Abstract
The field of nanomedicine has received much attention for its potential to allow for targeted identification and treatment of tumors, while sparing healthy tissue. This promise has yet to be clinically realized; instead nanomedicine has translated into clinical benefit via formulations that improve the pharmacokinetics and toxicity profiles of toxic chemotherapeutic agents. In this perspective, we highlight that several of the defining strategies for using nanoparticles intravenously to target solid tumors have limited supporting data in animal studies. Namely, it does not appear that reducing macrophage (and other cell type) uptake in vitro leads to better biodistribution in vivo, nor does increasing blood circulation time nor active targeting. We suggest instead that the coming decade will primarily see nanoparticles impact immunotherapy and local/pseudolocal cancer therapy.
Keywords: : biodistribution, liver accumulation, nanomedicine, nanoparticles, tumor targeting
The field of nanomedicine, the use of nanomaterials to treat disease, has received much attention for its potential to improve cancer imaging and therapy [1]. Ideally nanomedicines would allow for the targeted identification and treatment of a tumor, while sparing healthy tissue. The promise of nanomedicine as a ‘magic bullet’ against cancer has yet to be realized in the clinic though it has led to an explosion of work in development of nanoparticle-based medicines. Instead nanomedicine has translated into clinical benefit via formulations that improve the pharmacokinetics and toxicity profiles of toxic chemotherapeutic agents [2,3]. In this perspective, we highlight that several of the defining strategies for achieving ‘magic bullet’ distribution that have been attempted for the past several decades have not generated strong data to support continuing these approaches. Namely, it does not appear that reducing macrophage (and other cell type) uptake in vitro leads to better biodistribution in vivo, nor does increasing blood circulation time nor active targeting. We suggest instead that the coming decade will primarily see nanoparticles impact immunotherapy and local/pseudolocal cancer therapy.
Nanoparticles can be made of polymers, proteins, lipids or metals. Nonmetallic nanoparticles are the most advanced therapeutically comprising 15 out of the 16 currently approved nanoparticle-based therapies in the USA as of 2016 [5]. Despite their successful translation to the clinic, many of the basic questions on how these and other nanoparticles behave in the body have not been answered. This is likely due in large part to the fact that while polymeric nanoparticles are advanced clinically, they can be difficult to track in vivo since they are organic molecules that can be difficult to detect against the biological background and many of them are noncovalently assembled making it difficult to ascertain if they are assembled or dissociated. Thus, for tracking, they are commonly modified with exogenous labels which may perturb the behavior of the particle and may also become dissociated from the particle.
On the other hand, metallic nanoparticles, like gold nanoparticles or quantum dots, do not require an exogenous label; these particles generally remain intact following administration and are electron dense and easy to find in fixed cells using electron microscopy [6]. Additionally, it is possible to accurately quantify the levels of gold nanoparticles using inductively coupled plasma mass spectrometry [7] due to the lack of background levels of gold in healthy or particle-free tissue; therefore, it is straightforward to determine the biodistribution of these particles in mice after death. For these reasons, metallic nanoparticles can serve as model materials to study how nanoparticles behave in complex and dynamic biological systems. The focus of this perspective will be on gold nanoparticles for this reason. It is, of course, acknowledged that gold nanoparticles are an imperfect model system for the other classes of nanoparticles but some general lessons can be learned.
Biodistribution: challenges in targeting tumor in preference to liver
The study of nanoparticles as drug delivery vehicles has generally been motivated by targeting tumors with poorly formed and leaky vasculature. In 1986, Matsumura and Maeda described a phenomenon later termed the enhanced permeability and retention effect (EPR effect) in tumors [8]. The EPR effect describes the increased accumulation of particulates in the tumor due to a combination of fenestrations in the vasculature and poor lymphatic drainage from tumors. This EPR effect is considered the primary mechanism for the passive accumulation of nanoparticles in tumors in vivo [9]. The fenestrations in the blood vessels act as a sieve leading to nanoparticles being trapped in the perivascular space. Additionally, the impaired lymphatic drainage prevents the tumor from efficiently clearing the nanoparticles allowing nanoparticles to accumulate at the tumor in greater quantities than in healthy tissues. Many of the systems being developed today in the laboratory for tumor targeting rely exclusively on this passive accumulation of nanoparticles in the tumor. While laboratory development of nanomedicines depends on this EPR effect in rodents, the prevalence of this phenomenon in human tumors is unclear with clinical evidence suggesting that the extent of EPR depends highly on the tumor type [10,11]. Moreover, this passive accumulation also results in accumulation in the liver, due in part to the similar fenestrations in the blood vessels that are a part of the normal physiology of the liver.
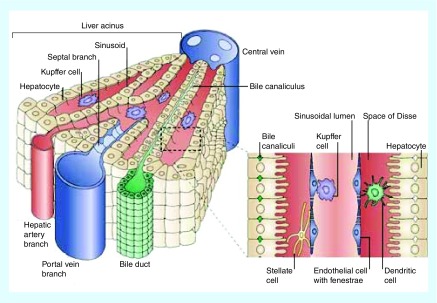
The liver functions to remove foreign material, including viruses, bacteria and nanoparticles from the bloodstream, a role for which its physiology is well adapted [12]. Fenestrations in the endothelial cells allow for foreign particulates to be trapped in a manner similar to the EPR effect. The physiology of the liver helps to explain why nonspecific accumulation of nanoparticles within the liver is a major barrier to clinical translation of nanomaterials. Once trapped in the liver, nanoparticles have been reported to interact with hepatocytes, liver sinusoidal endothelial cells, B cells and Kupffer cells [13] (Figure 1). Hepatocytes make up 60% of all the cells in the liver [14] and are responsible for the metabolism of small molecule drugs and metabolites from the gut [15]. Endothelial cells line the sinusoids. Fenestrations between the endothelial cells allow nutrients and blood to bathe the hepatocytes. Kupffer cells, the liver's resident macrophage population, are the primary phagocytic cells in the liver [15–17]. Kupffer cells make up 80–90% of the macrophages in the body [16]. Kupffer cells are known to phagocytose foreign particulates and are thought to be the cell type responsible for most of the liver accumulation [18–20]. As a direct consequence of their size, which can be advantageous for exploiting the EPR effect, there is high accumulation of nanoparticles in the livers of tumor bearing animals. In a long-term study of injected uncoated gold nanoparticles (3–7 nm), Singer et al. found that within 1 min of being injected 72% of the gold nanoparticles were found in the liver [21]. Liver accumulation is a predominant problem for nanomedicines administered intravenously.
Figure 1. . Liver anatomy.
The liver is comprised of hepatocytes, Kupffer cells and fenestrated endothelial cells. The sinusoid is supplied with blood from the hepatic artery.
Reprinted with permission from Nature Reviews Immunology [4] © Macmillan Publishers Ltd (2006).
Toward selective biodistribution
The field has extensively investigated and continues to investigate ways to control the biodistribution of nanoparticles and shift the localization from primarily liver to predominantly tumor by increasing the rate of tumor accumulation and decreasing the rate of liver accumulation. Overall, very modest progress has been made toward this goal. Here we discuss several strategies that have been attempted and then the hypotheses underlying them with an aim toward stimulating discussion of new hypotheses and approaches.
Toward selective biodistribution: active targeting
Active targeting is the use of a targeting ligand that binds preferentially to tumor cells to increase the rate of accumulation of nanoparticles in a tumor. Antibodies [22,23], peptides [24,25] and small molecules [26] have all been used to target nanoparticles to specific cells or tumors. In vitro targeted nanoparticles show a greater association with cells containing the target than the control nanoparticles [22]. However, experiments using gold nanoparticles in vivo show conflicting evidence for the efficacy of tumor targeting. Some have seen the amount of nanoparticles within the tumor increase when a targeting moiety is included, and others have seen no difference in the total amount of nanoparticles localized within a tumor when a targeting moiety is included [27–29]. One potential reason for the variability seen in targeting efficacy is the differences in model systems used. The most effective targeting strategies, which show modest increases in tumor accumulation with the inclusion of a targeting moiety, can be found in model systems using human targets in a mouse. Specifically the greatest increases in effect are seen with the use of human antibodies that are not crossreactive with proteins in the mouse (Table 1). However, when the targeting ligand is an antibody that binds to a target mouse protein no difference is seen between targeted and nontargeted nanoparticles, likely due to the fact that despite the target being overexpressed in the tumor the bulk of the target is found in healthy tissue since the tumor is so small relative to the rest of the animal (Table 2). While the use of a human target in a mouse model may be effective, the results seen do not take into account the expression of the target in other healthy tissues like would be seen in a clinical example. Performing these experiments in a mouse model with a mouse target suggests that targeting will rarely increase bulk tumor accumulation in humans though it may have effects in cellular localization.
Table 1. . Human targets and targeting efficacy in mouse models of cancer.
| Nanoparticle | Target | Tumor accumulation |
|---|---|---|
| Gold nanorod (HD = 51, AR = 3) [30] Gold nanorod (HD = 51, AR = 3) Gold nanorod (HD = 51, AR = 3) |
EGFR uPAR Untargeted |
65 ppm* (24 h) 70 ppm* (24 h) 45 ppm* (24 h) |
| Gold nanorod (HD = 70, AR = 4.2) [31] Gold nanorod (HD = 70, AR = 4.2) |
EGFR Untargeted |
50% i.d. (24 h) 10% i.d. (24 h) |
| Gold nanoparticles (5 nm) [32] Gold nanoparticles (5 nm) |
EGFR Untargeted |
4.5 mg Au/g tissue* 0.5 mg Au/g tissue* |
Studies in the table are limited to gold nanoparticle studies that quantified tumor accumulation via inductively coupled plasma methodologies and contained at least an untargeted control.
*Estimated from bar graphs in the publication.
Table 2. . Murine targets and targeting efficacy in mouse models of cancer.
| Nanoparticle | Target | Tumor accumulation |
|---|---|---|
| Gold nanorod (HD = 51, AR = 3) [30] Gold nanorod (HD = 51, AR = 3) |
αvβ3 integrin Untargeted |
15 ppm* (24 h) 45 ppm* (24 h) |
| Gold nanoparticle (155 nm) [27] Gold nanoparticle (85 nm) |
GRPR Untargeted |
0.48% i.d. (4 h) 0.5% i.d.* (4 h) |
| Gold nanoparticle (77.0 nm) [28] Gold nanoparticle (73.2 nm) |
Transferrin receptor Untargeted |
2.5% i.d.* (24 h) 2.5% i.d.* (24 h) |
| Gold nanoparticles (22 nm) [33] Gold nanoparticles (22 nm) |
αvβ3 integrin Untargeted |
0.16% i.d. (72 h) 0.16% i.d. (72 h) |
Studies in the table are limited to gold nanoparticle studies that quantified tumor accumulation via inductively coupled plasma methodologies and contained at least an untargeted control.
*Estimated from bar graphs in the publication.
Toward selective biodistribution: empirical passive targeting – nanoparticle size, shape & charge
Nanoparticles can be synthesized in a variety of shapes and sizes, and functionalized with different coatings that affect the charge of the material. Numerous researchers have investigated if there are certain shapes, sizes or charges that intrinsically result in selective tumor accumulation. Untangling the role of each property on biodistribution can be difficult as many of these parameters are linked, for example, the particle coating in combination with the size and shape will dictate both final hydrodynamic size and charge, and few studies test all of the permutations of all of these parameters. Indeed, in practice, most of this approach has consisted of empirically measuring biodistribution of different nanoparticles and attempting to derive rules based on the results.
With respect to size, for similarly functionalized nanoparticles, the smaller the hydrodynamic diameter of the nanoparticle the wider the distribution in vivo, with particles seen in the thymus, kidney, heart, lung and brain in addition to their presence in the liver and spleen [34]. As particle size increased the presence of nanoparticles were detected predominantly in the liver and spleen [14,34,35]. In tumor-bearing animals, several studies have shown that as particle size increases, tumor accumulation increases. One reason for this difference is that small nanoparticles can more easily diffuse in and out of the tumor while the larger particles are retained [36]. There is an upper limit to the size of nanoparticles that will accumulate in tumors, though this limit is dependent on the tumor model and the degree of fenestrations in the vasculature [36]. In general, increasing the particle size up to 200 nm can increase tumor accumulation and retention, although the majority of nanoparticles injected still accumulate in the liver and spleen [37].
For shape and charge, it is difficult to draw general conclusions as different papers using different animal models and different time scales observe different trends in accumulation results, though again overall the majority of the nanoparticles injected intravenously accumulate in the liver and spleen [38,39]. Indeed, there does not appear to be any size, shape or charge that is inherently tumor tropic, though neutral and zwitterionic particles may last longer in circulation than either negative or positively charged particles.
Toward selective biodistribution: rational passive targeting
Lacking a particular shape, size or charge that would convey tumor targeting, researchers have predominantly turned to rationally modifying the surfaces of nanoparticles in attempts to get selective tumor accumulation. Two hypotheses dominate current surface modification strategies for achieving tumor targeting:
Proteins rapidly coat nanoparticles after intravenous injection and these proteins strongly influence liver accumulation and biodistribution in general;
A portion of nanoparticles in the bloodstream will diffuse into the tumor space every time particles flow past the tumor [40,41]. Thus, reducing the rate of uptake by the liver to prolong blood circulation time will lead to enhanced tumor accumulation.
Motivated by these hypotheses, researchers have investigated many surface coatings for nanoparticles, commonly seeking to limit protein opsonization and extend blood circulation time. Here we briefly discuss the most common strategy, PEGylation, the emerging strategy of biomimicry and then comment on the underlying hypotheses.
Selective biodistribution: attempts based on PEGylation
PEGylation of nanoparticles can reduce the amount of macrophage uptake in vitro and can extend the circulation time of nanoparticles compared with uncoated nanoparticles in vivo [42,43]. The presence of PEG on a nanoparticle can reduce particle aggregation, protein opsonization and cell uptake as well as increase blood half-life and tumor accumulation.
PEG can reduce particle aggregation by physically blocking interactions between two nanoparticle surfaces [44]. By passivating the surface with a PEG layer, the nanoparticles are less likely to aggregate in high salt conditions like the blood stream. By preventing aggregation, cell uptake is also reduced [45]. In addition to preventing interactions between gold surfaces, PEG also acts as a shield that prevents the direct interaction of nanoparticles and proteins. By inhibiting the interactions of proteins and the gold nanoparticle surface there is a decrease in opsonization. Increasing the density of PEG on the surface, either by increasing the amount of PEG available or by backfilling with additional PEG, reduces the amount of proteins that adsorb to the surface of the nanoparticle [46,47]. There is a correlation between decreasing protein adsorption onto the particle and decreasing cell uptake in vitro [46].
Increasing the length of the PEG on the surface also leads to an increase in blood half-life [48]. It is hypothesized that this increase in blood half-life is due to a reduction in protein adsorption, which triggers a delay of phagocytosis. This delay in protein signaling allows the nanoparticle to evade the macrophages.
Eventually even densely PEGylated nanoparticles accumulate in the liver. It is unclear if this accumulation is a property of the initial nanoparticle formulation or due to alterations over time in the surface coating of the nanoparticles. It has been reported that physiological levels of cysteine and cystine can disrupt the PEGylation of a nanoparticle [49]. This disruption could trigger aggregation between particles remaining in the bloodstream or it could trigger opsonization on the particles over time. Similarly, it has also been reported that proteins are capable of binding to gold nanoparticles despite the presence of a PEG layer [50]. While the intact PEG layer may be able to prevent interactions between proteins and the nanoparticle surface, extended exposure to the plasma may disrupt the integrity of the PEG allowing for opsonization to occur. This could explain why there is a delay in the accumulation of PEGylated particles in the liver. It is also possible that uptake of PEGylated particles is not primarily driven by protein opsonization. Overall, while PEGylation may be useful at reducing macrophage uptake in the short term, it is not capable of avoiding the phagocytic cells for a long period of time.
Selective biodistribution: attempts based on biomimicry
A relatively new approach to extending circulation time is to use biological signals to shield the nanoparticles from macrophages. Particles containing a minimal self-peptide, based on the sequence of CD-47, successfully avoided uptake of macrophages in vitro; however, imaging experiments in vivo suggested that by 90 min whether the particles contained the self-peptide or a scrambled peptide the outcome was similar, the majority of the particles were still found in the liver [51]. Whole body imaging showed negligible differences between the self-peptide and control groups at 90 min, and ex vivo imaging of tumors showed less than 10% of the injected dose of the self-peptide particles were located in the tumor [51]. In this initial report on this interesting strategy, the final biodistribution was not too different from that observed for PEGylated particles. This strategy highlights the disconnect between in vitro cell uptake studies and in vivo biodistribution result and is evidence of the need to look beyond macrophage avoidance to improve biodistribution.
Other groups have used the membranes from erythrocytes or from leukocytes in order to coat their nanoparticles. Erythrocytes have much longer half-lives in the body than nanoparticles; in humans erythrocytes can last for over 100 days before they are recycled (40 days in mice) [52,53]. It was thought that by using the membranes of erythrocytes it would be possible to translate this long in vivo lifespan to nanoparticles. Extruding erythrocytes in the presence of nanoparticles created camouflaged nanoparticles which were detected in the blood of mice up to 72 h, longer than their PEGylated counterparts [54]. While the circulation time has been improved over PEGylated particles, these materials still are primarily found in the liver and the spleen after only 24 h and the quantity in those organs continues to increase over time [54]. In addition to using erythrocytes, leukocytes have been extruded to generate camouflaged nanoparticles. Again, the goal is to take advantage of signals in the cell membrane that prevent phagocytosis of healthy cells. When coated with J774 murine macrophage cell membranes, the nanoparticle was not phagocytosed by those cells in vitro [55]. In vivo, these particles did show a delay in liver uptake but only of 40 min [55]. Despite containing biological markers that may delay phagocytosis in vitro and delay liver accumulation in vivo, these nanoparticles have a similar final biodistribution pattern as PEGylated particles, though on a different time scale suggesting that it may be possible to delay the accumulation in the liver but not prevent it.
Toward selective biodistribution: commentary on underlying hypotheses
Role of protein opsonization in biodistribution
The general understanding in the field is that upon injection into the bloodstream uncoated nanoparticles will undergo rapid opsonization by serum proteins [56]. This opsonization can trigger phagocytosis by Kupffer cells, the liver's resident macrophage population and other macrophages in the body which will traffic the particles to the liver [42]. Delaying or preventing opsonization through chemical modifications of the nanoparticle surface has been a common strategy to avoid phagocytosis by macrophages. However, recent reports have also suggested that protein opsonization, with the appropriate proteins, is required to delay phagocytosis [57,58]. The conflicting evidence on the role of the protein corona in cell uptake has made it apparent that if the protein corona plays a large role in controlling biodistribution it is due to a number of factors. In some cases, the orientation/conformation of the protein corona has been shown to dictate cell uptake in vitro [59] and in others, proteins present in very small amounts in the corona have been implicated as the drivers of behavior [57,58,60]. Further work is essential to clarify the role of the protein corona. If proteins that are very minor components of the corona are indeed driving behavior, it may be very challenging to rationally design coatings to bind these proteins in the proper ratio.
Impact of longer blood circulation
It is routinely written that increasing the blood circulation time of nanoparticles will lead to greater tumor accumulation, but there is actually very limited evidence that this is true. There are reports of spherical gold nanoparticles with blood circulation half-lives of >50 h [48] and nanoworms with circulation times >1 week [61]; however, in these cases the animal either was healthy or the tumor accumulation was not measured. These are extreme examples of long circulating nanoparticles; many nanoparticles have blood half-lives <1 h. Several studies have noted that increasing the circulation time increases the amount of nanoparticles that accumulate in the tumor via the EPR effect [41,48,51]. There is, however, no mathematically described relationship between tumor accumulation and blood circulation time. Within an individual paper's particle system, there may be a trend between longer circulation time and greater tumor accumulation; however, this trend is difficult to apply more broadly as there is no consistency between particle size, shape, surface coating, and dose nor between the tumor type, mouse strain and immune status of the mice (Table 3). Additionally, a study with 2 nm functionalized gold nanoparticles with variable surface charge did not show a relationship between tumor accumulation and blood half-life. Negatively charged 2 nm gold nanoparticles with an 18-min half-life had a tumor accumulation of 15 μg Au/g tumor while neutral particles with a 5-h half-life had a tumor accumulation of only 5 μg Au/g tumor. This difference may be due in part to the differences in charge for each material. A study with spherical gold nanoparticles of different sizes showed an increase in tumor accumulation up to 26% injected dose·hour per gram of tumor as blood half-life increased to 16.5 h [48]. A third study failed to achieve >1% injected dose at the tumor regardless of the length of blood circulation time, though it did increase slightly from 0.3% with a 34 s half-life with BSA passivated silica-coated gold nanorods to 0.8% with an 18.6-min half-life with PEG passivated silica-coated gold nanorods [62]. In short, there is no predictive relationship currently between blood half-lives and tumor accumulation for different nanoparticles. The length of blood circulation time depends on a mixture of particle size, shape, charge, coating and dose, but the analysis of the results of several studies suggests that increasing the circulation time of a nanoparticle system does not necessarily increase tumor accumulation.
Table 3. . Blood half life and tumor accumulation.
| Nanoparticle | Surface coating | Blood half life | Tumor accumulation |
|---|---|---|---|
| 2 nm Gold nanoparticles [38] 2 nm Gold nanoparticles 2 nm Gold nanoparticles |
TEG-Hydroxyl TEG-Amine TEG-Carboxyl |
5 h 24 h 18 m |
25 μg† Au/g tumor 5 μg† Au/g tumor 15 μg† Au/g tumor |
| 22 nm Gold nanoparticles [48] 40 nm Gold nanoparticles 99 nm Gold nanoparticles 82 nm Gold nanoparticles 61 nm Gold nanoparticles |
PEG2000-SH PEG2000-SH PEG10,000-SH PEG10,000-SH PEG5000-SH |
2.5 h 4 h 7.2 h 11.6 h 16.5 h |
0.3% injected dose·h/g 15.8% injected dose·h/g 17.9% injected dose·h/g 20.4% injected dose·h/g 26.5% injected dose·h/g |
| Gold nanorods (hd = 42) [62] Gold nanorods (hd = 44) Gold nanorods (hd = 22) |
SiO2-PEG SiO2-BSA BSA |
18.6 m 3.3 m 34 s |
0.83% injected dose 0.45% injected dose 0.3% injected dose |
| Gold nanorods (13nm × 47nm) [63] | PEG | 17 h | 7% injected dose/g |
Studies in the table are limited to gold nanoparticle studies that used inductively coupled plasma methodologies to quantify both blood circulation and tumor accumulation.
†Estimated from bar graphs in the publication.
PEG: Polyethylene glycol, TEG: Tetraethylene glycol.
Concluding thoughts on biodistribution
Overall, despite careful control of the physical and chemical properties of nanoparticles and careful control over their surface coating, nanoparticles rarely have higher than 10% injected dose in the tumor with many reports of <5% injected dose [64]. This accumulation at the tumor is likely to overestimate the actual amount of nanoparticle that interacts with a cancer cell. In the tumor environment, nanoparticles could accumulate adjacent to the blood vessel or be phagocytosed by macrophages associated with the tumor [43,65].
While 10% of the injected dose at the tumor may be higher than free drug, the majority of nanoparticles injected, regardless of coating, accumulate in the liver. Using current strategies, it may be possible to delay liver accumulation, but the final biodistribution of nanoparticles is predominantly liver accumulation. In general, liver clearance is highly efficient for many materials in the body and escaping this clearance is restricted to a small minority of materials (e.g., red blood cells) through a tightly regulated process. It is unlikely that this problem will be solved by empirical testing of materials. We believe that if this clearance is to be overcome the field will have to turn to detailed studies of how different materials are entrapped in the liver.
Nanoparticle clearance
For all therapeutics, a key criterion is clearance and this is even more important for nanoparticles given their current predominant off-target accumulation in the liver and spleen. It should be noted that clearance is definitely more varied than accumulation between different classes of nanoparticles, that is, soft (polymers, proteins, lipids) versus hard (metallic) nanoparticles. In particular, many soft nanoparticles are inherently biodegradable and have very different clearance profiles than metallic nanoparticles [66]. For this perspective, we have chosen to remain focused on gold nanoparticles for consistency with the biodistribution section. The kidneys and the liver are the two main routes of elimination for injected nanomaterials and are discussed separately here.
Metallic nanoparticle clearance: kidneys
In the kidneys, the glomerulus filters the blood and blood particulates (including nanoparticles) through three membranous layers. Even though each layer has slits >30 nm wide, the overlapping of the slits results in a pore size of approximately 5 nm, allowing particulates smaller than this size to pass through the kidney [67]. In addition to the size selectivity, the glomerulus also is charge selective, permitting the passage of positively charged particles and proteins through the pores [68]. The charge selectivity in the glomerulus is one method by which the kidney selectively maintains some proteins in the bloodstream while clearing others in the urine. Studies on the actual size cutoff of renal clearance vary in their results. In one report, 3-nm gold nanoparticles were found in the urine of mice, while 5-nm gold particles were found primarily in the liver [69]. However, there have also been reports of 5.5 nm metallic nanoparticles, undergoing renal clearance in vivo, while sizes >6 nm were primarily taken up into the liver [70]. Other reports show urine accumulation of aluminum oxide nanoparticles of 10 nm [71] and even bundled carbon nanotubes >100 nm in length and >10 nm in width have been found in the urine [72]. This suggests that the renal clearance of the nanoparticles is likely more complicated than a simple size or charge cut off and may depend at least in part on the material, shape, size and coating of the nanoparticles. Since renal clearance involves the filtering and removing of nanomaterials from the blood, renal clearance occurs rapidly in vivo; for small particles, approximately 80% of the injected dose of 4.6-nm nanoparticles were cleared via the urine after 4 h [70]. While renal clearance is desirable since it would prevent accumulation of the nanoparticles in healthy organs, particles that clear rapidly via the kidneys do not typically accumulate in the tumor at high levels.
Nanoparticle clearance: liver
Like renal clearance, hepatic clearance involves filtering of the blood. As blood passes through the hepatic tissue via the portal vein, small molecule drugs, viruses, bacteria or nanoparticles of all sizes can be taken up by hepatocytes, sinusoidal endothelial cells and Kupffer cells in the liver [15,16]. Hepatocytes typically metabolize small molecule drugs and release the metabolized products either back into the bloodstream or into the bile [73]. Hepatocytes can endocytose nanoparticles, though at a much lower rate than macrophages. Once processed by hepatocytes, these nanoparticles are cleared through bile and into the feces [14,74]. However, hepatocyte uptake is only seen when large doses of nanoparticles have been given [75] or when macrophages have been chemically depleted [16]. Kupffer cells phagocytose bacteria, viruses and nanoparticles to remove them from the bloodstream [20,76]. Particles that undergo hepatic clearance are found in fecal matter; however, the conversion from liver accumulation to fecal matter is a slow process; a study by Sadauskas et al. found that 91% of the uncoated 40-nm gold nanoparticles that had accumulated in the liver on the first day after injection were still present in the liver 6 months later [77]. A more recent study in 2015 demonstrated that there was a 20% injected dose reduction in gold levels in the liver between day 1 and day 3 for 7-nm gold nanoclusters with a negative surface charge [62]. The turnover time of nanoparticles in the liver may be size dependent but is generally considered to be slow possibly due to the nanoparticles escaping from the Kupffer cells over time either through cell death or exocytosis. Studies have shown that Kupffer cells, the macrophages in the liver responsible for the phagocytosis of large nanoparticles, are completely replaced after 21 days [20,76]. As Kupffer cells die, the cells are consumed by new Kupffer cells; some particles may escape at that time and could accumulate in the bile. The turnover of Kupffer cells could explain the slow time scale of the hepatic clearance seen in vivo. Overall, the progression of nanoparticles through the liver has been little studied and it is unclear how it depends on nanoparticle characteristics such as size, charge, shape and more.
The clearance of nanoparticles from the body requires a delicate balance in timing. Nanoparticles that are cleared too quickly will not accumulate at the tumor site and will be excreted along with its cargo in the urine. On the other hand, nanoparticles that persist in the body could cause drug- or nanoparticle-related toxicity in organs such as the liver or kidneys – the organs responsible for filtering the blood to remove drugs and nanomaterials.
Conclusion
Nanoparticles have attracted a great deal of attention for targeted drug delivery to tumors. The vast majority of work has focused on intravenous administration to target solid tumors. While some success and clinical impact has been achieved, the overall efficiency of tumor delivery for the field has not markedly increased in recent years. By reviewing the biodistribution of gold nanoparticles, we have highlighted that there is contradictory data to support several strategies (EPR-based targeting, reducing macrophage [and other cell type] uptake, increasing blood circulation time, active targeting) that are widely touted. It is also briefly emphasized that while nanoparticles are predominantly cleared by the liver, much remains to be learned about this process.
Future perspective
The field of nanomedicine is continuing to grow and expand toward more clinical applications; however, there are important gaps in knowledge to overcome so that we can design materials that achieve improved tumor accumulation and whole body clearance. This goal has been particularly challenging to achieve for intravenously administered nanoparticles targeting solid tumors. One appealing strategy for more rapid clinical impact is to avoid this problem by using nanoparticles to improve local and pseudolocal (such as within the bladder or intraperitoneal cavity) drug delivery. This approach is expected to see increasing attention in the coming years, particularly in the setting of immunotherapy since nanoparticles are inherently predominantly taken up by macrophages.
Developing more effective solutions for the holy grail of selective solid tumor targeting following intravenous administration will require more systematic studies from the field. It is known that physiochemical characteristics of nanoparticles including size, shape, surface charge and surface coating as well as the parameters of the study such as animal species and strain, immune status, injected dose, disease status and studying timing can all influence the outcome of a biodistribution study. In order to more fully understand the interplay between all of these parameters we need to improve reporting of our biodistribution studies as well as include more time course studies to determine blood circulation time and monitor the kinetics of tumor accumulation. In order to compare across studies, discussions of nanoparticle dose should be included as the dose is likely to impact how long nanoparticles can circulate and how quickly nanoparticles accumulate in immune cells. Additionally it will be important to include appropriate control particles in order to determine if newer particles are able to improve upon PEGylated particles. Including a common control could also help to analyze the data that comes from different labs each working with different animals and disease models. More detailed studies on the mechanisms of accumulation in the liver and spleen are also needed. These studies are generally expensive, laborious and do not attract as much attention as therapeutic efficacy studies, but, as a field, we must understand the principles behind accumulation more deeply to develop new strategies for tumor targeting and liver/spleen avoidance [13].
The same is true for the other side of the equation. It may be that avoiding liver/spleen accumulation is essentially impossible for nanoparticles but that nanoparticles can be designed that clear more rapidly from these organs than from tumors. In the idealized version of this strategy, clearance from healthy tissue would be fast enough that the goal of selective drug action at the tumor would be achieved by clearance of the drug from healthy tissue before it could induce off-target toxicity. In recent years, a concerted effort has been made in this direction by studying degradable assemblies or aggregates of nanoparticles and monitoring their biodistribution [78] as well as clearance over time [69]. A primary goal of these studies is to understand, following liver accumulation, what subunit size nanoparticles need to be broken down into for clearance. Degradable assemblies or aggregates of nanoparticles are necessary to study this effect because, as discussed above, very small nanoparticles are primarily excreted through the kidneys and have low liver accumulation while larger nanoparticles accumulate in the liver but have minimal clearance. Thus, to understand what size particle could be processed out of liver cells and cleared it is necessary to assemble a larger construct that will accumulate in the liver cells but then break down into small subunits for clearance from those cells. These early studies have suggested that the subunit size needed for enhanced clearance is quite small (<3 nm) and clearance has remained quite slow for all systems following liver accumulation. Additional studies are required in order to optimize the degradation and clearance of these complex structures and better understand how nanoparticles are processed following liver accumulation.
Executive summary.
Design of nanoparticles for targeting tumors has primarily been based on exploiting the enhanced permeability and retention effect.
The enhanced permeability and retention effect in human tumors is much more limited than in mouse models and, at best, is highly heterogeneous.
Designing particles for accumulation based on leaky vasculature has led to materials that predominantly accumulate in the liver.
Three major strategies for improving biodistribution (reducing macrophage [and other cell type] uptake, increasing blood circulation time, active targeting) have not resulted in significant and general improvements in tumor accumulation.
Intavenous administration of nanoparticles for tumor targeting remains a challenging problem.
Clearance is markedly different for different types of nanoparticles. Generally for hard/metallic nanoparticles, renal clearance only occurs for very small particles, but there are exceptions. Most nanoparticles clear through the liver.
The precise mechanism of liver clearance is understudied.
Footnotes
Financial & competing interests disclosure
JM Berlin was supported in part by NIH R01CA197359 during the preparation of this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Duncan R. Nanomedicine gets clinical. Materials Today. 2005;8(8 Suppl.):16–17. [Google Scholar]
- 2.Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 2014;9:4357–4373. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragelle H, Danhier F, Préat V, Langer R, Anderson DG. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv. 2016:1–14. doi: 10.1080/17425247.2016.1244187. [DOI] [PubMed] [Google Scholar]; •• Highlights the current state of approved nanodrugs and highlights potential regulatory challenges.
- 4.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat. Rev. Immunol. 2006;6(3):244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 5.Stylianopoulos T, Jain RK. Design considerations for nanotherapeutics in oncology. Nanomedicine. 2015;11(8):1893–1907. doi: 10.1016/j.nano.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano EL, Romano M. Staphylococcal protein a bound to colloidal gold: a useful reagent to label antigen-antibody sites in electron microscopy. Immunochemistry. 1977;14(9):711–715. [Google Scholar]
- 7.Albanese A, Tsoi KM, Chan WCW. Simultaneous quantification of cells and nanomaterials by inductive-coupled plasma techniques. J. Lab. Autom. 2013;18(1):99–104. doi: 10.1177/2211068212457039. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Part 1):6387–6392. [PubMed] [Google Scholar]
- 9.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control. Rel. 2012;164(2):138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Clark AJ, Wiley DT, Zuckerman JE, et al. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc. Natl Acad. Sci. USA. 2016;113(14):3850–3854. doi: 10.1073/pnas.1603018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond.) 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsoi KM, Macparland SA, Ma X-Z, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016;15(11):1212–1221. doi: 10.1038/nmat4718. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses the distribution of intravenously injected nanoparticles in the liver and highlights the difficulty in acheiveing nanoparticle clearance from the liver. This article highlights the physical characteristics of circulation as well as the biological interactions that impair liver clearance.
- 14.Semmler-Behnke M, Kreyling WG, Lipka J, et al. Biodistribution of 1.4- and 18-nm gold particles in rats. Small. 2008;4(12):2108–2111. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- 15.Haschek WM, Rousseaux CG, Wallig MA. Fundamentals of Toxicologic Pathology (2nd Edition) Academic Press; San Diego, CA, USA: 2010. Chapter 9 – The liver; pp. 197–235. [Google Scholar]
- 16.Zhang Y-N, Poon W, Tavares AJ, Mcgilvray ID, Chan WCW. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. J. Control. Rel. 2016;240:332–348. doi: 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 2002;1:1–1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho W-S, Cho M, Jeong J, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009;236(1):16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Cho W-S, Cho M, Jeong J, et al. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2010;245(1):116–123. doi: 10.1016/j.taap.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Sadauskas E, Wallin H, Stoltenberg M, et al. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007;4:10. doi: 10.1186/1743-8977-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer JM, Adlersberg L, Sadek M. Long-term observation of intravenously injected colloidal gold in mice. J. Reticuloendothel. Soc. 1972;12(6):658–671. [PubMed] [Google Scholar]
- 22.Melancon MP, Lu W, Yang Z, et al. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol. Cancer Ther. 2008;7(6):1730–1739. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chattopadhyay N, Cai Z, Pignol J-P, et al. Design and characterization of HER-2-targeted gold nanoparticles for enhanced x-radiation treatment of locally advanced breast cancer. Mol. Pharm. 2010;7(6):2194–2206. doi: 10.1021/mp100207t. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Ma H, Zhang X, et al. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials. 2012;33(4):1180–1189. doi: 10.1016/j.biomaterials.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 25.Meyers JD, Cheng Y, Broome AM, et al. Peptide-targeted gold nanoparticles for photodynamic therapy of brain cancer. Part. Part. Syst. Charact. 2015;32(4):448–457. doi: 10.1002/ppsc.201400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathinaraj P, Lee K, Park S-Y, Kang I-K. Targeted images of KB cells using folate-conjugated gold nanoparticles. Nanoscale Res. Lett. 2015;10:5. doi: 10.1186/s11671-014-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chanda N, Kattumuri V, Shukla R, et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl Acad. Sci. USA. 2010;107(19):8760–8765. doi: 10.1073/pnas.1002143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi CHJ, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl Acad. Sci. USA. 2010;107(3):1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirollo KF, Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol. 2008;26(10):552–558. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Peng X, Wang Y, et al. A re-examination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano. 2010;4(10):5887–5896. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charan S, Sanjiv K, Singh N, et al. Development of chitosan oligosaccharide-modified gold nanorods for in vivo targeted delivery and noninvasive imaging by NIR irradiation. Bioconjug. Chem. 2012;23(11):2173–2182. doi: 10.1021/bc3001276. [DOI] [PubMed] [Google Scholar]
- 32.Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010;62(3):346–361. doi: 10.1016/j.addr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peiris PM, Deb P, Doolittle E, et al. Vascular targeting of a gold nanoparticle to breast cancer metastasis. J. Pharm. Sci. 2015;104(8):2600–2610. doi: 10.1002/jps.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Zhou C, Long M, Qin Y, Sun X, Zheng J. Luminescent gold nanoparticles with efficient renal clearance. Angewandte Chemie International Edition. 2011;50(14):3168–3172. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J. Control. Rel. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvizo RR, Miranda OR, Moyano DF, et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS One. 2011;6(9):e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirn S, Semmler-Behnke M, Schleh C, et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. e.V. 2011;77(3):407–416. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Sun J, Han J, He Z. Long-circulating targeted nanoparticles for cancer therapy. Current Nanosci. 2010;6(4):347–354. [Google Scholar]
- 41.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 42.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond.) 2011;6(4):715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotech. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doane T, Burda C. Nanoparticle mediated non-covalent drug delivery. Adv. Drug Deliv. Rev. 2013;65(5):607–621. doi: 10.1016/j.addr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albanese A, Chan WCW. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011;5(7):5478–5489. doi: 10.1021/nn2007496. [DOI] [PubMed] [Google Scholar]
- 46.Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012;134(4):2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 47.Dai Q, Walkey C, Chan WCW. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angewandte Chemie. 2014;53(20):5093–5096. doi: 10.1002/anie.201309464. [DOI] [PubMed] [Google Scholar]
- 48.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating tumor targeting efficiency of nanoparticles through design. Nano Letters. 2009;9(5):1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 49.Larson TA, Joshi PP, Sokolov K. Preventing protein adsorption and macrophage uptake of gold nanoparticles via a hydrophobic shield. ACS Nano. 2012;6(10):9182–9190. doi: 10.1021/nn3035155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maus L, Dick O, Bading H, Spatz JP, Fiammengo R. Conjugation of peptides to the passivation shell of gold nanoparticles for targeting of cell-surface receptors. ACS Nano. 2010;4(11):6617–6628. doi: 10.1021/nn101867w. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal ‘self’ peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science (N.Y.) 2013;339(6122):971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Röhme D. Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo . Proc. Natl Acad. Sci. USA. 1981;78(8):5009–5013. doi: 10.1073/pnas.78.8.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kay MM. Mechanism of removal of senescent cells by human macrophages in situ . Proc. Natl Acad. Sci. USA. 1975;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parodi A, Quattrocchi N, Van De Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nano. 2013;8(1):61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nano. 2013;8(10):772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 57.Ritz S, Schöttler S, Kotman N, et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules. 2015;16(4):1311–1321. doi: 10.1021/acs.biomac.5b00108. [DOI] [PubMed] [Google Scholar]
- 58.Schöttler S, Becker G, Winzen S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nano. 2016;11(4):372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 59.Fleischer CC, Payne CK. Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles. J. Phys. Chem. B. 2014;118(49):14017–14026. doi: 10.1021/jp502624n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walkey CD, Olsen JB, Song F, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8(3):2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 61.Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nano. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Bai R, Yang R, et al. Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration. Metallomics. 2015;7(3):516–524. doi: 10.1039/c4mt00340c. [DOI] [PubMed] [Google Scholar]
- 63.Maltzahn GV, Park J-H, Agrawal A, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer res. 2009;69(9):3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. [Google Scholar]
- 65.Nichols JW, Bae YH. Odyssey of a cancer nanoparticle: from injection site to site of action. Nano Today. 2012;7(6):606–618. doi: 10.1016/j.nantod.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li M, Panagi Z, Avgoustakis K, Reineke J. Physiologically based pharmacokinetic modeling of PLGA nanoparticles with varied mPEG content. Int. J. Nanomed. 2012;7:1345–1356. doi: 10.2147/IJN.S23758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang B, He X, Zhang Z, Zhao Y, Feng W. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acct Chem. Res. 2012;46(3):761–769. doi: 10.1021/ar2003336. [DOI] [PubMed] [Google Scholar]
- 68.Brenner BM, Bohrer MP, Baylis C, Deen WM. Determinants of glomerular permselectivity: insights derived from observations in vivo . Kidney Int. 1977;12(4):229–237. doi: 10.1038/ki.1977.107. [DOI] [PubMed] [Google Scholar]
- 69.Chou LYT, Zagorovsky K, Chan WCW. DNA assembly of nanoparticle superstructures for controlled biological delivery and elimination. Nat. Nano. 2014;9(2):148–155. doi: 10.1038/nnano.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soo Choi H, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat. Biotech. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pérez-Campaña C, Gómez-Vallejo V, Puigivila M, et al. Biodistribution of different sized nanoparticles assessed by positron emission tomography: a general strategy for direct activation of metal oxide particles. ACS Nano. 2013;7(4):3498–3505. doi: 10.1021/nn400450p. [DOI] [PubMed] [Google Scholar]
- 72.Singh R, Pantarotto D, Lacerda L, et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl Acad. Sci. USA. 2006;103(9):3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haschek WM, Rousseaux CG, Wallig MA. Fundamentals of Toxicologic Pathology (2nd Edition) Academic Press; San Diego, CA, USA: 2010. Kidney and lower urinary tract; pp. 261–318. [Google Scholar]
- 74.Lipka J, Semmler-Behnke M, Sperling RA, et al. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31(25):6574–6581. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Liu T, Choi H, Zhou R, Chen IW. RES blockade. A strategy for boosting efficiency of nanoparticle drug. Nano Today. 2015;10(1):11–21. [Google Scholar]; • Proposes a novel approach to improving nanoparticle biodistribution.
- 76.Crofton R, Diesselhoff-Den Dulk M, Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J. Exp. Med. 1978;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sadauskas E, Danscher G, Stoltenberg M, Vogel U, Larsen A, Wallin H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine. 2009;5(2):162–169. doi: 10.1016/j.nano.2008.11.002. [DOI] [PubMed] [Google Scholar]; • Reports the slow elimination of gold nanoparticles from the liver of a mouse.
- 78.Tam JM, Tam JO, Murthy A, et al. Controlled assembly of biodegradable plasmonic nanoclusters for near-infrared imaging and therapeutic applications. ACS Nano. 2010;4(4):2178–2184. doi: 10.1021/nn9015746. [DOI] [PMC free article] [PubMed] [Google Scholar]