Highlights
-
•
Delta brushes have been inconsistently described over the last five decades.
-
•
Their characteristics vary according to gestational age and vigilance state.
-
•
Delta brushes can be spontaneous or elicited by peripheral stimuli.
-
•
Different delta brush abnormalities are a marker of specific pathologies.
Keywords: Delta brushes, Spontaneous activity transients, Neonatal EEG
Abstract
Delta brushes are the hallmark of the EEG of premature infants. They are readily recognisable because of their characteristic appearance and are a key marker of neural maturation. However they are sometimes inconsistently described in the literature making identification of abnormalities challenging. The goal of this review is to provide an overview of research findings on this topic in the last five decades. Firstly, the characteristic features of delta brushes are described, including the developmental trajectory of their incidence and how they are modulated by vigilance state in normal neonates. Secondly, their clinical significance is discussed including how abnormalities in their incidence or appearance indicate particular pathophysiology. We propose that (i) the effect of age and vigilance state on the frequency, amplitude and topography of delta brushes, and (ii) heterogeneity within the cohorts of ‘normal’ premature infants studied, may explain the very variable descriptions of delta brush characteristics in the literature. By explicitly taking these factors into consideration to explain delta brush variability, the presented summary facilitates the clinical electrodiagnostic and prognostic use of delta brush abnormalities as a biomarker.
1. Definition and characteristics (Table 1)
Table 1.
Characteristics of delta brushes according to post-menstrual age.
| PMA (weeks) | 24–27 | 28–30 | 31–32 | 33–34 | 35–36 | 37–38 | 39–40 |
|---|---|---|---|---|---|---|---|
| Incidence | +/− | ++ | ++ | +++ | ++ | + | +/− |
| Frequency of delta wave | <1 Hz | <1 Hz | ⩽2 Hz | ⩽2 Hz | ⩽2 Hz | ⩽2 Hz | ⩽2 Hz |
| Amplitude of delta wave | ++ | +++ | +++ | ++ | ++ | + | + |
| Peak fast frequency | 12–14 Hz | 14–16 Hz | 14–20 Hz | 16–20 Hz | 16–20 Hz | 10–15 Hz | 10–15 Hz |
| Topography | Diffuse; central | Diffuse; central | Diffuse; central; temporal-occipital | Diffuse; central; temporal-occipital | Temporal occipital > central | Temporal-occipital | Temporal-occipital |
| Effect of vigilance state | AS > Wake > QS | AS > Wake > QS | AS > Wake > QS | QS > Wake = AS | QS > Wake = AS | QS mainly | QS only |
PMA = post-menstrual age; AS = active sleep; QS = quiet sleep.
The neonatal EEG is strikingly different from the EEG recorded in later infancy; it presents a range of distinctive features which change rapidly with maturation. The hallmark of the premature neonatal EEG is the delta brush; review of their incidence and appearance is an important part of the clinical neurophysiological assessment of hospitalised infants. In this review we will refer to the age of the infants in terms of post-menstrual age (PMA) which is gestational age measured from the time of the last menstrual period + postnatal age (Tsuchida et al., 2013).
1.1. Slow frequency component
Delta brushes are transient patterns characterised by a slow delta wave with superimposed fast activity. The underlying slow activity is up to 1.5 Hz with a lower threshold variously stated as 0.3 Hz (Volpe, 1995, André et al., 2010, Noachtar et al., 1999, Scher, 2006), 0.5 Hz (D’Allest and André, 2002) or 0.8 Hz (Tharp et al., 1981). The frequency of the slow activity increases with advancing PMA, but remains within the delta range, reaching 1–2 Hz by 32–34 weeks (D’Allest and André, 2002, André et al., 2010). The duration of delta brush activity is from 0.5 s to an upper limit of between 1.5 s (Conde et al., 2005) and 5 s from 31 weeks (Radvanyi-Bouvet et al., 1987). The amplitude of the delta wave ranges from 50 μV to an upper limit of 250–300 μV (Lamblin et al., 1999, André et al., 2010, Tsuchida et al., 2013) and peaks in amplitude at 30–31 weeks (D’Allest and André, 2002) before decreasing with age.
1.2. Fast frequency component
The delta wave of a delta brush has a superimposed set of fast frequencies, usually on a steeply ascending slope (Tolonen et al., 2007, Watanabe et al., 1999, Conde et al., 2005; Fig. 1b). This over-riding fast activity has been heterogeneously quantified as 8–20 Hz (Tharp et al., 1981, Biagioni et al., 1994), 8–22 Hz (Lombroso, 1979), 8–25 Hz (Milh et al., 2007), 10–20 Hz (Scher, 2006, Boylan, 2007), 14–24 Hz (Goldie et al., 1971), 18–22 Hz (Volpe, 1995, Koszer et al., 2006), >8 Hz (Lamblin et al., 1999, André et al., 2010) or as ‘alpha-beta’ which implies 8-30 Hz (Hellström-Westas and Rosén, 2010). The standardised neonatal EEG terminology of the American Clinical Neurophysiology Society (Tsuchida et al., 2013) defines the over-riding fast activity as being 8–12 Hz or 18–22 Hz, but references Lamblin et al.’s (1999) French language paper, which only specifies that the superimposed fast activity is >8 Hz. Spectral decomposition of neonatal EEG bursts reveals a rapid fall-off in power above 24 Hz (Myers et al., 2012) suggesting that frequencies above 24 Hz are not present to a significant extent, although minimal gamma-range frequencies are possible (Milh et al., 2007).
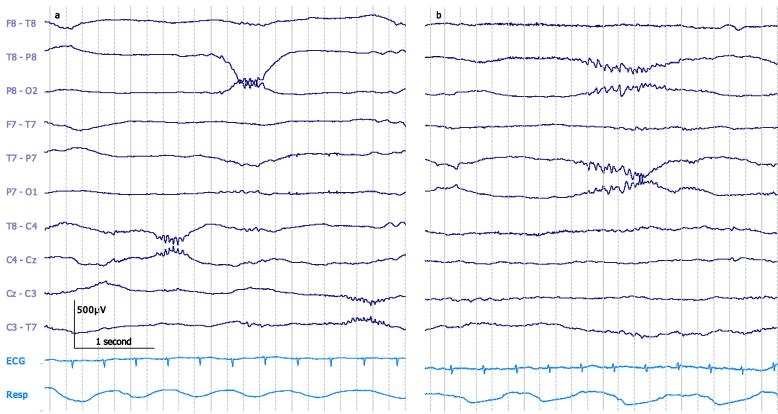
Fig. 1.
a: Pericentral and posterior temporal delta brushes in a 29 + 6 weeks PMA neonate during quiet sleep. b: Bilateral posterior temporal delta brush in a 34 + 6 weeks PMA neonate during active sleep. Both traces: bipolar montage. 30 μV/mm. 0.1–70 Hz bandpass; 50 Hz notch filter.
The peak frequency in the delta brush fast activity band increases from 12–14 Hz at 27 weeks, 14–16 Hz at 28–32 weeks (Anderson et al., 1985), peaking at 16–20 Hz at 32–35 weeks (Niedermeyer, 2005) and falling to 10–15 Hz from 36 weeks (Volpe, 1995, Niedermeyer, 2005). The frequency of fast activity is also affected by vigilance state: bursts during quiet sleep have a slightly lower peak frequency (Myers et al., 2012).
The amplitude of the superimposed fast activity is at least 10–20 μV (Lombroso, 1979, Conde et al., 2005) reaching up to 60 μV (Lamblin et al., 1999, André et al., 2010, Scher, 2006) or occasionally 100 μV (Tharp et al., 1981). Again, amplitude varies according to post-menstrual age; Volpe (1995) observes particularly high voltages at 34–35 weeks.
1.3. Incidence
Delta brushes can be present from 24 weeks (Tsuchida et al., 2013, Hahn and Tharp, 2005) but are usually seen from 28–30 weeks (Lamblin et al., 1999, Boylan et al., 2008, Niedermeyer, 2005, Vecchierini et al., 2007). Their peak incidence is at 32–35 weeks (Boylan et al., 2008, Hahn and Tharp, 2005, Lamblin et al., 1999, D’Allest and André, 2002, André et al., 2010, Hahn and Tharp, 2005) and they disappear between 38 and 42 weeks (Koszer et al., 2006, Hahn and Tharp, 2005, Boylan et al., 2008). During early prematurity they are more frequent during active than quiet sleep. From 29 to 34 weeks this changes and delta brushes are more frequent, and of higher amplitude, in quiet than active sleep (Goldie et al., 1971, Lombroso, 1979, Watanabe and Iwase, 1972, Scher, 2006, Koszer et al., 2006, Tharp et al., 1981, André et al., 2010). At later post-menstrual ages (34–42 weeks), although scattered delta brushes may be present during active sleep and wakefulness, quiet sleep is the only state in which they are prominent (Goldie et al., 1971, D’Allest and André, 2002, Niedermeyer, 2005, Statz et al., 1982). In general, the reduction of delta brush occurrence correlates with the emergence of clear vigilance state differentiation (Colonnese et al., 2010, Mirmiran et al., 2003), suggesting a relationship between these two developmental features.
Delta brushes can also occur during wakefulness, but have been scarcely reported, probably because premature infants only spend 2–4% of their time in this vigilance state (Curzi-Dascalova et al., 1993). A single study reports that the frequency of delta brushes during wakefulness is more than during quiet sleep but less than during active sleep until 33 weeks. From 34 to 42 weeks, delta brushes occur during wakefulness as often as during active sleep (Watanabe and Iwase, 1972).
1.4. Topographical distribution
Several authors have gone beyond assessing overall incidence of delta brushes across post-menstrual age and have described the peak incidence of particular delta brush topographies. Delta brushes are diffuse (Lamblin et al., 1999) or prominent pericentrally between 29–34 weeks (Volpe, 1995, Boylan, 2007), involving also temporal-occipital regions from 30–34 weeks (D’Allest and André, 2002, Hahn and Tharp, 2005, Arichi et al., 2016, André et al., 2010, Tolonen et al., 2007) (Fig. 1a and b). From 36 weeks, when delta brushes are starting to reduce overall, the pericentral topography is absent or extremely scarce (Volpe, 1995, Watanabe and Iwase, 1972). Meanwhile, temporal and occipital delta brushes persist until full-term age (37–42 weeks). Delta brushes in the frontal regions are the least common (Milh et al., 2007, Watanabe and Iwase, 1972, Whitehead et al., 2016), but occasionally occur up until 36 weeks (Watanabe and Iwase, 1972) (Fig. 2). Delta brush topographies can have specific frequency characteristics: pericentral delta brushes have a higher mean overriding fast frequency compared to other locations between 27 and 32 weeks (Anderson et al., 1985).
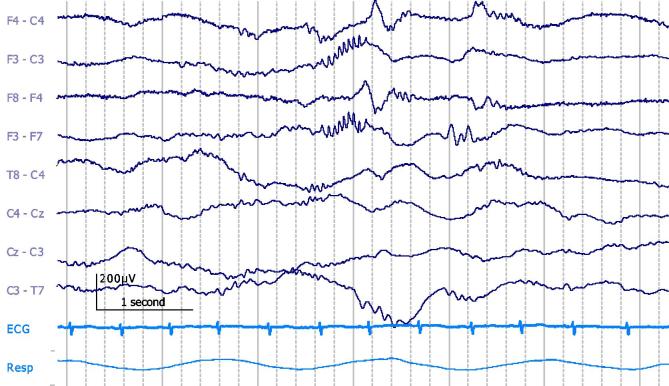
Fig. 2.
Frontal delta brush in 34 + 5 PMA neonate during active sleep. Bipolar montage. 20 μV/mm. DC-70 Hz bandpass; 50 Hz notch filter.
Only two studies have described differences in delta brush topography between vigilance states (Watanabe and Iwase, 1972, Whitehead et al., 2016). However, a fuller quantification of their topographic distribution may facilitate the monitoring of neurophysiological mechanisms for brain development (Myers et al., 2012). Between 31 and 42 weeks, temporal-occipital delta brushes are the most common topography during wakefulness and active sleep while pericentral delta brushes, when present, are mostly seen during quiet sleep (Whitehead et al., 2016, Watanabe and Iwase, 1972). Between 33 and 38 weeks, occipital delta brushes are the type least enhanced by quiet sleep (Watanabe and Iwase, 1972).
Delta brushes are usually considered as static events; few studies have addressed their propagation dynamics. They have been characterised as occurring asynchronously in homologous areas of the two hemispheres (Hahn and Tharp, 2005) or as local events that have a tendency to spread (Khazipov and Luhmann, 2006). Spontaneous activity transients (SATs), a term that encompasses delta brushes but also other mixed-frequency activity bursts, appear to propagate from temporal-occipital to frontal areas according to onset times at different EEG derivations (Vanhatalo et al., 2005, Vanhatalo and Kaila, 2010). Overall, SATs are found to be focal in premature neonates and to develop, towards full-term age, into a partially synchronous activity that involves both hemispheres (Tolonen et al., 2007).
2. Elicited delta brushes
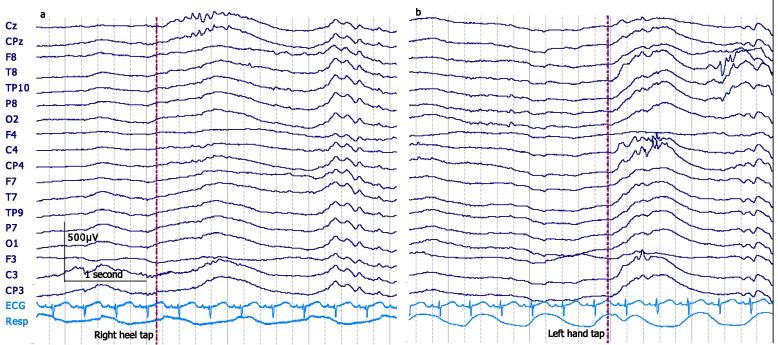
Delta brushes occur spontaneously, but can also be triggered by peripheral sensory stimuli. The topography of stimulated delta brushes has been shown to coarsely relate to stimulus modality. In neonates <35 weeks, visual stimuli elicit occipital delta brushes (Colonnese et al., 2010) and auditory stimuli evoke mid-temporal delta brushes (Chipaux et al., 2013). At 29–31 weeks, stroking the hand or foot elicits pericentral delta brushes preferentially located at the contralateral mid-central or vertex electrode respectively (Milh et al., 2007). Preliminary data obtained by our own group using tap stimulation indicate that delta brushes with the same somatotopic pattern can also be seen at 32–33 weeks (Fig. 3) (unpublished). Noxious heel lances can elicit delta brushes at the mid-temporal regions (Fabrizi et al., 2011) from 28 weeks; this activity is replaced by a mature vertex evoked potential between 32 and 35 weeks (Hartley et al., 2016, Fabrizi et al., 2011).
Fig. 3.
Elicited delta brushes in a 32 + 5 weeks PMA neonate during active sleep seen at the midline following right heel tap (a) and predominantly at the contralateral mid-central electrodes following a left palm tap (b). Both traces: Fz reference montage. 25 μV/mm. DC-70 Hz bandpass; 50 Hz notch filter.
3. Clinical significance of delta brushes (Table 2)
Table 2.
Delta brush abnormalities and their significance.
| Delta brush abnormality | Possible significance of delta brush abnormality |
|---|---|
| + add-on features | significance of add-on features |
| Absence or attenuation of alpha-beta frequencies, including delta brushes | Unclear clinical significance Acute-stage abnormality |
| + loss of theta frequencies | Poorer prognosis |
| Incidence of delta brushes ⩾2 weeks immature for PMA | Unclear clinical significance Chronic-stage dysmature abnormality: most associated with cognitive impairment |
| + follows >3 weeks acute EEG depression | Poorer prognosis |
| + >4 weeks dysmature | Poorer prognosis |
| + presence of seizures | Not predictive of poor outcome |
| +<37 weeks PMA | Interpret with caution, especially if <29 weeks |
| Deformed delta brushes | Unclear clinical significance Chronic-stage disorganised abnormality: most associated with white matter damage |
| + >4 weeks postnatal age | Poorer prognosis |
| + pronounced anterior spread | Associated with higher PVL grade |
| + temporal sawtooth waves or delta brushes past the age at which they are expected | Strengthens clinical suspicion of abnormal maturational process |
| Reduced amplitude 8–20 Hz compared to normal values | Effect of prematurity, especially if born <29 weeks; unclear clinical significance |
PMA = post-menstrual age.
Because delta brushes are a prominent feature of the EEG in premature neonates, deviation from their normal developmental trajectory and untimely absence or presence facilitate the recognition of aberrant EEG patterns and is diagnostically and prognostically useful (Table 2). EEG abnormalities can be separated into acute stage and chronic stage abnormalities.
3.1. Acute-stage abnormality: depressed EEG
EEG depression includes attenuated faster activity, as well as decreased continuity, and is often defined as an acute-stage EEG abnormality (Okumura et al., 2002). After a brain insult, alpha-beta rhythms like those in delta brushes are initially reduced. This is followed, in the most abnormal cases, with loss of theta rhythms, leaving only delta waves (Watanabe et al., 1999). Mild EEG depression suggests suboptimal clinical outcome when it lasts for over three weeks and/or is followed by an EEG with an immature appearance considering the neonate’s post-menstrual age (Hayakawa et al., 1997, Hayakawa et al., 1997). Acute-stage EEG abnormalities are seen in 65% of neonates with periventricular leukomalacia and are most specific (less false positives) if seen from postnatal day 5 (Okumura et al., 1999).
As delta brushes are an abundant feature of normal EEGs in premature neonates, their absence facilitates the recognition of a depressed EEG (Holmes and Lombroso, 1993). A low voltage pattern without delta brushes was found to be associated with major neurological sequelae in 4/5 infants. Conversely, in infants with the same pattern overlaid with some normal features, such as delta brushes, this was not predictive of outcome. For example, while 7/23 neonates had major sequelae, a further 7/23 (i.e. exactly the same proportion) developed normally (Tharp et al., 1981). There is a trend towards a greater incidence of delta brushes in premature neonates who had normal outcome but this only reached statistical significance in EEGs at 33–34 weeks (Biagioni et al., 1994). A diminished incidence of delta brushes in neonates with major ultrasound lesions is observed in the first week of life; this reduced occurrence of delta brushes is most marked in neonates born at 31–32 weeks in whom this finding persists until 36 weeks (Conde et al., 2005). A further study found that in 4/5 neonates with later cognitive impairment, EEG maturation had arrested between 28 and 34 weeks, including absence of the expected increase in delta brushes during this developmental period (Hayakawa et al., 1997, Hayakawa et al., 1997). The absence of delta brushes in EEGs recorded at 33–34 weeks is seen almost exclusively in infants who died or had abnormal sequelae but typically in combination with other grossly abnormal EEG features (Tharp et al., 1981). However, when delta brushes are absent at ages at which they are expected, some authors caution against necessarily interpreting this as abnormal (Boylan et al., 2008).
3.2. Chronic-stage abnormality: dysmaturity
EEGs are defined as dysmature if they present patterns which are at least 2 weeks immature for the neonate’s post-menstrual age (Holmes and Lombroso, 1993, André et al., 2010, Tsuchida et al., 2013, Hahn and Tharp, 2005) and this is considered a chronic-stage EEG abnormality (Watanabe et al., 1999). The number of delta brushes is one of the features used as a benchmark to determine EEG maturity. Normative values for delta brush incidence have been proposed (Lombroso, 1979, Watanabe and Iwase, 1972) to quantify the degree of abnormality (Holmes and Lombroso, 1993). Despite this, some authors argue that dysmaturity is difficult to ascertain in infants <37 weeks because the normal EEG during prematurity is more variable than at full-term age (Tharp, 1990). Most studies which refer to delta brushes assess absolute number or proportion of bursts containing fast activity. The amplitude of the underlying delta wave (Biagioni et al., 1994, Conde et al., 2005) and the over-riding fast (Biagioni et al., 1994) do not correlate with the presence of major ultrasound lesions or clinical outcome and are therefore not used as markers of maturity.
The presence of prominent delta brushes during quiet and active sleep in term-age neonates is considered ‘mildly abnormal’ and requires a follow-up EEG (Tharp, 1990). Persistent EEG dysmaturity in neonates, over serial recordings, is believed to indicate cortical (rather than white matter) injury and correlates with poor cognitive outcome (Lombroso, 1985, Holmes and Lombroso, 1993, Okumura et al., 2002) with a positive correlation between severity of dysmaturity and severity of outcome (Karch et al., 1981, Biagioni et al., 1996). The presence of severe dysmaturity has a high specificity (93.8%) but a low sensitivity (50%) for mild or severe neurological abnormalities at ⩾12 months (Biagioni et al., 1996). All five neonates with later cognitive impairment had delta brushes after 39 weeks along with other features of dysmaturity (Hayakawa et al., 1997, Hayakawa et al., 1997). Using delta brush incidence, alongside three other EEG measures, to assess dysmaturity, 83% of neonates with bronchopulmonary dysplasia associated with a dysmature EEG at 37–42 weeks had a poor outcome, compared to 23% with normal EEGs (Hahn and Tharp, 1990). On the other hand, EEG dysmaturity has been followed by normal outcome, but in only one study of three subjects (Tharp et al., 1981). The absence of all chronic-stage abnormalities in neonates born between 25 and 32 weeks is associated with entirely normal (92%) or borderline (6%) mental development (Okumura et al., 2002).
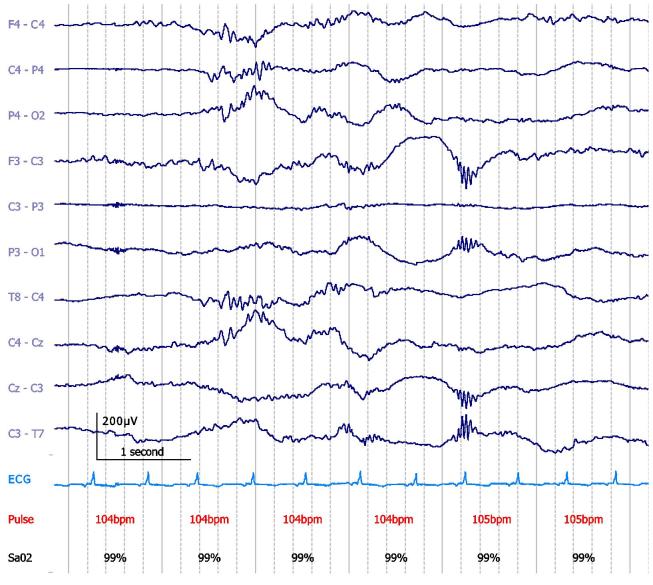
EEG burst rate peaks at a later age in neonates with hypoxic ischemic encephalopathy (HIE) compared to controls (Ranasinghe et al., 2015) but dysmaturity in full-term neonates with HIE can be associated with normal outcome (Holmes et al., 1982). Also in neonates with seizures, there is no relationship between EEG dysmaturity and outcome (Fig. 4) (Lombroso and Holmes, 1993), possibly because seizures can induce temporary dysmaturity of the background EEG (Lombroso, 1975).
Fig. 4.
Dysmature EEG with profuse delta brushes in a 37 + 3 weeks PMA neonate (day 1 of life) with intracerebral haemorrhage and right central-parietal seizures, prior to the establishment of sleep cycling. No psychoactive medications had yet been administered. At day 10, dysmaturity was still present. The neonate had a normal outcome at 2 years of age. Bipolar montage. 15 μV/mm. 0.1–70 Hz bandpass; 50 Hz notch filter.
3.3. Chronic stage abnormality: disorganised EEG
A pathological chronic-stage disorganised EEG presents abnormal morphology of background activities (Okumura et al., 2002). Deformed cog-shaped delta brushes are the most typical representation of this EEG abnormality (André et al., 2010, Watanabe et al., 1999, Tich et al., 2007, Kidokoro et al., 2006). They are defined as spindle-like, fast spiky wave bursts in the 13-20 Hz range (Kidokoro et al., 2006, André et al., 2010), they lack smoothness, their peak to peak amplitude is >400 μV and they have a wider base than normal delta brushes (Tich et al., 2007). Their presence necessitates follow-up EEGs, in which EEG dysmaturity such as persistent delta brushes or temporal sawtooth waves, will strengthen the preliminary diagnosis of an abnormal maturational process (Tich et al., 2007).
The disorganised pattern of deformed delta brushes is observed most frequently between postnatal days 6–21 in neonates born at 28–33 weeks (Okumura et al., 1999). A disorganised EEG is more likely to be associated with normal cognitive development than a dysmature EEG but is closely related to periventricular leukomalacia (Okumura et al., 2002). In neonates with a disorganised pattern 43–93% have periventricular leukomalacia and develop cerebral palsy according to the severity of the EEG abnormality (Okumura et al., 2002, Okumura et al., 1999, Kidokoro et al., 2006), particularly if it persists beyond four weeks of postnatal age (Hayakawa et al., 1997, Watanabe et al., 1999). The anterior spread of the deformed delta brushes correlates with the degree of periventricular leukomalacia (Kidokoro et al., 2006). However, ‘deformed’ delta brushes are also observed in healthy pre-term neonates. In fact in the frontal regions there is no difference in the amount of ‘deformed’ delta brushes observed between healthy neonates and those with periventricular leukomalacia (Kidokoro et al., 2006).
3.4. The effect of prematurity, alone, on delta brush characteristics
Most authors summarise that there is little or no difference between the EEGs of infants born at term and normal infants grown to term after premature delivery (Volpe, 1995, Tsuchida et al., 2013), although spectral analysis has shown lower EEG power in theta, alpha and beta bands for the prematurely born, while delta activity was comparable (Scher et al., 1992). Only one study specifically compared the characteristics of delta brushes in infants born very pre-term without major ultrasound lesions, with those in neonates of the same post-menstrual age who were born later (Conde et al., 2005). Delta brushes persisted for longer in infants born at <29 weeks and, in addition, the amplitude of the over-riding fast activity was reduced in those born <27 weeks. However, EEGs of neonates born at >29 weeks were no different from those of later-born control infants (Conde et al., 2005).
4. Conclusion
Delta brushes are the most well-known feature of the pre-term EEG. However, since the 1970s, there have been discrepancies between authors on key facts such as the age of their peak incidence and their most common topographies in normal neonates. This review highlights concordant findings in normal babies, while briefly summarising where authors differ. The effect of age and vigilance state on the frequency, amplitude and topography of delta brushes may explain their very variable descriptions in the literature. In addition, the wide variety of ‘normal’ findings in premature babies may arise from studies lacking the long-term follow-up necessary to establish that a baby is truly neurologically and cognitively normal (Biagioni et al., 1994). The second part of the review describes the clinical relevance of delta brushes. The clinical scenarios are discussed in which appreciation of delta brush abnormalities has been found to be diagnostically and/or prognostically useful. In summary, there is a large literature on delta brushes. Once delta brush variability is taken into consideration, their easy identification and striking abundance in the pre-term period makes them a viable biomarker of acute or chronic neurological impairment.
Conflict of interest
None.
Acknowledgements
We would like to thank Dr Janet Rennie, Dr Giles Kendall and Dr Sean Mathieson for sharing the neonatal case illustrated by Fig. 4. We would also like to thank the families who participated in our neonatal EEG research program and contributed to Fig. 1, Fig. 2, Fig. 3. This work was supported by the Medical Research Council (MR/L019248/1).
Contributor Information
Kimberley Whitehead, Email: k.whitehead@ucl.ac.uk.
Ronit Pressler, Email: r.pressler@ucl.ac.uk.
Lorenzo Fabrizi, Email: l.fabrizi@ucl.ac.uk.
References
- Anderson C.M., Torres F., Faoro A. The EEG of the early premature. Electroencephalogr. Clin. Neurophysiol. 1985;60(2):95–105. doi: 10.1016/0013-4694(85)90015-x. [DOI] [PubMed] [Google Scholar]
- André M., Lamblin M.-D., d’Allest A.M., Curzi-Dascalova L., Moussalli-Salefranque F., Tich S.N.T., Vecchierini-Blineau M.-F., Wallois F., Walls-Esquivel E., Plouin P. Electroencephalography in premature and full-term infants. Developmental features and glossary. Clin. Neurophysiol. 2010;40(2):59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Arichi, T., Barone, G., Whitehead, K., Lee, A., Padormo, F., Edwards, D., Fabrizi, L., Identification of cortical generators of spontaneous activity in the preterm brain with EEG-fMRI. Poster 1992 Human Brain Mapping congress 2016. https://ww5.aievolution.com/hbm1601/index.cfm?do=abs.pubSearchAbstracts.
- Biagioni E., Bartalena L., Biver P., Pieri R., Cioni G. Electroencephalographic dysmaturity in preterm infants: a prognostic tool in the early postnatal period. Neuropediatrics. 1996;27(6):311–316. doi: 10.1055/s-2007-973800. [DOI] [PubMed] [Google Scholar]
- Biagioni E., Bartalena L., Boldrini A., Cioni G., Giancola S., Ipata A.E. Background EEG activity in preterm infants: correlation of outcome with selected maturational features. Electroencephalogr. Clin. Neurophysiol. 1994;91(3):154–162. doi: 10.1016/0013-4694(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Boylan G., Murray D., Rennie J. The normal EEG and aEEG. In: Rennie J., Hagmann C., Robertson N., editors. Neonatal Cerebral Investigation. second ed. Cambridge University Press; 2008. [Google Scholar]
- Boylan G.B. Neurophysiology of the neonatal period. In: Pressler R., Binnie C., Cooper R., Robinson R., editors. Churchill Livingstone Elsevier; 2007. (Neonatal and Paediatric Clinical Neurophysiology). [Google Scholar]
- Chipaux M., Colonnese M.T., Mauguen A., Fellous L., Mokhtari M., Lezcano O., Milh M. Auditory stimuli mimicking ambient sounds drive temporal ‘delta-brushes’ in premature infants. PLoS ONE. 2013;8(11):e79028. doi: 10.1371/journal.pone.0079028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese M.T., Kaminska A., Minlebaev M., Milh M., Bloem B., Lescure S., Moriette G., Chiron C., Ben-Ari Y., Khazipov R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67(3):480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J.R.C., de Hoyos A.L.R., Martínez E.D., Campo C.G., Pérez A.M., Borges A.A.H. Extrauterine life duration and ontogenic EEG parameters in preterm newborns with and without major ultrasound brain lesions. Clin. Neurophysiol. 2005;116(12):2796–2809. doi: 10.1016/j.clinph.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Curzi-Dascalova L., Figueroa J.M., Eiselt M., Christova E., Virassamy A., Dàllest A.M., Guimarâes H., Gaultier C., Dehan M. Sleep state organization in premature infants of less than 35 weeks’ gestational age. Pediatr. Res. 1993;34(5):624–628. doi: 10.1203/00006450-199311000-00013. [DOI] [PubMed] [Google Scholar]
- D’Allest A.M., André M. Electroencephalography. In: Hanson M., Evrard P., Rodeck C., Lagercrantz H., editors. The Newborn Brain: Neuroscience and Clinical Applications. first ed. Cambridge University Press; 2002. [Google Scholar]
- Fabrizi L., Slater R., Worley A., Meek J., Boyd S., Olhede S., Fitzgerald M. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Curr. Biol. 2011;21(18):1552–1558. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie L., Svedsen-Rhodes U., Easton J., Roberton N.R.C. The development of innate sleep rhythms in short gestation infants. Dev. Med. Child Neurol. 1971;13(1):40–50. doi: 10.1111/j.1469-8749.1971.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Hahn J.S., Tharp B.R. The dysmature EEG pattern in infants with bronchopulmonary dysplasia and its prognostic implications. Electroencephalogr. Clin. Neurophysiol. 1990;76(2):106–113. doi: 10.1016/0013-4694(90)90208-2. [DOI] [PubMed] [Google Scholar]
- Hahn J.S., Tharp B.R. Neonatal and Paediatric Encephalography. In: Aminoff Michael J., editor. Electrodiagnosis in Clinical Neurology. fifth ed. Elsevier; 2005. [Google Scholar]
- Hartley C., Moultrie F., Gursul D., Hoskin A., Adams E., Rogers R., Slater R. Changing balance of spinal cord excitability and nociceptive brain activity in early human development. Curr. Biol. 2016;26(15) doi: 10.1016/j.cub.2016.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa F., Okumura A., Kato T., Kuno K., Watanabe K. Disorganized patterns: chronic-stage EEG abnormality of the late neonatal period following severely depressed EEG activities in early preterm infants. Neuropediatrics. 1997;28(5):272–275. doi: 10.1055/s-2007-973713. [DOI] [PubMed] [Google Scholar]
- Hayakawa F., Okumura A., Kato T., Kuno K., Watanabe K. Dysmature EEG pattern in EEGs of preterm infants with cognitive impairment: maturation arrest caused by prolonged mild CNS depression. Brain Dev. 1997;19(2):122–125. doi: 10.1016/s0387-7604(96)00491-3. [DOI] [PubMed] [Google Scholar]
- Hellström-Westas L., Rosén I. Electroencephalography and amplitude-integrated EEG. In: Lagercrantz H., Hanson M., Ment L., Peebles D., editors. The Newborn Brain: Neuroscience and Clinical Applications. second ed. Cambridge University Press; 2010. [Google Scholar]
- Holmes G.L., Lombroso C.T. Prognostic value of background patterns in the neonatal EEG. J. Clin. Neurophysiol. 1993;10(3):323–352. doi: 10.1097/00004691-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Holmes G., Rowe J., Hafford J., Schmidt R., Testa M., Zimmerman A. Prognostic value of the electroencephalogram in neonatal asphyxia. Electroencephalogr. Clin. Neurophysiol. 1982;53(1):60–72. doi: 10.1016/0013-4694(82)90106-7. [DOI] [PubMed] [Google Scholar]
- Karch D., Kindermann E., Arnold G. The prognostic significance of determining bioelectric brain maturity in newborn infants with perinatal complication. Klin. Padiatr. 1981;193(4):301–304. doi: 10.1055/s-2008-1034480. [DOI] [PubMed] [Google Scholar]
- Khazipov R., Luhmann H.J. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29(7):414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Kidokoro H., Okumura A., Watanabe K. Abnormal brushes in preterm infants with periventricular leukomalacia. Neuropediatrics. 2006;37(5):265–268. doi: 10.1055/s-2006-924614. [DOI] [PubMed] [Google Scholar]
- Koszer S.E., Moshé S.L., Holmes G.L. Visual analysis of the neonatal electroencephalogram. In: Holmes G.L., Moshé S.L., Royden Jones H. Jr., editors. Clinical Neurophysiology of Infancy, Childhood, and Adolescence. Butterworth Heinemann Elsevier; 2006. [Google Scholar]
- Lamblin M.-D., André M., Challamel M.J., Curzi-Dascalova L., d’Allest A.M., De Giovanni E., Moussalli-Salefranque F. Électroencéphalographie du nouveau-né prématuré et à terme. Aspects maturatifs et glossaire. Clin. Neurophysiol. 1999;29(2):123–219. doi: 10.1016/s0987-7053(99)80051-3. [DOI] [PubMed] [Google Scholar]
- Lombroso C.T. Neurophysiological observations in diseased newborns. Biol. Psychiatry. 1975;10(5):527–558. [PubMed] [Google Scholar]
- Lombroso C.T. Quantified electrographic scales on 10 pre-term healthy newborns followed up to 40–43 weeks of conceptional age by serial polygraphic recordings. Electroencephalogr. Clin. Neurophysiol. 1979;46(4):460–474. doi: 10.1016/0013-4694(79)90147-0. [DOI] [PubMed] [Google Scholar]
- Lombroso C.T., Holmes G.L. Value of the EEG in neonatal seizures. J. Epilepsy. 1993;6(1):39–70. [Google Scholar]
- Lombroso C.T. Neonatal polygraphy in full-term and premature infants: a review of normal and abnormal findings. J. Clin. Neurophysiol. 1985;2(2):105–156. doi: 10.1097/00004691-198504000-00002. [DOI] [PubMed] [Google Scholar]
- Milh M., Kaminska A., Huon C., Lapillonne A., Ben-Ari Y., Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb. Cortex. 2007;17(7):1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Mirmiran M., Maas Y.G.H., Ariagno R.L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003;7(4):321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- Myers M.M., Grieve P.G., Izraelit A., Fifer W.P., Isler J.R., Darnall R.A., Stark R.I. Developmental profiles of infant EEG: overlap with transient cortical circuits. Clin. Neurophysiol. 2012;123(8):1502–1511. doi: 10.1016/j.clinph.2011.11.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E. Maturation of the EEG: development of waking and sleep patterns. In: Neidermeyer E., Lopes Da Silva F., editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. fifth ed. Lippincott Williams and Wilkins; 2005. [Google Scholar]
- Noachtar, S., Binnie, C., Ebersole, J., Mauguière, F., Sakamoto, A., Westmoreland, B., 1999. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. In: Deuschl, G., Eisen, A. (Eds.), Recommendations for the Practice of Clinical Neurophysiology: Guidelines of the International Federation of Clinical Physiology (EEG Suppl. 52). [PubMed]
- Okumura A., Hayakawa F., Kato T., Kuno K., Watanabe K. Positive rolandic sharp waves in preterm infants with periventricular leukomalacia: their relation to background electroencephalographic abnormalities. Neuropediatrics. 1999;30(6):278–282. doi: 10.1055/s-2007-973505. [DOI] [PubMed] [Google Scholar]
- Okumura A., Hayakawa F., Kato T., Kuno K., Watanabe K. Developmental outcome and types of chronic-stage EEG abnormalities in preterm infants. Dev. Med. Child Neurol. 2002;44(11):729–734. doi: 10.1017/s0012162201002845. [DOI] [PubMed] [Google Scholar]
- Radvanyi-Bouvet M.-F., de Bethmann O., Monset-Couchard M., Fazzi E. Cerebral lesions in early prematurity: EEG prognostic value in the neonatal period. Brain Dev. 1987;9(4):399–405. doi: 10.1016/s0387-7604(87)80113-4. [DOI] [PubMed] [Google Scholar]
- Ranasinghe S., Or G., Wang E.Y., Levins A., McLean M.A., Niell C.M., Chau V. Reduced cortical activity impairs development and plasticity after neonatal hypoxia ischemia. J. Neurosci. 2015;35(34):11946–11959. doi: 10.1523/JNEUROSCI.2682-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M.S. Electroencephalography of the newborn: normal features. In: Holmes G.L., Moshé S.L., Royden Jones H. Jr., editors. Clinical Neurophysiology of Infancy, Childhood, and Adolescence. Butterworth Heinemann Elsevier; 2006. [Google Scholar]
- Scher M.S., Steppe D.A., Dahl R.E., Asthana S., Guthrie R.D. Comparison of EEG sleep measures in healthy full-term and preterm infants at matched conceptional ages. Sleep. 1992;15(5):442–448. doi: 10.1093/sleep/15.5.442. [DOI] [PubMed] [Google Scholar]
- Statz A., Dumermuth G., Mieth D., Duc G. Transient EEG patterns during sleep in healthy newborns. Neuropediatrics. 1982;13(3):115–122. doi: 10.1055/s-2008-1059609. [DOI] [PubMed] [Google Scholar]
- Tharp B.R. Electrophysiological brain maturation in premature infants: an historical perspective. J. Clin. Neurophysiol. 1990;7(3):302–314. doi: 10.1097/00004691-199007000-00002. [DOI] [PubMed] [Google Scholar]
- Tharp B.R., Cukier F., Monod N. The prognostic value of the electroencephalogram in premature infants. Electroencephalogr. Clin. Neurophysiol. 1981;51(3):219–236. doi: 10.1016/0013-4694(81)90136-x. [DOI] [PubMed] [Google Scholar]
- Tich S.N.T., d’Allest A.M., Touzery de Villepin A., de Belliscize J., Walls-Esquivel E., Salefranque F., Lamblin M.D. Pathological features of neonatal EEG in preterm babies born before 30 weeks of gestational age. Clin. Neurophysiol. 2007;37(5):325–370. doi: 10.1016/j.neucli.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Tolonen M., Palva J.M., Andersson S., Vanhatalo S. Development of the spontaneous activity transients and ongoing cortical activity in human preterm babies. Neuroscience. 2007;145(3):997–1006. doi: 10.1016/j.neuroscience.2006.12.070. [DOI] [PubMed] [Google Scholar]
- Tsuchida T.N., Wusthoff C.J., Shellhaas R.A., Abend N.S., Hahn C.D., Sullivan J.E., Nguyen S. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J. Clin. Neurophysiol. 2013;30(2):161–173. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S., Palva M., Andersson S., Rivera C., Voipio J., Kaila K. Slow endogenous activity transients and developmental expression of K+–Cl− Cotransporter 2 in the immature human cortex. Eur. J. Neurosci. 2005;22(11):2799–2804. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S., Kaila K. Emergence of spontaneous and evoked electroencephalographic activity in the human brain. In: Lagercrantz Hugo, Hugo Mark Hanson, Ment Laura, Peebles Donald., editors. The Newborn Brain: Neuroscience and Clinical Applications. second ed. Cambridge University Press; 2010. [Google Scholar]
- Vecchierini M.-F., André M., d’Allest A.M. Normal EEG of premature infants born between 24 and 30 weeks gestational age: terminology, definitions and maturation aspects. Clin. Neurophysiol. 2007;37(5):311–323. doi: 10.1016/j.neucli.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Volpe J. Neurology of the Newborn. third ed. W.B. Saunders Company; 1995. Specialised studies in the neurological evaluation. [Google Scholar]
- Watanabe K., Iwase K. Spindle-like fast rhythms in the EEGs of low-birthweight infants. Dev. Med. Child Neurol. 1972;14(3):373–381. doi: 10.1111/j.1469-8749.1972.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Hayakawa F., Okumura A. Neonatal EEG: a powerful tool in the assessment of brain damage in preterm infants. Brain Dev. 1999;21(6):361–372. doi: 10.1016/s0387-7604(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Whitehead, K., Laudiano-Dray, P., Meek, J., Fabrizi, L., Topography and function of spontaneous EEG transients in neonates is organised by vigilance state. Poster 1993 Human Brain Mapping congress 2016. https://ww5.aievolution.com/hbm1601/index.cfm?do=abs.pubSearchAbstracts.