Highlights
-
•
There is little standardization of EEG practices, when PNES is suspected.
-
•
Use of suggestion is controversial but potential concerns can be addressed.
-
•
Ictal assessment during PNES is essential and can strengthen the diagnosis.
-
•
Optimal formatting of the EEG report can improve communication between clinicians.
Keywords: Psychogenic nonepileptic seizures, Nonepileptic attack disorder, Suggestion, EEG
Abstract
The gold-standard for the diagnosis of psychogenic non-epileptic seizures (PNES) is capturing an attack with typical semiology and lack of epileptic ictal discharges on video-EEG. Despite the importance of this diagnostic test, lack of standardisation has resulted in a wide variety of protocols and reporting practices. The goal of this review is to provide an overview of research findings on the diagnostic video-EEG procedure, in both the adult and paediatric literature. We discuss how uncertainties about the ethical use of suggestion can be resolved, and consider what constitutes best clinical practice. We stress the importance of ictal observation and assessment and consider how diagnostically useful information is best obtained. We also discuss the optimal format of video-EEG reports; and of highlighting features with high sensitivity and specificity to reduce the risk of miscommunication. We suggest that over-interpretation of the interictal EEG, and the failure to recognise differences between typical epileptic and nonepileptic seizure manifestations are the greatest pitfalls in neurophysiological assessment of patients with PNES. Meanwhile, under-recognition of semiological pointers towards frontal lobe seizures and of the absence of epileptiform ictal EEG patterns during some epileptic seizure types (especially some seizures not associated with loss of awareness), may lead to erroneous PNES diagnoses. We propose that a standardised approach to the video-EEG examination and the subsequent written report will facilitate a clear communication of its import, improving diagnostic certainty and thereby promoting appropriate patient management.
1. Introduction
Psychogenic non-epileptic seizures (PNES) are episodic disturbances of normal functioning and reduced self-control associated with a range of motor, sensory and mental manifestations that superficially resemble epileptic seizures, but which are not caused by epileptic activity (Brown and Reuber, 2016). They are also known as non-epileptic attacks and, historically, pseudo- and hysterical seizures (Krumholz, 1999). Most fulfill the diagnostic criteria of dissociative seizures (World Health Organisation, 2016) or conversion seizures (American Psychiatric Association, 2013). In the UK, patients who have had a first seizure are typically referred by a general or emergency care practitioner to a neurologist for investigation (children or adolescents are usually referred to paediatricians) (NICE, 2012). Research on the recognition of PNES in primary and emergency care is lacking, but clinical experience suggests that almost all patients subsequently given this diagnosis are initially referred to specialists with a working diagnosis of “epileptic seizure(s)” or “syncope”.
1.1. Referral for EEG from the seizure specialist
The seizure specialist assessing a patient will initially aim to improve the working diagnosis by taking a more detailed history from patients and any seizure witnesses. However, a diagnosis of PNES on clinical grounds alone will remain uncertain (Malmgren et al., 2012). Semiological details are often misremembered and there are no pathognomonic clinical signs for the diagnosis of PNES (Asadi-Pooya and Sperling, 2015, Syed et al., 2011, Rugg-Gunn et al., 2001). Even if an event is witnessed, neurologists may assign an incorrect diagnosis in 11–25% of cases (Seneviratne et al., 2012, O’Sullivan et al., 2013). The diagnosis of PNES can only be considered as definitively documented when a typical attack has been captured by simultaneous video-EEG recording (LaFrance et al., 2013). A normal interictal EEG cannot be considered as strong evidence of a diagnosis of PNES (just like the detection of interictal EEG changes – even if epileptiform - does not prove a diagnosis of epilepsy in the absence of clinical symptoms in keeping with the EEG changes). However, while not diagnostic in isolation, the documented absence of interictal epileptiform abnormalities can support the clinical impression of PNES (LaFrance et al., 2013).
1.2. Provision of EEG
Given that the effectiveness of treatment for epilepsy or PNES depends almost entirely on a correct diagnosis, an ILAE Task Force has published guidance suggesting that patients with possible PNES should be referred for video-EEG to increase the level of diagnostic certainty, if this test is available (LaFrance et al., 2013). In the UK, 6% of routine EEG referrals include a differential diagnosis of PNES (Whitehead et al., 2016b). When all routine EEGs are considered, 1.5% capture a PNES (Angus-Leppan, 2007). This proportion rises to 6.7% when the EEG is requested for “suspected epilepsy” (Modur and Rigdon, 2008). This means that the investigation of PNES constitutes a significant portion of the neurophysiology workload. Despite the key role of the investigation and the frequency with which it is requested, little attention has been paid to the use of specific protocols for the examination of patients with suspected PNES, or to the interaction between EEG professionals and patients with possible PNES (Worsely et al., 2011). In particular, there is little guidance on EEG reporting when PNES is a differential diagnosis, despite recent recognition that the reporting of EEGs requires increased standardisation (Beniczky et al., 2013). The goal of this review is to describe best practice in the use of video-EEG in the diagnosis of PNES, identified from published literature.
2. Outpatient synchronized video-EEG
Hospital-based EEG with time-locked video recording is the ‘gold-standard’ investigation for the assessment of possible PNES (LaFrance et al., 2013). Ambulatory EEG without video is less well suited to the investigation of events of uncertain aetiology because of the lack of documentation of pre-, peri- and post-ictal behavior (Rowan et al., 1987). Even when separate video-recordings are used, these are typically not time-locked to the EEG. One consequence is that rhythmic changes on the EEG may be misinterpreted as epileptiform when they are artefactual in nature, for instance because it was not possible to ascertain that they were linked to limb movement or eyelid flutter. What is more, the seizure onset is often not captured on ad-hoc recordings by care-givers during ambulatory EEG. Ambulatory recordings are particularly inconclusive in the investigation of seizures one might not expect to be associated with ictal EEG changes (such as focal seizures without loss of awareness or hypermotor seizures) (Devinsky et al., 1988, Bare et al., 1994).
2.1. Taking a clinical history from the patient and any available eyewitness
The first steps of an EEG are to take written consent for the video recording, start the video running immediately (before electrode application), and to ask the patient to describe the semiology of the seizures they have been experiencing. This information is crucial when the judgement is made whether a captured event was typical of habitual attacks or not. The use of a structured questionnaire could facilitate the capture of a high level of detail, but such questionnaires would have to be completed prior to the application of EEG electrodes (Reuber et al., 2016). During the process of taking the patient’s clinical history it is important to establish whether there are multiple seizure types and to obtain detailed accounts of the subjective and objective manifestations of all seizure types the patient has experienced. It is helpful if the patient’s account of their seizures can be complemented by that of a seizure witness (Plug and Reuber, 2009).
2.2. Activation techniques
Routine video-EEG should include a portion of ‘resting’ EEG and the activation techniques hyperventilation and photic stimulation, unless contraindicated (ANS/BSCN, 2013, ANS/BSCN, 2015, American Clinical Neurophysiology Society, 2016b). Service evaluations have shown that, in patients referred for EEG with suspected PNES, 12/102 (11.8%) had a PNES elicited by hyperventilation and 15/133 (11.3%) had a PNES evoked by photic stimulation (Kane et al., 2014, Whitehead et al., 2016b). In one prospective study as many as 5/15 (33%) patients had a PNES during these activation procedures, when they were referred from a specialist outpatient clinic with probable PNES immediately after describing their symptoms to the doctor (McGonigal et al., 2002).
There is no consensus of published opinion on whether hyperventilation and photic stimulation should be abandoned if a PNES has occurred spontaneously, or whether they should be performed regardless (Luther et al., 1982; Benbadis et al., 2000, Ribaï et al., 2006). The latter course has the benefit of potentially demonstrating that patients are sensitive to suggestion, which may increase referring doctors’ certainty of the PNES diagnosis (Ribaï et al., 2006). In addition, provocation techniques may elicit previously unseen epileptiform discharges indicative of a reduced epileptic seizure threshold or mixed PNES/epilepsy, e.g. in a 12-year old girl who had multiple PNES during EEG monitoring but then had unequivocal generalised epileptiform activity elicited by photic stimulation (unpublished case report from the UK cohort described in Whitehead et al., 2016b).
It is important that the EEG recording continues until it is clear that any activation techniques performed have failed to elicit an attack. The latency of PNES after provocative procedures is under 5 min in 74–94% of cases (Ribaï et al., 2006, Benbadis et al., 2000), suggesting that the EEG recording can be stopped 10 min post-cessation of activation methods (Lancman et al., 1994, Walczak et al., 1994).
2.3. Effectiveness of suggestion/provocation procedures when PNES are suspected
Over the last decades a number of studies have demonstrated that the use of different suggestion/provocation techniques can increase the proportion of brief outpatient video-EEG recordings in which patients with clinically suspected PNES will have a typical seizure (Popkirov et al., 2015b).
Suggestion techniques include measures which direct the patient’s attention to their seizures. Some elements of suggestion overlap with best clinical practice when evaluating any patient with an EEG because of seizures: The patients’ attention will be directed to their seizure symptoms during the description of their seizures before the start of the recording. This effect may be enhanced if the recording physiologist takes notes and ‘reviews aloud’ to clarify details (Benbadis et al., 2000). The obligatory information given to all patients undergoing EEG about the risk of a seizure during the recording procedure (especially when activation procedures are used) may also have a suggestive effect. The completion of consent forms documenting that a patient has been informed about the risks associated with EEG recordings involving activation techniques is good practice and may further enhance the effects of the verbal interactions listed above.
Some practitioners have proposed the use of more overt verbal suggestion when PNES are suspected (Cohen and Suter, 1982, Dericioǧlu et al., 1999). This could involve stating how helpful the recording of a typical seizure would be or how activation techniques are likely to elicit a typical attack. Once the EEG recording has started (and especially when activation techniques are used) the verbal reinforcement of clinical symptoms and signs by explicit description of changes apparent to the examiner, or by interaction with the patient about any symptoms arising, has also been used (Benbadis et al., 2000).
One randomised controlled study demonstrated that the combination of such verbal suggestion methods with hyperventilation and photic stimulation in the presence of a doctor during a routine EEG may elicit seizures in 2/3 patients with suspected PNES whereas an EEG recording with the same activation procedures but without suggestion and without the presence of a doctor only captured seizures in 1/3 (in view of the small study size the between-group difference did not quite reach significance, p = 0.058). In this study suggestion was more effective in patients with a history of previous events in medical settings (McGonigal et al., 2002). These findings are in keeping with those of other studies which have demonstrated that, when a diagnosis of PNES is suspected, the use of suggestion with hyperventilation and photic stimulation will allow practitioners to capture PNES in 64–69% of patients (Popkirov et al., 2015b).
Some previous protocols of suggestion procedures have also involved verbal suggestive procedures intended to stop PNES (Cohen and Suter, 1982) on the basis that a seizure which could be provoked and stopped by suggestive intervention would be even more likely to be a PNES than one that had only been precipitated by such a procedure (Dericioǧlu et al., 1999, Walczak et al., 1994, Kanner et al., 2008).
In addition to verbal suggestion and EEG activation procedures practitioners have used a range of placebo interventions to provoke seizures when PNES are suspected, especially intravenous saline injections but also the placement of a tuning fork or a cold alcohol swab on the skin (Popkirov et al., 2015b). No studies have compared the yield of different seizure provocation methods using placebo and there is no evidence that these methods provoke PNES more often than the combination of verbal suggestion with routine EEG activation methods.
Despite the fact that suggestion may increase the diagnostic yield of video-EEG recordings, many guidelines do not explicitly advocate it (LaFrance et al., 2013, NICE, 2012). Consequently, there is no established protocol for its use and marked methodological heterogeneity (Popkirov et al., 2015b, Cuthill and Espie, 2005). What is more, there are two key concerns regarding the use of suggestion: The first relates to the ethics of using suggestive provocation procedures (Kanner et al., 2009, Bernat, 2010a, Eijkholt and Lynch, 2013), the second to the possibility of triggering non-habitual events.
2.4. Ethical considerations relating to suggestion/provocation procedures
Many argue that suggestion requires that the clinician deceive the patient. The patient is either explicitly (e.g. by stating that saline is a physiologically active substance that lowers the seizure threshold) or implicitly (e.g. by stating that hyperventilation “induces seizures” but not distinguishing between epilepsy and PNES) misled into believing that the technique is intended to bring about an epileptic seizure. Deception prevents informed consent to the diagnostic test, which in turn is variously held to violate the patient’s trust in the clinician (O’Neill, 2003, Kanner et al., 2009) or to violate the patient’s dignity or autonomy (Bernat, 2010b). Further, deception may jeopardise the therapeutic potential of clinician-patient relationships (Bernat, 2010b) or undermine broader social confidence in the motives and practices of health workers (Kanner et al., 2009). The potential for harm is exacerbated by the psychopathology of many patients with PNES, such as difficulty in maintaining long-term trusting relationships (Bernat, 2010b). While the prohibition against clinicians deceiving patients is frequently held to be absolute (Kanner et al., 2009, Bernat, 2010b), we view it as a strong obligation that may nonetheless be outweighed by competing considerations.
If the use of suggestion is considered appropriate in a given case, potential harms may be mitigated by reducing or removing any elements of deception. While there is no evidence directly comparing suggestion with or without deception, consent procedures that explicitly describe the role of suggestion in inducing PNES and not epilepsy appear still to provoke PNES reliably (McGonigal et al., 2002, Hoepner et al., 2013), indicating that in most clinical contexts suggestion may be employed without needing to deceive the patient.
A second ethical concern relates to potential harm arising directly from the seizure induction process. Violent attacks may lead to inadvertent self-injury, and some provocative techniques may induce epileptic seizures as well as PNES. This is in principle no different from any other diagnostic test – the potential long-term benefits of accurate diagnosis must be weighed against negative consequences associated with the procedure (e.g. radiation exposure from imaging). To perform this successfully requires having a clear idea of the intended response to positive or negative results on a test; an accurate diagnosis is of little use if the referring clinician takes no further responsibility to manage the diagnosed condition appropriately. It also requires attending to specific contextual features that may make certain procedures higher- or lower-risk, e.g. hyperventilation might be thought safe in most patients but where the procedure could have severe consequences – as in those with ischemic heart disease – the use of other suggestion techniques may be preferable.
In the absence of clear consensus guidance on the ethical use of suggestion in the diagnosis of PNES, it is not possible to offer simple “do or don’t” advice (DeMarco and Ford, 2006). Instead we propose that suggestion is only used after consideration on a case-by-case basis, according to the specifics of each situation in order to determine how best to promote the patient’s long-term interests. Ask: is deception really necessary for diagnostic benefit in this case? If so, do the potential benefits outweigh the potential harm of such deception? Answering these questions provides some direction.
2.5. Procedures for ensuring that captured events are typical of habitual seizures
In one study, hyperventilation and photic stimulation with suggestion triggered non-habitual psychogenic symptoms in 2/32 (6.3%) patients and non-habitual syncopal symptoms in a further 3/32 (9.4%) patients (Popkirov et al., 2015a). In comparison, just 0.9% of patients experience syncopal symptoms when undergoing hyperventilation without suggestion (Angus-Leppan, 2007). Practitioners have suggested that the risk of eliciting atypical events could be reduced by commenting on the emergence of clinical signs in a ‘dispassionate’, rather than reinforcing way (Walczak et al., 1994), however this might also reduce the effect of suggestion. The most important safeguard against a serious diagnostic error on the basis of an atypical event provoked by suggestion is the recording of a detailed description of the habitual episodes from the patient and from seizure witnesses (if at all possible). The presence of an eyewitness of previous seizures during the video-EEG recording should not be discouraged. They help to determine whether an attack is typical (McGonigal et al., 2004, Bazil et al., 1994). An additional benefit is that some patients with PNES have attacks more often, or only, in the presence of others (Leis et al., 1992, Rowan, 2000). If witnesses cannot be present during the recording procedure, it is best to show available witnesses a video of the captured seizure to ensure that it was a typical event.
To assess typicality, a scale of between 1 (totally atypical) and 10 (absolutely typical) with a cutoff score ≥7 points to identify a typical PNES may be used (Hoepner et al., 2013). In view of the fact that patients and relatives often have a different perspective on the seizures (Reuber et al., 2011, Whitehead et al., 2015a, Whitehead et al., 2015b), the views of both parties on how typical a particular attack was should be recorded. Another way of assessing typicality which has been proposed is to consider whether ‘all automatisms in induced seizures had been noted in at least some spontaneous seizures, and at least some automatisms in spontaneous seizures were noted in induced seizures’ (Walczak et al., 1994). However, patients and relatives may be keen to believe that the attack was ‘typical’, especially after a long period of monitoring without attacks. For this reason, ideally, the video of the event would be re-shown one month later to double-check it was typical (Ribaï et al., 2006).
2.6. Clinical testing during and after psychogenic non-epileptic seizures
The observation of a typical seizure during a video-EEG recording provides clinicians with an opportunity to carry out ictal testing. Awareness during an attack should be tested by asking the patient questions or giving them simple verbal commands (such as “stick out your tongue”) (Wilkus et al., 1984). Even if they are unable to respond, they should be given a word (e.g. a colour) to remember and asked later whether they recall this word and/or any other aspects of the attack (Bell et al., 1998, Lancman et al., 1994, King et al., 1982). The testing of awareness is the most important assessment during a PNES because the presence of symmetrical alpha activity during an attack with documented loss of awareness is very strongly suggestive of PNES (Lesser, 1996). In addition, testing for avoidance, such as resistance to eye opening or a controlled fall of the hand when it is dropped over the patient’s face, may demonstrate muscle tone and volitional movement in apparently atonic or dialeptic attacks (LaFrance et al., 2013, Reuber and Elger, 2003, Luther et al., 1982, Leis et al., 1992, DeToledo and Ramsay, 1996). In PNES, the amplitude or persistence of shaking may be modified by gently holding the involved limb or changing the patient’s position (Rowan, 2000). The physiologist should audibly describe any subtle features which may not be apparent on later review of the video-recording.
2.7. Duration of video-EEG recording
The minimum recommended duration of an outpatient EEG is twenty minutes (American Clinical Neurophysiology Society, 2016a, American Clinical Neurophysiology Society, 2016b), although there are several reports of the use of longer recording times in the investigation of PNES, ranging from 40–60 min to 2–8 h (McGonigal et al., 2004, Luther et al., 1982, Rowan et al., 1987, Kanner et al., 2008). One benefit of longer recordings is the possibility that a prolonged sleep period of at least one hour will be captured, meaning that all non-REM sleep stages can be screened for epileptiform abnormalities (Luther et al., 1982, Rowan et al., 1987). Secondly, a longer appointment may increase the chance of capturing a PNES or, ideally, multiple PNES which will offer additional data for review, and the opportunity to determine the stereotypy of events (Rowan, 2000, Seneviratne et al., 2010).
Two studies have directly compared the yield of PNES between an initial short recording, and an extended recording. In one, 9/22 (40.9%) patients who had their EEG extended from 2 to 4–6 h, had an event only in the extra part of the EEG, with no benefit to recording beyond 6 h (Chayasirisobhon et al., 1993). However, all patients in that study were partially sleep-deprived and asked to withhold the last dose of their anti-epileptic medication and it is not reported whether the events captured in the EEG from 2 h onwards were non-epileptic or epileptic. In the other study, patients with unclassified seizures, who were not deprived of sleep or their anti-epileptic medication, initially underwent a standard 30-min EEG with hyperventilation, photic stimulation (and suggestion if PNES was suspected). This was then extended for a further 3.5–4 h of resting EEG: 18/165 (10.9%) patients in whom the initial section of the EEG was negative had a PNES only in the extra part of the EEG (Modur and Rigdon, 2008).
Prolonged recordings can also yield PNES in the absence of activation techniques: resting EEGs of five hours duration captured a spontaneous PNES in 30% patients suspected to have PNES (Bhatia et al., 1997). In summary, there is some evidence that recordings extended for up to five hours will increase the likelihood of a PNES occurring, especially in patients with at least thrice-weekly to daily attacks for whom the yield can be as high as 50–74% respectively (Rowan et al., 1987). Nevertheless, relatively brief outpatient EEGs lasting 1–2 h which include hyperventilation, photic stimulation and suggestion, can elicit typical PNES in up to 66% patients suspected to have this disorder (Benbadis et al., 2004).
3. Video-EEG report
3.1. Video-EEG report: Clinical
It is helpful for the EEG report to begin with a brief ‘clinical history’ section detailing the referral diagnosis and a description of all of the patient’s current habitual events. This provides the opportunity to confirm or expand upon the semiological information documented elsewhere, and offer an update on the patient’s clinical situation which may have changed since the neurology/paediatrics clinic appointment after which the EEG was requested. In a review of 1000 EEGs acquired in 2002, data on the referral diagnosis was missing in many cases (Angus-Leppan, 2007), against recommended best practice (American Clinical Neurophysiology Society, 2016a). Such omissions could be made less likely by the use of a standardised pro forma report to prompt the physiologist, e.g. ‘The patient was referred with a differential diagnosis of [x]. His/her current events consist of [x]′.
The EEG report should list the clinical features of any captured event, including the semiology observed by the physiologist, and the subjective symptoms reported by the patient. The level of detail should allow the reader to compare this event to those described in the ‘clinical history’ part of the report. Particular attention should be paid to signs which reliably distinguish epileptic seizures from PNES, e.g. interrupted, variable in direction, progressive involvement of body parts not following an anatomical pattern (King et al., 1982, Benbadis et al., 1996, Rowan, 2000, Avbersek and Sisodiya, 2010). Semiologies with high specificity for PNES, e.g. side-to-side head or body movement (Avbersek and Sisodiya, 2010), could be highlighted in bold font. The description should capture whether or not a seizure occurred spontaneously or in response to suggestion/provocation and include an account of the patient’s responses to any ictal testing carried out. Given that ictal EEG may fail to show epileptiform activity in seizures without impairment of consciousness these observations have considerable impact on the reliability of the ictal EEG findings. It is beneficial to provide attack duration because increasing lengths of over two minutes (LaFrance et al., 2013, Lesser, 1996) make the episode less and less likely to be epileptic, and prolonged attacks may mark patients at risk of being incorrectly treated for status epilepticus (Dworetzky et al., 2010). It is useful to state the absence of stertorous breathing after a convulsive event because this has high sensitivity for a non-epileptic aetiology (Rosemergy et al., 2013).
The description should not only highlight differences between a captured seizure and an epileptic generalised tonic clonic seizure. PNES can superficially resemble other seizures including focal seizures of frontal and parietal lobe origin as well as myoclonic or absence seizures. In order to interpret the ictal EEG pattern, it is important for the reader to know whether the captured event could, for instance, have been a focal seizure of frontal lobe origin which would not uncommonly be expected to occur without ictal epileptiform changes in a scalp EEG recording (Kanner et al., 2008). Therefore, a seizure semiology suggestive of frontal lobe epilepsy e.g. bilateral asymmetric tonic posturing or sudden onset of brief complex movements, should be emphasised (Devinsky and Paraiso, 2000, Rowan, 2000).
3.2. Video-EEG report: Interictal EEG
The description of the interictal EEG should encompass accounts of both normal and, where relevant, abnormal rhythms. It is important to make clear whether any identified EEG abnormalities are epileptiform, e.g. spikes and sharp waves, or non-specific, e.g. sharpened theta activity (Noachtar and Rémi, 2009). Nonspecific EEG changes have been described in nearly 50% of patients with PNES, so such changes do not discriminate these patients well from those with epilepsy. Epileptiform discharges, however, are much rarer in patients with PNES and more closely associated with epilepsy (Reuber et al., 2002a, Reuber et al., 2002b; Widdess-Walsh et al., 2012). While, in isolation, they are never diagnostic of epilepsy, they should certainly be considered an indication that a patient may be at risk of experiencing epileptic seizures (LaFrance et al., 2013). Background epileptiform features in patients in whom no seizure has been captured or the presence of such features alongside a recording of a typical PNES may also indicate that further monitoring is required to investigate the possibility of coexistent epileptic and psychogenic seizures. This dual diagnosis is more likely in patients with intractable epilepsy, such as candidates for epilepsy surgery, in whom 1.9–2.8% have both types of seizures captured on video-EEG (Vega-Zelaya et al., 2014, Whitehead et al., 2015b, Reuber et al., 2002a, Reuber et al., 2002b), or in patients with learning disabilities (Duncan and Oto, 2008).
The review of interictal (and ictal) EEG requires a combination of reference and longitudinal and transverse bipolar montages (American Clinical Neurophysiology Society, 2016b). In PNES, the risk of misinterpretation of physiological interictal EEG phenomena is contributed to by commonly used neuroactive medications, which can cause nonspecific EEG changes. Commonly misinterpreted features include the description of sharpened or forward-spreading alpha activity as epileptiform (Benbadis and Tatum, 2003). In one study of patients with PNES who had been misdiagnosed as having refractory epilepsy, benign variants and sharpened slowing in the temporal regions had contributed to the misdiagnosis in 19/45 cases (Smith et al., 1999). Therefore, it is important that the neurophysiologist applies strict criteria before describing transients as epileptiform, e.g. standing out from the background, high amplitude, after-going slow wave (Benbadis and Tatum, 2003). Examples of epileptiform activity should be provided as time-locked annotations on the EEG record, facilitating re-review of the data. The full EEG should be archived – in one study just 15/41 ‘epileptiform’ EEGs were available for re-review (Benbadis and Tatum, 2003).
3.3. Video-EEG report: Ictal EEG
It should be stated whether the EEG is normal before, during and after a PNES (Dericioǧlu et al., 1999, Slater et al., 1995, Luther et al., 1982, Leis et al., 1992, Devinsky et al., 2011). Assessment of the pre-ictal EEG is important because non-epileptic events may occur as a dissociative or conversion response triggered by an epileptic seizure (Devinsky and Gordon, 1998). The clinical scenario of nonepileptic ‘elaboration’ of an epileptic seizure should be distinguished from that in which a patient has discrete epileptic and non-epileptic events (e.g. Whitehead et al., 2015b). EEG during clinical events should be closely reviewed for subtle epileptiform ictal activity such as suppression and low voltage beta or alpha activity (Slater et al., 1995, Mahowald and Schenck, 2000). Ill-defined epileptiform ictal patterns can be better appreciated by using a wide timebase and different display sensitivities (Mahowald and Schenck, 2000, Wilkus et al., 1984). If EEG changes are identified at the time of a clinical event then the video should be carefully reviewed, frame-by-frame if necessary, to ensure that the changes are not reflecting limb or eye movement artefact. In the case of a lateralised rhythm, it should be noted which side of the patient’s head has contact with the pillow. The report should also state explicitly if the ictal EEG could not be assessed (for instance because of extensive muscle and movement artefact). Close attention to the post-ictal EEG is necessary because it is possible for seizures to lack any accompanying ictal EEG changes but be succeeded by slowing or activation of spikes, especially in frontal lobe epilepsy (Whitehead et al., 2016a).
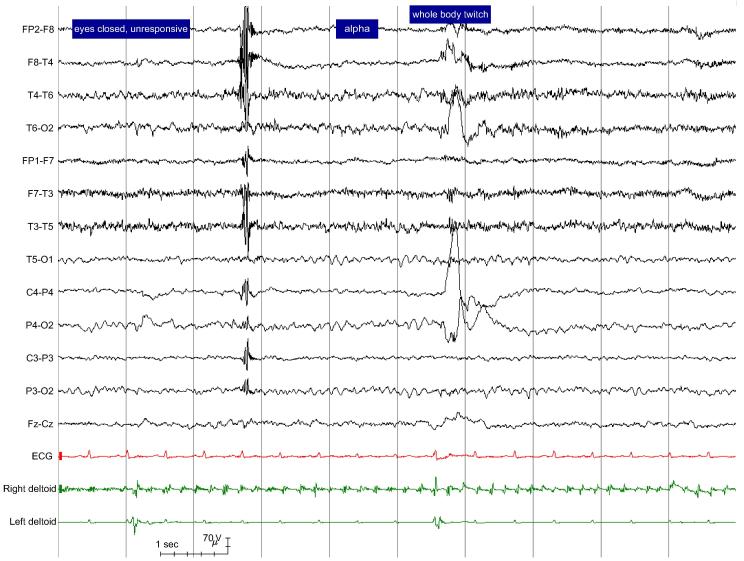
The strongest electro-clinical evidence to support a non-epileptic aetiology is the absence of an epileptiform ictal pattern in association with semiology, e.g. loss of awareness, which would be expected to be accompanied by such changes if they were caused by epilepsy, plus the presence of symmetrical alpha activity during and/or immediately after the attack (Chen and LaFrance, 2016, King et al., 1982, Niedermeyer, 2005, Rowan, 2000). It is important to make explicit the temporal relationship between any EEG pattern seen during the seizure (e.g. alpha activity) and semiology, e.g. by stating that a symmetrical alpha rhythm was present while the patient was unresponsive and there was shaking of all four limbs. If an attack arises shortly after a period of apparent sleep, the report should state whether this behavioural sleep is accompanied by EEG sleep phenomena or whether the EEG pattern was actually more consistent with wakefulness: if strictly defined, the observation of pre-ictal ‘pseudo-sleep’ has a high specificity for PNES (Benbadis et al., 1996). If an attack arises from EEG-documented sleep, stating the exact time EEG sleep phenomena are lost and how many seconds later the attack begins, can help to differentiate the attack from epileptic seizures which customarily arise abruptly from EEG-documented sleep (Orbach et al., 2003). The factual report may be supplemented by a screenshot of the EEG during the event (American Clinical Neurophysiology Society, 2016a; Fig. 1). Ideally, the full video-EEG of each attack, beginning at least two minutes prior to attack onset, is archived for future review and reference (Benbadis et al., 1996).
Fig. 1.
Adult female with history of 1) prolonged events of thrashing with loss of awareness 2) twitches 3) ‘absences’. Typical examples of attack types 1) and 2) were captured and found to be non-epileptic. Figure shows EEG during the offset of attack type 1) with symmetrical alpha rhythm, heart rate at the upper limit of the patient’s resting heart rate and rhythmic low amplitude non-evolving 5 Hz tremor on the right deltoid trace. This attack lasted for 33 min with intermittent cessation of shaking (e.g. first second of screenshot). Eyes were closed for the duration of the attack and resistance to eye opening was documented.
3.4. Video-EEG report: Ictal polygraphy
It is important not only to describe EEG changes but also to comment on the ictal and post-ictal heart rate in comparison to the baseline because the heart rate typically elevates more rapidly during epileptic seizures and remains elevated for longer in the postictal period, when compared to PNES (Reinsberger et al., 2012). Conversely the heart rate may also decrease during epileptic seizures, which is never seen in PNES. While it is best to observe ictal movements on video, movement artefact captured in EEG channels or, ideally, bilateral surface EMG recordings can be useful. Whereas the frequency of shaking movements in PNES remains relatively static or episodes of vigorous motor activity may be interspersed with ‘pauses’ of more than five seconds, the frequency of movements associated with epileptic seizures commonly change and evolve during the course of a seizure (Vinton et al., 2004, Devinsky and Paraiso, 2000, Beniczky et al., 2014). In psychogenic convulsive events, deltoid EMG amplitude is less, and the ratio of high frequency (tonic): low frequency (clonic) EMG activity is smaller, when compared to that typically observed in epileptic generalised tonic clonic seizures (Beniczky et al., 2014). In psychogenic myoclonus, surface EMG bursts typically last for 75–150 ms, while they last for just 20–50 ms in cortical myoclonus (Apartis, 2014). Therefore, in the routine setting, applying electrodes to just a single appropriate muscle can offer useful information, although full assessment of myoclonus would require sampling multiple agonist and antagonist muscles. Both psychogenic myoclonus and tremor may be modified by competitive rhythmic tasks, while their organic counterparts cannot (Apartis, 2014).
3.5. Other forms of physiological monitoring
Confirmatory studies following up recent work involving the diagnostic use of accelerometers or microphones to identify diagnostic movement or breathing patterns may improve the diagnostic utility of EEG monitoring in the future (Bayly et al., 2013, Rosemergy et al., 2013). Pulse oximetry monitoring may also play a role in the differentiation of epileptic and psychogenic seizures: oxygen saturation may decrease in tonic clonic epileptic seizures and usually normal in PNES (Marshall et al., 1991). However, to date, it is not known whether additional methods of physiological monitoring add to the diagnostic value of routine EEG recordings of patients with possible PNES, so these additional tests can currently not be recommended for general use outside research settings.
4. Special considerations in paediatrics
Many studies have shown that PNES are not uncommon in children. However, presentations of epilepsy and nonepileptic events are more often atypical, so initial misdiagnosis rates are high (Patel et al., 2007, Szabó et al., 2012, Malmgren et al., 2012). Whereas in adults physiological nonepileptic events are usually easy to distinguish from epilepsy or PNES, in paediatrics the differential diagnosis is wider and includes physiological episodes specific to childhood, such as breath holding spells and gastrooesophageal reflux associated with laryngospasm (Sandifer syndrome) (Benbadis, 2009). Furthermore, the observed semiology can be virtually identical in paediatric epilepsy syndromes and PNES, such as in Childhood or Juvenile Absence Epilepsy and behavioural vacant spells. Young children and those with learning disabilities in particular may not be able to describe their attacks, such that an accurate eyewitness account becomes essential. Video recording is an invaluable objective tool in this regard, such that concurrent video and EEG recording of the habitual attacks, which can then be reviewed with a parent or guardian, has become the diagnostic gold standard (Kotagal et al., 2002, LaFrance et al., 2013). It is important to bear in mind that over-read EEGs out-with the clinical context have historically been a common cause of misdiagnosis in children, particularly when maturational or physiological variants (e.g. hyperventilation-induced high-amplitude rhythmic slow activity) have been misinterpreted (Benbadis, 2009).
Young children have rather more non-motor or subtle pauci-kinetic behavioural psychogenic events than adults, such as staring/zoning out/vacant spells mistaken for absence seizures, or pseudo-syncope events mimicking dialeptic or atonic seizures (Szabó et al., 2012, Kane et al., 2015). Tremor or shuddering episodes are the most common motor psychogenic phenomena, although behavioural stereotypies/tics and hypermotor/hyperkinetic events do occur. Sleep disorders are quite common in children (e.g. parasomnias, hypnic jerks and confusional arousals) which can be misdiagnosed as seizures, but can usually be correctly diagnosed by overnight video-EEG (Kotagal et al., 2002). The co-occurrence of epilepsy and nonepileptic events can be particularly challenging; as many as 50% of children with intractable epilepsy have events that are misdiagnosed as seizures, in part due to over-reporting or ‘anchoring bias’ by their naturally concerned parents or guardians (Bye et al., 2000, Sohal et al., 2014, Kane et al., 2015). Another interesting observation from prospective data, in over 200 children with intractable epilepsy, is that co-existing nonepileptic events and PNES are more prevalent in boys under 10 years (ratio 2:1), but in girls over 10 years of age (ratio 2:1) (Kane et al., 2015), which is similar to reports by others (Kotagal et al., 2002, Patel et al., 2007). The importance of identifying PNES in children is the same as in adults, but early diagnosis may optimise therapeutic interventions and offer potential safeguarding opportunities.
5. Conclusion
The differentiation of PNES from epilepsy is challenging and relies on an integrated multidisciplinary approach which utilizes the expertise of the epileptologist and clinical neurophysiology physicians and physiologists (Gates, 2000, Mahowald and Schenck, 2000). One of the greatest dangers is the over-interpretation of benign EEG phenomena or movement artefacts as epileptiform. Other diagnostic errors arise from unclear accounts of ictal and post-ictal behavior, reports which omit useful information, or the inappropriate extrapolation of a clinical diagnosis from the recording of atypical or minor seizures. Increased standardization of EEG protocols and reporting practices have the potential to add value by improving clinical decision-making, ensuring that a high quality service is provided across centres and that patients’ exposure to potentially unethical practices is minimised. In the absence of guidance from national or international bodies (such as the ILAE) the evidence summarized in this review and the practice points highlighted in Table 1 should provide an initial outline to current best practice in this area.
Table 1.
Summary table of recommendations for the use of video-EEG when PNES are a possible diagnosis.
| Procedure | Proposals for best practice |
|---|---|
| Preparation |
|
| Suggestion |
|
| Ictal observation |
|
| Verification |
|
| EEG report |
|
| Archiving |
|
Conflict of interest
None.
References
- American Clinical Neurophysiology Society, 2016a. Guidelines for EEG Reporting.
- American Clinical Neurophysiology Society, 2016b. Minimum Technical Requirements for Performing Clinical EEG.
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM–5). [DOI] [PubMed]
- Angus-Leppan Heather. Seizures and adverse events during routine scalp electroencephalography: a clinical and EEG analysis of 1000 records. Clin. Neurophysiol. 2007;118(1):22–30. doi: 10.1016/j.clinph.2006.08.014. [DOI] [PubMed] [Google Scholar]
- ANS/BSCN, 2013. ANS/BSCN Guidelines for Hyperventilation During EEG Recordings. http://www.bscn.org.uk//data/files/Guidelines/Hypervent.pdf.
- ANS/BSCN, 2015. ANS/BSCN Guidelines for Photic Stimulation during EEG Recordings. http://www.bscn.org.uk//data/files/Guidelines/Photic.pdf.
- Apartis E. Clinical neurophysiology of psychogenic movement disorders: how to diagnose psychogenic tremor and myoclonus. Neurophys. Clin./Clin. Neurophys. 2014;44(4):417–424. doi: 10.1016/j.neucli.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Asadi-Pooya Ali A., Sperling Michael R. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60–65. doi: 10.1016/j.yebeh.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Avbersek Andreja, Sisodiya Sanjay. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J. Neurol. Neurosurg. Psychiatry. 2010;81(7):719–725. doi: 10.1136/jnnp.2009.197996. [DOI] [PubMed] [Google Scholar]
- Bare Mary A., Burnstine Thomas H., Fisher Robert S., Lesser Ronald P. Electroencephalographic changes during simple partial seizures. Epilepsia. 1994;35(4):715–720. doi: 10.1111/j.1528-1157.1994.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Bayly Jade, Carino John, Petrovski Slavé, Smit Michelle, Fernando Dilini A., Vinton Anita, Yan Bernard, Gubbi Jayavardhana R., Palaniswami Marimuthu S., O’Brien Terence J. Time-frequency mapping of the rhythmic limb movements distinguishes convulsive epileptic from psychogenic nonepileptic seizures. Epilepsia. 2013;54(8):1402–1408. doi: 10.1111/epi.12207. [DOI] [PubMed] [Google Scholar]
- Bazil C.W., Kothari M., Luciano D., Moroney J., Song S., Vasquez B., Weinreb H.J., Devinsky O. Provocation of nonepileptic seizures by suggestion in a general seizure population. Epilepsia. 1994;35(4):768–770. doi: 10.1111/j.1528-1157.1994.tb02509.x. [DOI] [PubMed] [Google Scholar]
- Bell William L., Park Yong D., Thompson Elizabeth A., Radtke Rodney A. Ictal cognitive assessment of partial seizures and pseudoseizures. Arch. Neurol. 1998;55(11):1456–1459. doi: 10.1001/archneur.55.11.1456. [DOI] [PubMed] [Google Scholar]
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav. 2009;15(1):15–21. doi: 10.1016/j.yebeh.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Benbadis Selim, Tatum William. Overintepretation of EEGs and misdiagnosis of epilepsy. J. Clin. Neurophysiol. 2003;20(1) doi: 10.1097/00004691-200302000-00005. [DOI] [PubMed] [Google Scholar]
- Benbadis Selim R., Lancman Marcelo E., King Lynn M., Swanson Sara J. Preictal pseudosleep a new finding in psychogenic seizures. Neurology. 1996;47(1):63–67. doi: 10.1212/wnl.47.1.63. [DOI] [PubMed] [Google Scholar]
- Benbadis S.R., Johnson K., Anthony K., Caines G., Hess G., Jackson C., Vale F.L., Tatum W.O., IV Induction of psychogenic nonepileptic seizures without placebo. Neurology. 2000;55(12):1904–1905. doi: 10.1212/wnl.55.12.1904. [DOI] [PubMed] [Google Scholar]
- Benbadis S.R., Siegrist K., Tatum W.O., Heriaud L., Anthony K. Short-term outpatient EEG video with induction in the diagnosis of psychogenic seizures. Neurology. 2004;63(9):1728–1730. doi: 10.1212/01.wnl.0000143273.18099.50. [DOI] [PubMed] [Google Scholar]
- Beniczky Sándor, Aurlien Harald, Brøgger Jan C., Fuglsang-Frederiksen Anders, Martins-da-Silva António, Trinka Eugen, Visser Gerhard. Standardized computer-based organized reporting of EEG: score. Epilepsia. 2013;54(6):1112–1124. doi: 10.1111/epi.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniczky Sándor, Conradsen Isa, Moldovan Mihai, Jennum Poul, Fabricius Martin, Benedek Krisztina, Andersen Noémi, Hjalgrim Helle, Wolf Peter. Quantitative analysis of surface electromyography during epileptic and nonepileptic convulsive seizures. Epilepsia. 2014;55(7):1128–1134. doi: 10.1111/epi.12669. [DOI] [PubMed] [Google Scholar]
- Bernat James L. The ethics of diagnosing nonepileptic seizures with placebo infusion. Virtual Mentor. 2010;12(11):854. doi: 10.1001/virtualmentor.2010.12.11.ccas3-1011. [DOI] [PubMed] [Google Scholar]
- Bernat The ethics of diagnosing nonepileptic seizures with placebo infusion. Virtual Mentor. 2010;12(11):854. doi: 10.1001/virtualmentor.2010.12.11.ccas3-1011. [DOI] [PubMed] [Google Scholar]
- Bhatia M., Sinha P.K., Jain S., Padma M.V., Maheshwari M.C. Usefulness of short-term video eeg recording with saline induction in pseudoseizures. Acta Neurol. Scand. 1997;95(6):363–366. doi: 10.1111/j.1600-0404.1997.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Brown Richard J., Reuber Markus. Towards an integrative theory of psychogenic non-epileptic seizures (PNES) Clin. Psychol. Rev. 2016;47:55–70. doi: 10.1016/j.cpr.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Bye A.M.E., Kok D.J.M., Ferenschild F.T.J., Vles J.S.H. Paroxysmal non-epileptic events in children: a retrospective study over a period of 10 years. J. Paediatr. Child Health. 2000;36(3):244–248. doi: 10.1046/j.1440-1754.2000.00496.x. [DOI] [PubMed] [Google Scholar]
- Chayasirisobhon S., Griggs L., Westmoreland S., Kim C.S. The usefulness of one to two hour video EEG monitoring in patients with refractory seizures. Clin. EEG. 1993;24(2):78–84. doi: 10.1177/155005949302400208. [DOI] [PubMed] [Google Scholar]
- Chen David, LaFrance Curt. Diagnosis and treatment of nonepileptic seizures. Continuum (Minneap Minn) 2016;22(1 Epilepsy):116–131. doi: 10.1212/CON.0000000000000282. [DOI] [PubMed] [Google Scholar]
- Cohen Robert J., Suter Cary. Hysterical seizures: suggestion as a provocative EEG test. Ann. Neurol. 1982;11(4):391–395. doi: 10.1002/ana.410110413. [DOI] [PubMed] [Google Scholar]
- Cuthill Fiona M., Espie Colin A. Sensitivity and specificity of procedures for the differential diagnosis of epileptic and non-epileptic seizures: a systematic review. Seizure. 2005;14(5):293–303. doi: 10.1016/j.seizure.2005.04.006. [DOI] [PubMed] [Google Scholar]
- DeMarco Joseph P., Ford Paul J. Balancing in ethical deliberation: superior to specification and casuistry. J. Med. Philosophy. 2006;31(5):483–497. doi: 10.1080/03605310600912675. [DOI] [PubMed] [Google Scholar]
- Dericioǧlu N., Saygi S., Ciǧer A. The value of provocation methods in patients suspected of having non-epileptic seizures. Seizure. 1999;8(3):152–156. doi: 10.1053/seiz.1999.0277. [DOI] [PubMed] [Google Scholar]
- DeToledo J.C., Ramsay R.E. Patterns of involvement of facial muscles during epileptic and nonepileptic events review of 654 events. Neurology. 1996;47(3):621–625. doi: 10.1212/wnl.47.3.621. [DOI] [PubMed] [Google Scholar]
- Devinsky Orrin, Gordon Elisabeth. Epileptic seizures progressing into nonepileptic conversion seizures. Neurology. 1998;51(5):1293–1296. doi: 10.1212/wnl.51.5.1293. [DOI] [PubMed] [Google Scholar]
- Devinsky Orrin, Paraiso Joel. Non-Epileptic Seizures. Second Ed. Butterworth Heinemann; 2000. Unusual epileptic events and non-epileptic seizures: differential diagnosis and coexistence. [Google Scholar]
- Devinsky Orrin, Kelley Kathy, Porter Roger J., Theodore William H. Clinical and electroencephalographic features of simple partial seizures. Neurology. 1988;38(9) doi: 10.1212/wnl.38.9.1347. 1347–1347. [DOI] [PubMed] [Google Scholar]
- Devinsky Orrin, Gazzola Deana, Curt LaFrance W. Differentiating between nonepileptic and epileptic seizures. Nature Rev. Neurol. 2011;7(4):210–220. doi: 10.1038/nrneurol.2011.24. [DOI] [PubMed] [Google Scholar]
- Duncan R., Oto M. Psychogenic nonepileptic seizures in patients with learning disability: comparison with patients with no learning disability. Epilepsy Behav. 2008;12(1):183–186. doi: 10.1016/j.yebeh.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Dworetzky Barbara A., Bubrick Ellen J., Szaflarski Jerzy P. Nonepileptic psychogenic status: markedly prolonged psychogenic nonepileptic seizures. Epilepsy Behav. 2010;19(1):65–68. doi: 10.1016/j.yebeh.2010.06.052. [DOI] [PubMed] [Google Scholar]
- Eijkholt Marleen, Lynch Timothy. Provoking pseudo-seizures: provocative placebo practices. AJOB Neurosci. 2013;4(3):33–35. [Google Scholar]
- Gates John. Non-Epileptic Seizures. Second ed. Butterworth Heinemann; 2000. Part summary: neurologic aspects of non-epileptic seizures in the adult and pediatric patient. [Google Scholar]
- Hoepner Robert, Labudda Kirsten, Schoendienst Martin, May Theodor W., Bien Christian G., Brandt Christian. Informing patients about the impact of provocation methods increases the rate of psychogenic nonepileptic seizures during EEG recording. Epilepsy Behav. 2013;28(3):457–459. doi: 10.1016/j.yebeh.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Kane Nick, Grocott Lesley, Kandler Ros, Lawrence Sarah, Pang Catherine. Hyperventilation during electroencephalography: safety and efficacy. Seizure. 2014;23(2):129–134. doi: 10.1016/j.seizure.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Kane Nick, Warren Elliott, Patel Jay, Wright Ingram, Carter Mike. The occurrence of non-epileptic events (NEE) in a pediatric population with medically intractable epilepsy enrolled on a pediatric epilepsy surgery program. J. Neurol. Disord. 2015;3(4) [Google Scholar]
- Kanner Andres, Curt LaFrance, Tim Betts. Epilepsy A Comprehensive Textbook. Second ed. Lippincott Williams & Wilkins; 2008. Psychogenic nonepileptic seizures. [Google Scholar]
- Kanner Andres M., Benbadis Selim R., Leeman Beth. Rebuttals and a final commentary. Epilepsy Behav. 2009;15(2):115–118. [Google Scholar]
- King Don W., Gallagher Brian B., Murvin Alice J., Smith Dennis B., Marcus Donald J., Hartlage Lawrence C., Charles Ward L. Pseudoseizures diagnostic evaluation. Neurology. 1982;32(1) doi: 10.1212/wnl.32.1.18. 18–18. [DOI] [PubMed] [Google Scholar]
- Kotagal Prakash, Costa Maristela, Wyllie Elaine, Wolgamuth Barbara. Paroxysmal nonepileptic events in children and adolescents. Pediatrics. 2002;110(4) doi: 10.1542/peds.110.4.e46. e46–e46. [DOI] [PubMed] [Google Scholar]
- Krumholz A. Nonepileptic seizures: diagnosis and management. Neurology. 1999;53(5 Suppl. 2):S76–S83. [PubMed] [Google Scholar]
- LaFrance W. Curt, Jr, Baker Gus A., Duncan Rod, Goldstein Laura H., Reuber Markus. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005–2018. doi: 10.1111/epi.12356. [DOI] [PubMed] [Google Scholar]
- Lancman Marcelo E., Asconapé Jorge J., Craven William J., Howard George., Kiffin Penry J. Predictive value of induction of psychogenic seizures by suggestion. Ann. Neurol. 1994;35(3):359–361. doi: 10.1002/ana.410350319. [DOI] [PubMed] [Google Scholar]
- Leis A. Arturo., Ross Mark A., Summers Alan K. Psychogenic seizures ictal characteristics and diagnostic pitfalls. Neurology. 1992;42(1) doi: 10.1212/wnl.42.1.95. 95–95. [DOI] [PubMed] [Google Scholar]
- Lesser Ronald P. Psychogenic Seizures. Neurology. 1996;46(6):1499–1507. doi: 10.1212/wnl.46.6.1499. [DOI] [PubMed] [Google Scholar]
- Luther J. Scott, McNamara James O., Carwile Sandra, Miller Patrica, Hope Victor. Pseudoepileptic seizures: methods and video analysis to aid diagnosis. Ann. Neurol. 1982;12(5):458–462. doi: 10.1002/ana.410120508. [DOI] [PubMed] [Google Scholar]
- Mahowald Mark, Schenck Carlos. Non-Epileptic Seizures. Second ed. Butterworth Heinemann; 2000. Parasomnia purgatory: epileptic/non-epileptic parasomnia interface. [Google Scholar]
- Malmgren K., Reuber M., Appleton R. Epilepsy (Oxford Textbook of Clinical Neurology Series) Oxford University Press; Oxford: 2012. Differential diagnosis of epilepsy. 81-94. [Google Scholar]
- Marshall H., Carew-McColl M., James M.R. Pulse oximetry during apparent tonic-clonic seizures. Lancet. 1991;337(8738):394–395. doi: 10.1016/0140-6736(91)91168-t. Originally published as Volume 1, Issue 8738. [DOI] [PubMed] [Google Scholar]
- McGonigal A., Oto M., Russell A.J.C., Greene J., Duncan R. Outpatient video EEG recording in the diagnosis of non-epileptic seizures: a randomised controlled trial of simple suggestion techniques. J. Neurol. Neurosurg. Psychiatry. 2002;72(4):549–551. doi: 10.1136/jnnp.72.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigal A., Russell A.J.C., Mallik A.K., Oto M., Duncan R. Use of short term video EEG in the diagnosis of attack disorders. J. Neurol. Neurosurg. Psychiatry. 2004;75(5):771–772. doi: 10.1136/jnnp.2003.024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modur Pradeep N., Rigdon Barbara. Diagnostic yield of sequential routine EEG and extended outpatient video-EEG monitoring. Clin. Neurophys. 2008;119(1):190–196. doi: 10.1016/j.clinph.2007.09.128. [DOI] [PubMed] [Google Scholar]
- NICE, 2012. Epilepsies: Diagnosis and Management. https://www.nice.org.uk/guidance/cg137/chapter/1-Guidance#diagnosis-2.
- Niedermeyer Ernst. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Fifth Ed. Lippincott Williams & Wilkins; 2005. Nonepileptic attacks. [Google Scholar]
- Noachtar Soheyl, Rémi Jan. The role of EEG in epilepsy: a critical review. Epilepsy Behav. 2009;15(1):22–33. doi: 10.1016/j.yebeh.2009.02.035. [DOI] [PubMed] [Google Scholar]
- O’Neill O. Some limits of informed consent. J. Med. Ethics. 2003;29(1):4–7. doi: 10.1136/jme.29.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan Sean S., Redwood Rebecca I., Hunt David, McMahon Elaine M., O’Sullivan Suzanne. Recognition of psychogenic non-epileptic seizures: a curable neurophobia? J. Neurol. Neurosurg. Psychiatry. 2013;84(2):228–231. doi: 10.1136/jnnp-2012-303062. [DOI] [PubMed] [Google Scholar]
- Orbach Darren, Ritaccio Anthony, Devinsky Orrin. Psychogenic, nonepileptic seizures associated with video-EEG–verified sleep. Epilepsia. 2003;44(1):64–68. doi: 10.1046/j.1528-1157.2003.29302.x. [DOI] [PubMed] [Google Scholar]
- Patel Hema, Scott Eric, Dunn David, Garg Bhuwan. Nonepileptic seizures in children. Epilepsia. 2007;48(11):2086–2092. doi: 10.1111/j.1528-1167.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- Plug L., Reuber M. Making the diagnosis in patients with blackouts: it’s all in the history. Pract. Neurol. 2009;9(1):4–15. doi: 10.1136/jnnp.2008.161984. [DOI] [PubMed] [Google Scholar]
- Popkirov Stoyan, Grönheit Wenke, Wellmer Jörg. Hyperventilation and photic stimulation are useful additions to a placebo-based suggestive seizure induction protocol in patients with psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:88–90. doi: 10.1016/j.yebeh.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Popkirov, Grönheit, Wellmer A systematic review of suggestive seizure induction for the diagnosis of psychogenic nonepileptic seizures. Seizure. 2015;31:124–132. doi: 10.1016/j.seizure.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Reinsberger Claus, Perez David L., Murphy Melissa M., Dworetzky Barbara A. Pre- and postictal, not ictal, heart rate distinguishes complex partial and psychogenic nonepileptic seizures. Epilepsy Behav. 2012;23(1):68–70. doi: 10.1016/j.yebeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Reuber Markus, Elger Christian E. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205–216. doi: 10.1016/s1525-5050(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Reuber M., Fernández G., Helmstaedter C., Qurishi A., Elger C.E. Evidence of brain abnormality in patients with psychogenic nonepileptic seizures. Epilepsy Behav. 2002;3(3):249–254. doi: 10.1016/s1525-5050(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Reuber Markus, Kurthen Martin, Fernández Guillén, Schramm Johannes, Elger Christian E. Epilepsy surgery in patients with additional psychogenic seizures. Arch. Neurol. 2002;59(1):82–86. doi: 10.1001/archneur.59.1.82. [DOI] [PubMed] [Google Scholar]
- Reuber Markus, Jamnadas-Khoda Jenny, Broadhurst Mark, Grunewald Richard, Howell Steve, Koepp Matthias, Sisodiya Sanjay, Walker Matthew. Psychogenic nonepileptic seizure manifestations reported by patients and witnesses. Epilepsia. 2011;52(11):2028–2035. doi: 10.1111/j.1528-1167.2011.03162.x. [DOI] [PubMed] [Google Scholar]
- Reuber Markus, Chen Min, Jamnadas-Khoda Jenny, Broadhurst Mark, Wall Melanie, Grünewald Richard A., Howell Stephen J., Koepp Matthias, Parry Steve, Sisodiya Sanjay, Walker Matthew, Hesdorffer Dale. Value of patient-reported symptoms in the diagnosis of transient loss of consciousness. Neurology. 2016;87:625–633. doi: 10.1212/WNL.0000000000002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaï P., Tugendhaft P., Legros B. Usefulness of prolonged video–EEG monitoring and provocative procedure with saline injection for the diagnosis of non epileptic seizures of psychogenic origin. J. Neurol. 2006;253(3):328–332. doi: 10.1007/s00415-005-0991-9. [DOI] [PubMed] [Google Scholar]
- Rosemergy Ian, Frith Richard, Herath Samantha, Walker Elizabeth. Use of postictal respiratory pattern to discriminate between convulsive psychogenic nonepileptic seizures and generalized tonic-clonic seizures. Epilepsy Behav. 2013;27(1):81–84. doi: 10.1016/j.yebeh.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Rowan James. Non-Epileptic Seizures. Second ed. Butterworth Heinemann; 2000. Diagnosis of non-epileptic seizures. [Google Scholar]
- Rowan A. James, Siegel Michael, Rosenbaum David H. Daytime intensive monitoring comparison with prolonged intensive and ambulatory monitoring. Neurology. 1987;37(3) doi: 10.1212/wnl.37.3.481. 481–481. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn F.J., Harrison N.A., Duncan J.S. Evaluation of the accuracy of seizure descriptions by the relatives of patients with epilepsy. Epilepsy Res. 2001;43(3):193–199. doi: 10.1016/s0920-1211(00)00209-6. [DOI] [PubMed] [Google Scholar]
- Seneviratne Udaya, Reutens David, D’Souza Wendyl. Stereotypy of psychogenic nonepileptic seizures: insights from video-EEG monitoring. Epilepsia. 2010;51(7):1159–1168. doi: 10.1111/j.1528-1167.2010.02560.x. [DOI] [PubMed] [Google Scholar]
- Seneviratne Udaya, Rajendran Deepa, Brusco Maria, Phan Thanh G. How good are we at diagnosing seizures based on semiology? Epilepsia. 2012;53(4):e63–e66. doi: 10.1111/j.1528-1167.2011.03382.x. [DOI] [PubMed] [Google Scholar]
- Slater Jeremy D., Brown Marland C., Jacobs William, Eugene Ramsay R. Induction of pseudoseizures with intravenous saline placebo. Epilepsia. 1995;36(6):580–585. doi: 10.1111/j.1528-1157.1995.tb02571.x. [DOI] [PubMed] [Google Scholar]
- Smith D., Defalla B.A., Chadwick D.W. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. QJM. 1999;92(1):15–23. doi: 10.1093/qjmed/92.1.15. [DOI] [PubMed] [Google Scholar]
- Sohal A., Khan A., Hussain N. Prolonged video-EEG in identifying paroxysmal nonepileptic events in children with epilepsy: a useful tool. J. Clin. Neurophysiol. 2014;31:149–151. doi: 10.1097/WNP.0000000000000035. [DOI] [PubMed] [Google Scholar]
- Syed Tanvir U., Curt LaFrance W., Kahriman Emine S., Hasan Saba N., Rajasekaran Vijayalakshmi, Gulati Deepak, Borad Samip. Can semiology predict psychogenic nonepileptic seizures? A prospective study. Ann. Neurol. 2011;69(6):997–1004. doi: 10.1002/ana.22345. [DOI] [PubMed] [Google Scholar]
- Szabó Léna, Siegler Zsuzsanna, Zubek László, Liptai Zoltán, Körhegyi Ivett, Bánsági Boglárka, Fogarasi András. A detailed semiologic analysis of childhood psychogenic nonepileptic seizures. Epilepsia. 2012;53(3):565–570. doi: 10.1111/j.1528-1167.2012.03404.x. [DOI] [PubMed] [Google Scholar]
- Vega-Zelaya, Lorena, Marta Alvarez, Elena Ezquiaga, Jaime Nogeiras, María Toledo, Rafael G. Sola, and Jesús Pastor, 2014. Psychogenic Non-Epileptic Seizures in a Surgical Epilepsy Unit: Experience and a Comprehensive Review. In: Epilepsy Topics, Prof. Mark Holmes (Ed.), InTech. doi:10.5772/57439.
- Vinton Anita, Carino John, Vogrin Simon, MacGregor Lachlan, Kilpatrick Christine, Matkovic Zelko, O’Brien Terence J. “Convulsive” nonepileptic seizures have a characteristic pattern of rhythmic artifact distinguishing them from convulsive epileptic seizures. Epilepsia. 2004;45(11):1344–1350. doi: 10.1111/j.0013-9580.2004.04704.x. [DOI] [PubMed] [Google Scholar]
- Walczak Thaddeus S., Williams Daniel T., Berten Wolfgang. Utility and reliability of placebo infusion in the evaluation of patients with seizures. Neurology. 1994;44(3 Part 1) doi: 10.1212/wnl.44.3_part_1.394. 394–394. [DOI] [PubMed] [Google Scholar]
- Whitehead Kimberley, Stone Jon, Norman Paul, Sharpe Michael, Reuber Markus. Differences in relatives' and patients' illness perceptions in functional neurological symptom disorders compared with neurological diseases. Epilepsy Behav. 2015;42:159–164. doi: 10.1016/j.yebeh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Whitehead Kimberley, O’Sullivan Suzanne, Walker Matthew. Impact of psychogenic nonepileptic seizures on epilepsy presurgical investigation and surgical outcomes. Epilepsy Behav. 2015;46:246–248. doi: 10.1016/j.yebeh.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Whitehead Kimberley, Gollwitzer Stephanie, Millward Helen, Wehner Tim, Scott Catherine, Diehl Beate. The additional lateralizing and localizing value of the postictal EEG in frontal lobe epilepsy. Clin. Neurophysiol. 2016;127(3):1774–1780. doi: 10.1016/j.clinph.2015.11.050. [DOI] [PubMed] [Google Scholar]
- Whitehead Kimberley, Sherratt Michael, Kandler Ros, Lawrence Sarah, Pang Catherine. Photic stimulation during electroencephalography: efficacy and safety in an unselected cohort of patients referred to uk neurophysiology departments. Seizure. 2016;34:29–34. doi: 10.1016/j.seizure.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Widdess-Walsh, Peter, Mostacci, Barbara, Tinuper, Paolo, Devinsky, Orrin, 2012. Psychogenic nonepileptic seizures. In: Hermann, Stefan, William, Theodore, (Eds.), Handbook of Clinical Neurology, Epilepsy, Part I, Third Series, vol. 107, Elsevier. [DOI] [PubMed]
- Wilkus Robert J., Dodrill Carl B., Thompson Paul M. Intensive EEG monitoring and psychological studies of patients with pseudoepileptic seizures. Epilepsia. 1984;25(1):100–107. doi: 10.1111/j.1528-1157.1984.tb04162.x. [DOI] [PubMed] [Google Scholar]
- World Health Organisation, 2016. International Statistical Classification of Diseases and Related Health Problems 10th Revision.
- Worsely Caroline, Whitehead Kimberley, Kandler Rosalind, Reuber Markus. Illness perceptions of health care workers in relation to epileptic and psychogenic nonepileptic seizures. Epilepsy Behav. 2011;20(4):668–673. doi: 10.1016/j.yebeh.2011.01.029. 10.1016/j.yebeh.2011.01.029. [DOI] [PubMed] [Google Scholar]