Summary
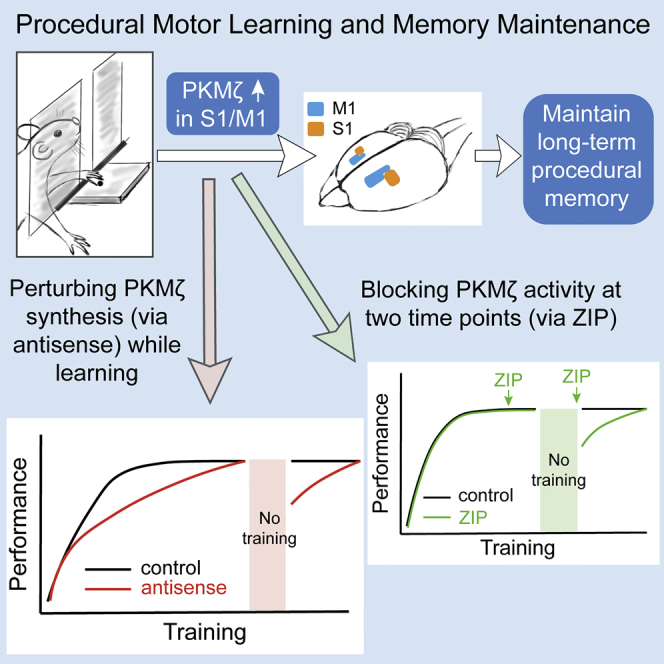
Procedural motor learning and memory are accompanied by changes in synaptic plasticity, neural dynamics, and synaptogenesis. Missing is information on the spatiotemporal dynamics of the molecular machinery maintaining these changes. Here we examine whether persistent increases in PKMζ, an atypical protein kinase C (PKC) isoform, store long-term memory for a reaching task in rat sensorimotor cortex that could reveal the sites of procedural memory storage. Specifically, perturbing PKMζ synthesis (via antisense oligodeoxynucleotides) and blocking atypical PKC activity (via zeta inhibitory peptide [ZIP]) in S1/M1 disrupts and erases long-term motor memory maintenance, indicating atypical PKCs and specifically PKMζ store consolidated long-term procedural memories. Immunostaining reveals that PKMζ increases in S1/M1 layers II/III and V as performance improved to an asymptote. After storage for 1 month without reinforcement, the increase in M1 layer V persists without decrement. Thus, the persistent increases in PKMζ that store long-term procedural memory are localized to the descending output layer of the primary motor cortex.
Subject Areas: Neuroscience, Behavioral Neuroscience, Molecular Neuroscience
Graphical Abstract
Highlights
-
•
Perturbing PKMζ synthesis in S1/M1 slows the formation of skilled motor memory
-
•
Blocking PKMζ activity specifically erases memories maintained without reinforcement
-
•
Skilled motor learning induces the increase of PKMζ in S1/M1 layers II/III and V
-
•
PKMζ sustains the engram for procedural motor memory in M1 layer V
Neuroscience; Behavioral Neuroscience; Molecular Neuroscience
Introduction
Motor learning is characterized by a slow improvement of the smoothness and accuracy of skilled movements, which, once established, are maintained for long periods of time without further practice (Dayan and Cohen, 2011). A skilled motor task in which rodents are trained to reach with their preferred forelimb through a small slot to grasp food pellets has been widely used to study the neural substrate underlying motor learning (Fu and Zuo, 2011, Kargo, 2004, Kleim, 2002, Kleim et al., 1998, Kleim et al., 2004, Luft et al., 2004, Monfils and Teskey, 2004, Rioult-Pedotti et al., 2007, Rioult-Pedotti et al., 2000, Rioult-Pedotti et al., 1998). Performance gains and the maintenance of proficiency on this task depend on the integrity of the sensorimotor cortex (Luft et al., 2004, Sanes and Donoghue, 2000, Whishaw, 2000, Whishaw et al., 2008). Plastic changes in sensorimotor cortex, including synaptic strength modification and structural remodeling, have been correlated with different phases of the learning process (Harms et al., 2008, Kleim et al., 2004, Monfils and Teskey, 2004, Rioult-Pedotti et al., 1998, Rioult-Pedotti et al., 2000, Rioult-Pedotti et al., 2007, Xu et al., 2009). Rioult-Pedotti and colleagues, for example, found that after 5 days of training, the synaptic efficacy of horizontal connections in primary motor cortex (M1) layer II/III increased significantly on the contralateral hemisphere to the preferred forelimb (Rioult-Pedotti et al., 1998), indicating a long-term potentiation (LTP)-like modification of synaptic transmission (Monfils and Teskey, 2004, Rioult-Pedotti et al., 2000). Both spine formation and elimination were seen immediately after the first motor learning session in mice (Xu et al., 2009) and were sustained for up to 20 days with continued daily training in rodents (Kleim et al., 1996, Xu et al., 2009). However, the molecular mechanisms that store motor memories in the sensorimotor cortex remain unknown.
The persistent increase in the autonomously active, atypical protein kinase C (aPKC) isoform PKMζ is both necessary and sufficient for maintaining LTP (Ling et al., 2002, Osten et al., 1996, Sacktor et al., 1993). PKMζ activity retains increased amounts of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptors (AMPARs) in postsynaptic sites to maintain synaptic potentiation (Migues et al., 2010, Sacktor, 2012). PKMζ also contributes to maintaining the structural modifications of dendritic spines and synapses (Chen et al., 2014, Shao et al., 2012), changes that have been extensively observed in the sensorimotor cortex after sensorimotor learning (Yu and Zuo, 2011). Inhibition of persistent aPKC activity in specific brain structures by zeta inhibitory peptide (ZIP) disrupts the maintenance of various types of memory, including hippocampus-dependent spatial memory (Pastalkova et al., 2006), basolateral amygdala-dependent fear memories (Gámiz and Gallo, 2011, Kwapis et al., 2012, Kwapis et al., 2009, Migues et al., 2010, Serrano et al., 2008), dorsal lateral striatum-dependent habit memory (Pauli et al., 2012), and insular cortex-dependent long-term associative conditioned taste aversion memory (Shema et al., 2007), as well as sensorimotor cortex-dependent motor learning in the reaching task (von Kraus et al., 2010). However, ZIP affects PKC isoforms in addition to PKMζ, particularly the other aPKC, PKCι/λ, which can compensate for PKMζ in PKMζ-knockout mice (Tsokas et al., 2016). Therefore, we started by investigating the role of PKMζ in motor learning with PKMζ-antisense oligodeoxynucleotides directed against the translational start site of the PKMζ mRNA, which specifically disrupts PKMζ synthesis and not PKCι/λ synthesis when applied during LTP in brain slices (Hsieh et al., 2017, Tsokas et al., 2016) and effectively blocks learning-induced increases in PKMζ when injected intracranially (Hsieh et al., 2017).
Results
Learning Phases of a Skilled Reaching Task
We used a skilled reaching task to study the role of PKMζ in sensorimotor learning and long-term memory maintenance. Rats were trained to reach with their preferred forelimbs through a small slot to grasp food pellets. Repeated training sessions were required to obtain good performance, which provided an extended time window to examine in detail any changes in sensorimotor cortex. The success rate, defined as % successful reaches/total reaching attempts, was used to evaluate task performance in a daily 30-min training session. A successful reach includes the following motor sequence: (1) lift and advance the preferred forelimb through the slot; (2) pronate and grasp food pellet; (3) retrieve without dropping the pellet (Klein et al., 2012).
Consistent with the model described by Monfils and Teskey (Monfils and Teskey, 2004), we observed that the acquisition and maintenance of skilled motor memory can be divided into four phases: (1) a skill acquisition phase (days 1–4), when success rate is comparatively low (<30%); (2) a performance improvement phase (days 5–9), when the success rate increases rapidly until plateauing (at ∼70%); (3) the proficiency maintenance phase, when performance is stable with continual training; and (4) a long-term memory storage phase, when performance is stable without training (Figure S1 and Transparent Methods). Because ZIP appears to specifically disrupt the long-term sensorimotor memory storage phase (von Kraus et al., 2010), we first focused on the effects of PKMζ-antisense on the acquisition and subsequent maintenance of sensorimotor learning and memories.
PKMζ-Antisense Slows the Performance Improvement Phase of Sensorimotor Learning
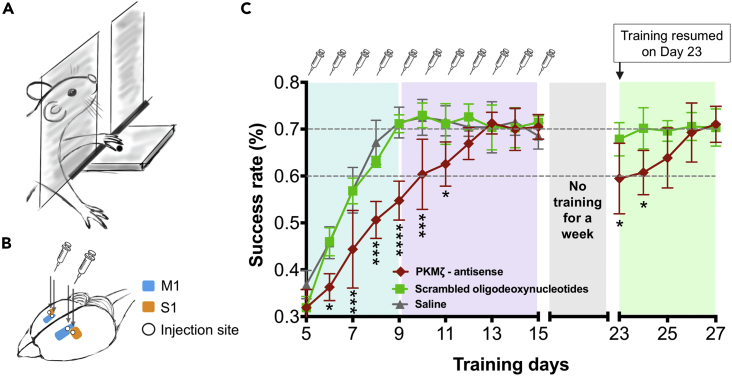
To determine the necessity of PKMζ in sensorimotor cortical networks during sensorimotor learning and memory maintenance, we utilized antisense oligodeoxynucleotides against the translation start site of PKMζ mRNA, which specifically and effectively block PKMζ synthesis during LTP in brain slices and learning in vivo (Hsieh et al., 2017, Tsokas et al., 2016). We injected PKMζ-antisense bilaterally in S1/M1 30 min before daily training and examined its effect on sensorimotor learning (Figures 1 and S2 and Transparent Methods). The antisense group (red) had a significantly lower learning rate compared with the control groups that received inactive scrambled oligodeoxynucleotide (green) or saline (gray) injections (Figure 1). Post hoc Tukey's tests after repeated two-way ANOVA showed significantly lower success rates for the antisense group compared with scrambled oligodeoxynucleotide and saline groups on days 6–11 (Figure 1). In the antisense group, the fast performance improvement phase was replaced with a slow learning curve that extended from days 5 to 11 (Figure 1). With continued training the success rate of the antisense group eventually reached the same asymptote as the other groups on day 12 (Figure 1). Rats with scrambled oligodeoxynucleotide or saline injections learned the skilled reaching task as efficiently as uninjected animals (Figures 1 and S1), indicating the surgery and intracortical injections did not affect their ability to acquire the skilled sensorimotor memory.
Figure 1.
PKMζ-Antisense Slows Sensorimotor Learning
(A) Illustration showing rats trained to reach through a slot with their preferred forelimb and grasp a food pellet on a platform outside of the behavioral chamber.
(B) Bilateral injection sites of M1 (blue) and S1 (orange).
(C) PKMζ-antisense, scrambled oligodeoxynucleotides, and saline were injected bilaterally 30 min before the skilled reaching session every day for 15 days (1 μL/hemisphere) in each group. The learning curves for animals that received PKMζ-antisense, scrambled oligodeoxynucleotide, or saline injections were marked in red (n = 6), green (n = 4), and gray (n = 5) (mean ± SD). Two-way ANOVA showed significant effect between injection groups on learning (F(2, 12) = 32.95; p < 0.0001). Post hoc Tukey's tests for multiple comparisons showed significant lower success rate for the antisense group compared with scrambled oligodeoxynucleotide group (antisense vs. scrambled) or saline group (antisense vs. saline). The p value for anti vs. scrambled: day 6 (*p = 0.0105), day 7 (***p = 0.0006), day 8 (***p = 0.0007), day 9 (****p < 0.0001), day 10 (***p = 0.0006), and day 11 (*p = 0.0278); the p value for antisense vs. saline: day 6 (p = 0.0072), day 7 (p = 0.0002), day 8 (p < 0.0001), day 9 (p < 0.0001), day 10 (p = 0.0004), and day 11 (p = 0.0092). The asterisks in the figure represent the p value of antisense vs. scrambled. The same groups of rats that had received daily PKMζ-antisense or scrambled oligodeoxynucleotide injection in S1/M1 from day 1 to 15 were tested again on days 23–27 after a 1-week period of no training. Student’s t-test showed significant lower success rates of rats with PKMζ-antisense compared with those with scrambled oligodeoxynucleotide injections on day 23 (*p= 0.0313) and day 24 (*p = 0.0183). (See Figure S3A for training from days 1 to 5.)
PKMζ-Antisense Disrupts the Stability of Long-Term Sensorimotor Memory
The injection of PKMζ-antisense in S1/M1 slowed the rate of learning in the proficiency acquisition phase, but with additional training days the antisense-injected animals eventually reached the same proficiency as the control animals. This result suggests there are two distinct mechanisms of motor learning—one that requires de novo PKMζ synthesis and a second that does not. Because our experiments involve continual training and reinforcement, we asked how well the PKMζ-independent mechanism maintains memory without this reinforcement. After asymptotic performance was reached, we ceased training and retested the rats 1 week later. The control, scrambled oligodeoxynucleotide group maintained peak performance even without reinforcement, whereas the PKMζ-antisense group showed significantly lower performance on days 23 and 24 (Figure 1). We then resumed daily training and found that the group previously injected with antisense relearned the task and reached the asymptotic success rate again on day 26. These results indicate that new PKMζ synthesis during learning is required specifically for the long-term storage of sensorimotor memory that is maintained without daily reinforcement.
ZIP Specifically Disrupts the Storage of Sensorimotor Memory Maintained without Reinforcement
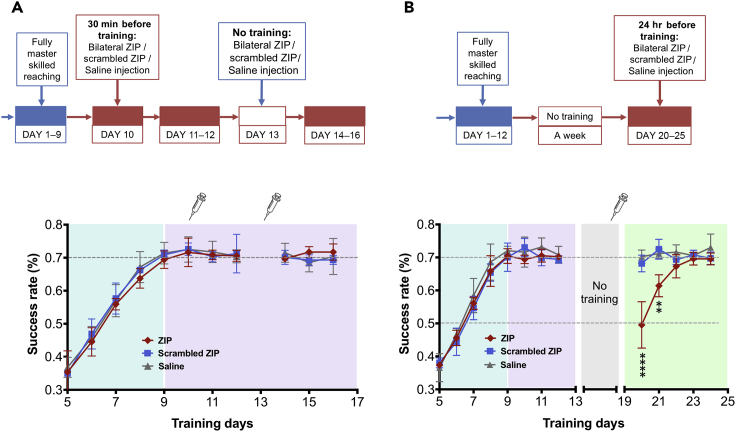
To test this hypothesis further, we examined the effect of the aPKC inhibitory peptide ZIP on sensorimotor memory maintenance after the skill was fully mastered and maintained in two protocols: one maintained with daily training and the other maintained without reinforcement for 1 week.
We first examined if long-term memory sustained by daily practice is disrupted by injections of ZIP. Rats were trained for 9 days to reach maximum proficiency in the skilled reaching task. One day after the last training session, ZIP, scrambled ZIP, or saline was injected bilaterally in S1/M1 (Figures 2A and S2 and Transparent Methods). Thirty minutes after injection, reaching proficiency was tested. The success rate of rats with ZIP injection was not significantly different from those with scrambled ZIP or saline injections (Figure 2A). The training was continued for the next 2 days and no difference was found. To further assess whether memories held by daily practice and reinforcement were not affected by ZIP, a second injection was given on day 13 in the same rats, and 1 day later the training resumed from days 14–16. Again, ZIP had no effect on the task performance (Figure 2A).
Figure 2.
ZIP Specifically Disrupts the Storage of Unreinforced Skilled Motor Memory
(A) Motor memory sustained by practice is PKMζ-independent. Rats were trained for 9 days until skilled reaching performance plateaued at ∼70% success rate. On day 10, the animals then were randomly divided into three groups that received ZIP, scrambled ZIP, or saline injections bilaterally 30 min before the skilled reaching task. The injection dosage of ZIP/scrambled ZIP was 1 μL per site (2 μL total in each hemisphere). Daily training was continued for days 11 and 12. On day 13, the same injections were made in the same rats as day 10 but without training after injection. Daily training was resumed from days 14 to 16. The learning curves for rats that received ZIP, scrambled ZIP, or saline injections are marked in red (n = 3), blue (n = 3), and gray (n = 5), respectively (mean ± SD). Two-way ANOVA showed no significant difference in the learning rate among the groups (F(2,8) = 0.6555, p = 0.5450). (See Figure S3B for training from days 1 to 5.)
(B) Motor memory sustained without practice is PKMζ-dependent. Rats were trained daily for 12 days followed by 1 week of regular housing. The animals then received ZIP, scrambled ZIP, or saline injections bilaterally on day 19 (2 μL/hemisphere). On day 20, daily training resumed. The learning curves for rats that received ZIP, scrambled ZIP, or saline injections are marked in red (n = 5), blue (n = 5), and gray (n = 5), respectively (mean ± SD). Two-way ANOVA (F(2,8) = 6.927, p = 0.0180), followed by post hoc Tukey's comparisons, showed a significantly lower success rate of ZIP on days 20 and 21 compared with scrambled ZIP (****p < 0.0001 and **p = 0.0024) or saline groups (p < 0.0001 and p = 0.0086). The asterisks in the figure represented the p value of ZIP vs. scrambled ZIP. (See Figure S3C for training from days 1 to 5.)
A second set of rats were trained to asymptotic performance and after 1 week of no training were divided into three groups that received ZIP, scrambled ZIP, or saline injection bilaterally in S1/M1 (Figures 2B and S2 and Transparent Methods). Rats intracortically injected with scrambled ZIP or saline controls showed no loss of proficiency in the reaching task when tested again on day 20 (1 day after injection). But in striking contrast to its lack of effect on memory sustained by reinforcement, the ZIP injection disrupted long-term motor memory that was maintained without reinforcement (Figure 2B). On resuming daily training from days 20 to 25, a relearning curve revealed that the group previously injected with ZIP acquired motor memory with a proficiency indistinguishable from the original learning curve, indicating no savings of motor memory after memory erasure by ZIP.
Sensorimotor Training Induces a Persistent Increase of PKMζ in Sensorimotor Cortex
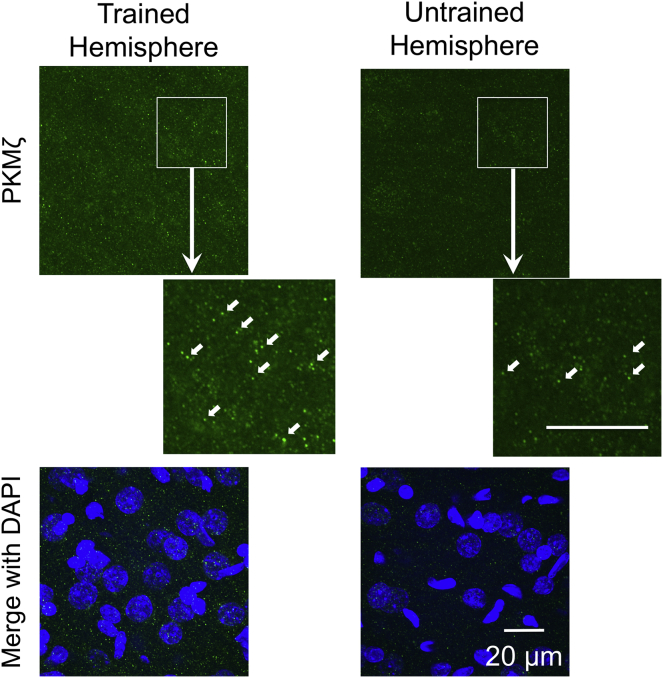
To localize the persistently increased PKMζ that stores unreinforced long-term motor memory, we next measured the changes of PKMζ expression throughout S1 and M1 forelimb regions during each learning phase. Confocal microscopy revealed PKMζ in the sensorimotor cortex is compartmentalized in small puncta (Figure 3), similar to its distribution in the hippocampus (Hernández et al., 2014). The puncta number and size were quantified in each hemisphere, and the interhemispheric ratios (trained/untrained hemispheres) were used to compare the amounts of PKMζ in each group with those in controls (naive rats) (Figures 4 and S5 and Transparent Methods).
Figure 3.
PKMζ Immunostaining of Sensorimotor Cortex
Immunostaining of PKMζ detected as small puncta (white arrows). Green, PKMζ staining; blue, DAPI staining; scale bar, 20 μm. Sample images were from M1 layer II/III of a rat after 9 days of training (magnification 120x).
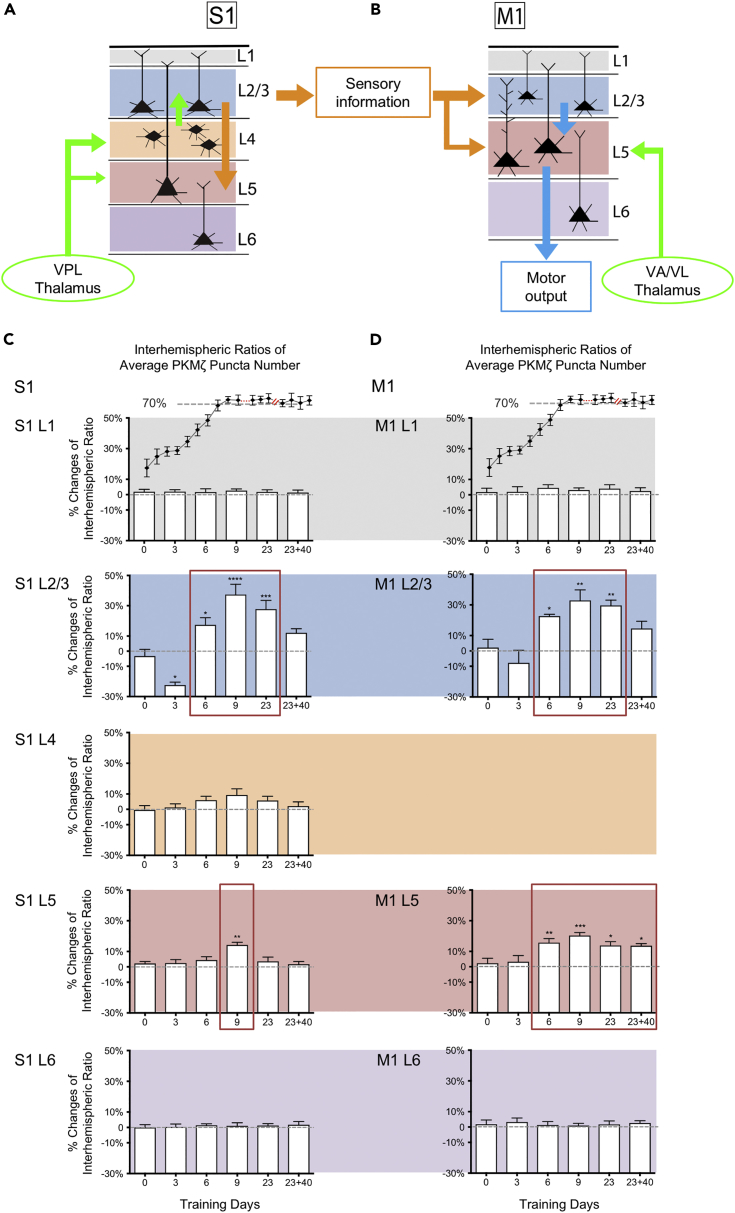
Figure 4.
Spatiotemporal Changes of PKMζ Puncta Number during Sensorimotor Learning and Memory
(A and B) Illustration of information propagation in S1 and M1 during sensorimotor learning.
(C and D) Layer-specific changes of PKMζ puncta number ratios in S1 and M1. For clarity, the learning curves of the skilled reaching task for the animals (same as Figure S1A) are shown as inserts. X axis, days of training; Y axis, % changes of interhemispheric ratio of average PKMζ puncta number (mean ±SEM). One-way ANOVAs showed significant changes in S1 layer II/III (F(5,26) = 20.55; p < 0.0001), S1 layer V (F(5,26) = 5.079; p = 0.0022), M1 layer II/III (F(5,26) = 8.764; p < 0.0001), and M1 layer V (F(5,26) = 6.857; p = 0.0003). Dunnett's multiple comparison tests showed differences between each training group and control (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). In contrast to puncta number, the interhemispheric ratios of the average PKMζ puncta size did not change significantly (see Figure S5). Different from PKMζ, PSD-95 only showed a transient increase at the end of the performance improvement phase (See Figure S6). The training paradigm and sample size are shown in Figure S4.
The results revealed that learning-induced changes in PKMζ, as well as the persistence of these changes during memory storage, are selective to distinct cortical layers. One-way ANOVAs showed significant changes of PKMζ puncta numbers in both S1 and M1 layers II/III and V. In the initial skill acquisition phase, which is not affected by PKMζ-antisense or ZIP, the interhemispheric ratios of PKMζ puncta number decreased significantly compared with control in S1 layer II/III (Figure 4C). In contrast, during the performance improvement phase, which is disrupted by these PKMζ antagonists, PKMζ increased in multiple cortical layers. After 6 and 9 days of training, PKMζ puncta numbers increased in S1 layer II/III, M1 layer II/III, and M1 layer V, and, after 9 days of training, in S1 layer V (Figures 4C and 4D). The increases reached a maximum on day 9 when memory expression reached asymptotic levels of performance. During the proficiency maintenance phase, when the animals continued daily training, the increased amounts of PKMζ in S1 layer II/III, M1 layer II/III, and M1 layer V were maintained, whereas those in S1 layer V returned to basal levels (Figures 4C and 4D).
During the long-term memory storage phase, i.e., after 40 days without training, the increases in PKMζ persisted specifically in M1 layer V (Figure 4D). As a control, we examined in all S1/M1 cortical layers a second protein, postsynaptic density protein-95 (PSD-95), which is associated with learning-induced structural changes in the sensorimotor cortex. In contrast to PKMζ, PSD-95 showed a transient increase at the end of the performance improvement training phase, which did not persist into the maintenance or storage phases (Figure S6). These results demonstrate that skilled motor training induces selective increases in PKMζ that can be either transient or highly stable at long timescales within specific layers of the sensorimotor cortex.
Discussion
Here we show that persistent increases in PKMζ maintain the long-term storage of skilled motor memory that is sustained without continual daily reinforcement. The locus of this persistent molecular storage mechanism during unrehearsed, long-term motor memory is the output cortical layer of M1.
Both PKMζ-specific antisense oligodeoxynucleotides and the aPKC-selective kinase inhibitor ZIP disrupted the maintenance of long-term skilled motor memory that is maintained without practice but not skilled motor memory that is reinforced daily. These results are in line with earlier findings with ZIP on conditioned taste aversion (CTA), in which the inhibitor erased the stored CTA memory but had no effect on CTA memories that were recently reconsolidated by re-exposure to the conditioning stimulus (Levitan et al., 2016). ZIP, however, cannot exclude the possibility of an additional role for PKCι/λ (Tsokas et al., 2016). Because both PKMζ-antisense and ZIP specifically disrupt memory maintained without reinforcement (Figures 1C and 2B), our results indicate that another mechanism of memory can maintain the expression of enhanced motor performance for ∼2 days during continual daily practice/reinforcement (Figure 2A). Such mechanisms might include the less persistent action of other PKC isoforms or other kinases such as CaMKII.
The changes of PKMζ in the forelimb contralateral S1 layer II/III are biphasic. During early skill acquisition, PKMζ is initially downregulated in S1 layer II/III (Figure 4C). This downregulation may represent a weakening of the sensory map or sensorimotor associations during the initial sensorimotor training. Downregulation of PKMζ is associated with long-term depression (Hrabetova and Sacktor, 1996, Hrabetova and Sacktor, 2001), and, therefore, the initial decrease of PKMζ in S1 layer II/III might represent an LTD-like process involving a weakening of pre-existing neuronal networks. Because rats were still actively exploring the training chamber and food pellet, each reaching attempt at this phase could induce new sensory stimuli patterns, and the comparatively low success rate might act as negative feedback to disrupt previously acquired sensorimotor associations. In contrast to the rapid acquisition of the stable motor engram in the performance improvement phase, this initial phase of motor learning is unaffected by PKMζ antagonists. Notably, there is a pause in performance improvement at day 4 that appears to separate this initial skill acquisition phase and the performance improvement phase (Figure S1) (Monfils and Teskey, 2004, von Kraus et al., 2010, Wang et al., 2011).
After initially downregulating in the skill acquisition phase, the amount of PKMζ rebounds to increase above baseline during the performance improvement phase that begins on day 5. These increases are selective to S1/M1 layers II/III and V (Figures 4C, 4D, and S5). The timing of the increase of PKMζ parallels that of the LTP-like potentiation of synaptic transmission observed in motor cortex after skill learning (Monfils and Teskey, 2004, Rioult-Pedotti et al., 2000). PKMζ-antisense specifically delays the acquisition of this performance improvement phase, in line with the critical role of PKMζ in maintaining LTP.
The persistence of these learning-induced increases in PKMζ is layer-specific, revealing the location of the molecular mechanism storing very long-term skilled motor memory. Even after 40 days without reinforcement, the increases of PKMζ in layer V of motor cortex are stable. In contrast, the initial PKMζ increases observed at the end of the performance improvement phase in the sensory cortex and layers II/III of the motor cortex return toward baseline. These results indicate that the persistent molecular mechanism specifically associated with stable skilled motor memory is within the cellular output circuitry of the primary motor cortex. The location of the stable PKMζ increases in layer V of M1 is in line with work showing that this rodent reaching task induces synaptic plasticity of thalamocortical pathways that target cortical layer V neurons, which then project to C8 and distal forepaw muscles used in learned grasping (Biane et al., 2016). Further research will be required to localize within M1 layer V neurons the PKMζ that persistently potentiates synaptic transmission and thus stores long-term procedural memory.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Dr. Changchi Hsieh, Dr. Janina Ferbinteanu, and Dr. Panayiotis Tsokas for technical advice and all the members of the Francis laboratory for discussion and support. This study was supported by grants from DAPAR (www.darpa.mil) (Award#60806; Project#:108723), NINDS (R01NS092894) (J.T.F.), IBR-SUNY Downstate graduate fund (J.T.F and J.H.G.), and by grants from NIMH (2R37 MH057068 and R01 115304) and NIDA (R01 DA034970) (T.C.S.).
Author Contributions
Conceptualization, J.T.F. and T.C.S.; Methodology, P.P.G. and J.T.F.; Investigation, P.P.G.; Writing, P.P.G., J.T.F., J.H.G., and T.C.S.; Funding Acquisition, J.T.F., J.H.G., and T.C.S.; Supervision, J.T.F., J.H.G., and T.C.S.
Declaration of Interests
The authors declare no competing interests.
Published: July 27, 2018
Footnotes
Supplemental Information includes Transparent Methods and six figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.07.002.
Contributor Information
Todd Charlton Sacktor, Email: tsacktor@downstate.edu.
Joseph Thachil Francis, Email: joey199us@gmail.com.
Supplemental Information
References
- Biane J.S., Takashima Y., Scanziani M., Conner J.M., Tuszynski M.H. Thalamocortical projections onto behaviorally relevant neurons exhibit plasticity during adult motor learning. Neuron. 2016;89:1173–1179. doi: 10.1016/j.neuron.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Cai D., Pearce K., Sun P.Y.-W., Roberts A.C., Glanzman D.L. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife. 2014;3:e03896. doi: 10.7554/eLife.03896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E., Cohen L.G. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M., Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gámiz F., Gallo M. Intra-amygdala ZIP injections impair the memory of learned active avoidance responses and attenuate conditioned taste-aversion acquisition in rats. Learn. Mem. 2011;18:529–533. doi: 10.1101/lm.2253311. [DOI] [PubMed] [Google Scholar]
- Harms K.J., Rioult-Pedotti M.S., Carter D.R., Dunaevsky A. Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J. Neurosci. 2008;28:5686–5690. doi: 10.1523/JNEUROSCI.0584-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A.I., Oxberry W.C., Crary J.F., Mirra S.S., Sacktor T.C. Cellular and subcellular localization of PKMζ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130140. doi: 10.1098/rstb.2013.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S., Sacktor T.C. Bidirectional regulation of protein kinase M zeta in the maintenance of long-term potentiation and long-term depression. J. Neurosci. 1996;16:5324–5333. doi: 10.1523/JNEUROSCI.16-17-05324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S., Sacktor T.C. Transient translocation of conventional protein kinase C isoforms and persistent downregulation of atypical protein kinase Mzeta in long-term depression. Brain Res. Mol. Brain Res. 2001;95:146–152. doi: 10.1016/s0169-328x(01)00185-1. [DOI] [PubMed] [Google Scholar]
- Hsieh C., Tsokas P., Serrano P., Hernández A.I., Tian D., Cottrell J.E., Shouval H.Z., Fenton A.A., Sacktor T.C. Persistent increased PKMζ in long-term and remote spatial memory. Neurobiol. Learn. Mem. 2017;138:135–144. doi: 10.1016/j.nlm.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo W.J. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J. Neurosci. 2004;24:5560–5569. doi: 10.1523/JNEUROSCI.0562-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim J. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol. Learn. Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kleim J.A., Barbay S., Nudo R.J. Functional reorganization of the rat motor cortex following motor skill learning. J. Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleim J.A., Lussnig E., Schwarz E.R., Comery T.A., Greenough W.T. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J. Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim J.A., Hogg T.M., VandenBerg P.M., Cooper N.R., Bruneau R., Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Sacrey L.-A.R., Whishaw I.Q., Dunnett S.B. The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neurosci. Biobehav. Rev. 2012;36:1030–1042. doi: 10.1016/j.neubiorev.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Kwapis J.L., Jarome T.J., Gilmartin M.R., Helmstetter F.J. Intra-amygdala infusion of the protein kinase Mzeta inhibitor ZIP disrupts foreground context fear memory. Neurobiol. Learn. Mem. 2012;98:148–153. doi: 10.1016/j.nlm.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis J.L., Jarome T.J., Lonergan M.E., Helmstetter F.J. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav. Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D., Fortis-Santiago Y., Figueroa J.A., Reid E.E., Yoshida T., Barry N.C., Russo A., Katz D.B. Memory retrieval has a dynamic influence on the maintenance mechanisms that are sensitive to inhibitory peptide (ZIP) J. Neurosci. 2016;36:10654–10662. doi: 10.1523/JNEUROSCI.1568-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling D.S.F., Benardo L.S., Serrano P.A., Blace N., Kelly M.T., Crary J.F., Sacktor T.C. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat. Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Luft A.R., Buitrago M.M., Ringer T., Dichgans J., Schulz J.B. Motor skill learning depends on protein synthesis in motor cortex after training. J. Neurosci. 2004;24:6515–6520. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migues P.V., Hardt O., Wu D.C., Gamache K., Sacktor T.C., Wang Y.-T., Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat. Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Monfils M.H., Teskey G.C. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125:329–336. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Osten P., Valsamis L., Harris A., Sacktor T.C. Protein synthesis-dependent formation of protein kinase Mzeta in long-term potentiation. J. Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E., Serrano P., Pinkhasova D., Wallace E., Fenton A.A., Sacktor T.C. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pauli W.M., Clark A.D., Guenther H.J., O'Reilly R.C., Rudy J.W. Inhibiting PKMζ reveals dorsal lateral and dorsal medial striatum store the different memories needed to support adaptive behavior. Learn. Mem. 2012;19:307–314. doi: 10.1101/lm.025148.111. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M.S., Donoghue J.P., Dunaevsky A. Plasticity of the synaptic modification range. J. Neurophysiol. 2007;98:3688–3695. doi: 10.1152/jn.00164.2007. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M.S., Friedman D., Donoghue J.P. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M.S., Friedman D., Hess G., Donoghue J.P. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Sacktor T.C., Osten P., Valsamis H., Jiang X., Naik M.U., Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc. Natl. Acad. Sci. USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor T.C. Memory maintenance by PKMζ–an evolutionary perspective. Mol. Brain. 2012;5:31. doi: 10.1186/1756-6606-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J.N., Donoghue J.P. Plasticity and primary motor cortex. Annu. Rev. Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Serrano P., Friedman E.L., Kenney J., Taubenfeld S.M., Zimmerman J.M., Hanna J., Alberini C., Kelley A.E., Maren S., Rudy J.W. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R., Sacktor T.C., Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shao C.Y., Sondhi R., van de Nes P.S., Sacktor T.C. PKMζ is necessary and sufficient for synaptic clustering of PSD-95. Hippocampus. 2012;7:1501–1507. doi: 10.1002/hipo.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P., Hsieh C., Yao Y., Lesburguères E., Wallace E.J.C., Tcherepanov A., Jothianandan D., Hartley B.R., Pan L., Rivard B. Compensation for PKMζ in long-term potentiation and spatial long-term memory in mutant mice. Elife. 2016;5:12677. doi: 10.7554/eLife.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kraus L.M., Sacktor T.C., Francis J.T. Erasing sensorimotor memories via PKMzeta inhibition. PLoS One. 2010;5:e11125. doi: 10.1371/journal.pone.0011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Conner J.M., Rickert J., Tuszynski M.H. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc. Natl. Acad. Sci. USA. 2011;108:2545–2550. doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw I.Q. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q., Alaverdashvili M., Kolb B. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav. Brain Res. 2008;192:124–136. doi: 10.1016/j.bbr.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Xu T., Yu X., Perlik A.J., Tobin W.F., Zweig J.A., Tennant K., Jones T., Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zuo Y. Spine plasticity in the motor cortex. Curr. Opin. Neurobiol. 2011;21:169–174. doi: 10.1016/j.conb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.