as much quantitation as possible, for clinicians to figure out the whole spectrum of paraclinical tests in light of the clinical evaluation. Today’s electrodiagnostic tools allow for assessment of many sensory and motor segments, including central tracts and peripheral nerves and, therefore, there are many possibilities for a dedicated electrodiagnostic expert to find the clinical-neurophysiological correlate of the disorder in question.
Unfortunately, though, time constraints in busy clinics and patient compliance generally limits the number of electrodiagnostic tests that can be performed. Furthermore, the expert in electrodiagnostic medicine knows that the results obtained make sense only if the tests performed were based on a clinical logic. To do that, the electrodiagnostic practitioner has to use data from patient’s history and physical examination to decide on the steps of further testing and finally issue a clinically relevant report. Often, the referral note is not sufficient. The examiner should be aware of the possibility that some relevant symptoms and signs, not necessarily stated in the referral, are very relevant for the outcome of the study. Indeed, the electrodiagnostic examiner is the one ultimately responsible for the report, whatever be the syndrome that led to the patient’s presentation for the examination. In fact, a good electrodiagnostic practitioner should have a thorough technical and clinical expertise, together with the necessary writing skills to be able to transmit to the referring physician his/her opinion beyond just cold data. Good practice in electrodiagnostic medicine contemplates as much refraining from raising clinically unfounded suspicions as not letting pass by clinically undiagnosed syndromes in which the electrodiagnostic tests play a relevant role.
Among the syndromes challenging the capacity of the electrodiagnostic practitioner are the medically unexplainable symptoms that affect the nervous system (Carson et al., 2000). The term psychogenic has been used for many years to describe this type of disorder but the more convenient term ‘functional disorders’ is now recommended (Stone and Carson, 2011). That a given disorder is functional and not derived from a recognizable neurological disorder is not a straightforward diagnosis. The clinical expression of some neurological disorders may be modified by will or adaptation, either amplifying or reducing the symptoms known to characterize the disorder. Compensatory mechanisms may be at play, which may modify the clinical expression in a way unknown to the patient, and sometimes also to the physician. On some occasions, the presence of a psychogenic disorder masks the diagnosis of a neurological disease with similar symptoms, as has been the case in a percentage of patients in many series of patients with functional neurological disorders reported so far. Finally, symptoms may derive from true psychiatric dysfunctions such as conversion disorder or hypochondria, or from disease-unrelated conditions, such as factitious disorders or malingering (Hallett, 2006, Hallett, 2010). Table 1 summarizes the classification of the medically unexplained syndromes, according to the certainty of the diagnosis.
Table 1.
Classification of the medically unexplained syndromes.
| Category | Definition |
|---|---|
| Possible | Symptoms consistent and congruous with a known disease but signs of obvious emotional disturbance or secondary gain |
| Probable | Symptoms consistent and congruous with a known disease but the patient has traits of psychogenicity or a psychiatric disorder |
| Clinically established | Symptoms are inconsistent and incongruent and the patient shows psychogenic signs, somatizations or psychiatric condition |
| Documented | Symptoms are completely relieved by placebo or the patient is witnessed to be free of symptoms when feeling unobserved |
Adapted from Williams et al. (1995).
Most reports on medically unexplainable symptoms in neurology have dealt with functional movement disorders, which have attracted the attention of researchers for a long time (Fahn and Williams, 1988, Koller et al., 1989, Lang et al., 1995, Stone et al., 2002, Stone et al., 2005, Hallett, 2006, Hallett, 2010, Edwards and Bhatia, 2012, Ricciardi et al., 2015a). Research in this area has led to the development of interesting electrodiagnostic tests to uncover the voluntary nature of some apparently involuntary movements (Hallett, 2010). This is the case for myoclonus (Thompson et al., 1992, Brown and Thompson, 2001), tremor (O’Suilleabhain and Matsumoto, 1998, Kumru et al., 2004) and in some forms of dystonia (Schwingenschuh et al., 2011, Macerollo et al., 2015a). These patients are commonly referred for specific electrodiagnostic studies, in search of laboratory support for a clinically-based diagnostic suspicion of functional disorder. This is indeed an attractive challenge for researchers in the various neurophysiological techniques that characterize voluntary and involuntary movements. However, the electrodiagnostic examiner often faces situations in which patients complain of weakness or sensory deficit with uncertain diagnosis. In many countries, the physician referring the patient for an electrodiagnostic study may not be a neurologist, and he/she may hope that the electrodiagnostic examination shows clearly if the motor or sensory deficit described by the patient is related or not to a known neurological disorder, to decide on the next step. It is therefore the responsibility of the electrodiagnostic examiner to use all clues available to build up his/her opinion on the case, including history and physical examination data (Hallett, 2016) to finally write a sensible report on the patient’s case. What follows is a review of the clinical and electrodiagnostic clues that the electrodiagnostic expert may use to determine whether or not the patient’s symptoms of weakness and sensory deficit are related to a neurological disorder or are non-neurological in nature.
2. Functional weakness
According to Stone et al. (2010), functional weakness is defined as weakness that is both internally inconsistent and incongruent with any recognizable neurological disease. They described the incidence, demographic and clinical characteristics of 107 cases. They found that patients with functional weakness were as disabled as patients with weakness due to neurological disease. The most common manifestation of functional weakness is hemiparesis (79% of the 107 cases studied by Stone et al. (2010)). However, there may be many other forms of presentation, including weakness of distal or proximal limb segments. There are many exploratory tricks to uncover functional weakness (Stone et al., 2012, Tremolizzo et al., 2014). Close observation of how the patient activates the supposedly paretic limb during postural tasks may already be very informative. When the suspected psychogenic weakness affects one leg only, the Hoover’s maneuver and the abductor’s sign (Sonoo, 2004) may be very helpful. In Hoover’s maneuver, the subject fails to press against the bed with the healthy leg when requested to raise the weak one. In the abductor’s sign, the subject fails to fix the non paretic leg in a neutral position when requested to abduct the weak leg, while he/she is holding the paretic leg in a fixed straight position when requested to abduct the non-paretic one (see Sonoo (2004 for a graphical explanation of the maneuver). When supposedly psychogenic weakness affects both feet, some useful clinical information can be obtained by observing whether or not synkinetic movements occur in the upper limbs in the attempts to dorsiflex the toes. This is indeed the case shown in Fig. 1, where the patient with true weakness of dorsiflexion of the feet showed an involuntary extension of the hand and fingers accompanying his unsuccessful efforts to counteract the resistance offered by the examiner’s hands opposing feet dorsiflexion. This indicates that the patient was indeed trying hard to perform the requested task. Such synkinesis is usually absent in patients who fake weakness and, therefore, its presence may be taken as a sign of true weakness. Similar signs can be used for upper limbs where homonymous synkinetic movements have been described in the contralateral hand when trying hard to abduct the index finger against resistance in unilateral tasks (Tinazzi et al., 2008). The electrodiagnostic approach to examine weakness entails various steps, outlined below.
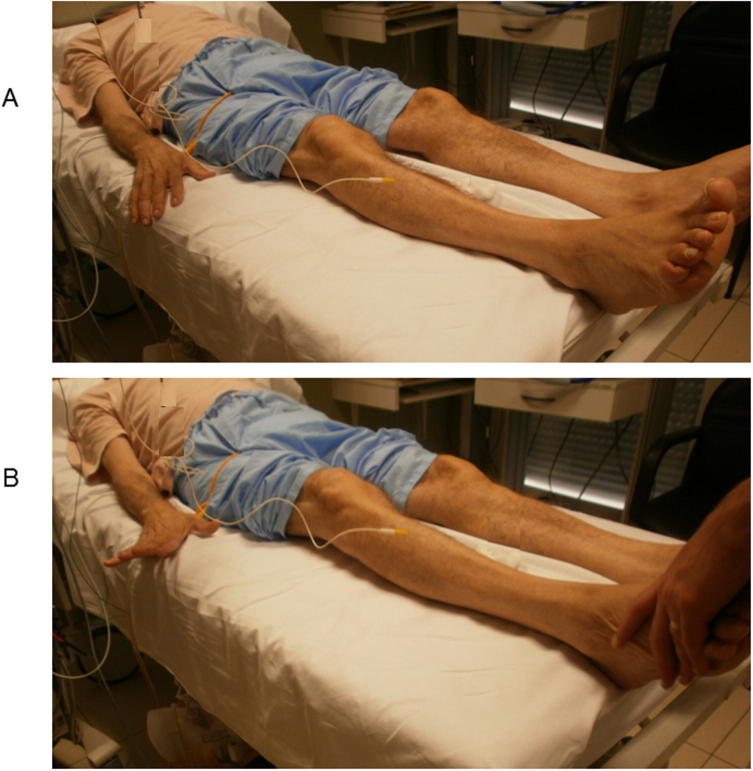
Fig. 1.
Ipsilateral upper limb synkinesis in weak foot dorsiflexion. The patient was a 62 y.o. man with severe polyneuropathy associated with liver cirrhosis and cryoglobulinemia. His strength for foot and toes dorsiflexion were 3 over 5 (barely able to raise them against gravity). When trying hard (lower picture), his fingers raised involuntarily (arrow).
2.1. Motor nerve conduction studies
It is convenient to begin the electrodiagnostic examination for the study of weakness by assessing possible peripheral neuropathy as its cause. The amplitude of the compound muscle action potential (CMAP) reflects the number of motor units activated at the site of the stimulus. However, it may be difficult to know if loss of amplitude can explain the symptoms in a given case. Obviously, the examiner should have normative values obtained with the same technique and examination conditions and, thus, determine if the amplitude values obtained in that case are significantly different from the norm. However, it has to be taken into account that the CMAP recorded from a given muscle does not represent all muscles contributing to the movement or task where weakness can be observed. This is, for instance, the case with dorsiflexion of the foot and toes. The main agents for these tasks are the tibialis anterior and the extensor digitorum (and hallucis) longus. The extensor digitorum brevis (EDB) is the muscle where conduction of the common peroneal nerve is usually assessed, but it contributes only slightly to toe dorsiflexion. In fact, weak foot dorsiflexion is compatible with a normal CMAP in the EDB, as in a case of L5 radiculopathy, while preserved foot dorsiflexion can occur with an absent CMAP in the EDB, as in a case of vasculitis involving just the most distal segment of the peroneal nerve (Fig. 2).
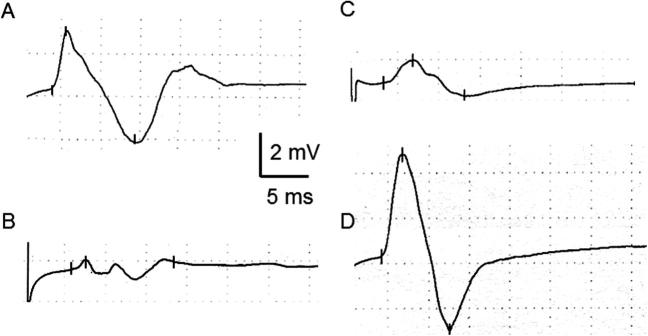
Fig. 2.
Peroneal nerve compound muscle action potentials (CMAP) recorded over the tibialis anterior (TA) after stimulation at the fibular head (A and C) and over the extensor digitorum brevis (EDB) after stimulation at the ankle (B and D). Recordings come from two different persons. The patient on the left (normal CMAP in TA but reduced in EDB) suffered from mononeuritis multiplex in the context of a connectivopathy-related vasculitic lesion and had marked atrophy of the extensor digitorum brevis but preserved dorsiflexion foot and toes strength (5/5). The patient on the right side (normal CMAP in EDB but reduced in TA) suffered from L5 radiculopathy and had marked atrophy of the tibialis anterior, with a foot dorsiflexion strength of 4/5.
The shape of the CMAP is a measure of dispersion of the nerve volley. Weakness may result from lack of synchronization of inputs to muscle fibers of different motor units, as in nerve demyelination. Slowness of nerve conduction velocity (NCV) may or may not be present but, usually, a slow NCV does not explain per se clinically relevant weakness (Cros and Triggs, 1996, Gordon and Wilbourn, 2001). To evaluate dispersion, the examiner has to take into account a few points. One is the distance between stimulation and recording because dispersion increases with distance (Taylor, 1993). Finding some irregularities in the waveform to proximal nerve stimulation may not be enough to consider the nerve studied as abnormal. When in doubt, the evaluation of the F wave becomes handy because it will show increased chronodispersion if the irregularities in the CMAP waveform are relevant (Vucic et al., 2007, Harbo et al., 2008). Conduction block may give rise to weakness more often than dispersion. This can be the case in early Guillain–Barré syndrome, where the CMAP obtained to distal stimulation may be preserved even in cases with complete paralysis caused by proximal conduction block. Again, the evaluation of the F wave is necessary in these cases. Certainly, the examiner cannot draw firm conclusions from a normal CMAP to distal stimulation. Normal shape, lack of dispersion and conduction block as well as normal F wave latency, perseverance and chronodispersion are more important data for reaching the conclusion of normal motor nerves in a given territory.
2.2. Transcranial magnetic stimulation
Weakness is more likely to happen with lesions involving the corticospinal tract. There are many maneuvers to use in the physical examination of a suspected disorder of corticospinal conduction. However, pyramidal signs may not be present or may be disguised by the concomitant presence of other neurological or non-neurological lesions. Therefore, it is convenient to use neurophysiological tools to assess corticospinal tract function. The most useful technique is transcranial magnetic stimulation (TMS), which consists of a non-painful activation of the corticospinal system to collect motor evoked potentials (MEPs) from the muscles suspected to show the dysfunction. For detailed methodological issues and guidelines for clinical applicability, the reader is addressed to the many reviews on the subject, such as Rossi et al. (2009), Groppa et al. (2012), Rossini et al. (2015) or others.
A useful measure to take from TMS studies is central motor conduction time, which is the subtraction of the peripheral conduction time from the latency of the responses to cortical stimulation. Again, every examiner should have normative reference values for comparison. The assessment may be more straightforward when the weakness is unilateral because of comparison to the contralateral side (Fig. 3). Unfortunately, weakness does not correlate with the abnormalities in central conduction time and there is even the possibility that some motor impairment is due to premotor disorders (Chamorro et al., 1997). However, in most instances, normal MEP amplitude and central motor conduction time can rule out the involvement of the pyramidal tract as a cause of weakness. In doubtful situations, it may be convenient to ask the patient to perform a sustained muscle contraction. Several signs of voluntary facilitation can be detected in the MEP, including shortening of onset latency, increase of peak-to-peak amplitude and lengthening of duration. Some of these signs may be absent in patients with functional paralysis, particularly the absence of an increase in duration of the MEP beyond that at rest, one of the most significant differences with respect to healthy subjects (Brum et al., 2016). TMS during voluntary contraction allows analysis of the silent period, and this may also give hints for suspected psychogenicity, due to an irregularly sustained level of muscle contraction. In this case, the duration of the silent period may vary substantially from one trial to the next. Obviously, clinical assessment is again of paramount importance, not to mistake an impaired voluntary drive for psychogenicity in patients with basal ganglia diseases, such as Parkinson’s disease, Huntington’s disease or dystonia, where irregularities in muscle contraction and in the duration of the silent period to TMS have also been described (Priori et al., 1994, Modugno et al., 2001, Rona et al., 1998).
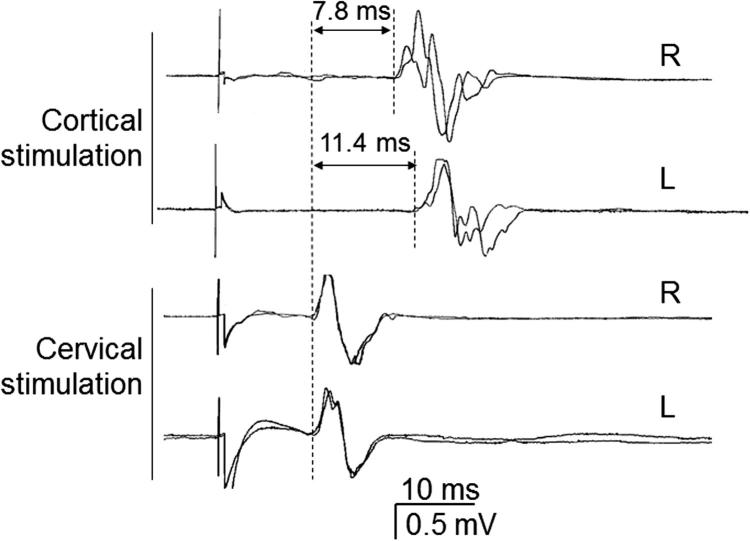
Fig. 3.
Differences in motor central conduction time (CCT) between right and left sides in a patient with weakness in his left arm due to a small right cortical infarct. CCT values, calculated by subtracting the latency of the responses obtained to foraminal cervical stimulation from those obtained to cortical stimulation, are indicated for each side.
2.3. Needle electromyography
One of the most troubling neurological disorders presenting with generalized weakness is motoneuron disease. It may not be the most frequent presentation, but patients with motoneuron disease may have only weakness, and there may be few or no relevant physical signs if fasciculations are not evident. Certainly, subtle clinical signs may not be passed unnoticed by an expert neurologist, but the suspicion of functional weakness may be raised in cases with equivocal history data. It is not uncommon that an electromyographer receives a patient with undiagnosed weakness and he/she is the first to include motoneuron disease in the list of diagnoses to take into account. This is a situation in which the electromyographer needs all information available to reach the correct diagnosis and is arguably the area where the diagnostic errors derived from EMG examinations have important clinical consequences. Errors can occur in both directions. Reporting a normal examination in a patient with motoneuron disease may delay the diagnosis (Nzwalo et al., 2014), while overinterpreting some EMG data to give the erroneous positive diagnosis of motoneuron disease in a patient with another less alarming neurological disorder could lead to unnecessary worry, concern and expense.
Needle EMG examinations are necessary for the differential diagnosis of weakness. The finding of denervation signs at rest is undoubtedly the most important of all data gathered from needle EMG. In this respect, it is adequate to remind that fasciculation potentials are considered a sign of denervation in patients with suspected motoneuron disease, according to the recently introduced Awaji criteria (De Carvalho et al., 2008, Costa et al., 2012, De Carvalho and Swash, 2013). Muscles to be examined should be decided according to clinical presentation but the electromyographer may recognize that EMG of some muscles is usually more informative than others. For instance, the finding of a slightly reduced interference pattern in certain muscles such as gastrocnemii or quadriceps may not necessarily put them into the category of denervated muscles. However, these findings in other muscles such as the tibialis anterior, should be considered relevant and lead to physical re-examination and history re-evaluation in the case of suspected functional weakness. For the evaluation of the eventual neurogenic deficit during contraction, the examiner has to take into account that pyramidal tract dysfunction may mask the deficit because of inconsistent and insufficient motor drive to α motoneurons during voluntary contraction. Such inadequate activation of α motoneurons is unfortunately similar to what would happen in patients with functional weakness. The bottom line is that the expected increase in motor unit firing frequency in a patient with early motoneuron disease may not always be apparent.
3. Sensory deficit
Patients with functional neurological disorders may present with sensory deficit. This may be congruous or not with a neurological distribution. Physical examination would easily rule out a neurological disorder if the sensory deficit is inconsistent with a known neurological syndrome. Neurophysiological assessment may certainly help by recording the sensory nerve action potentials (SNAPs) of the nerves supposedly damaged to compare them with those not involved in the same person. However, the examiner should take into account the possibility of referred sensations, which are typically dysesthetic. These may occur in some known situations such as the upper-limb dysesthesias related to heart disease, but it may occur in less well-known situations. Hip damage may create a referred sensation along the leg, distributed in the antero-lateral side of the thigh, mimicking meralgia paresthetica. And facial pain/paresthesias can originate from irritation of the trigeminal nerve at the alveolar or dental branches. No neurophysiological studies are available at present to disclose irritation of a nerve giving rise to these “positive” referred sensations.
A very relevant dysfunction in the sensory domain has been recently described in patients with functional movement disorders, which could explain some of the features of clinical presentation in these patients. This is the loss of sensory attenuation during performance of voluntary movements (Pareés et al., 2014). Reduction of intensity of sensation during self-generated movements allows for developing a normal sense of agency. The absence of such a reduction may explain why patients with functional movement disorders feel the movements as involuntary (Edwards et al., 2011, Kranick et al., 2013). Other disorders of sensation have been reported in patients with functional movement disorders, namely the alteration of interoceptive awareness (Ricciardi et al., 2015b) and the difficulty in recognizing their own emotional state, or alexithymia (Demartini et al., 2014). Such high order sensory disturbances may be important for the pathophysiology of functional neurological disorders but, in agreement with its own nature, they are unnoticed and, therefore, unreported, by the patients themselves. Reporting sensory deficit is a common manifestation of functional disorders whether combined or not with weakness. Distracting the patient’s attention while testing eventual reactions to sensory stimuli in an area supposed to be devoid of sensation is a clinical trick that can be useful in some instances but neurophysiological examination is indeed very relevant in this domain.
3.1. Sensory nerve conduction studies
Assessment of sensory nerve conduction is a basic study to perform in any patient reporting sensory deficit. Routinely, the examination of common nerves of the lower and upper limbs (sural, superficial peroneal, median and ulnar should be done bilaterally). If there is suspicion of distal neuropathies it may be adequate to study more distal nerves in the feet (dorsal sural, the distal branch of the deep peroneal nerve or medial/lateral plantar nerves). Focal loss of sensation is not a common report in functional neurological disorders but the examiner should anyway pay attention to an eventual asymmetric decrease of sensory nerve action potential (SNAP) amplitude, since mononeuritis multiplex could actually explain some apparently incongruous sensory signs combining hypesthesia and pain in unrelated regions (Collins et al., 2013). If the sensory deficit relates to proximal lesions, SNAPs of distal limb nerves may remain preserved. This is usually the case with radiculopathies due to a herniated disk, where the lesion is frequently at a preganglionic level, except when the disk protrusion occupies the inter-vertebral foramen and damages the sensory ganglion or its blood supply. In acute plexopathies, such as Parsonage–Turner syndrome, the examiner has to be careful to let sufficient time pass before diagnosing a postganglionic lesion since sensory denervation, whenever it takes place, might appear only after a significant delay (10–12 days). There are many different forms of presentation of a Parsonage–Turner syndrome and, in some of them, the expected postganglionic involvement does not affect any nerve available to electrodiagnostic studies (except for somatosensory evoked potentials).
Normal tendon jerks require normal sensory conduction up to the spinal cord or brainstem, in accordance to the type of reflex examined. Therefore, if a patient complains of absent sensation and the tendon jerks are normal (or enhanced), the cause of the defective sensation must be in the central nervous system. If in doubt, the use of an oscilloscope sweep-triggering hammer may be of interest to obtain the latency of the muscle response to the tendon tap (Péréon et al., 2004). In limb muscles, the presence of a normal silent period to electrical stimulation indicates normal afferent inputs not only for large fibers but also for small fibers (Kofler, 2003, Lopergolo et al., 2015). Cutaneous reflexes such as the long latency reflexes of hand muscles (Deuschl and Lücking, 1990, Chen and Ashby, 1993) may also be useful to disclose absence of damage in the segments of the sensory system involved in the reflex. For cranial nerve involvement, the examiner can use the cutaneous reflexes conveyed by the trigeminal nerve (Valls-Solé, 2005). The assessment should not be limited to the blink reflex recorded in the orbicularis oculi to supraorbital nerve stimuli since this can be normal in patients complaining of defective sensation in the lower face (for instance, in a case of connective tissue disease). The masseteric inhibitory reflex is more appropriate in those cases. Arguably, the most ubiquitous reflex response obtainable in the human body to any type of sensory stimulation is the sudomotor skin response (Vetrugno et al., 2003). This is recorded from the palm of the hand, referenced to the dorsum. In a case of suspected sensory deficit in a certain region of the body, application of a light electrical stimulus or a mechanical stimulus in that region should induce a normal sudomotor skin response in patients complaining of sensory deficit unrelated to a neurological disorder – a kind of lie-detector test (Ambach et al., 2008).
3.2. Somatosensory evoked potentials
Damage in the afferent spinal pathways could give rise to sensory deficit mostly in the lower limbs but also in the upper limbs if the lesion is at the cervical level. This is usually clinically evident but there can be situations in which the clinical deficit is not so obvious. The recording of somatosensory evoked potentials (SEPs) to electrical nerve stimulation may reveal conduction block along the dorsal columns. Normality of the SEPs should not be equated to absence of sensory deficit. Because of the well-known amplification of the signal along synapses leading the impulses toward the central nervous system, the SEPs may be normal even in patients with relevant sensory deficit. SEPs will be normal also in patients with damage in the antero-lateral pathway, as in syringomyelia (Treede et al., 1991) or the Wallenberg syndrome (Veciana et al., 2005). In these cases, nociceptive evoked potentials are needed to reveal the dysfunction. The most commonly used stimuli for the assessment of the nociceptive pathway are laser, which lead to laser evoked potentials (Treede, 2003) and thermodes that lead to contact heat evoked potentials (Atherton et al., 2007). Both of them are useful for the study of the nociceptive pathway but the examiner has to be aware that either laser or contact heat evoked potentials are actually long-latency evoked potentials, with more variability and, therefore, less precise latency values, than short-latency somatosensory evoked potentials. Nevertheless, they still are nowadays one of the most adequate means to document dysfunction in patients with neuropathic pain of peripheral or central origin (Baumgärtner et al., 2012).
Long-latency potentials involve some cerebral processing of sensory stimuli. Such processing may be diminished by diminished attention to the stimulus, as may occur in some patients with posttraumatic stress disorder (Felmingham et al., 2002). Instead, patients with factitious disorders or malingering, who voluntarily try to avoid any reaction to the stimulus may nevertheless generate an event-related potential to the avoidance reaction, and this remains time-locked to the stimulus. The P300 is generated in a cognitive task in which the subject has to pay attention and react to the presentation of an infrequent stimulus, which characteristics differ from those of a frequent one. In cases in which the subject tries actively to avoid any reaction, the P300 still appears with no differences in amplitude in comparison to trials with reaction (Zarkowski et al., 2007, Hoover et al., 2014).
3.3. Prepulse and gating effects
A sensory stimulus activates axon terminals, nerves and spinal tracts to reach cerebral sensory centers. Along this path, the traveling impulse generates action potentials and somatosensory evoked potentials that can be recorded with appropriately located recording electrodes. Apart from that, they may also generate reflex responses in the motor system at different points along the path if the sensory stimulus is of an intensity above threshold for activation of the motor system. Two of these responses are the blink reflex and the startle reaction. Both, the generation of long latency SEPs and the elicitation of reflex responses result from processing of the sensory stimulus within the central nervous system, one toward the brain cognitive areas, another toward the motor system. The concepts of prepulse and gating refer to how another sensory stimulus affects such processing. Prepulse effects are those observed in the response to a suprathreshold stimulus (pulse) when it is preceded by a stimulus of the same or other modality, subthreshold for elicitation of a motor response (prepulse). Gating effects are those observed in the somatosensory evoked potentials related to a certain stimulus when another stimulus is applied time-locked to them (afferent gating) or when the subject is moving (efferent gating). Inhibition is the best known aspect of the two phenomena.
Prepulse inhibition is an ubiquitous phenomenon that is characterized by the inhibition of the blink reflex and the startle reaction induced by either auditory or somatosensory stimuli (Valls-Solé et al., 1999). The effect means that the stimulus used as prepulse, which may be auditory, somatosensory, nociceptive or others (Valls-Solé, 2012), has reached the central nervous system and has been effectively integrated in prepulse circuits. Therefore, the method can be used to test sensation in the specific sensory modality of the prepulse stimulus and in the precise site where it has been applied. The method cannot tell, though, anything about conscious perception since the time interval for the effects to occur (usually 100 ms for auditory or electrical stimuli) remains in the preconscious domain. Furthermore, it is a qualitative phenomenon, which, per definition, occurs with the activation of only a small number of afferent axons, insufficient to generate a response. Inhibitory prepulse is sometimes used interchangeably with afferent gating to refer to the effects of a sensory stimulus on the cerebral evoked potentials generated by another stimulus. This may be the case for the P50 potential to auditory stimuli (Oranje et al., 2006). However, sensory gating and prepulse inhibition may be differentially affected in some conditions, such as schizophrenia (Braff et al., 2007). We have referred above to a type of sensory abnormality in patients with functional movement disorders that consists in the lack of sensory attenuation during voluntary movement. In fact, it has been shown in these patients there may be no amplitude reduction of the somatosensory evoked potentials when a stimulus is applied just after onset of a voluntary movement (Macerollo et al., 2015b). Gating mechanisms are influenced by the subject’s emotional state (Cromwell and Atchley, 2015), which may contribute to explaining the abnormalities in high-level processing of sensory information in these patients. The study of the sense of agency during voluntary movements is likely a key point in further understanding the pathophysiological mechanisms of psychogenic disorders.
4. Conclusions
The results of an electrodiagnostic examination make sense only if they are based on clinical logic. With this reasoning in mind, the electrodiagnostic practitioner should use not only data from electrodiagnostic testing, but also from patient’s history and physical examinations, to decide on the steps of further testing and finally issue a clinically relevant report. One of the most important assets of an electrodiagnostic examination is the avoidance of bias and of errors related to a premature clinical diagnosis. The electrodiagnostic examination requires undressing of some body parts and probing nerve function with stimuli inducing movements or generating sensory experiences. Therefore, the electrodiagnostic expert is in the position to notice subtle signs that could have passed unnoticed in previous possibly more superficial clinical examinations. In this context, the electrodiagnostic expert should consider the possibility of false weakness and sensory deficit or, to be more comprehensive, that some of the weakness and sensory deficit that the patient refers may not be related to a known neurological disorder. Electrodiagnostic practitioners should not be misled by clinical appearance in potentially fake symptoms or signs but, instead, identify them and try to document the condition on the bases of a good combination of clinical and technical knowledge, and use reason to avoid producing confounding EMG reports. If and when detected, it is not the function of the electrodiagnostic examiner to search for the possible cause of the disorder. Instead, the interpretation of the results should be related to pathophysiological mechanisms explaining symptoms and signs.
In some countries, patients referred for electrodiagnostic studies may not have yet had a thorough physical neurological examination that usually sets the bases for a suspected disease. In this case, to be of help to the patient and to the referring physician, the electrodiagnostic examiner must be proactive in the diagnosis and not limit his/her intervention to the requested tests if there are other tests needed to confirm the clinical suspicion of an alternative diagnosis. The results of the electrodiagnostic examination may come as a plus on the data obtained with the clinical examination. If suspicion of neurologically unexplained weakness or sensory deficit has arisen, the examiner should find means to warn the referring physician about the possibility of a functional disorder as the basis of the patient’s symptoms, beyond just reporting the crude and cold data obtained from the electrodiagnostic examination.
Conflict of interest statement
The author declares no conflict of interest.
References
- Ambach W., Stark R., Peper M., Vaitl D. Separating deceptive and orienting components in a concealed information test. Int. J. Psychophysiol. 2008;70:95–104. doi: 10.1016/j.ijpsycho.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Atherton D.D., Facer P., Roberts K.M., Misra V.P., Chizh B.A., Bountra C., Anand P. Use of the novel contact heat evoked potential stimulator (CHEPS) for the assessment of small fibre neuropathy: correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol. 2007;7:21. doi: 10.1186/1471-2377-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgärtner U., Greffrath W., Treede R.D. Contact heat and cold, mechanical, electrical and chemical stimuli to elicit small fiber-evoked potentials: merits and limitations for basic science and clinical use. Neurophysiol. Clin. 2012;42:267–280. doi: 10.1016/j.neucli.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Braff D.L., Light G.A., Swerdlow N.R. Prepulse inhibition and P50 suppression are both deficient but not correlated in schizophrenia patients. Biol. Psychiatry. 2007;61:1204–1207. doi: 10.1016/j.biopsych.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Brown P., Thompson P.D. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov. Disord. 2001;16:595–599. doi: 10.1002/mds.1145. [DOI] [PubMed] [Google Scholar]
- Brum M., Cabib C., Valls-Solé J. Clinical value of the assessment of changes in MEP duration with voluntary contraction. Front. Neurosci. 2016;9:505. doi: 10.3389/fnins.2015.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson A.J., Ringbauer B., Stone J., McKenzie L., Warlow C., Sharpe M. Do medically unexplained symptoms matter? A prospective cohort study of 300 new referrals to neurology outpatient clinics. J. Neurol. Neurosurg. Psychiatry. 2000;68:207–210. doi: 10.1136/jnnp.68.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A., Marshall R.S., Valls-Solé J., Tolosa E., Mohr J.P. Motor behavior in stroke patients with isolated medial frontal ischemic infarction. Stroke. 1997;28:1755–1760. doi: 10.1161/01.str.28.9.1755. [DOI] [PubMed] [Google Scholar]
- Chen R., Ashby P. Reflex responses in upper limb muscles to cutaneous stimuli. Can. J. Neurol. Sci. 1993;20:271–278. doi: 10.1017/s0317167100048174. [DOI] [PubMed] [Google Scholar]
- Collins M.P., Arnold W.D., Kissel J.T. The neuropathies of vasculitis. Neurol. Clin. 2013;31:557–595. doi: 10.1016/j.ncl.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Costa J., Swash M., de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis: a systematic review. Arch. Neurol. 2012;69:1410–1416. doi: 10.1001/archneurol.2012.254. [DOI] [PubMed] [Google Scholar]
- Cromwell H.C., Atchley R.M. Influence of emotional states on inhibitory gating: animals models to clinical neurophysiology. Behav. Brain Res. 2015;276:67–75. doi: 10.1016/j.bbr.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros D., Triggs W.J. Guillain-Barré syndrome: clinical neurophysiologic studies. Rev. Neurol. 1996;152:339–343. [PubMed] [Google Scholar]
- De Carvalho M., Swash M. Fasciculation potentials and earliest changes in motor unit physiology in ALS. J. Neurol. Neurosurg. Psychiatry. 2013;84:963–968. doi: 10.1136/jnnp-2012-304545. [DOI] [PubMed] [Google Scholar]
- De Carvalho M., Dengler R., Eisen A., England J.D., Kaji R., Kimura J., Mills K., Mitsumoto H., Nodera H., Shefner J., Swash M. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- Demartini B., Petrochilos P., Ricciardi L., Price G., Edwards M.J., Joyce E. The role of alexithymia in the development of functional motor symptoms (conversión disorder) J. Neurol. Neurosurg. Psychiatry. 2014;85:1132–1137. doi: 10.1136/jnnp-2013-307203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G., Lücking C.H. Physiology and clinical applications of hand muscle reflexes. Electroencephalogr. Clin. Neurophysiol. Suppl. 1990;41:84–101. doi: 10.1016/b978-0-444-81352-7.50012-1. [DOI] [PubMed] [Google Scholar]
- Edwards M.J., Moretto G., Schwingenschuh P., Katschnig P., Bhatia K.P., Haggard P. Abnormal sense of intention preceding voluntary movement in patients with psychogenic tremor. Neuropsychologia. 2011;49:2791–2793. doi: 10.1016/j.neuropsychologia.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Edwards M.J., Bhatia K.P. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250–260. doi: 10.1016/S1474-4422(11)70310-6. [DOI] [PubMed] [Google Scholar]
- Fahn S., Williams D.T. Psychogenic dystonia. Adv. Neurol. 1988;50:431–455. [PubMed] [Google Scholar]
- Felmingham K.L., Bryant R.A., Kendall C., Gordon E. Event-related potential dysfunction in posttraumatic stress disorder: the role of numbing. Psychiatry Res. 2002;109:171–179. doi: 10.1016/s0165-1781(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Gordon P.H., Wilbourn A.J. Early electrodiagnostic findings in Guillain-Barré syndrome. Arch. Neurol. 2001;58:913–917. doi: 10.1001/archneur.58.6.913. [DOI] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V., Kaelin-Lang A., Mima T., Rossi S., Thickbroom G.W., Rossini P.M., Ziemann U., Valls-Solé J., Siebner H.R. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Physiology of psychogenic movement disorders. J. Clin. Neurosci. 2010;17:959–965. doi: 10.1016/j.jocn.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Psychogenic movement disorders: a crisis for neurology. Curr. Neurol. Neurosci. Rep. 2006;6:269–271. doi: 10.1007/s11910-006-0015-x. [DOI] [PubMed] [Google Scholar]
- Hallett M. Functional (psychogenic) movement disorders – clinical presentations. Parkinsonism Relat. Disord. 2016;22(Suppl. 1):S149–S152. doi: 10.1016/j.parkreldis.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbo T., Andersen H., Jakobsen J. Length-dependent weakness and electrophysiological signs of secondary axonal loss in chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2008;38:1036–1045. doi: 10.1002/mus.21000. [DOI] [PubMed] [Google Scholar]
- Hoover S., Zottoli T.M., Grose-Fifer J. ERP correlates of malingered executive dysfunction. Int. J. Psychophysiol. 2014;91:139–146. doi: 10.1016/j.ijpsycho.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Kofler M. Functional organization of exteroceptive inhibition following nociceptive electrical fingertip stimulation in humans. Clin. Neurophysiol. 2003;114:973–980. doi: 10.1016/s1388-2457(03)00060-9. [DOI] [PubMed] [Google Scholar]
- Koller W., Lang A., Vetere-Overfield B., Findley L., Cleeves L., Factor S., Singer C., Weiner W. Psychogenic tremors. Neurology. 1989;39:1094–1099. doi: 10.1212/wnl.39.8.1094. [DOI] [PubMed] [Google Scholar]
- Kranick S.M., Moore J.W., Yusuf N., Martinez V.T., Lafaver K., Edwards M.J. Action-effect binding is decreased in motor conversion disorder: implications for sense of agency. Mov. Disord. 2013;28:1110–1116. doi: 10.1002/mds.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H., Valls-Solé J., Valldeoriola F., Marti M.J., Sanegre M.T., Tolosa E. Transient arrest of psychogenic tremor induced by contralateral ballistic movements. Neurosci. Lett. 2004;370:135–139. doi: 10.1016/j.neulet.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Lang A.E., Koller W.C., Fahn S. Psychogenic parkinsonism. Arch. Neurol. 1995;52:802–810. doi: 10.1001/archneur.1995.00540320078015. [DOI] [PubMed] [Google Scholar]
- Lopergolo D., Isak B., Gabriele M., Onesti E., Ceccanti M., Capua G., Fionda L., Biasiotta A., Di Stefano G., La Cesa S., Frasca V., Inghilleri M. Cutaneous silent period recordings in demyelinating and axonal polyneuropathies. Clin. Neurophysiol. 2015;126:1780–1789. doi: 10.1016/j.clinph.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Macerollo A., Batla A., Kassavetis P., Parees I., Bhatia K.P., Edwards M.J. Using reaction time and co-contraction to differentiate acquired (secondary) from functional ‘fixed’ dystonia. J. Neurol. Neurosurg. Psychiatry. 2015;86:933–934. doi: 10.1136/jnnp-2014-309040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macerollo A., Chen J.C., Pareés I., Kassavetis P., Kilner J.M., Edwards M.J. Sensory attenuation assessed by sensory evoked potentials in functional movement disorders. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modugno N., Currà A., Giovannelli M., Priori A., Squitieri F., Ruggieri S., Manfredi M., Berardelli A. The prolonged cortical silent period in patients with Huntington’s disease. Clin. Neurophysiol. 2001;112:1470–1474. doi: 10.1016/s1388-2457(01)00599-5. [DOI] [PubMed] [Google Scholar]
- Nzwalo H., de Abreu D., Swash M., Pinto S., de Carvalho M. Delayed diagnosis in ALS: the problem continues. J. Neurol. Sci. 2014;343:173–175. doi: 10.1016/j.jns.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Oranje B., Geyer M.A., Bocker K.B., Leon Kenemans J., Verbaten M.N. Prepulse inhibition and P50 suppression: commonalities and dissociations. Psychiatry Res. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- O’Suilleabhain P.E., Matsumoto J.Y. Time-frequency analysis of tremors. Brain. 1998;121:2127–2134. doi: 10.1093/brain/121.11.2127. [DOI] [PubMed] [Google Scholar]
- Pareés I., Brown H., Nuruki A., Adams R.A., Davare M., Bhatia K.P., Friston K., Edwards M.J. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain. 2014;137:2916–2921. doi: 10.1093/brain/awu237. [DOI] [PubMed] [Google Scholar]
- Péréon Y., Nguyen The Tich S., Fournier E., Genet R., Guihéneuc P. Electrophysiological recording of deep tendon reflexes: normative data in children and in adults. Neurophysiol. Clin. 2004;34:131–139. doi: 10.1016/j.neucli.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Priori A., Berardelli A., Inghilleri M., Accornero N., Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain. 1994;117:317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- Ricciardi L., Demartini B., Morgante F., Parees I., Nielsen G., Edwards M.J. Symptom severity in patients with functional motor symptoms: patient’s perception and doctor’s clinical assessment. Parkinsonism Relat. Disord. 2015;21:529–532. doi: 10.1016/j.parkreldis.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Ricciardi L., Demartini B., Crucianelli L., Krahé C., Edwards M.J., Fotopoulou A. Interoceptive awareness in patients with functional neurological symptoms. Biol. Psychol. 2015;113:68–74. doi: 10.1016/j.biopsycho.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Rona S., Berardelli A., Vacca L., Inghilleri M., Manfredi M. Alterations of motor cortical inhibition in patients with dystonia. Mov. Disord. 1998;13:118–124. doi: 10.1002/mds.870130123. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A., Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingenschuh P., Katschnig P., Edwards M.J., Teo J.T., Korlipara L.V., Rothwell J.C., Bhatia K.P. The blink reflex recovery cycle differs between essential and presumed psychogenic blepharospasm. Neurology. 2011;76:610–614. doi: 10.1212/WNL.0b013e31820c3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J. Neurol. Neurosurg. Psychiatry. 2004;75:121–125. [PMC free article] [PubMed] [Google Scholar]
- Stone J., Wojcik W., Durrance D., Carson A., Lewis S., MacKenzie L., Warlow C.P., Sharpe M. What should we say to patients with symptoms unexplained by disease? The “number needed to offend”. BMJ. 2002;325:1449–1450. doi: 10.1136/bmj.325.7378.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Smyth R., Carson A., Lewis S., Prescott R., Warlow C., Sharpe M. Systematic review of misdiagnosis of conversion symptoms and “hysteria”. BMJ. 2005;331:989. doi: 10.1136/bmj.38628.466898.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Warlow C., Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537–1551. doi: 10.1093/brain/awq068. [DOI] [PubMed] [Google Scholar]
- Stone J., Carson A. Functional neurologic symptoms: assessment and management. Neurol. Clin. 2011;29:1–18. doi: 10.1016/j.ncl.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Stone J., Warlow C., Sharpe M. Functional weakness: clues to mechanism from the nature of onset. J. Neurol. Neurosurg. Psychiatry. 2012;83:67–69. doi: 10.1136/jnnp-2011-300125. [DOI] [PubMed] [Google Scholar]
- Taylor P.K. CMAP dispersion, amplitude decay, and area decay in a normal population. Muscle Nerve. 1993;16:1181–1187. doi: 10.1002/mus.880161107. [DOI] [PubMed] [Google Scholar]
- Thompson P.D., Colebatch J.G., Brown P., Rothwell J.C., Day B.L., Obeso J.A., Marsden C.D. Voluntary stimulus-sensitive jerks and jumps mimicking myoclonus or pathological startle syndromes. Mov. Disord. 1992;7:257–262. doi: 10.1002/mds.870070312. [DOI] [PubMed] [Google Scholar]
- Tinazzi M., Simonetto S., Franco L., Bhatia K.P., Moretto G., Fiaschi A., Deluca C. Abduction finger sign: a new sign to detect unilateral functional paralysis of the upper limb. Mov. Disord. 2008;23:2415–2419. doi: 10.1002/mds.22268. [DOI] [PubMed] [Google Scholar]
- Treede R.D., Lankers J., Frieling A., Zangemeister W.H., Kunze K., Bromm B. Cerebral potentials evoked by painful, laser stimuli in patients with syringomyelia. Brain. 1991;114:1595–1607. doi: 10.1093/brain/114.4.1595. [DOI] [PubMed] [Google Scholar]
- Treede R.D. Neurophysiological studies of pain pathways in peripheral and central nervous system disorders. J. Neurol. 2003;250:1152–1161. doi: 10.1007/s00415-003-0237-7. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L., Susani E., Riva M.A., Cesana G., Ferrarese C., Appollonio I. Positive signs of functional weakness. J. Neurol. Sci. 2014;340:13–18. doi: 10.1016/j.jns.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J. Neurophysiological assessment of trigeminal nerve reflexes in disorders of central and peripheral nervous system. Clin. Neurophysiol. 2005;116:2255–2265. doi: 10.1016/j.clinph.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J. Assessment of excitability in brainstem circuits mediating the blink reflex and the startle reaction. Clin. Neurophysiol. 2012;123:13–20. doi: 10.1016/j.clinph.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J., Valldeoriola F., Molinuevo J.L., Cossu G., Nobbe F. Prepulse modulation of the startle reaction and the blink reflex in normal human subjects. Exp. Brain Res. 1999;129:49–56. doi: 10.1007/s002210050935. [DOI] [PubMed] [Google Scholar]
- Veciana M., Valls-Solé J., Rubio F., Callén A., Robles B. Laser evoked potentials and prepulse inhibition of the blink reflex in patients with Wallenberg’s syndrome. Pain. 2005;117:443–449. doi: 10.1016/j.pain.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Vetrugno R., Liguori R., Cortelli P., Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin. Auton. Res. 2003;13:256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- Vucic S., Black K., Chong P.S., Cros D. Multifocal motor neuropathy with conduction block: distribution of demyelination and axonal degeneration. Clin. Neurophysiol. 2007;118:124–130. doi: 10.1016/j.clinph.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Williams D.T., Ford B., Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv. Neurol. 1995;65:231–257. [PubMed] [Google Scholar]
- Zarkowski P., Esparza B., Russo J. Validation of a rational malingering test using evoked potentials. J. Clin. Neurophysiol. 2007;24:413–418. doi: 10.1097/WNP.0b013e31812f6be9. [DOI] [PubMed] [Google Scholar]