Highlights
-
•
Many patterns – the “ictal-interictal continuum” – are associated with seizures to varying degrees.
-
•
The degree to which these patterns should be treated is not known.
-
•
We review significance of these patterns, and propose an approach to treatment.
Keywords: Ictal-interictal continuum, Periodic discharges, Rhythmic delta activity
Abstract
Seizures contribute to patient mortality and are usually treated aggressively. Rhythmic and periodic patterns – the “ictal-interictal continuum” – are often associated with seizures, yet the optimum method of treating these patterns is not known: should they be aggressively suppressed, or monitored without treatment? Understanding which patterns are more strongly associated with seizures and which are highly associated with mortality is important to help the clinician decide how to treat these findings. We present an overview of the etiologies, association with seizures, and mortality of periodic and rhythmic patterns, and one approach to treatment.
1. Introduction
Seizures and status epilepticus carry a high risk of morbidity and mortality. A large seizure burden is associated with unfavorable outcomes after hospitalization; in some settings, every additional hour of seizure recorded on EEG increases the risk of later disability and mortality (De Marchis et al., 2016, Payne et al., 2014). Therefore, recognition of seizures and EEG patterns strongly associated with seizures, is vitally important.
Some EEG patterns are recognizably ictal (i.e., patterns with clinical and EEG improvement after IV AED, epileptic discharges occurring at >2.5 Hz, or those with typical spatiotemporal evolution (Beniczky et al., 2013)), and some are clearly non-ictal. However, neurophysiologists frequently encounter more ambiguous patterns, with periodic discharges or rhythmic activity found in more than one-third of patients undergoing continuous EEG monitoring in a large multicenter database (Lee et al., 2016). This activity lies on a spectrum now known as the “ictal-interictal continuum (Chong and Hirsch, 2005)” (Fig. 1). These patterns present a challenging management situation, especially in comatose patients.
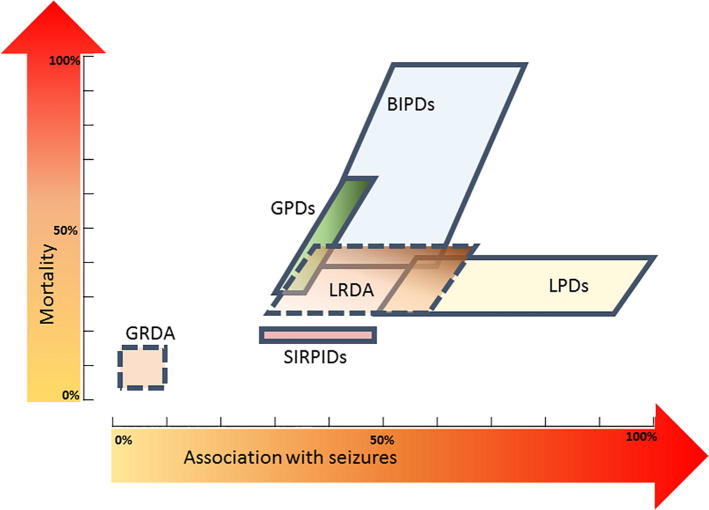
Fig. 1.
Quantitative ictal-Interictal Continuum. This figure (inspired by Chong and Hirsch, 2005) shows the reported relative association with seizures and published mortality of patterns on the ictal-interictal continuum. On the x-axis is increasing association with seizures; on the y-axis is increasing mortality. LPDs = lateralized periodic discharges; BIPDs = bilateral independent periodic discharges; GPDs = generalized periodic discharges; LRDA = lateralized rhythmic delta activity; GRDA = generalized rhythmic delta activity; SIRPIDs = stimulus-induced rhythmic, periodic, or ictal discharges. Mortality rates not available for GRDA and LRDA.
We will review here the prevalence, etiologies, and outcomes of these patterns (Table 1), as well as an approach to treatment.
Table 1.
Reported etiologies, association with seizures, and mortality of periodic and rhythmic patterns.
| Feature | Common causes | Association with seizures | Mortality | |
|---|---|---|---|---|
| Lateralized Periodic Discharges (LPDs) | Stroke Tumor Infection |
Hemorrhage | 50–100% | 24–41% |
| Bilateral Independent Periodic Discharges (BIPDs) | Stroke Anoxic injury Metabolic disorders |
Infection Tumors |
43–78% | 39–100% |
| Generalized Periodic Discharges (GPDs) | Metabolic Sepsis Anoxic |
Stroke | 29–50% | 30–64% |
| Lateralized Rhythmic Delta Activity (LRDA) | Hemorrhage Stroke Tumor |
TBI Infection |
25–63% | |
| Generalized Rhythmic Delta Activity (GRDA) | Encephalopathy Stroke Hemorrhage |
Tumor Infection Drug induced |
No additional association with seizures compared to controls without GRDA | |
| Stimulus-Induced Rhythmic, Periodic, or Ictal Discharges (SIRPIDs) | Hemorrhage Anoxic injury Drug toxicity |
Metabolic TBI |
27–51% One large study found no increase in seizures when features had stimulus-induced compared to spontaneous patterns |
17% |
2. Lateralized periodic discharges (LPDs)
2.1. Definition
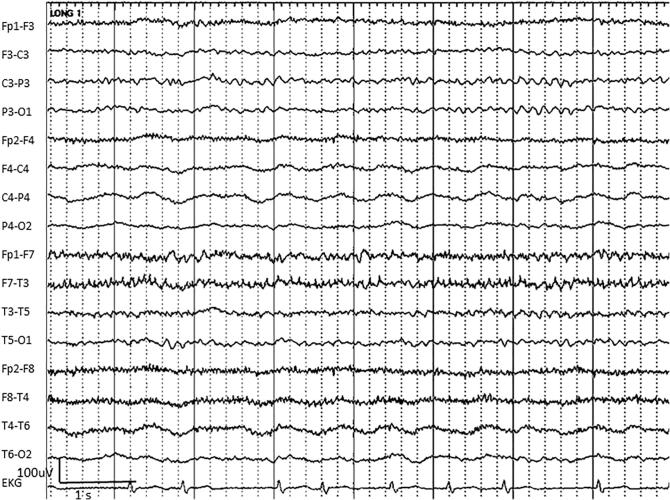
The discharges formerly known as “PLEDs” (periodic lateralized epileptiform discharges), lateralized periodic discharges (LPDs) are unilateral, relatively uniform discharges (often with sharp or spike morphology), typically ranging from 100–300 uV (Schomer and Lopes da Silva, 2011), recurring at regular intervals up to 3 per second, with a clear return to baseline between adjacent discharges (Hirsch et al., 2013) (Fig. 2) .
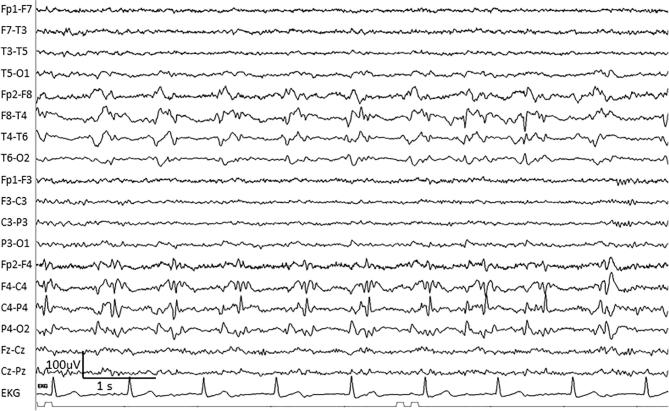
Fig. 2.
An example of right-sided lateralized periodic discharges (LPDs) in a 70-year-old woman with CNS lymphoma. Note the fast component with LPDs, which may be termed LPDs + F (LPDs plus fast).
2.2. Prevalence
LPDs are a relatively uncommon finding in the general population. In unselected patients undergoing EEG (including outpatients), the prevalence of LPDs is 0.4–1% (Fitzpatrick and Lowry, 2007, Pohlmann-Eden et al., 1996). In hospitalized patients undergoing continuous EEG monitoring, the prevalence is 6.2%–8.6% (Lee et al., 2016, Sen-Gupta et al., 2014, Swisher et al., 2015). Periodic patterns tend to flock together: LPDs are also seen in 21.5% of patients with generalized periodic discharges (GPDs) undergoing prolonged cEEG monitoring (Foreman et al., 2012).
2.3. Etiologies
The most common causes of LPDs are stroke, infection, tumor, and hemorrhage (Fitzpatrick and Lowry, 2007, García-Morales et al., 2002, Gurer et al., 2004, Walsh and Brenner, 1987), and LPDs can be seen after either an acute insult or in the setting of a chronic condition (San juan Orta et al., 2009). Intracranial hemorrhage commonly produces LPDs (particularly when involving the cortex), and LPDs occur in 13% of these patients (Claassen et al., 2007).
Most often, LPDs arise from dysfunction in both the cortical gray and subcortical white matter (Gurer et al., 2004, San juan Orta et al., 2009), but periodic discharges including LPDs can also occur in the setting of toxic or metabolic insult, even in the absence of a focal lesion (Fitzpatrick and Lowry, 2007, García-Morales et al., 2002).
2.4. Association with seizures
LPDs are often temporally associated with clear-cut seizures. Depending on the study, the incidence of clinical or electrographic seizures in the setting of LPDs is 50–100% (García-Morales et al., 2002, Gaspard et al., 2013, Pohlmann-Eden et al., 1996, Swisher et al., 2015). The question of whether LPDs themselves represent ictal patterns is debated; when focal twitching or movements are time-locked to a discharge, LPDs are considered ictal (Walsh and Brenner, 1987); clear focal motor seizures including epilepsia partialis continua (EPC) may be time- and location-correlated to observed LPDs (Fitzpatrick and Lowry, 2007). However, in a study of with and without motor manifestations, Sen-Gupta et al. found that LPDs with associated motor findings were more likely to arise from central head regions, and concluded that motor findings are not a reliable determinant of whether LPDs are “ictal” or “non-ictal.” They argue that LPDs occurring in other locations would be expected to have other clinical manifestations (such as confusion or aphasia), or to be asymptomatic (Sen-Gupta et al., 2014), and that LPDs without motor manifestations may also be ictal patterns.
LPDs have been further differentiated based on associated morphology: In 1991, Reiher et al. drew a distinction between PLEDs associated with brief, stereotyped rhythmic discharges, termed “PLEDs plus”, and PLEDs without rhythmic discharges, termed “PLEDs proper” (Reiher et al., 1991). Seizures and status epilepticus were significantly more likely to be seen in patients with PLEDs plus (74% of patients) than with PLEDS proper (6% of patients). “PLEDs plus” as a high-risk group for seizures has been confirmed subsequently (Fitzpatrick and Lowry, 2007), most recently in a large multicenter study of 4772 patients undergoing cEEG. Patients with LPDs-plus had a significantly higher association with seizures than did patients with LPDs alone, with odds ratios of 13.35 and 6.68 respectively (Rodriguez Ruiz et al., 2016).
LPDs with superimposed fast or rhythmic characteristics are more likely to respond to medication: in one series, the 7 of 23 patients who showed clinical and EEG improvement after receiving AEDs for LPDs all had “LPDs plus” (Pedersen et al., 2013).
The frequency of LPDs also provides information about their association with seizures: while LPDs at any frequency carry a risk of seizures, the risk increases with higher frequencies, reaching an odds ratio of 16.4 for LPDs >2 Hz (Rodriguez Ruiz et al., 2016).
2.5. Imaging and supportive tests
Metabolic imaging with SPECT during LPDs has been reported to show hypoperfusion (Hisada et al., 2000) in some cases, leading to more conservative treatment, or hyperperfusion (Claassen, 2009) in others, which may influence more aggressive treatment, as increased blood flow on SPECT is a sensitive marker for ictal activity in patients with epilepsy (Devous et al., 1998).
FDG-PET also may show hypermetabolism or hypometabolism in the region of LPDs, and may change from hypermetabolic to hypometabolic during a patient’s course as LPDs resolve (Claassen, 2009, Handforth et al., 1994). This may represent the changing nature of LPDs during hospital course, though hyper- or hypometabolism could also reflect recent seizures prior to imaging or surrounding brain injury.
In a study of patients with periodic discharges who also received FDG-PET, 9 patients with LPDs had PET, of which 7/9 (77.8%) had focal hypermetabolism (Struck et al., 2016); in 8/9 (including all with hypermetabolism), the pattern was thought to represent electrographic or electroclinical status epilepticus based on motor correlate or response to medication.
On MRI, the degree of ADC change correlates to neuronal cell loss in animal studies (Engelhorn et al., 2007); ADC changes and restricted diffusion is seen after SE. One small series of 10 patients with LPDs (5 with seizures and 5 without) found that patients with LPDs only had no evidence of restricted diffusion (Narayanan, 2016), which suggests that LPDs do not produce the same degree of neuronal injury as do seizures.
Neuron-specific enolase (NSE), a marker of brain injury, is elevated after both convulsive and non-convulsive seizures (Rabinowicz et al., 1995), but has not yet been widely used in patients with periodic or rhythmic patterns. NSE levels did not rise despite ongoing LPDs in one reported patient (Claassen, 2009).
2.6. Outcomes
LPDs have a significant association with increased mortality. The mortality of patients with LPDs ranges from 24–41% (Fitzpatrick and Lowry, 2007, San juan Orta et al., 2009, Walsh and Brenner, 1987), with a “poor” outcome in 68% (Sen-Gupta et al., 2014) and only 21% achieving independent functional status after 1 year (San juan Orta et al., 2009).
The underlying etiology of LPDs greatly influences outcome (Walsh and Brenner, 1987). Patients with an underlying neoplasm have higher rates of functional dependence after one year compared to those with a vascular etiology (San juan Orta et al., 2009). In one study of 79 patients with LPDs, the risk of death was lower when LPDs were due to chronic etiology versus an acute etiology (OR 0.14, 95% CI 0.03–0.72). In addition, the risk of death was lower when LPDs had associated seizures than in LPDs without seizures (OR 0.21, 95% CI 0.04–0.97) (San juan Orta et al., 2009). However, patients with status epilepticus who had LPDs following resolution of status have higher mortality than those who did not (40% mortality in patients with LPDs) (Jaitly et al., 1997).
A case-control study of 37 patients with LPDs, age and etiology-matched to control patients, found that LPDs were significantly associated with worsened outcome, seizures, and impairment of consciousness, even in the absence of acute brain injury (Sainju et al., 2015). Patients with intracerebral hemorrhage have a worse prognosis when LPDs are present (OR 10.1 for poor outcome with LPDs (Claassen et al., 2007)). In patients with poor-grade subarachnoid hemorrhage, the presence of LPDs is highly associated with a worse outcome (OR 18.8 for poor outcome) (Claassen et al., 2006).
Interestingly, there is some evidence that patients with isolated periodic discharges (PDs) are more likely to die than are to those with PDs who do have seizures on EEG. One study found an odds ratio for death of 0.21 in patients with PDs and seizures compared to those with PDs alone (San juan Orta et al., 2009); this may reflect more severe injury in those with PDs in isolation, or may reflect a transient or less severe nature of PDs associated with seizures.
In patients without a history of seizures prior to hospitalization, the majority of those with LPDs go on to have later seizures (56%–67% of patients) (Schraeder and Singh, 1980, Walsh and Brenner, 1987).
In most cases, LPDs are an acute and transient finding, resolving after 8–10 days (Fitzpatrick and Lowry, 2007, García-Morales et al., 2002). LPDs may in fact be seen in a stage of untreated prolonged status epilepticus, following continuous ictal activity (Treiman et al., 1990). They may represent an acute phase of the healing process or a stage in the recovery (or path to) seizures, resolving by 2 weeks after the acute event. However, in rare instances LPDs will be a chronic finding, and have been observed chronically in asymptomatic patients for up to 20 years (Westmoreland et al., 1986).
3. Bilateral independent periodic discharges (BIPDs)
3.1. Definition
Rarer than their related cousin LPDs, BIPDs may carry a poorer prognosis. BIPDs are asynchronous, repetitive, independent left and right hemispheric discharges, often with a sharp or spike morphology, typically ranging from 100–300 uV (Schomer and Lopes da Silva, 2011), which recur at regular intervals up to 3 per second, with a clear period between adjacent discharges (Hirsch et al., 2013) Fig. 3).
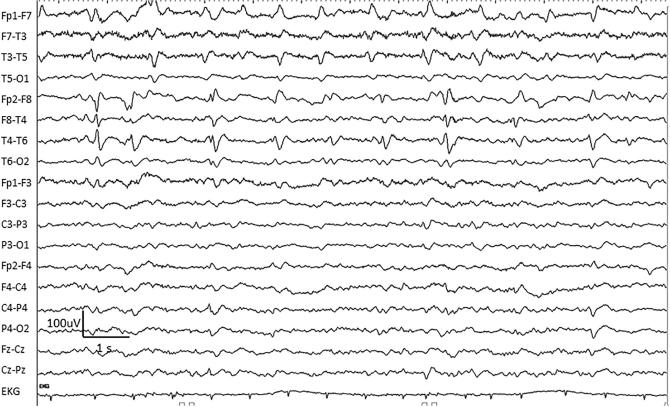
Fig. 3.
An example of bilateral independent periodic discharges (BIPDs) in a 68-year-old woman with scleroderma and HSV encephalitis.
3.2. Prevalence
BIPDs are far less common than LPDs; only 0.2% of unselected patients undergoing EEG have BIPDs (Fitzpatrick and Lowry, 2007). In a large, multicenter study of all patients undergoing continuous EEG monitoring, BIPDs were present in 0.4% of patients (and in only 4% of those with epileptiform discharges) (Lee et al., 2016); other studies have reported BIPDs in 3.5% of critically ill patients undergoing cEEG (Claassen et al., 2004). Etiology matters: BIPDs are seen in 9.5% of patients with CNS infection (Carrera et al., 2008), but in only 1% of patients with intracerebral hemorrhage (Treiman et al., 1990). BIPDs may also keep company with other periodic discharges, found in 10.5% of patients with GPDs (Foreman et al., 2012); patients may also have only unilateral LPDs at other times (Fitzpatrick and Lowry, 2007).
3.3. Etiologies
BIPDs are usually found in settings of acute and subacute injury, rather than chronic conditions (San juan Orta et al., 2009). The most common causes reported are infections, anoxic injury (de la Paz and Brenner, 1981), stroke, tumors (San juan Orta et al., 2009), and metabolic disorders (Pedersen et al., 2013); more rare causes include Hashimoto’s encephalitis (Fitzpatrick and Lowry, 2007) and lupus (Aye et al., 2013).
3.4. Association with seizures
BIPDs also have a high association with seizures, which are reported in 43–78% of patients with BIPDs (de la Paz and Brenner, 1981, Fitzpatrick and Lowry, 2007, San juan Orta et al., 2009); 100% association with seizures was seen in 4 patients with BIPDs and CNS infection (Carrera et al., 2008). Patients with BIPDs often have a poor neurologic exam, and 48% are comatose (compared to 17% of patients with LPDs). Focal neurologic findings on exam are rare (San juan Orta et al., 2009); however, focal unilateral and bilateral independent focal motor activity has been reported with over half the patients with BIPDs (Fitzpatrick and Lowry, 2007).
3.5. Imaging
In one study, focal findings on imaging were less common in patients with BIPDs (25%) compared to LPDs (74%) (Pedersen et al., 2013). Similar to LPDs, the majority of patients with BIPDs have both cortical and subcortical abnormalities (San juan Orta et al., 2009).
Fewer cases of metabolic imaging have been published in patients with BIPDs than with LPDs. One patient with lupus and BIPDs in the frontal regions and underlying white matter disease had reduced blood flow in the areas of BIPDs on SPECT (Aye et al., 2013); reduced blood flow with BIPDs has been reported in one other case (Fushimi et al., 2003).
3.6. Outcomes
BIPDs have been thought of as a marker of more severe disease and as an indicator of worse prognosis than LPDs. The reported mortality ranges from 39–100 (Pedersen et al., 2013, San juan Orta et al., 2009).
The largest comparisons of BIPDs to LPDs have shown higher mortality in BIPDs. De la Paz et al. found a 61% mortality in 18 patients with BIPDs, more than twice the 29% found in the 45 patients with LPDs(de la Paz and Brenner, 1981). In a retrospective study of 21 patients with BIPDs, the mortality was 52%, much higher than the overall mortality rate of patients with LPDs (27%) (Fitzpatrick and Lowry, 2007). In a separate study including 23 patients with BIPDs, 39.1% had a fatal outcome, while only 21.7% had an independent recovery; this was significantly worse than the 24–29% mortality in patients with LPDs and GPDs (San juan Orta et al., 2009).
Rare cases of long-term, “benign” BIPDs are reported (Fushimi et al., 2003).
4. Generalized periodic discharges (GPDs)
4.1. Definition
GPDs at first appear closely related to LPDs and BIPDs; however, metabolic illnesses more commonly give rise to GPDs. GPDs are bilaterally synchronous, repetitive discharges (often with a sharp or spike morphology), typically with amplitudes >100 uV, repeating at regular intervals at up to 3 per second, with a clear period between adjacent discharges (Hirsch et al., 2013) (Fig. 4).
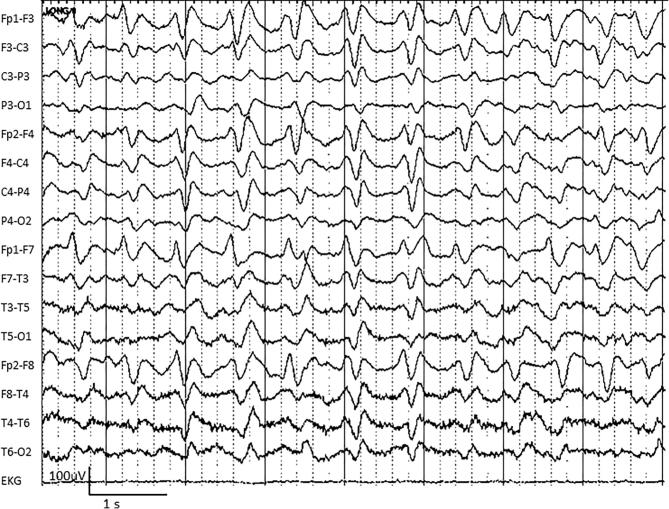
Fig. 4.
An example of generalized periodic discharges (GPDs) in a 54-year-old man with sepsis and renal failure.
4.2. Prevalence
A large review of 3064 patients undergoing cEEG found GPDs in 138 (4.5%) (Foreman et al., 2012); other studies have found a much lower prevalence, from 0.8–1.8% (Lee et al., 2016, Swisher et al., 2015). GPDs often coexist with LPDs.
4.3. Etiologies
The majority of patients with GPDs have a toxic-metabolic illness or sepsis, and many have coexisting brain injury as well (Foreman et al., 2012, Husain et al., 1999, San juan Orta et al., 2009, Yemisci et al., 2003). GPDs were present in only 6% of patients with intracerebral hemorrhage in one study (Claassen et al., 2007). Compared to LPDs, an acute insult (rather than a chronic injury) is more common with GPDs (San juan Orta et al., 2009). Patients with GPDs are usually comatose or stuporous, comatose 70% of the time in one study (Foreman et al., 2012, San juan Orta et al., 2009).
4.4. Association with seizures
The rates of seizures with GPDs are significant though not quite as high as the seizure rates of BIPDs and LPDs. The largest series report the incidence of seizures with GPDs as between 29–50% (Foreman et al., 2012, San juan Orta et al., 2009, Swisher et al., 2015), and these are often nonconvulsive. In a case-control study of 200 patients on cEEG with GPDs and 200 without, seizures were much more prevalent in the GPD group (46%) compared to the controls (34%) (Foreman et al., 2012). An even greater difference was seen in the rates of patients with nonconvulsive status epilepticus (NCSE): 22% of the patients with GPDs had NCSE, compared to 7% of controls. Higher rates of concomitant seizures are seen with higher frequencies of GPDs; while GPDs <1.5 Hz were not significantly associated with seizures in one large multicenter study, GPDs occurring at 1.5–2 Hz had an odds ratio of 2.3 for association with seizures and of 3.3 at high frequencies (≥2 Hz) (Rodriguez Ruiz et al., 2016).
4.5. Imaging
Similar to the other periodic findings described, a combination of subcortical and cortical injuries are common in GPDs (Yemisci et al., 2003), though isolated subcortical lesions are present in 30% (San juan Orta et al., 2009).
PET is less commonly reported in patients with GPDs than with LPDs. Three of 5 patients with GPDs had hypermetabolism on PET (Struck et al., 2016); each of the 3 with hypermetabolism had concern for electrographic or electroclinical status epilepticus based on response to medications or other clinical findings.
4.6. Outcomes
The mortality in patients with GPDs is quite high: 30%–64% (Foreman et al., 2012, Husain et al., 1999, San juan Orta et al., 2009). A poor outcome has been seen in 51.5% in one study (Foreman et al., 2012), and only 18% reached an independent functional capacity at one year of follow up (San juan Orta et al., 2009). However, some mortality may be due to the frequently coexisting seizures, and mortality is related to underlying etiology: in one large study, after controlling for the presence of seizures (which predict worse outcome), patients with GPDs did not have a worse outcome than were controls matched by age and etiology (Foreman et al., 2012).
4.7. Triphasic waves
A subtype of GPDs with “triphasic morphology” were first described in the 1950 s (Kaplan and Sutter, 2015). Triphasic morphology refers to “repetitive electrographic elements consisting of three phases, each longer than the preceding one: a surface positive high-amplitude (>70 uV) wave preceded and followed by negative waves with smaller amplitude” (Sutter et al., 2013). However, accurately identifying triphasic waves is challenging: one study of 11 cEEG reviewers assessing 20 samples of GPDs found only fair inter-rater agreement (κ = 0.33) in whether or not GPDs had a triphasic morphology (Foreman et al., 2016).
Traditionally described with hepatic insufficiency, triphasic waves (TWs) have been strongly associated with liver and multi-organ failure, especially at high levels of urea and ammonia (Sutter et al., 2013). However, TWs are also seen in other scenarios such as hypernatremia, hypothyroidism, sepsis, lithium toxicity, and hypertensive encephalopathy (Faigle et al., 2013, Kaplan and Sutter, 2015). One recent study of GPDs found that those with triphasic morphology were just as likely to be associated with seizures as were GPDs without (Foreman et al., 2016).
In a large series of patients with encephalopathy, Sutter et al. found that patients with TWs have a high mortality rate (OR 4.5 for in-hospital death) (Sutter et al., 2013).
5. Lateralized rhythmic delta activity (LRDA)
5.1. Definition
Less clearly related to the periodic patterns, lateralized rhythmic activity carries a similar risk of seizures to its periodic cousins, LPDs. LRDA is a unilateral repetitive waveform, with nearly uniform duration and morphology, recurring at up to 3 Hz without a measurable inter-waveform interval(Hirsch et al., 2013) (Fig. 5). LRDA tends to occur in shorter runs (often lasting less than one minute) than do LPDs(Gaspard et al., 2013).
Fig. 5.
An example of right-sided lateralized rhythmic delta activity (LRDA) in a 64-year-old man with right middle cerebral artery stroke. Note the abnormal EKG rhythm as well.
5.2. Prevalence
One series found LRDA in 27/558 (4.7%) of acutely ill patients on continuous EEG monitoring (Gaspard et al., 2013); a large proportion (44%) of these cases are also associated with LPDs (Gaspard et al., 2013).
5.3. Etiologies
The most common conditions for LRDA reported are intracerebral and subarachnoid hemorrhages; other conditions include stroke, tumor, traumatic brain injury, and infection (Gaspard et al., 2013). A high proportion (70%) of patients with LRDA have a focal abnormality on neurologic examination, concordant with the side of LRDA (Gaspard et al., 2013).
5.4. Association with seizures
One study found seizures in the majority of patients with LRDA (Gaspard et al., 2013), most of which were nonconvulsive. In that study, the rate of seizures was even higher in patients with LRDA (63%) than with LPDs (57%) (Gaspard et al., 2013).
Similarly to LPDs and GPDs, faster rates of LRDA have a higher risk of associated seizures. Rodriguez-Ruiz et al. found that LRDA is associated with seizures in 25–44% of patients, with a higher risk of seizures seen at higher frequencies (significant associations found at rates of 1.5–2 Hz (OR 1.8) and ≥2 Hz (OR 4.0); Rodriguez Ruiz et al., 2016).
5.5. Imaging
LRDA is frequently seen in lesions involving cortex or subcortical white matter (Gaspard et al., 2013); nearly all patients with LRDA have focal injury on the side of the rhythmic activity. Patients with LRDA more commonly have a focal lesion involving the cortex than do patients with nonrhythmic focal slowing (Gaspard et al., 2013).
5.6. Outcome
To date, no studies have reported the outcome of patients with LRDA, though it may be similar to that of patients with LPDs given the similar association with seizures.
6. Generalized rhythmic delta activity (GRDA)
6.1. Definition
Perhaps the most benign inhabitant of the ictal-interictal zone, GRDA is a bilateral, bisynchronous and symmetric repetitive waveform, often >100 uV in amplitude, with nearly uniform duration and morphology recurring at up to 3 Hz (without a measurable inter-waveform interval) (Hirsch et al., 2013) (Fig. 6). Prior to the ACNS guidelines for terminology standardization in 2012, the term frontal intermittent rhythmic delta activity (FIRDA) was frequently used, as GRDA is commonly maximal in the frontal regions.
Fig. 6.
An example of generalized rhythmic delta activity (GRDA) in a 79-year-old man with sepsis.
6.2. Prevalence
GRDA was reported in 16.1% of patients undergoing continuous EEG monitoring in one large study of 4772 patients (Rodriguez Ruiz et al., 2016); observed rates reported for FIRDA in unselected patient groups are 0.6–6% (Accolla et al., 2011, Watemberg et al., 2002).
6.3. Etiologies
When described as FIRDA, the most common etiologies have been reported to be brain tumor surgery and cerebrovascular diseases (Kubota and Ohnishi, 1997, Sutter et al., 2013). In one series, the majority (55%) of patients had a structural lesion, while an infectious condition, renal disease, or sedative medication were commonly associated (Accolla et al., 2011).
6.4. Association with seizures
A large, multicenter study of 1513 critically ill patients with periodic or rhythmic activity found that GRDA was not associated with an increased risk of seizures, even at higher frequencies (>2 Hz) or when accompanied by sharp morphology (Rodriguez Ruiz et al., 2015).
However, in certain patient groups, GRDA may have a different implication. Patients with NMDA encephalitis often have GRDA or GRDA with overlying fast activity, known as “extreme delta brush;” GRDA and EDB are argued to represent ictal patterns in these patients.
6.5. Imaging
Although GRDA and FIRDA are bilateral or generalized patterns, the underlying brain lesion (when present) is more frequently lateralized than midline (lateralized in 77% of cases) (Accolla et al., 2011).
One of 4 patients (25%) with GRDA who underwent FDG-PET had focal hypermetabolism on PET; in the one patient with hypermetabolism, there was concern for electrographic status epilepticus based on >3 Hz frequency of GRDA at times (Struck et al., 2016). The patients with frequency of GRDA <3 Hz had no hypermetabolism.
6.6. Outcome
The outcome after GRDA is related to the underlying etiology. Outcomes after FIRDA are relatively good, with 69% of patients with FIRDA returning home after hospital discharge in one study (Sutter et al., 2013).
7. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs)
7.1. Definition
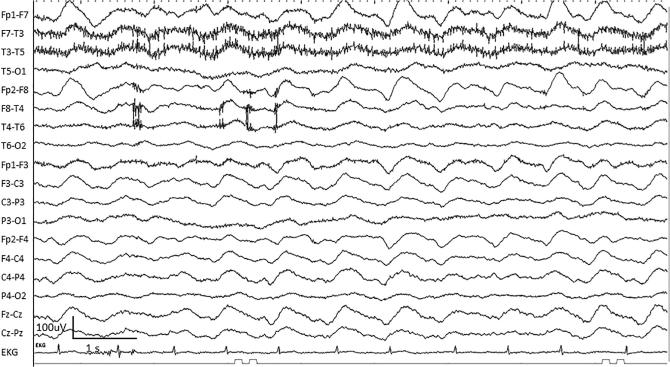
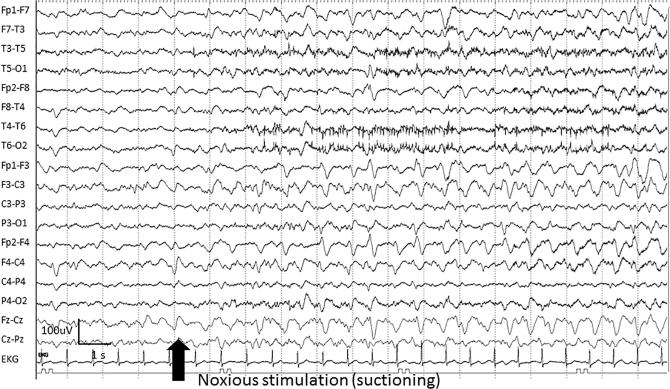
A special subtype of patterns in the ictal-interictal continuum, SIRPIDs are rhythmic, periodic, or ictal-appearing patterns consistently elicited by stimulation of the patient (i.e. suctioning, noise, or physical examination of the patient) (Hirsch et al., 2004) (Fig. 7). The stimulus-induced nature may be indicated by adding “SI” to a following term such as SI-GPDs or SI-LRDA under the ACNS standardized terminology guidelines (Hirsch et al., 2013). However, as SIRPIDs are studied separately in a number of publications (Alvarez et al., 2013, Braksick et al., 2016), we will consider these stimulus-induced patterns together.
Fig. 7.
An example of stimulus-induced generalized periodic discharges (SI-GPDs) evolving to generalized rhythmic delta activity (GRDA) in a 68-year-old woman with tuberculosis meningitis.
7.2. Prevalence
SIRPIDs are a relatively common phenomenon in the critically ill, found in 10–34% of patients undergoing EEG monitoring (Braksick et al., 2016, Hirsch et al., 2004, Ong et al., 2012).
7.3. Etiologies
SIRPIDs also stem from many etiologies, the most common being intracerebral hemorrhage, anoxic brain injury, metabolic disturbances, traumatic brain injury, and drug toxicity (Braksick et al., 2016, Hirsch et al., 2004, Van Straten et al., 2014).
7.4. Association with seizures
Most studies report a strong association of SIRPIDs with seizures, with 27–51% of patients found to have coexisting spontaneous seizures (Braksick et al., 2016, Hirsch et al., 2004) (and 4/4 patients with SIRPIDs had NCSE in one small study) (Van Straten et al., 2014). However, the largest series of patients on cEEG monitoring found no increased association of seizures in patients with stimulus-induced compared to spontaneous patterns (Rodriguez Ruiz et al., 2016). When present, focal seizure onset locations often have no relation to the location of focal SIRPIDs (Hirsch et al., 2004).
7.5. Imaging
In the three reports of patients with SIRPIDs who received SPECT, there was no increase in cerebral blood flow during SIRPIDs (Smith et al., 2014, Zeiler et al., 2011). This included one patient with motor weakness during the discharges (Zeiler et al., 2011), and has been taken as evidence to support more conservative management rather than aggressive treatment to suppress SIRPIDs.
7.6. Outcomes
Focal SIRPIDs have been associated with a poor outcome when present in patients with intracerebral hemorrhage (Claassen et al., 2007). When present after cardiac arrest, SIRPIDs seen during the hypothermia period (but not during normothermia) are associated with increased mortality; patients with SIRPIDs were also more likely to have a discontinuous EEG background (a known poor predictive feature) (Alvarez et al., 2013). SIRPIDs were more commonly seen in patients who died in the hospital than in patients who survived in one study; however, after adjusting for EEG reactivity, age, and anoxic brain injury, SIRPIDs were independently associated with increased mortality (Braksick et al., 2016).
8. Treatment approach
No clear guidelines exist for how to treat these ictal-interictal rhythmic and periodic patterns. However, management can be tailored to the on the clinical scenario and supportive studies (when available). Increased seizure burden is associated with worse outcome in particular patient populations (De Marchis et al., 2016, Payne et al., 2014); as some periodic and rhythmic patterns are highly associated with seizures, the case for treatment is strong.
If periodic patterns correspond to clinical signs such as motor activity, the case for treating with AEDs is strong; when found in a comatose patient or patient with altered mental status but no motor signs, the need for aggressive treatment is less clear. Seizure prophylaxis with one AED is usually reasonable, but how aggressively should the clinician treat ongoing LPDs? The underlying etiology of the patient’s disease, the comorbidities and resuscitation status of the patient, and overall prognosis (independent of the EEG pattern) all must play a role in the decision whether or not to aggressively treat or suppress periodic discharges. Supportive studies such as PET or SPECT may help clarify the need for treatment (Claassen, 2009). When patterns are of unclear significance, nonsedating AEDs should be used preferentially, and the patient’s clinical status taken into account to avoid the need for intubation. If the patient can tolerate a benzodiazepine, a trial of intravenous (IV) benzodiazepine may help clarify the significance of the pattern (if there is a clinical and/or electrographic improvement), but continuous IV medications are not recommended for these unclear patterns.
Most seizures occur within 24 h of cEEG (Swisher et al., 2015), but in some high-risk populations such as intracerebral hemorrhage, more than one-fourth of seizures occur after the first 24 h (Claassen et al., 2007). When high-risk periodic or rhythmic patterns are seen, monitoring for at least 24–48 h to assess for seizures is reasonable.
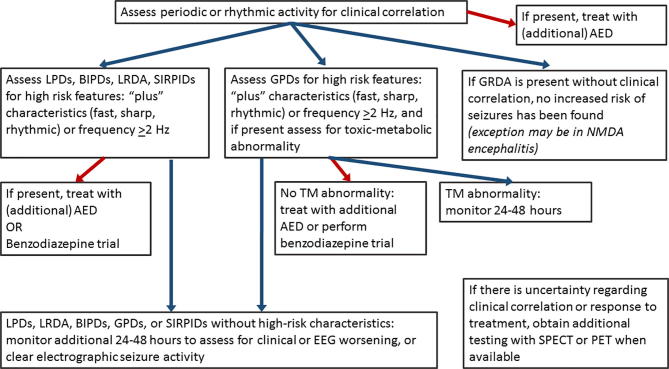
We therefore suggest the following approach to treatment of patterns on the ictal-interictal continuum – see Fig. 8 (modified from Rodriguez (Rodríguez et al., 2016) and Claassen (Claassen, 2009)):
-
1)
Assess for clinical correlation to the rhythmic or periodic pattern and if present, treat with an (additional) AED.
-
2)
Assess for any of the high-risk characteristics: LPDs, LRDA, BIPDs, or SIRPIDs with “plus” characteristics (sharp, fast, or rhythmic activity), or LRDA > 2 Hz; if present, treat with an (additional) AED or perform benzodiazepine trial, and monitor for additional 24–48 h; continue treatment if there is clinical and EEG improvement.
If GPDs with high risk characteristics (>2 Hz, or additional rhythmic or fast activity) are present: assess for underlying toxic-metabolic abnormality and if found, monitor 24–48 h; if no toxic-metabolic etiology is present, treat with an (additional) AED, or perform benzodiazepine trial to assess for clinical improvement.
-
3)
If LPDs, LRDA, BIPDs, GPDs, or SIRPIDs without high-risk characteristics are present, monitor for an additional 24–48 h to assess for clinical or EEG worsening, or clear electrographic seizure activity (and treat with AED if found).
-
4)
If there is uncertainty regarding clinical correlation or response to treatment, additional testing with functional imaging (SPECT or PET) when available is recommended; increased blood flow with the rhythmic or periodic pattern supports treatment.
-
5)
For GRDA, no increased risk of seizures has been found; in the absence of clinical correlation, usually no treatment is advised (the exception may be NMDA encephalitis (Kirkpatrick et al., 2011)).
Fig. 8.
Suggested algorithm for approach to patterns on the ictal-interictal continuum. AED = antiepileptic drug; LPDs = lateralized periodic discharges; BIPDs = bilateral independent periodic discharges; GPDs = generalized periodic discharges; LRDA = lateralized rhythmic delta activity; GRDA = generalized rhythmic delta activity; SIRPIDs = stimulus-induced rhythmic, periodic, or ictal discharges; TM = toxic-metabolic.
9. Concluding remarks
Denizens of the ictal-interictal continuum are challenging to encounter and must be carefully evaluated to determine whether high-risk features occur. In most cases, prolonged (24–48 h) monitoring is warranted, as is empiric AED treatment for some high-risk patterns. Functional imaging may help determine the significance of the pattern if clinical correlation and EEG response to treatment are ambiguous.
Conflict of interest
None.
References
- Accolla E.A., Kaplan P.W., Maeder-Ingvar M., Jukopila S., Rossetti A.O. Clinical correlates of frontal intermittent rhythmic delta activity (FIRDA) Clin. Neurophysiol. 2011;122:27–31. doi: 10.1016/j.clinph.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Alvarez V., Oddo M., Rossetti A.O. Stimulus-induced rhythmic, periodic or ictal discharges (SIRPIDs) in comatose survivors of cardiac arrest: characteristics and prognostic value. Clin. Neurophysiol. 2013;124:204–208. doi: 10.1016/j.clinph.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Aye S., Lim K., Ramli N., Tan C. Periodic lateralized epileptiform discharges (PLEDs) in cerebral lupus correlated with white-matter lesions in brain MRI and reduced cerebral blood flow in SPECT. Lupus. 2013;22:510–514. doi: 10.1177/0961203312474705. [DOI] [PubMed] [Google Scholar]
- Beniczky S., Hirsch L.J., Kaplan P.W., Pressler R., Bauer G., Aurlien H., Brøgger J.C., Trinka E. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54:28–29. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- Braksick S.A., Burkholder D.B., Tsetsou S., Martineau L., Mandrekar J., Rossetti A.O. Associated factors and prognostic implications of stimulus-induced rhythmic, periodic, or ictal discharges. JAMA Neurol. 2016;73:585. doi: 10.1001/jamaneurol.2016.0006. [DOI] [PubMed] [Google Scholar]
- Carrera E., Claassen J., Oddo M., Emerson R.G., Mayer S.A., Hirsch L.J. Continuous electroencephalographic monitoring in critically ill patients with central nervous system infections. Arch. Neurol. 2008;65:1612–1618. doi: 10.1001/archneur.65.12.1612. [DOI] [PubMed] [Google Scholar]
- Chong D.J., Hirsch L.J. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J. Clin. Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- Claassen J. How I treat patients with EEG patterns on the ictal-interictal continuum in the neuro ICU. Neurocrit. Care. 2009;11:437–444. doi: 10.1007/s12028-009-9295-8. [DOI] [PubMed] [Google Scholar]
- Claassen J., Hirsch L.J., Frontera J.A., Fernandez A., Schmidt M., Kapinos G., Wittman J., Connolly E.S., Emerson R.G., Mayer S.A. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit. Care. 2006;4:103–112. doi: 10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- Claassen J., Jetté N., Chum F., Green R., Schmidt M., Choi H., Jirsch J., Frontera J.A., Connolly E.S., Emerson R.G., Mayer S.A., Hirsch L.J. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- Claassen J., Mayer S.A., Kowalski R.G., Emerson R.G., Hirsch L.J. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- de la Paz D., Brenner R.P. Bilateral independent periodic lateralized epileptiform discharges. Clinical significance. Arch. Neurol. 1981;38:713–715. doi: 10.1001/archneur.1981.00510110073012. [DOI] [PubMed] [Google Scholar]
- De Marchis G.M., Pugin D., Meyers E., Velasquez A., Suwatcharangkoon S., Park S. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253–260. doi: 10.1212/WNL.0000000000002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devous M.D., Thisted R.A., Morgan G.F., Leroy R.F., Rowe C.C. SPECT brain imaging in epilepsy: a meta-analysis. J. Nucl. Med. 1998;39:285–293. [PubMed] [Google Scholar]
- Engelhorn T., Weise J., Hammen T., Bluemcke I., Hufnagel A., Doerfler A. Early diffusion-weighted MRI predicts regional neuronal damage in generalized status epilepticus in rats treated with diazepam. Neurosci. Lett. 2007;417:275–280. doi: 10.1016/j.neulet.2007.02.072. [DOI] [PubMed] [Google Scholar]
- Faigle R., Sutter R., Kaplan P.W. Electroencephalography of encephalopathy in patients with endocrine and metabolic disorders. J. Clin. Neurophysiol. 2013;30:505–516. doi: 10.1097/WNP.0b013e3182a73db9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick W., Lowry N. PLEDs: clinical correlates. Can. J. Neurol. Sci. 2007;34:443–450. [PubMed] [Google Scholar]
- Foreman B., Claassen J., Abou Khaled K., Jirsch J., Alschuler D.M., Wittman J. Generalized periodic discharges in the critically ill: a case-control study of 200 patients. Neurology. 2012;79:1951–1960. doi: 10.1212/WNL.0b013e3182735cd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman B., Mahulikar A., Tadi P., Claassen J., Szaflarski J., Halford J.J. Generalized periodic discharges and “triphasic waves”: a blinded evaluation of inter-rater agreement and clinical significance. Clin. Neurophysiol. 2016;127:1073–1080. doi: 10.1016/j.clinph.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Fushimi M., Matsubuchi N., Sekine A., Shimizu T. Benign bilateral independent periodic lateralized epileptiform discharges. Acta Neurol. Scand. 2003;108:55–59. doi: 10.1034/j.1600-0404.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- García-Morales I., García M.T., Galán-Dávila L., Gómez-Escalonilla C., Saiz-Díaz R., Martínez-Salio A., de la Peña P., Tejerina J.A. Periodic lateralized epileptiform discharges: etiology, clinical aspects, seizures, and evolution in 130 patients. J. Clin. Neurophysiol. 2002;19:172–177. doi: 10.1097/00004691-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Gaspard N., Manganas L., Rampal N., Petroff O.A.C., Hirsch L.J. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013;70:21–24. doi: 10.1001/jamaneurol.2013.3475. [DOI] [PubMed] [Google Scholar]
- Gurer G., Yemisci M., Saygi S., Ciger A. Structural lesions in periodic lateralized epileptiform discharges (PLEDs) Clin. EEG Neurosci. 2004;35:88–93. doi: 10.1177/155005940403500207. [DOI] [PubMed] [Google Scholar]
- Handforth A., Cheng J.T., Mandelkern M.A., Treiman D.M. Markedly increased mesiotemporal lobe metabolism in a case with PLEDs: further evidence that PLEDs are a manifestation of partial status epilepticus. Epilepsia. 1994;35:876–881. doi: 10.1111/j.1528-1157.1994.tb02526.x. [DOI] [PubMed] [Google Scholar]
- Hirsch L.J., Claassen J., Mayer S.A., Emerson R.G. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia. 2004;45:109–123. doi: 10.1111/j.0013-9580.2004.38103.x. [DOI] [PubMed] [Google Scholar]
- Hirsch L.J., LaRoche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T., Mani R., Arif H., Jette N., Minazad Y., Kerrigan J.F., Vespa P., Hantus S., Claassen J., Young G.B., So E., Kaplan P.W., Nuwer M.R., Fountain N.B., Drislane F.W. American clinical neurophysiology society’s standardized critical care EEG terminology. J. Clin. Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- Hisada K., Morioka T., Nishio S., Muraishi M., Yamamoto T., Yoshida T., Fukui M. Magnetoencephalographic analysis of periodic lateralized epileptiform discharges (PLEDs) Clin. Neurophysiol. 2000;111:122–127. doi: 10.1016/s1388-2457(99)00184-4. [DOI] [PubMed] [Google Scholar]
- Husain A.M., Mebust K.A., Radtke R.A. Generalized periodic epileptiform discharges: etiologies, relationship to status epilepticus, and prognosis. J. Clin. Neurophysiol. 1999;16:51–58. doi: 10.1097/00004691-199901000-00005. [DOI] [PubMed] [Google Scholar]
- Jaitly R., Sgro J.A., Towne A.R., Ko D., DeLorenzo R.J. Prognostic value of EEG monitoring after status epilepticus: a prospective adult study. J. Clin. Neurophysiol. 1997;14:326–334. doi: 10.1097/00004691-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Kaplan P.W., Sutter R. Affair with triphasic waves—their striking presence, mysterious significance, and cryptic origins. J. Clin. Neurophysiol. 2015;32:401–405. doi: 10.1097/WNP.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M.P., Clarke C.D., Sonmezturk H.H., Abou-Khalil B. Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti-NMDA receptor antibody encephalitis. Epilepsy Behav. 2011;20:392–394. doi: 10.1016/j.yebeh.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Kubota F., Ohnishi N. Study on FIRDA and 3 Hz rhythmic slow wave bursts occurring in the frontal area of epileptic patients. Clin. Electroencephalogr. 1997;28:112–116. doi: 10.1177/155005949702800209. [DOI] [PubMed] [Google Scholar]
- Lee J.W., LaRoche S., Choi H., Rodriguez Ruiz A.A., Fertig E., Politsky J.M. Development and feasibility testing of a critical care EEG monitoring database for standardized clinical reporting and multicenter collaborative research. J. Clin. Neurophysiol. 2016;33:133–140. doi: 10.1097/WNP.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan J. Can diffusion-weighted imaging be used as a tool to predict seizures in patients with PLEDS? Epileptic Disord. 2016 doi: 10.1684/epd.2016.0868. [DOI] [PubMed] [Google Scholar]
- Ong C., Gilmore E., Claassen J., Foreman B., Mayer S.A. Impact of prolonged periodic epileptiform discharges on coma prognosis. Neurocrit. Care. 2012;17:39–44. doi: 10.1007/s12028-012-9728-7. [DOI] [PubMed] [Google Scholar]
- Payne E.T., Zhao X.Y., Frndova H., McBain K., Sharma R., Hutchison J.S., Hahn C.D. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G.L., Rasmussen S.B., Gyllenborg J., Benedek K., Lauritzen M. Prognostic value of periodic electroencephalographic discharges for neurological patients with profound disturbances of consciousness. Clin. Neurophysiol. 2013;124:44–51. doi: 10.1016/j.clinph.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Pohlmann-Eden B., Hoch D.B., Cochius J.I., Chiappa K.H. Periodic lateralized epileptiform discharges–a critical review. J. Clin. Neurophysiol. 1996;13:519–530. doi: 10.1097/00004691-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Rabinowicz A.L., Correale J.D., Bracht K.A., Smith T.D., DeGiorgio C.M. Neuron-specific enolase is increased after nonconvulsive status epilepticus. Epilepsia. 1995;36:475–479. doi: 10.1111/j.1528-1157.1995.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Reiher J., Rivest J., Grand’Maison F., Leduc C.P. Periodic lateralized epileptiform discharges with transitional rhythmic discharges: association with seizures. Electroencephalogr. Clin. Neurophysiol. 1991;78:12–17. doi: 10.1016/0013-4694(91)90013-t. [DOI] [PubMed] [Google Scholar]
- Rodríguez V., Rodden M.F., LaRoche S.M. Ictal-interictal continuum: a proposed treatment algorithm. Clin. Neurophysiol. 2016;127:2056–2064. doi: 10.1016/j.clinph.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez Ruiz, A.A., Vlachy, J., Lee, J.W., Gilmore, E., Ayer, T., Arif, H., Gaspard, N., Hirsch, L., Laroche, S.M., 2015. Periodic and rhythmic patterns in the critically ill: characteristics associated with seizures. Philadelphia, PA.
- Rodriguez Ruiz A., Vlachy J., Lee J.W., Gilmore E.J., Ayer T., Haider H.A. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2016;70:1288–1295. doi: 10.1001/jamaneurol.2016.4990. [DOI] [PubMed] [Google Scholar]
- Sainju R.K., Manganas L.N., Gilmore E.J., Petroff O.A., Rampal N., Hirsch L.J., Gaspard N. Clinical correlates and prognostic significance of lateralized periodic discharges in patients without acute or progressive brain injury. J. Clin. Neurophysiol. 2015;32:495–500. doi: 10.1097/WNP.0000000000000206. [DOI] [PubMed] [Google Scholar]
- San juan Orta D., Chiappa K.H., Quiroz A.Z., Costello D.J., Cole A.J. Prognostic implications of periodic epileptiform discharges. Arch. Neurol.2009;66:177–193. doi: 10.1001/archneurol.2009.137. [DOI] [PubMed] [Google Scholar]
- Schomer D., Lopes da Silva F. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. [Google Scholar]
- Schraeder P.L., Singh N. Seizure disorders following periodic lateralized epileptiform discharges. Epilepsia. 1980;21:647–653. doi: 10.1111/j.1528-1157.1980.tb04318.x. [DOI] [PubMed] [Google Scholar]
- Sen-Gupta I., Schuele S.U., Macken M.P., Kwasny M.J., Gerard E.E. “Ictal” lateralized periodic discharges. Epilepsy Behav. 2014;36:165–170. doi: 10.1016/j.yebeh.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Smith C.C., Tatum W.O., Gupta V., Pooley R.A., Freeman W.D. SPECT-negative SIRPIDs. J. Clin. Neurophysiol. 2014;31:e6–e10. doi: 10.1097/WNP.0000000000000090. [DOI] [PubMed] [Google Scholar]
- Struck A.F., Westover M.B., Hall L.T., Deck G.M., Cole A.J., Rosenthal E.S. Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit. Care. 2016;24:324–331. doi: 10.1007/s12028-016-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R., Stevens R.D., Kaplan P.W. Clinical and imaging correlates of EEG patterns in hospitalized patients with encephalopathy. J. Neurol. 2013;260:1087–1098. doi: 10.1007/s00415-012-6766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher C.B., Shah D., Sinha S.R., Husain A.M. Baseline EEG pattern on continuous ICU EEG monitoring and incidence of seizures. J. Clin. Neurophysiol. 2015;32:147–151. doi: 10.1097/WNP.0000000000000157. [DOI] [PubMed] [Google Scholar]
- Treiman D.M., Walton N.Y., Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- Van Straten A.F., Fesler J.R., Hakimi R., Sheng T., Thompson D.M., Hakimi A.S. SIRPIDs. J. Clin. Neurophysiol. 2014;31:418–421. doi: 10.1097/WNP.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Walsh J.M., Brenner R.P. Periodic lateralized epileptiform discharges – long-term outcome in adults. Epilepsia. 1987;28 doi: 10.1111/j.1528-1157.1987.tb03684.x. [DOI] [PubMed] [Google Scholar]
- Watemberg N., Alehan F., Dabby R., Lerman-Sagie T., Pavot P., Towne A. Clinical and radiologic correlates of frontal intermittent rhythmic delta activity. J. Clin. Neurophysiol. 2002;19:535–539. doi: 10.1097/00004691-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Westmoreland B.F., Klass D.W., Sharbrough F.W. Chronic periodic lateralized epileptiform discharges. Arch. Neurol. 1986;43:494–496. doi: 10.1001/archneur.1986.00520050066024. [DOI] [PubMed] [Google Scholar]
- Yemisci M., Gurer G., Saygi S., Ciger A. Generalised periodic epileptiform discharges: clinical features, neuroradiological evaluation and prognosis in 37 adult patients. Seizure. 2003;12:465–472. doi: 10.1016/s1059-1311(02)00351-5. [DOI] [PubMed] [Google Scholar]
- Zeiler S.R., Turtzo L.C., Kaplan P.W. SPECT–negative SIRPIDs argues against treatment as seizures. J. Clin. Neurophysiol. 2011;28(493–496):1. doi: 10.1097/WNP.0b013e318231c00a. [DOI] [PubMed] [Google Scholar]