Abstract
Despite a long history of use for rectal and vaginal drug delivery, the current worldwide market for suppositories is limited primarily due to a lack of user acceptability. Therefore, virtually no rational pharmaceutical development of antiviral suppositories has been performed. However, suppositories offer several advantages over other antiviral dosage forms. Current suppository designs have integrated active pharmaceutical ingredients into existing formulation designs without optimization. As such, emerging suppository development has been focused on improving upon the existing classical design to enhance drug delivery and is poised to open suppository drug delivery to a broader range of drugs, including antiretroviral products. Thus, with continuing research into rational suppository design and development, there is significant potential for antiretroviral suppository drug delivery.
Keywords: : antiviral, drug delivery, formulation strategies, pessary, suppository
Suppositories: then & now
Suppositories are a dosage form designed to deliver drugs through rectal and vaginal routes of administration. They evolved as a more convenient alternative form of drug delivery from liquid enema formulations. In fact, the term suppositorium comes from the Latin word supponere, meaning ‘substitute’ [1]. While commonly perceived to be for rectal administration only, suppositories are also appropriate for vaginal administration. Pessaries are often used to describe vaginal suppositories. The Latin term pessarium derived from the Greek word pesos, which means ‘oval stone’ was used to describe the shape.
Suppositories and pessaries as drug delivery vehicles are not new dosage forms. Rectal drug delivery is one of the world's oldest strategies for drug dosing and rectally applied products have existed for hundreds of years. Suppositories have been referenced in the Hebrew Scriptures. Even vaginally applied pessaries are equally as old with documentation reported in Egyptian papyruses. Hippocrates wrote of various acorn-based medicines delivered rectally and vaginally for local pharmacologic effects [2].
Initially, rectal suppositories were composed of bases of baked honey, soap, tallow or horn impregnated with medicinal substances. In the late 18th century, cocoa butter was substituted as the primary base. The first record of the inclusion of an active pharmaceutical ingredient (API) in a suppository was in 1841 with the introduction of opium to cocoa butter. Suppositories during this time were approximately 5 g in size. In 1897, a combination of gelatin, glycerin and water was utilized for the first time. Fatty bases (triglycerides) were introduced as suppository bases during World War II due to shortages in cocoa butter. Following World War II, most commercial suppositories continued to be comprised of these hard fats as their primary bases. Also, during this time, the size of the suppositories was reduced to ∼2 g. Today, suppositories on the commercial market continue to adhere to these general product characteristics. The large majority of suppositories on the market are utilized for topical relief (paracetamol and caffeine [3]) or for incontinence (bisacodyl, lactulose and glycerin [3]). However, the US FDA lists several other drug products which are approved for rectal administration to treat ulcerative colitis (mesalamine, hydrocortisone) [3] and as an antipsychotic drug (prochlorperazine) [3]. For vaginal suppositories, the FDA lists compounds to treat vaginal yeast infections and bacterial vaginosis (clotrimazole, itraconazole) [4] and for hormone replacement therapies (progesterone) [4]. In Europe and Japan, several other drugs have been approved for rectal administration including products for epilepsy (diazepam) and pain (ibuprofen). In summary, the primary current approved uses of suppositories are laxative, analgesic, anti-inflammatory and antiemetic [5].
However, despite its age and current use today, the definition of a suppository is remarkably nonspecific. From the US, European and Japanese Pharmacopoeias (USP, EP and JP, respectively), only the EP has a specific chapter on rectal dosage forms [6]. The USP and JP only define a suppository as a dosage form adapted for application into the rectum [7,8]. And while more commonly used rectally, the JP does include vaginal administration into the monograph which defines a suppository. Thus suppositories are defined as a route of administration characterized by administration into the rectum or vagina to provide local or systemic effect [8]. Yet, in this open formulation space for suppository design, suppositories still primarily follow traditional designs and criteria rather than developing systematic and rationally designed formulations. Therefore, it is under these open guidelines that the development of suppositories into viable antiviral drug delivery dosage forms is being conducted today.
The suppository dosage form
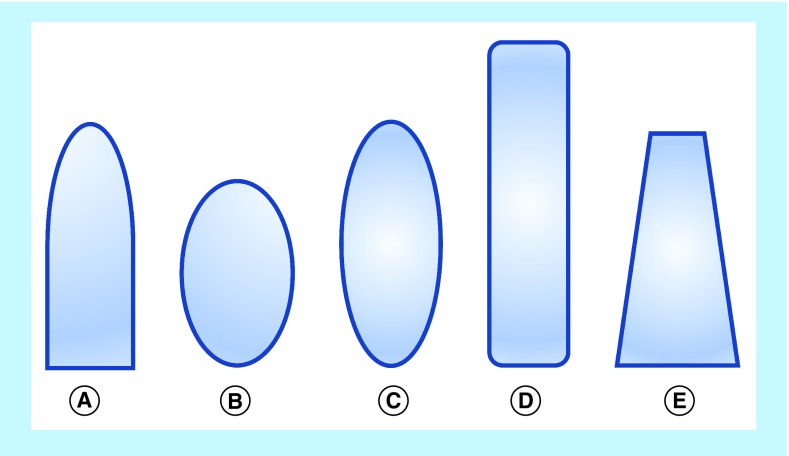
Suppositories have classically been cylindrical in geometry, longer than wide, with the most common shape being the ‘bullet’ or ‘torpedo’ shape. However, other commonly used shapes for suppositories include round and elongated ovals, tampon and ‘teardrop’ or ‘cone’ (Figure 1). These suppositories can be composed of, but not limited to, cocoa butter, coconut oil, glycerinated gelatin, hydrogenated vegetable oils and hard fats, polyethylene glycols (PEGs) and fatty acid esters of PEG. With a combination of these excipient bases, suppositories have fallen into one of two major types: lipophilic based or hydrophilic based. The lipophilic fat-based suppositories melt at body temperature to release drug to the body. They readily solubilize typical insoluble small-molecule drugs and require no localized fluids to spread and release drug. Typically, such suppositories are ideal for the rectum where there is little available fluid in the lower large intestinal tract. The hydrophilic water-based suppositories are unaffected by body temperature and require water to dissolve the suppository and release the drug. In contrast to the fat-based suppositories, hydrophilic suppositories can more easily support drug delivery of soluble drug compounds and use the body fluids to dissolve the suppository to transport the drug into the body. Such water-based suppositories are appropriate for vaginal application where there is more local fluid. Therefore, these two different types of suppositories have allowed this dosage form to become increasingly specific for drug delivery.
Figure 1. . Typical suppository shapes.
(A) Bullet or torpedo; (B) round oval; (C) elongated oval; (D) tampon; (E) teardrop or cone.
Regardless of indication, such as constipation, yeast infection, pain relief, viral prevention or antiviral treatment, drug delivery from a suppository, like all topically applied dosage forms, occurs primarily by passive diffusion for both vaginal and rectal administration. The bioavailability of drugs following rectal administration can be somewhat unpredictable compared with vaginal application, because of interindividual variations and the venous drainage of the rectum. In general, the rate and extent of drug absorption is lower than the oral route, mainly due to the smaller surface area available for absorption.
The lower and middle rectal (inferior and middle hemorrhoidal) veins flow into the interior vena cava, therefore this blood goes directly to the heart and into the general circulation. In contrast, the upper rectal (superior hemorrhoidal) vein flows into the portal vein and, therefore, this blood passes through the liver before reaching the heart. This means that rectally delivered drug products can enter the general circulation either directly or by passing through the highly metabolizing liver. Drugs absorbed in the middle and lower part of the rectum will pass directly into the general circulation and avoid first-pass metabolism in the liver. Bioavailability from the upper part of the rectum will be low for certain drugs, as much will be metabolized by the liver during its first pass and only a proportion of the drug molecules (if they are of the high clearance type) will enter the general circulation intact.
Investigations have shown that avoiding first-pass metabolism through the liver is possible by keeping the dosage form, and thus the released drug, in the lower part of the rectum. Compared with the small intestine, this situation is very favorable as most gastrointestinal veins drain into the portal vein.
Vaginal administration is also well suited for systemic drug delivery as the vaginal tissue contains readily available blood vessels. Comprised of three layers, top epithelial layer, muscular coat and the tunica adventitia, the vaginal rugae and microridges on the epithelial surface permit the vagina to expand, increasing the surface area of the vagina and thus enhancing the drug absorption of vaginally administered products. This route of administration also offers similar advantages as rectal administration, such as avoiding first-pass metabolism, reduction in gastrointestinal and hepatic side effects and local targeting of drugs to the reproductive organs.
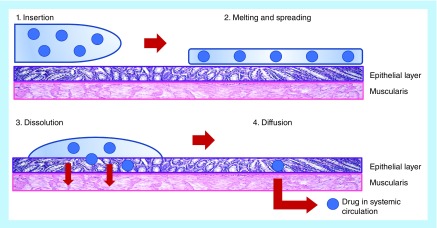
Therefore, despite the unique environments present in the vagina versus the rectum, insertion of a suppository results in the same chain of events leading to the absorption of the drug (Figure 2). A suppository will first dissolve in the fluid or melt on the mucous layer depending on whether it is hydrophilic or lipophilic. Due to osmotic effects of the dissolving vehicle, water is drawn to the rectum or vagina, and as the suppository dissolves or melts and spreads, drugs dissolved in the suppository will diffuse out toward the mucosal epithelial surfaces. Drugs suspended in the suppository base will first need to leave the vehicle (if it is water immiscible) under the influence of either gravity or ambulation and then begin solubilizing in fluid. For lipophilic melting suppositories, there is no need for fluid to be present to soften and spread the suppository. As the suppository liquifies under heat, the same drug transport observed in dissolving suppositories will occur in melting suppositories.
Figure 2. . Illustration of suppository delivery.
Suppositories as HIV prevention drug-delivery vehicles
In the field of HIV-prevention strategies, despite the implementation of the oral pre-exposure prophylaxis Truvada®, topical vaginal and rectal microbicides remain a significant arm in the antiretroviral therapy (ART)-based prevention pipeline. These topical delivery vehicles are being developed as strategies to provide additional affordable and effective options for at-risk populations. However, when considering topical drug delivery for both vaginal and rectal administration, the environment and population in which the product will act must be considered when choosing an appropriate dosage form. Therefore, a ‘one size fits all’ topical HIV prevention dosage form may not be possible. Several formulations may be necessary to accommodate the regional preferences and acceptability of end users. In addition to acknowledging user preferences, a successful topical ART requires the systematic integration of drug candidates with excipients in a scientifically rational way in order to produce a safe and stable product which serves to deliver the drug candidate efficiently to the appropriate target site at the appropriate and effective concentration. From the preformulation development, the appropriate dosage form is dependent upon factors such as the physicochemical characteristics and antiviral mechanism of action of the drug product under development.
Currently, there are several strategies being developed and investigated for vaginal and rectal topical antiviral drug delivery [9–17]. Semisolid dosage forms, or topical gels, are the most common products developed for vaginal and rectal delivery and several of these semisolid products have progressed to late phase clinical development, including tenofovir (TFV) gel [18], dapivirine (DPV) gel [17] and a dual vaginal and rectal IQP-0528 containing DuoGel [15,19]. Despite wide-spread use and the completion of many preclinical and clinical evaluations of gel formulations, the semisolid nature of the products result in leakage or general ‘messiness’ being a common problem experienced. Recently in the HIV microbicide VOICE trial [20], it was reported that while the efficacy of the TFV gel was high, adherence to gel use was significantly low, rendering the overall effectiveness of the gel product <40% [21–23]. Because of the low adherence rate, further development of semisolid gels as a topical antiviral drug delivery formulation has dramatically been deprioritized by funding organizations such as the NIH and the Bill and Melinda Gates Foundation. As such, solid dosage forms, including intravaginal rings and quick dissolving films, have been proposed as replacement formulations and continue to see development [14,24–28]. However, rings and films are not dosage forms that are an appropriate fit for rectal drug delivery.
To address the issues with adherence to semisolids and to also overcome the rectal limitations of other solid dosage forms, suppositories are being developed for anti-HIV drug delivery. Suppositories as a strategy in HIV prevention has several advantages over current and developing dosage forms. Administration of drugs through suppositories is compatible to both topical and systemic drug delivery. Suppositories have demonstrated the ability to deliver analgesics to local tissue as well as systemically deliver pain relief. Suppositories are often used as an alternative to oral administration to deliver drugs to patients that have difficulty swallowing or adhering to pills. Additionally, suppositories have pharmacokinetic advantages by increasing bioavailability to absorbable tissue, bypassing first pass metabolism, the ability to deliver high doses of drug and avoidance of irritation to the gastric mucosa. Suppositories also represent a dosage form that is suitable for both vaginal and rectal administration within a single dosage form without additional applicators, equipment or special storage conditions. The adaptable lipophilic/hydrophilic nature of suppository formulations allow for a greater number of antiviral drugs to be incorporated than current semisolid formulations. However, despite having several advantages, suppositories are not a popular route of administration for various obvious and less obvious reasons. Suppositories have varying levels of acceptability around the world depending upon geographic location and culture, and overall, they do not represent a large proportion of the available dosage forms [29,30].
In general, the acceptability and adherence to a product is a critical issue for all microbicide development. As such, understanding the qualitative and quantitative user sensory perception and experience data and how they influence formulation may be directed in identifying the most favorable characteristics desired by the targeted users to create an optimal topical antiviral product [31–35]. The lack of product efficacy in the VOICE and FACTS 001 trials of a vaginal gel delivering TFV attributed, in large part, to poor adherence by trial participants illustrate the importance of considering user sensory perception and experience data [21,23]. With the growing appreciation of the significant need to prevent rectal transmission events [10,36–38], acceptability and willingness to use rectal products must be considered in the formulation design. Simple translation of a vaginal formulation to a rectally applied product will likely not result in the development of a successful rectal delivery vehicle and product. Thus, while topical drug delivery is effective in preventing infection, adherence remains a great challenge. Improved dosage forms and regimens might mitigate this. Solid disintegrating vehicles such as suppositories occupy the design space between gels and rings and thus suppositories are well suited for both rectal and vaginal topical drug delivery. Despite being an ancient form of drug delivery, there has been little quantitative investigation in the design and development of suppositories when compared with every other dosage form. This review aims to investigate the challenges and current work in designing and developing suppositories for anti-HIV vaginal and rectal administration.
The challenges of the suppository as an anti-HIV drug-delivery vehicle
The primary challenge in suppository development for HIV prevention is overcoming the history of suppository shape and composition. Despite its long history of usage, the physical characteristics and composition of suppositories have gone without robust scientific evaluation or justification. From their inception, the most common shape for suppositories has been the ‘bullet’ or ‘torpedo’ shape. The size of suppositories has traditionally been 5 g until the mid-20th century when suppository sizes were reduced to 2 g for adults. For composition, suppositories have traditionally had lipophilic bases that melt at human body temperature. However, in the 19th century, in order to lower their sensitivity to higher ambient temperatures, hydrophilic excipients such as glycerin and gelatin were included. The addition of these water-based excipients, and water-based polymers later in the 20th century, also allowed the suppository to utilize body fluids to assist in drug delivery to the body.
Intuitive ease of insertion appears to be the driving factor in suppository shape, and a study into the modes of insertion initially suggested that the bullet or torpedo shape helped facilitate the device insertion and efficacy [39]. However, the results of this study have been challenged due to insufficient clinical data [40]. In the end, the size and shape of suppositories appear to have negligible impact on the effectiveness of drug delivery as several publications and the market itself have demonstrated that suppositories are capable of delivering a wide range of APIs to treat a wide range of ailments without challenging the current physical characteristics established for suppositories [5,41–45]. Yet, suppositories represent a very small portion of the drug market worldwide and there have been few investigations into the physical characteristics of suppositories on drug delivery.
Suppository use often provokes strong opinions in end users. Often, their popularity and overall acceptability is subject to regional or cultural attitudes and customs. Compared with the USA, the European markets show a much higher acceptability to overall suppository use and this is reflected in the greater number of products commercially available and translational research on APIs using suppositories as delivery vehicles. Therefore, it is perhaps unsurprising that the primary challenge of suppository development for antiviral drug delivery is not its effectiveness as a drug delivery vehicle but in their acceptability or ‘willingness to use’ the a product from the end users [46]. For suppositories, the most influential characteristic that affects acceptability would be their physical characteristics. Recently, studies into the shape of vaginal suppositories for a potential anti-HIV dosage form demonstrated that changes in shape influenced a woman's willingness to try the product. While maintaining the general proportions of the traditional bullet suppository, several other shapes were investigated including round ovals, long ovals, teardrop, and tampon shapes. The study concluded that the most favorable shapes were the bullet and long oval, but suggest this may be due simply to familiarity and not actual preferences. The study also showed that the shape of the suppository strongly influenced opinions on size and firmness even when volume and formulation remained constant. Depending on the shapes, the study participants identified specific sizes and firmness appropriate for each [47]. It is critical to note that this study only evaluated a willingness to try and not acceptability of the suppository dosage form; however, it does demonstrate an attempt at understanding how suppository shape influences potential acceptability.
With interest in suppositories as a dosage form for antiviral drug delivery increasing, it becomes important to address these issues of acceptability. For antiviral microbicides, it was shown that the acceptability of a product involves user characteristics and context along with dosage form characteristics [48]. In addition to shape, the color and odor [49], lubrication properties [50] and perception or other sensory issues with product placement in the vagina or rectum [51] have an impact on product acceptability. These studies demonstrate that the shape of suppositories is important to user acceptability and are quantifiable and suggest that a product can be designed to match overall increased acceptability instead of a product only for users predisposed to suppository use.
The traditional suppository includes lipophilic excipients that have a melting point at or below human body temperature. The most widely used fatty acid base is theobroma oil (cocoa butter). Theobroma oil is a triglyceride vegetable fat extracted from cocoa beans that is comprised primarily of palmitic acid, stearic acid and oleic acid. Theobroma oil is well tolerated by the rectal mucosa [52]. At typical room temperatures of 15–25°C (59–77°F), theobroma oil is a hard, amorphous solid, but at 30–35°C (86–95°F) (i.e., body temperature) it melts to a bland, nonirritating oil. Therefore, in warm climates, these suppositories are required to be stored under refrigeration. However, the most prominent disadvantage of theobroma oil is its polymorphism. Theobroma oil makes an ideal melting suppository base due to a melting point near human body temperature. In this state, theobroma oil is in its β-crystalline form. However, if the theobroma oil is overheated, the structure is converted to a much less stable β‘-crystal form with a melting point of 26°C. Therefore, the use of theobroma oil for suppositories must be carefully monitored. A secondary disadvantage of theobroma oil is its cost in production and worldwide availability. For these reasons, transition away from theobroma oil occurred during World War II when a lack of availability made the use of theobroma oil prohibitive. Today the cost of theobroma oil is high (and increasing) making the development of alternative triglycerides attractive.
As an alternative to theobroma oil, the development of synthetic triglycerides is influenced by changing economics. While they are currently more expensive than theobroma oil, they do not have the primary physicochemical disadvantage of theobroma oil – polymorphism. Therefore, they are often presented as meeting the melting characteristics of theobroma oil while eliminating the variability of polymorphism. For example, Fattibase, manufactured by Paddock Laboratories (Dublin, Ireland), is a preblended suppository base that consists of triglycerides from palm, palm kernel and coconut oils. Wecobee, manufactured by Stepan Company (IL USA), is a series of suppository bases derived from coconut oil triglycerides. The various types of Wecobee products have varying melting points designed for different applications. Witepsol, manufactured by Medisca, Inc. (Montreal, Canada), is another mixture of synthetic triglycerides with a melting point near human body temperature. These theobroma oil alternatives, among others such as Hydrokote (ABITEC; OH, USA) and Suppocire (Gattefosse; Saint-Priest, France), are all designed to address the variability of polymorphism observed with the use of theobroma oil.
The hydrophilic class of suppositories include excipients that dissolve in the presence of water. These include primarily glycerin, gelatin and water soluble polymers such as PEG. Suppositories based on glycerin are most known for use in the treatment of constipation. Glycerin is a water-soluble polyol compound that is hyperosmolar. Its mechanism as a suppository base is to draw moisture from the surrounding tissue to facilitate the dissolution of the suppository. As a result, gelatin suppositories dissolve slowly and provide long term release of active ingredients. While this action is beneficial when the desired effect is laxative, such hyperosmolar compounds prove problematic when formulating for antiviral prevention or treatment. The moisture drawn into the rectum or vagina via osmosis leaves the epithelial and underlying stromal tissue drier and susceptible to microscopic lacerations which may cause irritation and enhance infection by microorganisms. Additionally, glycerin in the vagina can lead to increased occurrences of yeast infections and possible bacterial vaginosis [53]. Gelatin or hydrogel-based suppositories have minimal effects on tissue osmolality so they do not draw additional fluid from the underlying mucous membrane [54]. This makes them useful for vaginal application where fluid is readily available for dissolving, but less so for rectal use where there is very little fluid available in the lower intestinal tract. Hydrogel-based suppositories are very hygroscopic and thus must be stored in sealed containers or packaging to prevent absorption of the atmospheric moisture and dissolve.
Water soluble polymers such as PEG also have properties suitable for both vaginal and rectal suppositories. They are chemically stable, nonirritating, miscible with water and mucous secretions, and widely available. Based upon molecular weight, PEG-based suppositories can be manufactured to exhibit a wide range of firmness, dissolving rates, and melting points. Essentially, as PEG chains increase, the firmness and melting points increase while the dissolution rate decreases. While possible to be used alone, suppositories are often a combination of varying PEG polymers. The characteristics of the suppository, including firmness, dissolving rate, melting point, are directly influenced by the exact combination and composition of PEG polymers used. Therefore, PEG offers a very design-oriented capability for suppository drug delivery. Since the water-based suppositories dissolve in body fluids and do not require body heat to melt the suppository, they can be formulated with much higher melting points to be used in environments with higher ambient temperatures.
The content of the suppository is not only influenced by the target environment, but is also influenced by the API to be delivered. For solid dosage forms, it is often preferred to suspend the intended API within a suppository base where it is not solubilized to facilitate more rapid drug release from the dosage form. Therefore, APIs that are Biopharmaceutic Classification System (BCS) Class I (high permeability, high solubility) are more suited for lipophilic-based suppositories. APIs that are BCS Class II (high permeability, low solubility) are more suited to hydrophilic-based suppositories [55]. In the field of antiviral drug development, particularly HIV drug development, the majority of existing and newly developing APIs are insoluble, small-molecular weight compounds [56,57]. Therefore, suppository development for antiviral drug delivery may begin with water-based dissolving suppositories for vaginal and rectal drug delivery.
Current suppository formulation strategies for antiviral drug delivery
Despite the long history of suppository use for both vaginal and rectal drug delivery, the development of suppositories for antiviral drug delivery is in its infancy. Numerous studies, particularly in the development of anti-HIV drugs, demonstrate the potential for both vaginal and rectal drug delivery to prevent and treat HIV infections [58]. So, it is not surprising that it is primarily a lack of user perception and acceptability rather than pharmaceutical feasibility that has limited wide spread development of the suppository as a drug delivery vehicle.
Current suppository development strategies primarily involve utilizing the available suppository dosage forms that exist on the market and integrating the antiviral APIs into them. However, there is little published research regarding the development of suppository-based drug delivery. Currently, the only published development is for a vaginal suppository containing Tenofovir delivered in carrageenan-based vaginal suppositories. Mimicking existing suppository formulations, the carrageenan-based vaginal suppositories showed efficacious release via diffusion and matrix erosion in water or by diffusion out of the matrix in vaginal fluid simulant and semen simulant fluid [59]. In the development of an antiviral rectal suppository, a positive response to rectal microbicides among the ‘men who have sex with men’ (MSM) community was reported, suggesting that rectal microbicides have a potential market in the MSM population and may have an important role in HIV/STI prevention [29,60]. Since the real challenge to ART suppositories may be social rather than pharmacological, further studies may be considered to combine clinical acceptability studies with clinical research together to understand the true feelings and perceptions of the MSM population when they use the products [29,30,61,62]. Additionally, acceptability studies confirm that the bullet and long oval shape are favored [47]. The firmness of suppositories also effected preferences with brittle suppositories being the more preferred [63].
Emerging suppository design & formulation
As research interest grows in formulating antiretroviral APIs into suppositories, it is not only important to understand the physical characteristics of what makes a suppository accepted, but the rate of drug release and pharmacokinetics are critical in developing a successful antiviral product. Therefore, emerging research has been focusing on the physicochemical characteristics of the dosage form into rationally design an ART suppository rather than incorporating current and novel API into existing formulation designs.
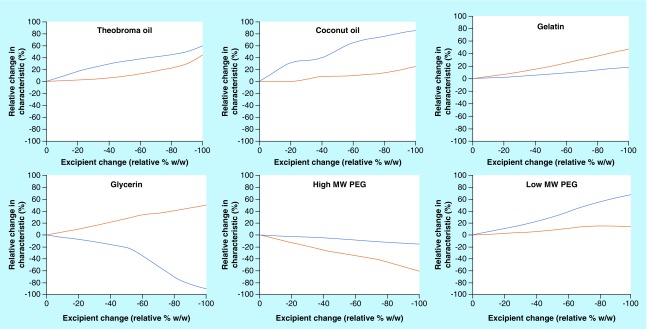
With solid dosage forms like suppositories, drug release and pharmacokinetics are strongly dependent upon the firmness and dissolving/melting rate which control spreading and drug release, respectively. In the current commercial market for suppositories as drug delivery vehicles, the pharmaceutical properties of rectal suppositories have not been critically investigated due to the high therapeutic indices of the APIs utilized (caffeine, glycerin, ibuprofen) for conditions that are not infectious in nature (pain relief, incontinence, anti-inflammatory). This is also true for drugs formulated in vaginal suppositories, where conditions unique to women (yeast infection, hormone replacement, vaginal candidiasis) have been treated with suppositories (clotrimazole, itraconazole, progesterone, miconazole). Therefore, to understand how suppositories can be effectively used to deliver ART drugs, understanding the role that the excipient has on drug release is critical. Recent studies have begun investigations on the effect of various hydrophilic and lipophilic excipients on the firmness and dissolving/melting rate of suppositories (Figure 3) [64]. From a starting formulation containing equal parts of several hydrophilic (high molecular weight [MW] PEG, low MW PEG, gelatin and glycerin) and lipophilic (theobroma oil and coconut oil) excipients, a baseline test formulation was investigated to measure how altering the relative concentrations of the excipient would affect the suppository firmness and disintegration (dissolving/melting rate). As the amount of theobroma oil was reduced from the baseline suppository formulation to 0% w/w, the firmness of the suppository increased by 40% and the disintegration rate increased by 60%. Therefore, the data suggest that as the relative amount theobroma oil is reduced in the formulation, the suppository will be firmer and will disintegrate faster. This trend is also observed with coconut oil, gelatin and low molecular weight PEGs where firmness increased by 20, 50 and 18%, respectively, and the disintegration rate increased by 85, 20 and 70%, respectively. Conversely for high molecular weight PEGs, as the amount of PEG is reduced from the baseline formulation to 0% w/w, the firmness of the suppository decreased by 60% and the disintegration rate decreased by 18%. Finally, as the amount of glycerin was reduced from the baseline suppository formulation to 0% w/w, a divergent behavior was observed where the firmness increased by 50% but the disintegration rate decreased by 90%. The results of this study indicate that the content and composition of the suppository excipients can have a significant impact of the physical properties of the suppository.
Figure 3. . Effects of excipient composition on base suppository firmness and disintegration.
From a base soluble suppository formulation, the relative composition (% w/w) of each excipient was varied while the other excipients remained at base concentrations. The changes in firmness and disintegration time relative to the base formulation was measured. X-axis is the relative change in excipient (% w/w). Y-axis is the relative change in characteristic (%): disintegration time (blue) and firmness (orange).
Despite being a solid dosage form, leakage from the vagina or rectum due to the suppository is a significant concern in user acceptability. The mechanism of drug delivery from a suppository essentially involves conversion of the solid to a semisolid followed by conversion to a liquid form as the suppository either dissolves or melts. Although liquification assists with the spread of the API throughout the vaginal or rectal canal, a consequence of this liquid state involves the potential for leakage. In vaginal administration, the volume of local vaginal fluid is relatively high depending on the individual and may result in higher leakage probabilities in some but not necessarily all women. Leakage has been reported by most women as a major acceptability concern. In product acceptability studies for cellulose sulfate vaginal gel and KY gel, 20% of the women in the study reported issues with leakage that would discourage future gel use [65–67]. Conversely, in rectal administration, semisolid fluid formulations resulted in a retrograde motion up the rectum [68,69]. While ostensibly helping drug delivery by allowing spread and greater coverage, the retrograde motion may spread the drug to the upper hemorrhoidal vein, subjecting the drug to potential first pass metabolism. Therefore, suppository development involving the incorporation of mucoadhesive excipients such as Carbopol (lubrizol) provides the melting/dissolving suppository with added yield-stress characteristics and thus the constituents of the suppository will only flow and spread under stresses. This would prevent leakage from the vagina due to gravity and avoid retrograde flow leading to first pass metabolism [70].
In HIV microbicide prevention strategies, systemic delivery is unimportant (and perhaps contraindicated), but API needs to be delivered to the topical epithelial and stromal tissue of the vagina and rectum where infection with HIV occurs. The active drug is required to be at the appropriate concentration and appropriate target tissue prior to virus introduction via semen. For rectal and vaginal formulations, this requires a balance between coverage (without leakage) and effective drug delivery. Mucoadhesive suppositories have been investigated to improve the delivery and bioavailability of drugs to the target tissue in the vagina and rectum [71]. These suppositories consist of a mucoadhesive front section comprised of a wax and a mucoadhesive back layer containing the API. The front layer limits leakage and spreading by anchoring the suppository to the area of administration and the terminal layer modulates drug release to ensure efficacious drug delivery is achieved.
For both systemic and topical use, drug delivery strategies resulting in sustained drug release have become increasingly important and it is thought that users would better adhere to delivery strategies which require less frequent dosing. Studies involving the development of sustained release suppositories have shown success with the incorporation of alginic acid to deliver prolonged doses of morphine in rabbits [72]. Also, the inclusion of high melting point solid fats such as bees wax has shown the ability to maintain plasma concentrations over significant periods of time [73]. These strategies may prove useful for the delivery of antiviral products to the vagina and rectum for prolonged drug delivery.
Future perspective
Despite significant advancements and continuous development in treatment strategies for antiviral preventative/treatment such as HIV, a successful optimized treatment continues to remain elusive. The current landscape of HIV development has shown that the current set of formulations has potential user adherence and compliance issues [20–23]. As a result, there is a critical need to develop new alternative HIV prevention strategies and therapies. Suppositories are being developed as an alternative prevention strategy to overcome the issues encountered in all previous topical pre-exposure prophylaxis dosage forms. While pharmacologically advantageous over other topical and oral formulations, suppositories have their own acceptability issues, which need to be addressed before a successful formulation can be developed. Little has been done to rationally develop the suppository as an optimal antiviral formulation, therefore future research into the understanding dosage form characteristics and their effect on drug delivery kinetics and user acceptability is critical. Transitioning from ART to highly active antiretroviral therapies (HAART) may aid in adapting to the shifting and evolving field of antiviral prevention and treatment. However, HAART still have their own complications with multidrug-resistant virus strains, toxicity, drug–drug interactions, difficult treatment regimens and inadequate pharmacology [74–76]. Suppositories have shown adaptability in drug delivery in the past and may provide a dosage form that may be amenable to current and future HAART drug delivery strategies.
Conclusion
The landscape of the HIV prevention and treatment field is constantly changing as new data emerge. As such the field needs to be able to adapt to those needs. Current development has moved away from traditional semisolid formulations such as gels and creams. Therefore, other dosage forms, such as solid suppositories have gained significant interest. However, in the antiviral research and development field, there has been little advancement in the field of suppository dosage forms. Suppositories as a drug delivery vehicle, while an established form, is a new formulation in the context of antiviral drug delivery. And while suppositories may be better known for their disadvantage than for advantages, understanding those disadvantages is key in overcoming them. The key disadvantage is willingness to use. By investigating the characteristics that users will prefer, a dosage form with the highest acceptability can be designed. Suppositories offer several advantages over most dosage forms due to their low cost, ease of administration and adaptable formulation form. By designing formulations for HIV/AIDS prevention and treatment instead of using classical forms, it is possible that suppositories may represent a successful formulation worldwide.
Executive summary.
Suppositories are an ancient dosage form that effectively delivers pharmaceutical products to the vagina and rectum and has remained remarkably unchanged over the centuries. Despite this, suppositories occupy a very small niche in the worldwide pharmaceutical market due to low user acceptance of the products.
The suppository dosage form
Traditionally cylindrical in shape, suppositories can be classified into two base types: lipophilic and hydrophilic. Lipophilic suppositories are primarily fat- or wax-based and melt at human body temperature. Hydrophilic suppositories are polymer or glycerin based and require fluids to dissolve.
Suppository drug delivery is via passive diffusion from the dosage form into the vaginal or rectal tissue.
The primary advantages of suppositories over other dosage forms include reduced first pass metabolism, both topical and systemic effect, accommodates patients who have difficulty swallowing pills, and increased bioavailability of drugs.
The primary disadvantage of suppositories is overcoming user perception and acceptance of the dosage form.
Suppositories as HIV prevention drug-delivery vehicles
Currently very little research on the design and development of suppositories as an antiviral vehicle for either topical or systemic drug delivery to the vagina or rectum has been performed. The growing focus and importance of disease prevention in the ‘men who have sex with men’ community has increased interest in rectally applied dosage forms, and new research into the design and development of antiretroviral therapeutic (ART) suppositories has emerged.
The challenges of the suppository as an anti-HIV drug-delivery vehicle
The shape and size of suppositories have remained largely unchanged over the years and have had little impact on improving the willingness of individuals to use the products. The shape of suppositories has classically been cylindrical in geometry, longer than wide, with the most common products utilizing ‘bullet’ or ‘torpedo’ shapes.
Similarly, the content of the suppository base has also seen little change and optimization over the years. Suppositories can be categorized as melting (lipophilic) or dissolving (hydrophilic) suppositories.
Current suppository formulation strategies for antiviral drug delivery
Currently there are no suppository products available for the prevention or treatment of viral infections such as HIV or other sexually transmitted organisms.
Emerging suppository design & formulation
Despite the long history of suppository use for both vaginal and rectal drug delivery, the development of suppositories for antiviral drug delivery remains in its infancy. While current suppository development strategies primarily utilize the pre-existing and available suppository dosage forms on the market with integration of the antiviral active pharmaceutical ingredients (APIs), emerging research has been focusing on the physicochemical characteristics of the dosage form to rationally design a new and novel ART suppository.
Emerging research into developing suppositories into ART drug delivery vehicles include: evaluation of the design space to better understand how the individual excipients affect API dissolution and pharmacokinetics, incorporation of mucoadhesive components to address leakage and confine delivery to specific areas of the vagina or rectum, mechanisms to improve delivery and bioavailability and the development of sustained release strategies.
Footnotes
Financial & competing interests disclosure
Portions of the work presented in this review were funded by the National Institutes of Health (NIH) grant number U19 AI101961. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Aiache JM, Renoux R, Fistre D, editors. History of the Suppository Form. J.R. Prous Publishers; Barcelona, Spain: 1984. [Google Scholar]
- 2.Shah SM, Sultan AH, Thakar R. The history and evolution of pessaries for pelvic organ prolapse. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006;17(2):170–175. doi: 10.1007/s00192-005-1313-6. [DOI] [PubMed] [Google Scholar]
- 3.FDA. U.S. Food and Drug Administration: Rectal Drugs. www.fda.gov 2017(03–01)
- 4.FDA. U.S. Food and Drug Administration: Vaginal Drugs. www.fda.gov 2017(4/20)
- 5.Jannin V, Lemagnen G, Gueroult P, Larrouture D, Tuleu C. Rectal route in the 21st Century to treat children. Adv. Drug. Deliv. Re.v. 2014;73:34–49. doi: 10.1016/j.addr.2014.05.012. [DOI] [PubMed] [Google Scholar]; •• A comprehensive review of suppositories for pediatric administration and the role of suppositories in the world market.
- 6.Pharmacopoeia E. European Directorate for the Quality of Medicines and Healthcare. Rectal Preparations EP 01/2008:1145. 2011 [Google Scholar]
- 7.Pharmacopoeia US. The U.S. Pharmacopoeia. Nomenclature Guidlines <1121>. 2016:36. [Google Scholar]
- 8.Pharmacopoeia J. The Japanese Pharmacopoeia: 17th Edition. Monogrpahs for Preparations. 2016:18–19. [Google Scholar]
- 9.Ham AS, Ugaonkar SR, Shi L, et al. Development of a combination microbicide gel formulation containing IQP-0528 and tenofovir for the prevention of HIV infection. J. Pharm. Sci. 2012;101(4):1423–1435. doi: 10.1002/jps.23026. [DOI] [PubMed] [Google Scholar]
- 10.Dezzutti CS, Rohan LC, Wang L, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J. Antimicrob. Chemother. 2012;67(9):2139–2142. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS ONE. 2010;5(2):e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akil A, Parniak M, Dezzutti C, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv. Transl. Res. 2011;1(3):209–222. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta KM, Pearce SM, Poursaid AE, et al. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J. Pharm. Sci. 2008;97(10):4228–4239. doi: 10.1002/jps.21331. [DOI] [PubMed] [Google Scholar]
- 14.Ham AS, Rohan LC, Boczar A, Yang L, Buckheit K, Buckheit RW., Jr Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm. Res. 2012 doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ham AS, Nugent ST, Peters JJ, et al. The rational design and development of a dual chamber vaginal/rectal microbicide gel formulation for HIV prevention. Antiviral. Res. 2015;120:153–164. doi: 10.1016/j.antiviral.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dezzutti CS, Yandura S, Wang L, et al. Pharmacodynamic activity of dapivirine and maraviroc single entity and combination topical gels for HIV-1 prevention. Pharm. Res. 2015;32(11):3768–3781. doi: 10.1007/s11095-015-1738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das Neves J, Martins JP, Sarmento B. Will dapivirine redeem the promises of anti-HIV microbicides? Overview of product design and clinical testing. Adv. Drug. Deliv. Rev. 2016;103:20–32. doi: 10.1016/j.addr.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Naicker N, Naidoo A, Werner L, et al. Efficacy and safety of tenofovir-containing antiretroviral therapy in women who acquired HIV while enrolled in tenofovir gel prophylaxis trials. Antivir. Ther. 2016 doi: 10.3851/IMP3106. [DOI] [PubMed] [Google Scholar]
- 19.Pereira LE, Mesquita PM, Ham A, et al. Pharmacokinetic and pharmacodynamic evaluation following vaginal application of IQB3002, a dual-chamber microbicide gel containing the nonnucleoside reverse transcriptase inhibitor IQP-0528 in Rhesus macaques. Antimicrob. Agents. Chemother. 2015;60(3):1393–1400. doi: 10.1128/AAC.02201-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees H, Delany-Moretlwe S, Baron D, et al. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. CROI. 2015 [Google Scholar]; • A report on the clinical trial of a vaginally administered microbicide in African women. The results illustrate the need to consider user acceptability in HIV prevention design.
- 22.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Der Straten A, Stadler J, Montgomery E, et al. Women's experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Hu M, Shi Y, et al. Vaginal microbicide film combinations of two reverse transcriptase inhibitors, EFdA and CSIC, for the prevention of HIV-1 sexual transmission. Pharm. Res. 2015;32(9):2960–2972. doi: 10.1007/s11095-015-1678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grammen C, Van Den Mooter G, Appeltans B, et al. Development and characterization of a solid dispersion film for the vaginal application of the anti-HIV microbicide UAMC01398. Int. J. Pharm. 2014;475(1–2):238–244. doi: 10.1016/j.ijpharm.2014.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Peitzmeier SM, Tomko C, Wingo E, et al. Acceptability of microbicidal vaginal rings and oral pre-exposure prophylaxis for HIV prevention among female sex workers in a high-prevalence US city. AIDS Care. 2017:1–5. doi: 10.1080/09540121.2017.1300628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark JT, Clark MR, Shelke NB, et al. Engineering a segmented dual-reservoir polyurethane intravaginal ring for simultaneous prevention of HIV transmission and unwanted pregnancy. PLoS ONE. 2014;9(3):e88509. doi: 10.1371/journal.pone.0088509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N. Engl. J. Med. 2016;375(22):2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pines HA, Gorbach PM, Weiss RE, et al. Acceptability of potential rectal microbicide delivery systems for HIV prevention: a randomized crossover trial. AIDS Behav. 2013;17(3):1002–1015. doi: 10.1007/s10461-012-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman PA, Cameron MP, Roungprakhon S, Tepjan S, Scarpa R. Acceptability and preferences for hypothetical rectal microbicides among a community sample of young men who have sex with men and transgender women in Thailand: a discrete choice experiment. AIDS Behav. 2016;20(11):2588–2601. doi: 10.1007/s10461-015-1258-9. [DOI] [PubMed] [Google Scholar]
- 31.Vargas SE, Fava JL, Severy L, et al. Psychometric properties and validity of a multi-dimensional risk perception scale developed in the context of a microbicide acceptability study. Arch. Sex. Behav. 2016;45(2):415–428. doi: 10.1007/s10508-015-0619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weld ED, Hiruy H, Guthrie KM, et al. A comparative pre-Phase I study of the impact of gel vehicle volume on distal colon distribution, user experience, and acceptability. AIDS. Res. Hum. Retroviruses. 2016;33(5):440–447. doi: 10.1089/aid.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrow KM, Underhill K, Van Den Berg JJ, Vargas S, Rosen RK, Katz DF. User-identified gel characteristics: a qualitative exploration of perceived product efficacy of topical vaginal microbicides. Arch. Sex. Behav. 2014;43(7):1459–1467. doi: 10.1007/s10508-013-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow K, Ruiz M. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12(2):272–283. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Exner TM, Correale J, Carballo–Diéguez A, et al. Women's anal sex practices: implications for formulation and promotion of a rectal microbicide. AIDS Education and Prevention. 2008;20(2):148–159. doi: 10.1521/aeap.2008.20.2.148. [DOI] [PubMed] [Google Scholar]
- 36.Mcgowan I, Dezzutti C. Rectal microbicide development. Curr. Top. Microbiol. Immunol. 2014;383:117–136. doi: 10.1007/82_2013_325. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A discussion on the need and current development of rectal microbicides to prevent HIV infection in the growing ‘men who have sex with men’ community.
- 37.Gorbach PM, Weiss RE, Fuchs E, et al. The slippery slope: lubricant use and rectal sexually transmitted infections: a newly identified risk. Sex. Transm. Dis. 2012;39(1):59–64. doi: 10.1097/OLQ.0b013e318235502b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mcgowan I. Rectal microbicides: can we make them and will people use them? AIDS Behav. 2011;15(0):66–71. doi: 10.1007/s10461-011-9899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abd-El-Maeboud KH, El-Naggar T, El-Hawi EMM, Mahmoud SaR, Abd-El-Hay S. Rectal suppository: commonsense and mode of insertion. Lancet. 1999;338(8770):798–800. doi: 10.1016/0140-6736(91)90676-g. [DOI] [PubMed] [Google Scholar]
- 40.Bradshaw A, Price L. Rectal suppository insertion: the reliability of the evidence as a basis for nursing practice. J. Clin. Nurs. 2007;16(1):98–103. doi: 10.1111/j.1365-2702.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 41.Saleem MA, Taher M, Sanaullah S, et al. Formulation and evaluation of tramadol hydrochloride rectal suppositories. Indian. J. Pharm. Sci. 2008;70(5):640–644. doi: 10.4103/0250-474X.45405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wada K, Uehara S, Ishii A, et al. A Phase II clinical trial evaluating the preventive effectiveness of lactobacillus vaginal suppositories in patients with recurrent cystitis. Acta. Med. Okayama. 2016;70(4):299–302. doi: 10.18926/AMO/54508. [DOI] [PubMed] [Google Scholar]
- 43.Naz S, Memon NY, Sattar A, Baloch R. Diclofenac rectal suppository: an effective modality for perineal pain. J. Pak. Med. Assoc. 2016;66(8):1005–1008. [PubMed] [Google Scholar]
- 44.Ye X, Yin H, Lu Y, Zhang H, Wang H. Evaluation of hydrogel suppositories for delivery of 5-aminolevulinic acid and hematoporphyrin monomethyl ether to rectal tumors. Molecules. 2016;21(10) doi: 10.3390/molecules21101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verdenelli MC, Cecchini C, Coman MM, et al. Impact of probiotic SYNBIO® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr. Microbiol. 2016;73(4):483–490. doi: 10.1007/s00284-016-1085-x. [DOI] [PubMed] [Google Scholar]
- 46.Lowry M. Rectal drug administration in adults: how, when, why. Nurs. Times. 2016;112(8):12–14. [PubMed] [Google Scholar]
- 47.Li B, Zaveri T, Ziegler GR, Hayes JE. Shape of vaginal suppositories affects willingness-to-try and preference. Antiviral. Res. 2013;97(3):280–284. doi: 10.1016/j.antiviral.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A study into determining the willingness to try a vaginal suppository in relation to the shape and size of the suppository.
- 48.Morrow KM, Hendrix C. Clinical evaluation of microbicide formulations. Antiviral Res. 2010;88:S40–S46. doi: 10.1016/j.antiviral.2010.09.008. Supplement(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrow K, Rosen R, Richter L, et al. The acceptability of an investigational vaginal microbicide, PRO 2000 gel, among women in a Phase I clinical trial. J. Women's Health. 2003;12(7):655–666. doi: 10.1089/154099903322404302. [DOI] [PubMed] [Google Scholar]
- 50.Whitehead SJ, Kilmarx PH, Blanchard K, et al. Acceptability of Carraguard vaginal gel use among Thai couples. AIDS. 2006;20(17):2141–2148. doi: 10.1097/QAD.0b013e32801086c9. [DOI] [PubMed] [Google Scholar]
- 51.Mahan ED, Morrow KM, Hayes JE. Quantitative perceptual differences among over-the-counter vaginal products using a standardized methodology: implications for microbicide development. Contraception. 2011;84(2):184–193. doi: 10.1016/j.contraception.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreton RC. Suppository Bases, Hard Fat. Pharmaceutical Press; PA, USA: 2012. pp. 945–950. [Google Scholar]
- 53.Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh M. Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet. Gynecol. 2013;121(4):773–780. doi: 10.1097/AOG.0b013e31828786f8. [DOI] [PubMed] [Google Scholar]
- 54.Porter TL, Stewart R, Reed J, Morton K. Models of hydrogel swelling with applications to hydration sensing. Sensors (Basel, Switzerland) 2007;7(9):1980–1991. doi: 10.3390/s7091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 56.Lane T, Pettifor A, Pascoe S, Fiamma A, Rees H. Heterosexual anal intercourse increases risk of HIV infection amoung young South African men. AIDS. 2006;20(1):123–125. doi: 10.1097/01.aids.0000198083.55078.02. [DOI] [PubMed] [Google Scholar]
- 57.Buckheit RW, Jr, Roberson JL, Lackman-Smith C, Wyatt JR, Vickers TA, Ecker DJ. Potent and specific inhibition of HIV envelope-mediated cell fusion and virus binding by G quartet-forming oligonucleotide (ISIS 5320) AIDS Res. Hum. Retroviruses. 1994;10(11):1497–1506. doi: 10.1089/aid.1994.10.1497. [DOI] [PubMed] [Google Scholar]
- 58.Buckheit R, Jr, Watson K, Morrow K, Ham A. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Res. 2010;85:142–158. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaveri T, Hayes JE, Ziegler GR. Release of tenofovir from carrageenan-based vaginal suppositories. Pharmaceutics. 2014;6(3):366–377. doi: 10.3390/pharmaceutics6030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang G, Zhang H, Yu H, Zhao Z, Yang J, Zhang M. Hypothetical rectal microbicide acceptability and factors influencing it among men who have sex with men in Tianjin, China. PLoS ONE. 2016;11(5):e0156561. doi: 10.1371/journal.pone.0156561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones DL, Weiss SM, Chitalu N, Bwalya V, Villar O. Acceptability of microbicidal surrogates among Zambian women. J. Sex. Transm. Dis. 2008;35(2):147–153. doi: 10.1097/OLQ.0b013e3181574dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cranston RD, Lama JR, Richardson BA, et al. MTN-017: a rectal Phase 2 extended safety and acceptability study of tenofovir reduced-glycerin 1% gel. Clin. Infect. Dis. 2017;64(5):614–620. doi: 10.1093/cid/ciw832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaveri T, Primrose RJ, Surapaneni L, Ziegler GR, Hayes JE. Firmness perception influences women's preferences for vaginal suppositories. Pharmaceutics. 2014;6(3):512–529. doi: 10.3390/pharmaceutics6030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ham AS, Nugent ST, Beveridge E, et al. HIV R4P. Chicago, IL, USA: October 17–21, 2016. Designing the suppository: a rational formulation design for rectal and vaginal administration. Presented at. [Google Scholar]
- 65.Holt BY, Morwitz VG, Ngo L, et al. Microbicide preference among young women in California. J. Women's Health. 2006;15(3):281–294. doi: 10.1089/jwh.2006.15.281. [DOI] [PubMed] [Google Scholar]
- 66.Malonza IM, Mirembe F, Nakabiito C, et al. Expanded Phase I safety and acceptability study of 6% cellulose sulfate vaginal gel. AIDS (London, England) 2005;19(18):2157–2163. doi: 10.1097/01.aids.0000194797.59046.8f. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz JL, Mauck C, Lai JJ, et al. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind Phase I safety study. Contraception. 2006;74(2):133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Cao YJ, Caffo BS, Fuchs EJ, et al. Quantification of the spatial distribution of rectally applied surrogates for microbicide and semen in colon with SPECT and magnetic resonance imaging. Br. J. Clin. Pharmacol. 2012;74(6):1013–1022. doi: 10.1111/j.1365-2125.2012.04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendrix CW, Fuchs EJ, Macura KJ, et al. Quantitative imaging and sigmoidoscopy to assess distribution of rectal microbicide surrogates. Clin. Pharmacol. Ther. 2008;83(1):97–105. doi: 10.1038/sj.clpt.6100236. [DOI] [PubMed] [Google Scholar]
- 70.Yahagi R, Machida Y, Onishi H. Mucoadhesive suppositories of ramosetron hydrochloride utilizing Carbopol. Int. J. Pharm. 2000;193(2):205–212. doi: 10.1016/s0378-5173(99)00338-5. [DOI] [PubMed] [Google Scholar]
- 71.Yahagi R, Onishi H, Machida Y. Preparation and evaluation of double-phased mucoadhesive suppositories of lidocaine utilizing Carbopol and white beeswax. J. Control. Rel. 1999;61(1–2):1–8. doi: 10.1016/s0168-3659(99)00111-x. [DOI] [PubMed] [Google Scholar]
- 72.Kawashima S, Inoue Y, Shimeno T, Fujiwara H. Studies on sustained-release suppositories. III. Rectal absorption of morphine in rabbits and prolongation of its absorption by alginic acid addition. Chem. Pharm. Bull. (Tokyo) 1990;38(2):498–505. doi: 10.1248/cpb.38.498. [DOI] [PubMed] [Google Scholar]
- 73.Takatori T, Shimono N, Higaki K, Kimura T. Evaluation of sustained release suppositories prepared with fatty base including solid fats with high melting points. Int. J. Pharm. 2004;278(2):275–282. doi: 10.1016/j.ijpharm.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 74.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283(3):381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 75.Richman DD. HIV therapeutics. Science. 1996;272(5270):1886–1888. doi: 10.1126/science.272.5270.1886. [DOI] [PubMed] [Google Scholar]
- 76.Trabattoni D, Lo Caputo S, Biasin M, et al. Modulation of human immunodeficiency virus (HIV)-specific immune response by using efavirenz, nelfinavir, and stavudine in a rescue therapy regimen for HIV-infected, drug-experienced patients. Clin. Diagn. Lab. Immunol. 2002;9(5):1114–1118. doi: 10.1128/CDLI.9.5.1114-1118.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]