Highlights
-
•
LICI repeatability showed a large variation at the subject level and ISI level.
-
•
Good repeatability at group level decreased when including inter-subject variation.
-
•
Added value of robot-guided coil positioning seems limited for paired pulse TMS.
Keywords: Transcranial magnetic stimulation, TMS, Repeatability, Long intracortical inhibition, LICI, Coil positioning
Abstract
Objectives
Transcranial magnetic stimulation (TMS) is widely used to assess cortical excitability. To detect changes in excitability with longitudinal studies, it is important to validate the repeatability of excitability measures within a subject between different sessions. Repeatability studies on long intracortical inhibition (LICI) are limited and reported agreement ranges from poor to good. This study aims to evaluate the repeatability of LICI in healthy subjects using paired pulse TMS. In addition, it investigates whether LICI repeatability differs for manual and robot-guided coil positioning.
Methods
Thirty healthy subjects (10 males, mean age 28.4 ± 8.2 years) were studied twice, approximately one week apart. Both motor cortices were stimulated with 50 paired pulses (intensity 120% of resting motor threshold) at interstimulus intervals (ISIs): 50, 100, 150, 200, 250 and 300 ms. In twenty subjects a figure-of-eight coil was positioned and held in place manually during both sessions, while in ten subjects a robot-navigated arm was used. LICI repeatability was assessed using the intraclass correlation coefficient (ICC).
Results
For manual and robot-guided coil positioning we found a large variation in repeatability at the subject level and ISI level, ranging from poor to good agreement. On a group level, we found good repeatability for averaged LICI curves (manual: ICC = 0.91, robot-guided: ICC = 0.95), which decreased when individual curves were correlated between sessions (manual: ICC = 0.76, robot-guided: ICC = 0.84).
Conclusion
For a correct interpretation of longitudinal study outcomes it is important to know the subject specific LICI repeatability and to analyze each ISI individually. Furthermore, the added value of robot-guided coil positioning for paired pulse TMS seems limited.
Significance
The large variation in LICI repeatability at the subject level and ISI level should be taken into account in longitudinal studies, while robot-guided coil positioning seems unnecessary.
1. Introduction
Since the introduction of transcranial magnetic stimulation (TMS) in 1985 as a method to directly stimulate the human motor cortex (Barker et al., 1985), TMS has been widely used to assess cortical excitability (Ferreri and Rossini, 2013, Kujirai et al., 1993, Valls-Solé et al., 1992). Single pulse TMS measures the global excitability of cortical interneurons, corticospinal pathways and spinal motor neurons (Abbruzzese and Trompetto, 2002, Valls-Solé et al., 1992), where paired pulse TMS focuses more on the excitability of cortical neurons only (Abbruzzese and Trompetto, 2002, Kujirai et al., 1993). By varying the interval between the paired pulses, information can be obtained from excitatory and inhibitory networks. Short intracortical inhibition (SICI) is observed when applying a sub-threshold conditioning pulse 1–5 ms before a supra-threshold test pulse (Kujirai et al., 1993). Increasing this interstimulus interval (ISI) to 6–30 ms results in intracortical facilitation (ICF) (Kujirai et al., 1993, Ziemann et al., 1996). Long intracortical inhibition (LICI) occurs when applying a supra-threshold conditioning pulse 50–400 ms before a supra-threshold test pulse (Valls-Solé et al., 1992). It is thought that suppression and facilitation of the test response arises from inhibitory and excitatory mechanisms at the level of the cerebral cortex rather than the spinal cord (Di Lazzaro et al., 1998, Hanajima et al., 1998, Kujirai et al., 1993). Gamma-aminobutyric acid (GABA)-A receptor mediated inhibitory mechanisms are likely to contribute to SICI (Hanajima et al., 1998, Ilić et al., 2002, Kujirai et al., 1993), GABA-B receptor mediated inhibition to LICI (McDonnell et al., 2006, Werhahn et al., 1999), and strong N-methyl-d-aspartate (NMDA) receptor mediated facilitation (Schwenkreis et al., 1999, Ziemann et al., 1998) combined with weaker GABA-A receptor mediated inhibition to ICF (Hanajima et al., 1998). As paired pulse TMS provides a direct measure of cortical excitability, it is a commonly used paradigm in a variety of neurological conditions like Alzheimer’s disease, amyotrophic lateral sclerosis, chronic pain, epilepsy, migraine, Parkinson’s disease and stroke (Chen et al., 2008, Ni and Chen, 2015).
Longitudinal studies can be used to monitor the disease process, or to evaluate the effect of a (therapeutic) intervention. Individual subjects are followed over time, instead of comparing groups of subjects at a specific moment in time as in transversal (cross-sectional) studies (Badawy et al., 2012, Kimiskidis et al., 2004). At present, a transversal design can only differentiate patients from healthy subjects at a group level, due to the high inter-subject variability of excitability measures (Boroojerdi et al., 2000, Du et al., 2014, Orth et al., 2003, Wassermann, 2002). To detect individual changes in cortical excitability with a longitudinal design, it is important to validate the repeatability of excitability measures within the same subject between different TMS sessions (Badawy et al., 2012, Fleming et al., 2012, Hermsen et al., 2016). Although the inter-session variability is lower than the inter-subject variability (Boroojerdi et al., 2000, Du et al., 2014, Orth et al., 2003, Wassermann, 2002), mixed degrees of repeatability are found in healthy subjects for paired pulse TMS. Studies assessing SICI and ICF reported low to moderate inter-session variability (Badawy et al., 2012, Boroojerdi et al., 2000, Orth et al., 2003). Furthermore, test–retest reliability varied from moderate to good for SICI (Du et al., 2014, Fleming et al., 2012, Hermsen et al., 2016, Maeda et al., 2002), and from poor to good for ICF (Du et al., 2014, Fleming et al., 2012, Hermsen et al., 2016, Maeda et al., 2002).
Repeatability studies on LICI are limited. Initially, Farzan et al. (2010) reported high test–retest reliability (Farzan et al., 2010), and Badawy et al. (2012) low inter-session variability for LICI (Badawy et al., 2012). However, recently Du et al. (2014) found poor reliability for long ISIs (30–500 ms) when applying a sub-threshold conditioning pulse followed by a supra-threshold test pulse (Du et al., 2014). Although LICI is generally induced by two supra-threshold pulses, this latter study indicates that LICI repeatability might not be as optimal as initially shown and needs to be further investigated.
Inter-session variability observed in longitudinal studies is assumed to be due, at least partially, to inaccuracies in positioning and handling of the TMS coil. Navigation methods can be used to ensure accurate coil positioning within and between consecutive sessions (Lefaucheur, 2010). The easiest and most conventional method of coil positioning is to use signature outputs, like motor responses or phosphenes, to identify the cortical area for stimulation (Barker et al., 1985, Lefaucheur, 2010, Rossini et al., 2015). More accurate methods include robot-guided positioning or stereotaxic neuronavigation (Lefaucheur, 2010, Sparing et al., 2008).
In this study we evaluate the repeatability of LICI in healthy subjects using paired pulse TMS. In addition, we investigate whether LICI repeatability differs for manual and robot-guided positioning of the TMS coil.
2. Materials and methods
The study protocol (trial ID: NL49854.044.14) was approved by the local medical ethics committee (Medisch Spectrum Twente, Enschede, the Netherlands) and was in accordance with the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013). We followed the guidelines for the use of TMS in clinical practice and research (Rossi et al., 2009).
2.1. Subjects
Healthy adults (18 years or older) with no personal history of epilepsy or brain lesion(s) were included. Subjects were excluded if they were taking pro-epileptogenic medication, had implanted devices (cochlear implant or deep brain stimulator), had metal objects in their brain or skull, or had a history of spinal cord surgery. In addition, females were excluded if there was a possibility of pregnancy.
All included subjects gave written informed consent and filled out the Screening Questionnaire before TMS (Rossi et al., 2011) and the Dutch Handedness Questionnaire (van Strien, 2003, van Strien, 1992).
2.2. TMS protocol
Subjects were seated comfortably in a chair, with their hands pronated in a relaxed position. They were instructed to keep their eyes open and their head in a fixed position. Subjects underwent the same TMS session twice, under equal circumstances: same investigators, measurement set-up and moment of the day. The second session took place approximately one week later (mean 7.5 days, range 6–15 days).
Paired biphasic TMS pulses, with a pulse duration of 400 μs, were given by a Magstim Rapid2 Stimulator (The Magstim Company Ltd, Whitland, United Kingdom). Both motor hot spots of the abductor digiti minimi (ADM) muscle were stimulated at each ISI with 50 paired pulses. We always started at the left hemisphere, after which the right hemisphere was stimulated. At each side, we first randomly applied ISIs 200, 250 and 300 ms, followed by ISIs 50, 100 and 150 ms in a random order. Because of technical limitations most subjects of the robot-guided coil positioning group could not be stimulated at ISI 50 ms. A random interval of approximately 4 s (range 3.5–4.5 s) was kept between pairs of consecutive pulses. Both the conditioning and test pulse were given at an intensity of 120% the resting motor threshold (rMT). rMT was defined as the minimum stimulation intensity needed to induce at least five motor evoked potentials (MEPs), with a peak-to-peak amplitude of at least 50 μV, out of ten consecutive pulses (Groppa et al., 2012, Rossini et al., 2015).
2.3. Coil positioning
The figure-of-eight aircooled 70 mm coil (The Magstim Company Ltd, Whitland, United Kingdom) was placed tangentially with the handle pointing backwards and laterally at an angle of 45° from the midline. In twenty subjects the coil was positioned and held in place manually during both sessions, always by the same investigator. In the other ten subjects, coil positioning was performed by a robot-navigated system (Advanced Neuro Technology, Enschede, the Netherlands). Subjects were tracked by a Polaris infrared camera system (Northern Digital, Waterloo, Canada), using a headband with four passive reflective markers. A head model was created using a general magnetic resonance image and by collecting three landmarks and approximately 300 additional points on the scalp with a tracking pointer. The location of the ADM hot spot was defined manually and indicated on the head model. A robotic arm, containing the coil, was used for positioning and displacements from the indicated location were detected and actively corrected to ensure accurate coil positioning during the entire session.
2.4. Electromyogram recording and analysis
The electromyogram (EMG) was recorded, from the ADM and abductor pollicis brevis (APB) muscles, with two surface Ag/AgCl electrodes placed in a belly-tendon montage. Although we stimulated the ADM hot spot, we simultaneously recorded the activity of the APB muscle. The ground electrode was placed on the dorsal side of the left hand. EMG was sampled at a frequency of either 2048 Hz (robot-guided coil positioning) or 5000 Hz (manual coil positioning) and recorded using an additional amplifier (TMSi, Oldenzaal, the Netherlands).
Even though subjects were asked to fully relax their ADM and APB muscles, recordings were afterwards checked for muscle pre-activation. Trials containing EMG activity larger than 50 μV in the 50 ms preceding the conditioning pulse were excluded. If more than 25 of the original 50 repetitions were discarded, that specific ISI was not taken into account during further analysis.
The amount of inhibition was determined separately for each subject, ISI and stimulated hemisphere. First, we calculated the mean peak-to-peak amplitude of the conditioning and test response, by taking the average over the fifty (or less) repetitions. Next, we calculated the ratio between this mean amplitude of the second test response (TR) and this mean amplitude of the first conditioning response (CR), expressed as a percentage: TR/CR (%) (Valls-Solé et al., 1992). In each subject we ended up with two LICI ratios for each ISI: one ratio for the dominant and one ratio for the non-dominant hemisphere. This ratio represents inhibition for values below 100% and facilitation for values above 100%.
2.5. Statistical analysis
The intraclass correlation coefficient (ICC) was used to estimate the agreement between repeated sessions; model ICC (3,1): two-way mixed single measures, absolute agreement (Shrout and Fleiss, 1979). Repeatability of LICI was assessed on three levels: 1) ISI, 2) subject and 3) group level.
At the ISI level, we correlated the individual LICI ratios of all subjects measured at a particular ISI during the first session, with all the individual ratios from the second session. We did this for the LICI ratios measured at the dominant or non-dominant hemisphere only, and for the ratios of both hemispheres pooled (two LICI ratios per subject per session).
At the subject level, we correlated the individual LICI ratios of all ISIs measured in a particular subject during the first session, with all the individual ISI ratios from the second session. For this, we pooled the LICI ratios of all ISIs measured at both hemispheres for each subject (two LICI ratios per ISI per session).
At the group level, we calculated for each ISI the mean LICI ratio over all subjects, and evaluated these averaged LICI curves. We correlated the mean LICI ratios of all ISIs measured during the first session, with the mean ratios from the second session. We did this for the mean LICI ratios measured at the dominant or non-dominant hemisphere only, and for the mean ratios of both hemispheres pooled (two LICI ratios per ISI per session). Additionally, we correlated the individual LICI ratios of all ISIs measured in all subjects during the first session, with all the individual ISI ratios from the second session. Again, we did this for each hemisphere separately, and for the ratios of both hemispheres pooled (two LICI ratios per ISI per subject per session).
As ISI 50 ms was not applied in most subjects from the robot-guided coil positioning group, this interval was not included into the ISI and group level analysis in this group. ICC varies between 0 and 1, where 1 represents perfect repeatability. Consistent with Du et al. (2014), we considered ICC values above 0.8 as good, values from 0.6 to 0.8 as moderate and values below 0.6 as poor repeatability (Du et al., 2014).
3. Results
Thirty-four healthy subjects were included in this study. Four subjects were excluded from analysis: one subject was not feeling well during the first session, in one subject it was not possible to perform the second session 1–2 weeks later due to illness, and two subjects had a rMT above 83% of maximum stimulator output, making stimulation at 120% rMT not possible. Except from the first excluded subject, all participants tolerated the paired pulse protocol well and no adverse events happened.
Thirty subjects (10 males, mean age 28.4 ± 8.2 years; range 20–51 years, 27 right-handed) completed the entire study. In twenty subjects coil positioning was performed manually, hereafter referred to as ‘manual group’. Robot-guided coil positioning was applied in ten subjects, referred to as ‘robot group’.
As we continuously stimulated the ADM hot spot and similar results were obtained for the ADM and APB muscles, only outcomes of the ADM muscle are presented below.
3.1. Repeatability of resting motor threshold
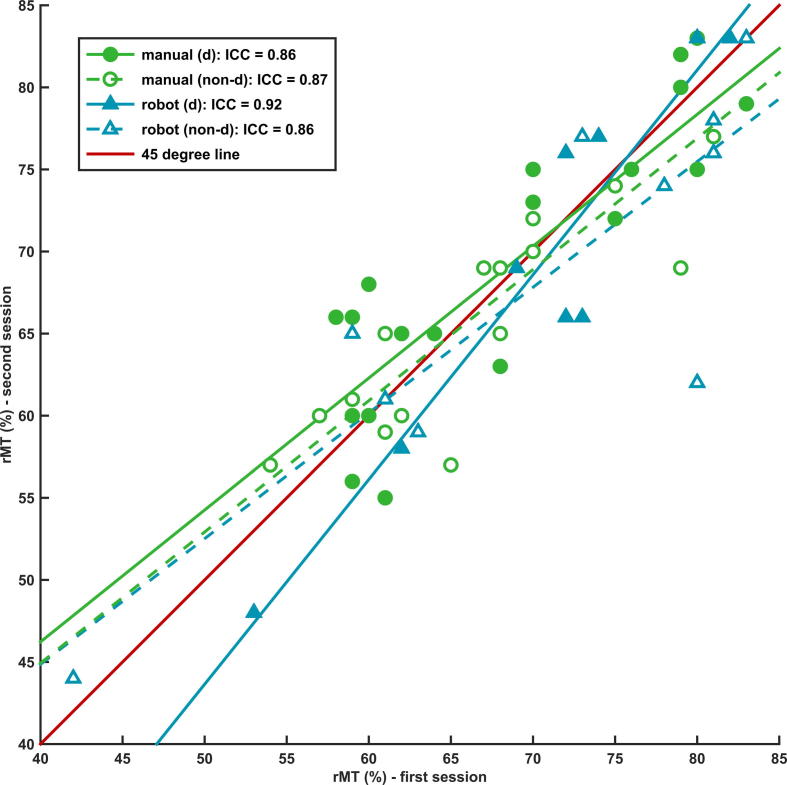
The averaged rMT values of the first and second session are given in Table 1. The ICC showed good repeatability for rMT between repeated sessions in both the manual group (dominant hemisphere: ICC = 0.86, non-dominant hemisphere: ICC = 0.87, overall: ICC = 0.86) and robot group (dominant hemisphere: ICC = 0.92, non-dominant hemisphere: ICC = 0.86, overall: ICC = 0.88), see Fig. 1.
Table 1.
Overview of rMT values (mean ± standard deviation (SD)) of both TMS sessions, separated for manual and robot-guided coil positioning at the dominant and non-dominant hemisphere. rMT is the percentage of maximum stimulator output (1.5 T).
| Coil positioning (hemisphere) | Session 1 rMT (%) | Session 2 rMT (%) |
|---|---|---|
| Manual (dominant) | 67.9 ± 8.5 | 68.6 ± 7.9 |
| Manual (non-dominant) | 67.5 ± 8.0 | 66.9 ± 7.4 |
| Robot-guided (dominant) | 70.8 ± 8.8 | 69.6 ± 11.6 |
| Robot-guided (non-dominant) | 70.1 ± 13.4 | 67.9 ± 11.8 |
Fig. 1.
Repeatability of resting motor threshold. The intraclass correlation coefficient (ICC) showing good repeatability for rMT between the first and second session in the manual (green dots) and robot group (blue triangles); d = dominant hemisphere, non-d = non-dominant hemisphere. The red line represents the 45° line through the origin: perfect repeatability. rMT is the percentage of maximum stimulator output (1.5 T). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Repeatability of long intracortical inhibition
3.2.1. Interstimulus interval level
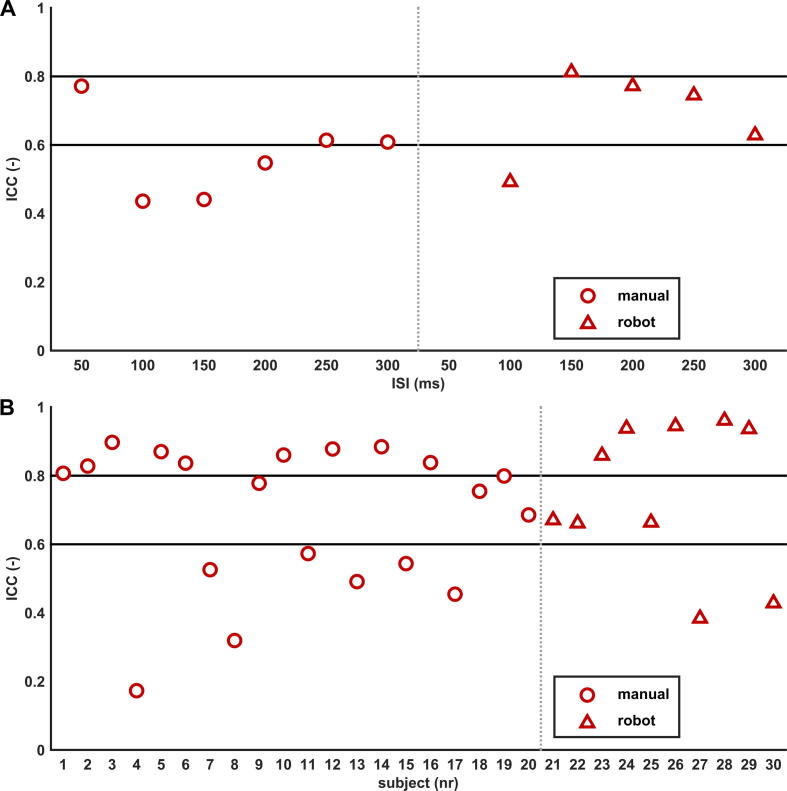
Correlating, for each ISI, the inhibition ratios of all subjects measured during the first and second session showed a large variation in repeatability at the ISI level. In the manual group agreement varied from poor to moderate levels (range ICC: 0.30–0.78), and in the robot group from poor to good levels (range ICC: 0.20–0.92), see Table 2. No statistical differences in repeatability were found between the dominant and non-dominant hemisphere in both groups (manual: p-value = 0.83, robot: p-value = 0.46). When inhibition ratios of both hemispheres were pooled, repeatability was poorest for ISIs 100 and 150 ms (overall: ICC = 0.44) in the manual group and best for ISI 50 ms (overall: ICC = 0.77). In the robot group, repeatability was poorest for ISI 100 ms (overall: ICC = 0.49) and best for ISI 150 ms (overall: ICC = 0.81), see Fig. 2A.
Table 2.
Overview of the intraclass correlation coefficient (ICC) showing the repeatability of inhibition ratios between the first and second session for each interstimulus interval (ISI), separated for manual and robot-guided coil positioning at the dominant and non-dominant hemisphere.
| ISI (ms) | Manual (dominant) | Manual (non-dominant) | Robot-guided (dominant) | Robot-guided (non-dominant) |
|---|---|---|---|---|
| 50 | 0.78 | 0.77 | – | – |
| 100 | 0.39 | 0.45 | 0.70 | 0.20 |
| 150 | 0.30 | 0.56 | 0.85 | 0.78 |
| 200 | 0.56 | 0.51 | 0.61 | 0.87 |
| 250 | 0.61 | 0.62 | 0.61 | 0.92 |
| 300 | 0.66 | 0.47 | 0.38 | 0.81 |
Fig. 2.
Repeatability of LICI at subject and ISI level. The intraclass correlation coefficient (ICC) showing the repeatability of inhibition ratios between the first and second session for A. each interstimulus interval (ISI) and B. each subject. Repeatability of the manual group is shown in red dots and of the robot group in red triangles; inhibition ratios of both hemispheres pooled. The horizontal black lines, at ICC = 0.6 and 0.8, represent thresholds for moderate and good repeatability. Overall, a large variation in repeatability is seen for ISIs (2A) and subjects (2B), ranging from poor to good levels of agreement. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Subject level
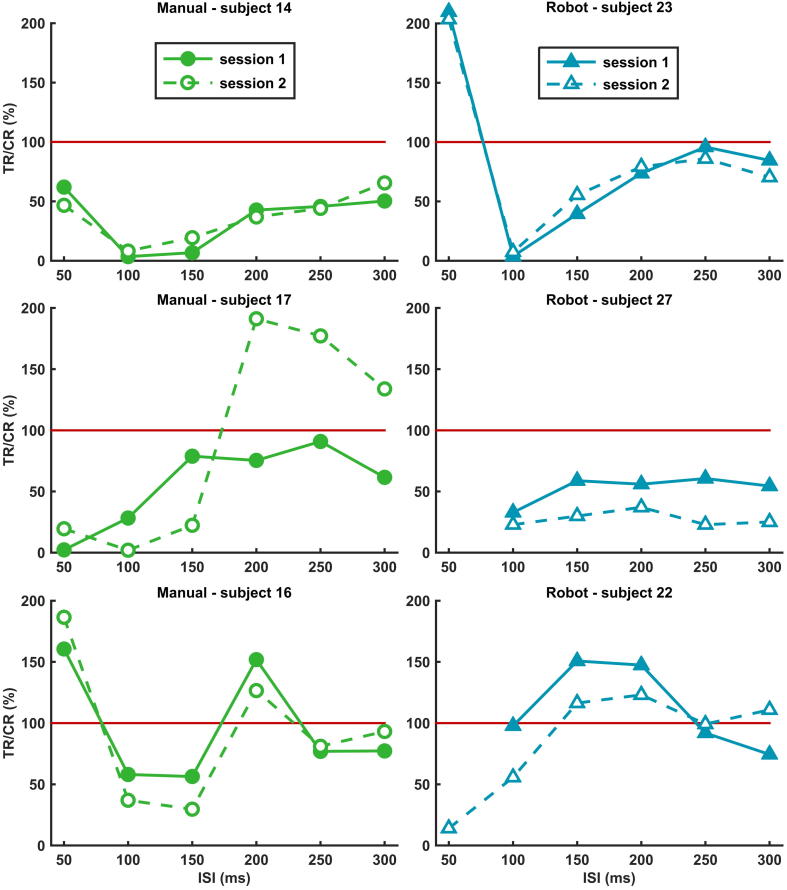
To assess the repeatability of LICI at an individual level, we pooled the inhibition ratios of all ISIs measured at both hemispheres for each subject. There was a large variation in LICI curves and LICI repeatability between subjects, see Figs. 2B and 3. In the manual group, 45% of the subjects showed good (ICC > 0.8), 20% moderate (0.6 ⩽ ICC ⩽ 0.8) and 35% poor (ICC < 0.6) repeatability. In the robot group, 50% showed good, 30% moderate and 20% poor repeatability.
Fig. 3.
Repeatability of LICI at subject level. Examples showing the large variation in repeatability between subjects of the manual (in green) and robot group (in blue). At the top, two examples of subjects where LICI curves showed good repeatability between both sessions. In the middle, examples of poor repeatability and at the bottom examples where LICI curves are vertically shifted between sessions while retaining their shape. Values below the red line (100%) represent inhibition and values above facilitation; TR = test response, CR = conditioning response, ISI = interstimulus interval. All examples are from the dominant hemisphere. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Group level
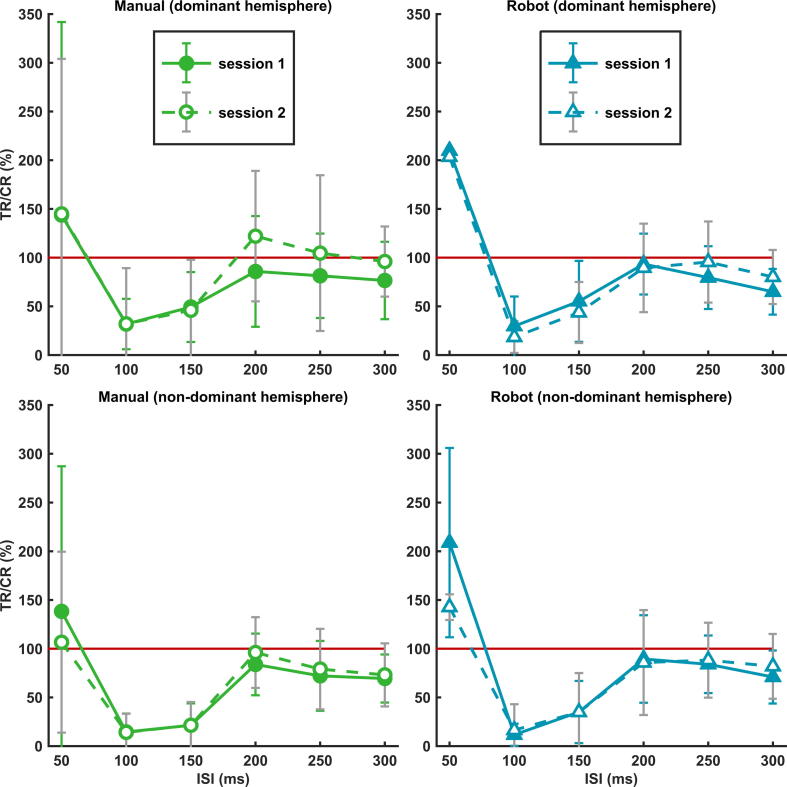
The averaged LICI curves of all subjects for both sessions are shown in Fig. 4, separated for both hemispheres in the manual and robot group. The LICI curves show great similarities; facilitation for ISI 50 ms and inhibition for ISIs 100–300 ms. The only exceptions are ISIs 200 and 250 ms, where slight facilitation was measured in the manual group at the dominant hemisphere during the second session. Correlating the mean LICI ratios of all ISIs measured during the first and second session, showed good repeatability in the manual (dominant hemisphere: ICC = 0.89, non-dominant hemisphere: ICC = 0.94, overall: ICC = 0.91) and robot group (dominant hemisphere: ICC = 0.91, non-dominant hemisphere: ICC = 0.98, overall: ICC = 0.95). However, when individual LICI ratios of all ISIs measured in all subjects of each group were correlated between sessions, the manual group showed moderate (dominant hemisphere: ICC = 0.73, non-dominant hemisphere: ICC = 0.80, overall: ICC = 0.76) and the robot group good LICI repeatability (dominant hemisphere: ICC = 0.81, non-dominant hemisphere: ICC = 0.86, overall: ICC = 0.84).
Fig. 4.
Repeatability of LICI at group level. The averaged LICI curves (mean ± SD) of all subjects for both sessions, separated for manual (in green) and robot-guided coil positioning (in blue) at the dominant (at the top) and non-dominant hemisphere (at the bottom). Values below the red line (100%) represent inhibition and values above facilitation; TR = test response, CR = conditioning response, ISI = interstimulus interval. All LICI curves showed good repeatability and great similarities; facilitation for ISI 50 ms and inhibition for ISIs 100–300 ms (exceptions are ISIs 200 and 250 ms in the manual group during the second session at the dominant hemisphere). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study we evaluated the repeatability of LICI in healthy subjects using paired pulse TMS. For manual and robot-guided coil positioning we found a large variation in repeatability for individual subjects and ISIs, ranging from poor to good levels of agreement. On a group level, good repeatability was found for the averaged LICI curves, which decreased when individual curves were correlated between sessions. Similar results were obtained for the ADM and APB muscles. In addition, rMT showed good repeatability in both groups.
4.1. Repeatability at interstimulus interval level
In the manual group, repeatability varied from poor to moderate levels at the ISI level. Repeatability was poorest for ISIs 100 and 150 ms (overall: ICC = 0.44) and best for ISI 50 ms (overall: ICC = 0.77). In the robot group, repeatability ranged from poor to good; poorest for ISI 100 ms (overall: ICC = 0.49) and best for ISI 150 ms (overall: ICC = 0.81). These outcomes are in-between the findings by Du et al. (2014) and Badawy et al. (2012). The first study reported poor agreement for ISIs 30–500 ms; even ICC < 0.1 for ISIs 120–500 ms (Du et al., 2014). The higher repeatability reported by us might be the result of a difference in the calculation of the inhibition ratio. We compared the test response to the conditioning response, instead of to the unconditioning single pulse response, which reflects a more direct modulation effect. Furthermore, in their study LICI was not induced by two supra-threshold pulses. Badawy et al. (2012) reported good agreement for ISIs 50–400 ms (range rho-c: 0.93–0.95); highest for ISI 50 ms (Badawy et al., 2012). The fact that their outcomes were based on a group analysis, while we correlated inhibition ratios of individual subjects between sessions, seems to explain their high agreement levels. We found similar repeatability at a group level, which decreased when including the inter-subject variability at the ISI level. Although Badawy et al. (2012) reported correlation quantified by rho-c (Badawy et al., 2012), we used ICC, as this is a more common method to estimate repeatability (Nickerson, 1997). Except for a term that decreases with increasing number of subjects, both coefficient equations are identical (Lin, 1989, Nickerson, 1997). Our data was only marginally affected when using rho-c.
The observed large variation in repeatability between ISIs indicates that it is preferred to analyze each ISI individually, instead of combining ISIs. Which repeatability for each ISI is required ultimately depends on the research question and study population. For example, in epilepsy research it appears that especially ISIs 2, 5, 250 and 300 ms differ significantly between patients and controls (de Goede et al., 2016). Therefore repeatability should be optimal for these particular ISIs, but not necessarily for the others.
We can only speculate about what causes the large variation in repeatability. Recently, Opie et al. (2016) suggested that there might be a difference between ISIs in the relative contribution of inhibitory mechanisms associated with LICI. They speculated that activation of both pre- and post-synaptic GABA-B receptors may contribute to LICI at ISI 100 ms, whereas at ISI 150 ms solely pre-synaptic GABA-B receptors are activated (Opie et al., 2016). In addition, the variation in repeatability may be partly due to habituation or loss of attention that might have occurred during the TMS session. Repeatability was especially poor for ISI 100 ms, an interval that was applied in the second part of the session. However, better agreement levels were found for ISIs 50 and 150 ms, which were also applied during the second part.
4.2. Repeatability at subject level
LICI curves and corresponding LICI repeatability varied widely between subjects, with repeatability ranging from poor to good. Du et al. (2014) also described a large variation in response profiles of healthy subjects. Despite a large variance across subjects, they stated that individuals appear to have a unique inhibition-facilitation profile that remains relatively stable during repeated sessions (Du et al., 2014). Indeed, Fig. 3 shows examples of good repeatability (at the top) and cases where LICI curves are vertically shifted while retaining their shape (at the bottom). However, in other subjects the poor to moderate repeatability was the result of two completely different shaped curves.
Our study indicates that LICI repeatability is subject specific and shows a high inter-subject variability, just as other excitability measures (e.g. the LICI curve). This observed large variation may limit the applicability of TMS as a tool to monitor the disease process or to evaluate the effect of an intervention. For the interpretation of longitudinal study results, it is necessary to know the subject specific LICI repeatability to prevent erroneous interpretation that may result from large variability. Furthermore, studies with multiple repeated sessions and longer inter-session periods are needed to investigate the long-term repeatability and stability of LICI. For example, Kimiskidis et al. (2004) demonstrated the stability of the corticomotor threshold on an individual and group level, using seven sessions over a period of five years (Kimiskidis et al., 2004).
We performed the same TMS session twice, under equal circumstances. Approximately half of the subjects showed similar LICI curves both times, while the other half had two (completely) different curves. The large variation in repeatability, might be due to a difference in coil positioning between sessions. Perhaps positioning is more critical in certain subjects because of their individual sulcus anatomy. Other explanations for a poor LICI repeatability could be a difference in mental state between sessions, or differences in sleep pattern, and/or intake of neuroactive substances (like alcohol, caffeine or nicotine) during the periods before the two sessions. Furthermore, females were measured during different phases of their menstrual cycle, as approximately one week was kept between repeated sessions. Despite inconclusive findings (Cerqueira et al., 2006, Conte et al., 2007, Hattemer et al., 2007, Huber et al., 2013, Kähkönen et al., 2003, Lang et al., 2008, Orth et al., 2005, Silvanto and Pascual-Leone, 2008, Smith et al., 1999, Ziemann et al., 1995, Zoghi et al., 2015), all these factors are potential confounders of cortical excitability that may contribute to poor repeatability. We have deliberately chosen not to compensate for these factors because, in order for TMS to become a clinical tool, relevant changes in cortical excitability should outweigh these potential confounders.
4.3. Repeatability at group level
Repeatability was good for the averaged LICI curves: manual (overall: ICC = 0.91) and robot group (overall: ICC = 0.95). These findings are similar to the low inter-session LICI variability reported by Badawy et al. (2012): range rho-c: 0.93–0.95 (Badawy et al., 2012), and the high test–retest reliability in the motor cortex for LICI reported by Farzan et al. (2010): Cronbach’s alpha = 0.88 (Farzan et al., 2010). Due to the averaging over subjects, it was still possible to find very high repeatability at a group level. However, repeatability decreased when individual LICI curves were correlated between sessions: manual (overall: ICC = 0.76) and robot group (overall: ICC = 0.84).
4.4. Repeatability of manual and robot-guided coil positioning
Minimal differences in LICI repeatability were found when comparing manual to robot-guided coil positioning. Although repeatability was slightly higher for robot-guided positioning, also in this group poor repeatability was found for individual subjects and ISIs. Fleming et al. (2012) did not find any significant differences in SICI or ICF reliability between a hand-held and navigated figure-of-eight coil (Fleming et al., 2012). Neuronavigation did not further improved SICI and ICF reliability, just as in our study robot-guided coil positioning did not improved LICI repeatability. Hence, the added value of neuronavigation or robot-guided coil positioning in paired pulse TMS studies seems limited and unnecessary. This would make the applicability of TMS as a tool for diagnostics and/or therapy evaluation easier. However, it should be noted that subjects participated in either the manual or robot-guided coil positioning group, and did not underwent both types of coil positioning. As no direct comparison was made, this study only provides indirect evidence for the limited added value of robot-guided coil positioning.
4.5. Limitations
To assess the LICI repeatability at the subject level and group level, inhibition ratios of multiple ISIs and both hemispheres were pooled. As outcomes from the dominant and non-dominant hemisphere or from different ISIs are (likely) dependent within subjects, data pooling violates the ICC assumption of independency. Thus, data pooling might lead to type I errors.
To compensate for muscle pre-activation, we rejected trials containing EMG activity in the 50 ms preceding stimulation. We only checked for EMG activity prior to the conditioning pulse, as for ISIs 50 and 100 ms the period between the end of the MEP and the second test pulse was too short. If less than 25 trials remained, that specific ISI was not taken into account. Although it is unknown how many pulses are needed for LICI estimation, a minimum of 20 and 25 pulses is needed for reliable SICI and ICF estimation (Chang et al., 2016), and at least 20–30 trials for single pulse TMS (Goldsworthy et al., 2016). Overall, less than 4% of the ISIs were not taken into account, of which 60% belonged to one subject (nr. 4). We compensated for muscle pre-activation because, due to spinal facilitation, MEP amplitudes are larger in contracted than in relaxed muscles (Abbruzzese and Trompetto, 2002, Hess et al., 1987, Hess et al., 1986, Wassermann et al., 2008), resulting in an overestimation of the peak-to-peak amplitude. However, since we calculated the ratio between mean MEP amplitudes (TR/CR (%)), EMG trial rejection might not even be necessary in case the conditioning and test response are equally affected by muscle pre-activation. Paired pulse studies are needed to investigate the influence of muscle pre-activation on inhibition ratios.
Another limitation is that robot-guided coil positioning was applied without neuronavigation. Navigation based on neuro-imaging enables coil positioning based on the underlying brain anatomy by selecting the stimulation regions on the image data. However, Fleming et al. (2012) found that neuronavigation did not further improved SICI and ICF reliability (Fleming et al., 2012).
5. Conclusion
A large variation in repeatability was found at the level of individual subjects and ISIs. While good repeatability was found for averaged LICI curves on a group level, it decreased when the inter-subject variability was taken into account. For the applicability of TMS as a clinical tool, the focus should move from a group level towards the level of an individual patient. For a correct interpretation of longitudinal study outcomes it is important to know the subject specific LICI repeatability and to analyze each ISI individually. The limited added value of robot-guided coil positioning in paired pulse TMS studies, makes it easier to use TMS as a tool for diagnostics and/or therapy evaluation.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Acknowledgements
The authors wish to thank Carin Eertman and Esther ter Braack for their assistance during the paired pulse TMS measurements, and all the subjects for their participation.
This study was financed by the Dutch TWIN foundation for neuromodulation. The funding source played no role in the design of the study, collection, analysis and interpretation of the data, and writing of the manuscript.
Contributor Information
Annika A. de Goede, Email: a.a.degoede@utwente.nl.
Michel J.A.M. van Putten, Email: m.j.a.m.vanputten@utwente.nl.
References
- Abbruzzese G., Trompetto C. Clinical and research methods for evaluating cortical excitability. J. Clin. Neurophysiol. 2002;19:307–321. doi: 10.1097/00004691-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Badawy R.A.B., Jackson G.D., Berkovic S.F., Macdonell R.A.L. Inter-session repeatability of cortical excitability measurements in patients with epilepsy. Epilepsy Res. 2012;98:182–186. doi: 10.1016/j.eplepsyres.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Barker A.T., Jalinous R., Freeston I.L. Non-invasive magnetic stimulation of human motor cortex. The Lancet. 1985;325:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B., Kopylev L., Battaglia F., Facchini S., Ziemann U., Muellbacher W. Reproducibility of intracortical inhibition and facilitation using the paired-pulse paradigm. Muscle Nerve. 2000;23:1594–1597. doi: 10.1002/1097-4598(200010)23:10<1594::aid-mus19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Cerqueira V., de Mendonça A., Minez A., Dias A.R., de Carvalho M. Does caffeine modify corticomotor excitability? Neurophysiol. Clin. Neurophysiol. 2006;36:219–226. doi: 10.1016/j.neucli.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Chang W.H., Fried P.J., Saxena S., Jannati A., Gomes-Osman J., Kim Y.-H. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin. Neurophysiol. 2016 doi: 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Cros D., Curra A., Di Lazzaro V., Lefaucheur J.-P., Magistris M.R. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Conte A., Gilio F., Iezzi E., Frasca V., Inghilleri M., Berardelli A. Attention influences the excitability of cortical motor areas in healthy humans. Exp. Brain Res. 2007;182:109–117. doi: 10.1007/s00221-007-0975-3. [DOI] [PubMed] [Google Scholar]
- de Goede A.A., ter Braack E.M., van Putten M.J.A.M. Single and paired pulse transcranial magnetic stimulation in drug naïve epilepsy. Clin. Neurophysiol. 2016;127:3140–3155. doi: 10.1016/j.clinph.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Restuccia D., Oliviero A., Profice P., Ferrara L., Insola A. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp. Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Du X., Summerfelt A., Chiappelli J., Holcomb H.H., Hong L.E. Individualized brain inhibition and excitation profile in response to paired-pulse TMS. J. Mot. Behav. 2014;46:39–48. doi: 10.1080/00222895.2013.850401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan F., Barr M.S., Levinson A.J., Chen R., Wong W., Fitzgerald P.B. Reliability of long-interval cortical inhibition in healthy human subjects: a TMS-EEG study. J. Neurophysiol. 2010;104:1339–1346. doi: 10.1152/jn.00279.2010. [DOI] [PubMed] [Google Scholar]
- Ferreri F., Rossini P.M. TMS and TMS-EEG techniques in the study of the excitability, connectivity, and plasticity of the human motor cortex. Rev. Neurosci. 2013;24 doi: 10.1515/revneuro-2013-0019. [DOI] [PubMed] [Google Scholar]
- Fleming M.K., Sorinola I.O., Newham D.J., Roberts-Lewis S.F., Bergmann J.H.M. The effect of coil type and navigation on the reliability of transcranial magnetic stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2012;20:617–625. doi: 10.1109/TNSRE.2012.2202692. [DOI] [PubMed] [Google Scholar]
- Goldsworthy M.R., Hordacre B., Ridding M.C. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–209. doi: 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R., Ugawa Y., Terao Y., Sakai K., Furubayashi T., Machii K. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J. Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattemer K., Knake S., Reis J., Rochon J., Oertel W.H., Rosenow F. Excitability of the motor cortex during ovulatory and anovulatory cycles: a transcranial magnetic stimulation study. Clin. Endocrinol. (Oxf.) 2007;66:387–393. doi: 10.1111/j.1365-2265.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- Hermsen A.M., Haag A., Duddek C., Balkenhol K., Bugiel H., Bauer S. Test–retest reliability of single and paired pulse transcranial magnetic stimulation parameters in healthy subjects. J. Neurol. Sci. 2016;362:209–216. doi: 10.1016/j.jns.2016.01.039. [DOI] [PubMed] [Google Scholar]
- Hess C.W., Mills K.R., Murray N.M. Responses in small hand muscles from magnetic stimulation of the human brain. J. Physiol. 1987;388:397. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C.W., Mills K.R., Murray N.M. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci. Lett. 1986;71:235–240. doi: 10.1016/0304-3940(86)90565-3. [DOI] [PubMed] [Google Scholar]
- Huber R., Maki H., Rosanova M., Casarotto S., Canali P., Casali A.G. Human cortical excitability increases with time awake. Cereb. Cortex. 2013;23:1–7. doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić T.V., Meintzschel F., Cleff U., Ruge D., Kessler K.R., Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J. Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähkönen S., Wilenius J., Nikulin V.V., Ollikainen M., Ilmoniemi R.J. Alcohol reduces prefrontal cortical excitability in humans: a combined TMS and EEG study. Neuropsychopharmacology. 2003;28:747–754. doi: 10.1038/sj.npp.1300099. [DOI] [PubMed] [Google Scholar]
- Kimiskidis V.K., Papagiannopoulos S., Sotirakoglou K., Kazis D.A., Dimopoulos G., Kazis A. The repeatability of corticomotor threshold measurements. Neurophysiol. Clin. Neurophysiol. 2004;34:259–266. doi: 10.1016/j.neucli.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kujirai T., Caramia M.D., Rothwell J.C., Day B.L., Thompson P.D., Ferbert A. Corticocortical inhibition in human motor cortex. J. Physiol. 1993;471:501. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N., Hasan A., Sueske E., Paulus W., Nitsche M.A. Cortical hypoexcitability in chronic smokers? A transcranial magnetic stimulation study. Neuropsychopharmacology. 2008;33:2517–2523. doi: 10.1038/sj.npp.1301645. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J.-P. Why image-guided navigation becomes essential in the practice of transcranial magnetic stimulation. Neurophysiol. Clin. Neurophysiol. 2010;40:1–5. doi: 10.1016/j.neucli.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Lin L.I.-K. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255. [PubMed] [Google Scholar]
- Maeda F., Gangitano M., Thall M., Pascual-Leone A. Inter-and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin. Neurophysiol. 2002;113:376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- McDonnell M.N., Orekhov Y., Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Ni Z., Chen R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl. Neurodegener. 2015;4 doi: 10.1186/s40035-015-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson C.A.E. A note on “a concordance correlation coefficient to evaluate reproducibility”. Biometrics. 1997;53:1503. [PubMed] [Google Scholar]
- Opie G.M., Rogasch N.C., Goldsworthy M.R., Ridding M.C., Semmler J.G. Investigating TMS–EEG indices of long-interval intracortical inhibition at different interstimulus intervals. Brain Stimulat. 2016 doi: 10.1016/j.brs.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Orth M., Amann B., Ratnaraj N., Patsalos P.N., Rothwell J.C. Caffeine has no effect on measures of cortical excitability. Clin. Neurophysiol. 2005;116:308–314. doi: 10.1016/j.clinph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Orth M., Snijders A.H., Rothwell J.C. The variability of intracortical inhibition and facilitation. Clin. Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Screening questionnaire before TMS: an update. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011;122:1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di I. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: An updated report from an I.F.C.N. Committee Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkreis P., Witscher K., Janssen F., Addo A., Dertwinkel R., Zenz M. Influence of the N-methyl-d-aspartate antagonist memantine on human motor cortex excitability. Neurosci. Lett. 1999;270:137–140. doi: 10.1016/s0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Silvanto J., Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.J., Keel J.C., Greenberg B.D., Adams L.F., Schmidt P.J., Rubinow D.A. Menstrual cycle effects on cortical excitability. Neurology. 1999;53 doi: 10.1212/wnl.53.9.2069. 2069–2069. [DOI] [PubMed] [Google Scholar]
- Sparing R., Buelte D., Meister I.G., Pauš T., Fink G.R. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum. Brain Mapp. 2008;29:82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J., Pascual-Leone A., Wassermann E.M., Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr. Clin. Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- van Strien, J.W., 2003. The Dutch Handedness Questionnaire. URL: hdl.handle.net/1765/956 (accessed 10.10.16).
- van Strien J.W. Classificatie van links-en rechtshandige proefpersonen [Classification of left- and right-handed research participants] Ned. Tijdschr. Voor Psychol. 1992;47:88–92. [Google Scholar]
- Wassermann E., Epstein C., Ziemann U. OUP Oxford; 2008. Oxford Handbook of Transcranial Stimulation. [Google Scholar]
- Wassermann E.M. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin. Neurophysiol. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Werhahn K.J., Kunesch E., Noachtar S., Benecke R., Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U., Chen R., Cohen L.G., Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Lönnecker S., Paulus W. Inhibition of human motor cortex by ethanol – a transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Rothwell J.C., Ridding M.C. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 1996;496:873. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi M., Vaseghi B., Bastani A., Jaberzadeh S., Galea M.P. The effects of sex hormonal fluctuations during menstrual cycle on cortical excitability and manual dexterity (a pilot study) PLoS ONE. 2015;10:e0136081. doi: 10.1371/journal.pone.0136081. [DOI] [PMC free article] [PubMed] [Google Scholar]