Highlights
-
•
sLORETA analyses performed on 14 healthy adults at rest and during an arithmetic task.
-
•
Theta and alpha directed connectivity revealed ACC and left IPL as hubs during task.
-
•
Information flow between left IFG and STG suggested a feedback loop.
Keywords: Quantitative EEG, sLORETA, iCoh, Directional connectivity, Frontal midline theta, Attention network, Mental arithmetic, Fronto-parietal network, Directional flow, Attention task, Granger causality
Abstract
Objectives
The aim of this paper is to investigate cortical electric neuronal activity as an indicator of brain function, in a mental arithmetic task that requires sustained attention, as compared to the resting state condition. The two questions of interest are the cortical localization of different oscillatory activities, and the directional effective flow of oscillatory activity between regions of interest, in the task condition compared to resting state. In particular, theta and alpha activity are of interest here, due to their important role in attention processing.
Methods
We adapted mental arithmetic as an attention ask in this study. Eyes closed 61-channel EEG was recorded in 14 participants during resting and in a mental arithmetic task (“serial sevens subtraction”). Functional localization and connectivity analyses were based on cortical signals of electric neuronal activity estimated with sLORETA (standardized low resolution electromagnetic tomography). Functional localization was based on the comparison of the cortical distributions of the generators of oscillatory activity between task and resting conditions. Assessment of effective connectivity was based on the iCoh (isolated effective coherence) method, which provides an appropriate frequency decomposition of the directional flow of oscillatory activity between brain regions. Nine regions of interest comprising nodes from the dorsal and ventral attention networks were selected for the connectivity analysis.
Results
Cortical spectral density distribution comparing task minus rest showed significant activity increase in medial prefrontal areas and decreased activity in left parietal lobe for the theta band, and decreased activity in parietal-occipital regions for the alpha1 band. At a global level, connections among right hemispheric nodes were predominantly decreased during the task condition, while connections among left hemispheric nodes were predominantly increased. At more detailed level, decreased flow from right inferior frontal gyrus to anterior cingulate cortex for theta, and low and high alpha oscillations, and increased feedback (bidirectional flow) between left superior temporal gyrus and left inferior frontal gyrus, were observed during the arithmetic task.
Conclusions
Task related medial prefrontal increase in theta oscillations possibly corresponds to frontal midline theta, while parietal decreased alpha1 activity indicates the active role of this region in the numerical task. Task related decrease of intracortical right hemispheric connectivity support the notion that these nodes need to disengage from one another in order to not interfere with the ongoing numerical processing. The bidirectional feedback between left frontal-temporal-parietal regions in the arithmetic task is very likely to be related to attention network working memory function.
Significance
The methods of analysis and the results presented here will hopefully contribute to clarify the roles of the different EEG oscillations during sustained attention, both in terms of their functional localization and in terms of how they integrate brain function by supporting information flow between different cortical regions. The methodology presented here might be clinically relevant in evaluating abnormal attention function.
1. Introduction
High time resolution EEG recordings can provide complementary and new information about localized brain function and functional connectivity underlying cognitive processes that require sustained attention (see e.g. (Milz et al., 2014, Sauseng et al., 2007a)).
There are two well-known rhythms that have been extensively studied in this respect.
Alpha oscillations, which are prominent during resting, eyes closed conditions will tend to decrease in regions related to attention and cognitive processing (Pfurtscheller and Da Silva, 1999). The other outstanding signature of general sustained attention is the well-known frontal midline theta, typically abbreviated as FmT (Ishihara and Yoshi, 1972). Increased FmT has been found to be related not only to sustained attention but also to a very broad set of cognitive events, which nevertheless all require sustained attention (Mitchell et al., 2008). For instance, Cavanagh and Frank review FmT in its relation to general cognitive control mechanisms (Cavanagh and Frank, 2014), while Hsieh and Ranganath (2014) review its relation to working memory. In addition, there is evidence showing that FmT plays important roles in memory and emotion processing (Mitchell et al., 2008).
The importance of alpha and theta band activity during sustained attention tasks and their relation to cognitive memory performance has been reviewed and discussed (Clayton et al., 2015, Klimesch, 1999, Nishida et al., 2015).
Another important property of alpha and theta oscillations is that they are thought to support top-down processing, providing large scale integration across different cortical regions (Buzsáki and Wang, 2012, von Stein and Sarnthein, 2000). This property can be expressed, for instance, in the form of an inhibition of distant task-irrelevant regions by means of an increase of alpha activity (De Pesters et al., 2016).
Non-invasive scalp EEG recordings can be used for the computation of cortical electric neuronal activity by using sLORETA (Pascual-Marqui, 2002b). These high-time resolution signals of cortical activity provide information for studying frequency resolved functional localization and functional connectivity at the cortical level. In particular, effective direct and directional connectivity as a function of frequency will be assessed with a recently developed method known as iCoh (isolated effective coherence) (Pascual-Marqui et al., 2014), which has also been successfully applied in a recent functional magnetic resonance imaging (fMRI) study (Lin et al., 2015). The iCoh method has the advantage of providing a correct frequency decomposition of the flow of oscillatory activity between relevant brain regions. The “effective connectivity” nature of the information provided by the iCoh method is due to the fact that only “direct” paths of connections between pairs of regions are estimated, with the exclusion of indirect connection paths. Furthermore, iCoh provides two estimators for the strength of oscillatory information flow between each pair of regions: one for each direction, i.e., from region “A” to “B”, and distinctly from region “B” to “A”.
The question remains as to which cortical regions of interest (ROIs) should be used for the connectivity analyses to be carried out using the sLORETA-iCoh methodology. For this purpose, we can base our choice of ROIs on the patterns and dynamics of metabolic activity as reported in sustained attention tasks in fMRI studies. A large number of fMRI studies have shed light on the integrative aspect of brain function, resulting in the discovery of resting state networks such as the default mode network and the attention networks (Buckner et al., 2008). These studies have contributed to the understanding of the role of important brain regions during both resting and during sustained attention states.
Regarding the choice of a mental test that requires sustained attention, we selected a mental arithmetic task, in particular, the serial sevens subtraction task, where the subject is instructed to covertly count backwards starting at a relatively large number, in steps of seven (Manning, 1982, Rueckert et al., 1996, Smith, 1967). The serial sevens subtraction test is strongly motivated by its widespread use in a clinical setting, being part of the MMSE test (Folstein et al., 1975), specifically used for assessing attention. There are many published fMRI studies on brain activation for this simple task, and in general for many other numerical tasks, which have shown involvement of prefrontal and parietal regions (Dehaene et al., 2004, Rickard et al., 2000). Additionally, mental arithmetic does not require vocalization, which would produce large artifacts in the EEG recordings.
Regarding event related potentials, instead of state dependent EEG, Hinault and Lemaire (Hinault and Lemaire, 2016) published a recent review on the timing and elucidation of arithmetic strategies.
It is the aim of this paper to compare the electric neuronal activity of the cortex, based on the spatial analysis of EEG, in a mental arithmetic task that requires sustained attention to the resting state condition. The questions of interest are:
-
1.
The localizations (which cortical regions) of oscillatory activity (which frequencies) that are different when comparing task condition to the resting state condition.
-
2.
Given a set of cortical regions of interest, which directional effective connections, at which frequencies, are different when comparing task condition to the resting state condition.
It is expected that the results will clarify the roles of the different EEG oscillations during sustained attention, both in terms of their functional localization and in terms of how they integrate brain function by supporting information flow between different cortical regions.
2. Methods
2.1. Subjects
Fourteen healthy right handed participants with normal medical histories (male: 11, female: 3, mean age ±S.D. = 24.9 ± 3.4) enrolled through an advertisement placed at Kansai Medical University. Handedness was based on self-report of preferred hand use, and complemented with verification that the subjects had no childhood history of forced change from left to right. The Institutional Ethical Review Board of Kansai Medical University approved the study and written informed consent as required by the Helsinki Declaration was obtained from the participants. Five thousand yen of gift certificates were paid to each subject as travel cost.
2.2. EEG recordings
EEG was recorded from 61 scalp electrodes of the International 10/10 System, at 500 Hz sampling rate and band-pass filtered from 0.016 to 166 Hz (hardware implemented at the level of the analog-to digital conversion of the amplifier circuitry), using the EEG-1200 NIHON KOHDEN system (NIHON KOHDEN, Tokyo, Japan). Electrode impedances were kept below 20 KOhm, as recommended by the manufacturer of the high-impedance amplifiers.
The electrode names used were: Fp1, Fp2, F7, F8, F3, F4, FC5, FC6, FC1, FC2, T7/T3, T8/T4, C3, C4, CP5, CP6, CP1, CP2, P7/T5, P8/T6, P3, P4, O1, O2, AF7, AF8, AF3, AF4, F5, F6, F1, F2, FC3, FC4, C5, C6, C1, C2, CP3, CP4, P5, P6, P1, P2, PO3, PO4, FT7, FT8, TP7, TP8, PO7, PO8, AFz, Fpz, Fz, Cz, Pz, POz, Oz, CPz, and FCz. The physical reference for the recordings was linked earlobes. However, all analyses performed throughout are reference-invariant, since the effect of the reference electrode is appropriately modeled and accounted for in the analyses, and eliminated as a nuisance parameter (Pascual-Marqui, 2002a, Pascual-Marqui, 2002b).
Eyes closed EEG was recorded under two conditions, as described in detail below: resting state and a mental arithmetic task. For each condition, artifact free EEG epochs were selected for further analysis. Artifact detection and removal used a subset of features described in Nolan et al. (2010), plus additional measures. In a first step, non-re-referenced recordings were FFT filtered to the 1.5–30 Hz band. Next, for each channel, medians and median absolute deviations were computed for amplitude, skewness, kurtosis, and spectral power in delta, alpha, and beta bands. Next, for all electrodes, global field power (Lehmann and Skrandies, 1980). Median and median absolute deviations were calculated. Robust z-scores, based on medians and median absolute deviations (Daszykowski et al., 2007), were then calculated and used to detect possible artifacts. This method simply marked EEG epochs as possible outliers, which were then visually inspected for final approval (rejection as true positive, or acceptance as false positive).
All recordings of EEG were performed before noon in order to standardize vigilance of participants. Participants were asked to sit on chair comfortably in a dimmed room during all recordings of EEG.
2.3. Recording conditions
EEG data was recorded in two conditions (Resting state and Arithmetic task). At first, spontaneous resting EEG with eyes closed was recorded for two minutes in all participants. After that, participants were instructed to perform the arithmetic task; two minutes of EEG with eyes closed during the mental and covert serial subtraction in steps of seven from five hundred. This task is called “serial sevens”, which is the one of most popular and studied tests (Manning, 1982, Smith, 1967).
2.4. Cortical signals of electric neuronal activity with sLORETA
The artifact free EEG recordings are used for computing the time varying cortical distribution of electric neuronal activity, by means of sLORETA (Pascual-Marqui, 2002b). This method provides time varying images of appropriately standardized current density values on 6239 cortical grey matter voxels, sampled on a 5 mm resolution grid, using the MNI152 anatomical template (Fuchs et al., 2002, Mazziotta et al., 2001). sLORETA has received both theoretical (Greenblatt et al., 2005, Sekihara et al., 2005) and experimental validation regarding its main property of correct localization (Pascual-Marqui et al., 2009), and sLORETA analyses were carried out with the free academic software package available at http://www.uzh.ch/keyinst/loreta.htm.
2.4.1. Functional localization of oscillatory activity specific to different brain conditions
Images for the electric neuronal generators of oscillatory activity corresponding to a set of frequency bands of interest were computed.
The image corresponding to the generation of alpha activity (8–12 Hz, for instance) is obtained as follows. First, the EEG recordings are FFT filtered to the alpha frequency band. Next, for each time sample of the filtered EEG recording, sLORETA is applied, and the image of squared current density values (i.e. instantaneous power) is computed. Finally, the average image across all time samples of squared current density values corresponds to the generators of the oscillatory activity (in this example being the alpha generators). In practice, equivalent results are obtained in a much more efficient manner by computations in the frequency domain, as explained in detail in Frei et al. (2001).
Note that these images correspond to the spectral density of the current density time series at each of the 6239 voxels. These images serve as the basis for the comparisons between different brain states.
All analyses were performed for the following three frequency bands, defined as theta (4.0–8.0 Hz), alpha 1 (8.0–10.0 Hz), and alpha 2 (10.0–12.0 Hz). The subdivision of the broad alpha band into low and high alpha sub-bands was obtained from factor analysis of EEG spectra (Herrmann et al., 1979, Kubicki et al., 1979, Shackman et al., 2010). Further validation for the functional differentiation of the alpha subbands were found in a simultaneous EEG-fMRI study (Jann et al., 2009).
2.4.2. Frequency decomposition of causal effective connectivity between nodes of the attention networks
Multiple time series of electric cortical activity can be used for assessing the properties of a cortical network: the strength, directionality, and spectral characteristics (i.e., which oscillations are preferentially transmitted) of the connections. In this study, causal effective connectivity as a function of frequency was computed by means of the isolated effective coherence (iCoh) (Pascual-Marqui et al., 2014).
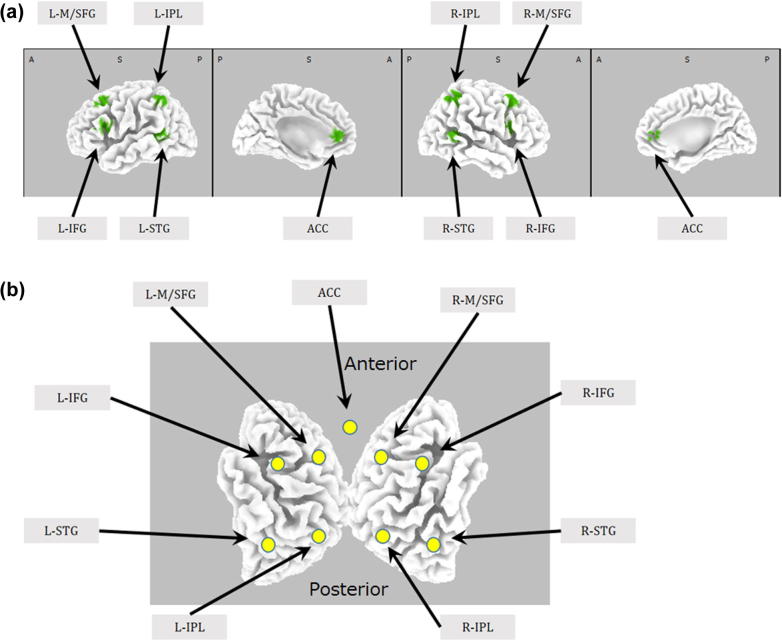
Based on the nature of the brain states studied here, nine regions of interest (ROIs) were selected, comprising the following nodes from the dorsal and ventral attention networks (Corbetta and Shulman, 2002, Fox et al., 2006): right and left Middle/Superior Frontal Gyrus (M/SFG), right and left Inferior Parietal Lobe (IPL), right and left Inferior Frontal Gyrus (IFG), right and left Superior Temporal Gyrus (STG), and Anterior Cingulate Cortex (ACC). Table 1 lists the names, abbreviations, and MNI-coordinates of the regions of interest, and Fig. 1 shows their anatomical locations. Current density (i.e. cortical electric neuronal activity) time series using sLORETA, at the coordinates reported in Table 1, were used for connectivity analyses, which means that each region of interest consisted of a single voxel. However, due to the low spatial resolution of sLORETA, each time series is actually a local spatial average, which in turn implies that the signals correspond to a small volume surrounding the single voxel. Technical details and quantification of the spatial spread of sLORETA signals are presented in an earlier paper (Pascual-Marqui, 2002b).
Table 1.
Regions of interest used in the connectivity analysis.
| Region | Abbreviation | Brodmann area | MNI coordinates (mm) |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Anterior Cingulate Cortex | ACC | 32 | 0 | 45 | 10 |
| L-Superior Temporal Gyrus | L-STG | 22 | −55 | −50 | 10 |
| R-Superior Temporal Gyrus | R-STG | 22 | 55 | −50 | 10 |
| L-Inferior Frontal Gyrus | L-IFG | 44 | −50 | 15 | 20 |
| R-Inferior Frontal Gyrus | R-IFG | 44 | 50 | 15 | 20 |
| L-Inferior Parietal Lobe | L-IPL | 40 | −45 | −50 | 55 |
| R-Inferior Parietal Lobe | R-IPL | 40 | 45 | −50 | 55 |
| L-Middle/Superior Frontal Gyrus | L-M/SFG | 8 | −40 | 20 | 50 |
| R-Middle/Superior Frontal Gyrus | R-M/SFG | 8 | 40 | 20 | 55 |
L: left, R: right.
Fig. 1.
Anatomical and schematic locations of the 9 Regions of Interest (ROI) used in the connectivity analysis. The abbreviations for the anatomical names are described in Table 1. a) Anatomical locations of the ROIs. b) Schematic representation of the ROIs.
Connectivity analysis for these nine regions, using the iCoh method, consisted of computing a total of 9 × 8 × 3 = 216 connectivity strength values, for each subject, for each condition, corresponding to all direction-specific pairs of regions (9 × 8 = 72), and to each of three frequency bands previously described (i.e. theta, alpha 1 and alpha 2).
2.4.3. Statistical methods of analysis
In general, all statistical tests are carried out based on the method of non-parametric randomization of the maximum-statistic, which has the advantage of correcting for multiple testing, and of not relying on Gaussianity (Blair and Karniski, 1994, Karniski et al., 1994, Nichols, 2012, Nichols and Holmes, 2002).
A brief description of the non-parametric randomization method follows. Further technical details can found in the literature, see e.g. (Nichols and Holmes, 2002). Consider, for instance, the data represented as , consisting of variables, measured on subjects, under two conditions and . Note that there are two distinct cases regarding the variables. In one case, the tests of interest involve the comparison of spectral power of cortical current density, thus producing R = 6239 × 3 variables, corresponding to 6239 cortical voxels, for three frequency bands (theta, alpha1 and alpha2). In a second case, the tests of interest involve the comparison of frequency dependent directed connectivity strengths (i.e. strength of directed information flow) between each distinct pair of regions of interest, thus producing R = (9 × 8) × 3 variables, corresponding to (9 × 8) distinct directed connections between nine ROIs, for three frequency bands (theta, alpha1, and alpha2).
The aim is the discovery of the variables (e.g. spectral power or connection strength) that are significantly different between the two conditions. For this purpose, the simple variable-by-variable t-statistic can be used as a statistical measure of “distance” between the two conditions, although other choices of statistics may also be used. From the set of “R” t-statistics (one for each variable), the absolute maximum is chosen. Then its empirical probability distribution is estimated by repeatedly randomizing the conditions “c”, and recalculation the maximum-t’s under the null hypothesis of “no difference between conditions”. This empirical probability gives the threshold with correction for multiple (“R” tests) testing, as explained in (Nichols and Holmes, 2002). The correction is exact (in the sense of Fisher’s exact test) for a large number of randomizations, regardless of the original probability distribution of the variables.
In the case of voxel-by-voxel statistics for functional localization, global subject-wise scaling (i.e. normalization) was performed prior to comparisons between conditions, in order to eliminate a source of non-relevant (i.e. non-physiological) variability typical of EEG spectral power. In particular, for each subject and each condition separately, the normalization factor is defined as the average spectral power across all voxels (6239) and frequency bands (three). A detailed description of different forms of image normalization methods typically used in functional localization studies can be found in (Frackowiak et al., 2004).
A second form of statistical test is based on the exceedance proportion, i.e. the number of variables (voxels or connections) above a certain threshold (Friston et al., 1990, Friston et al., 1991, Petersson et al., 1999). The non-parametric randomization methodology previously described can be directly applied in this case, providing the probability distribution for the proportions. This type of test is suitable for detecting sets of connections that jointly form functionally relevant networks. All results reported throughout this study are significant at the p = .05 level.
3. Results
3.1. Functional localization of oscillatory activity for mental arithmetic task compared to resting state
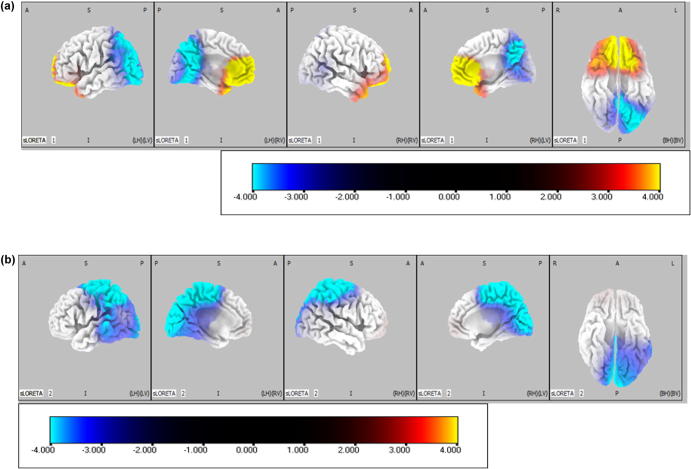
Cortical theta band oscillation, given by the spectral power of the cortical current density, was significantly increased in medial prefrontal areas and decreased at the left parietal lobe in the arithmetic task compared to resting state (Fig. 2a). In addition, alpha1 band oscillation was decreased in parietal occipital lobes in arithmetic task compared to resting state (Fig. 2b). There was no significant difference of current density between arithmetic task and resting state in alpha 2 band.
Fig. 2.
Statistical comparison of current density images: arithmetic task minus resting state. Warm colored areas (yellow) correspond to significantly (p = .05) higher activity under the arithmetic task, and cold colored areas (cyan) correspond to significantly (p = .05) higher activity under the resting state. A: anterior. S: superior. P: posterior. I: inferior. R: right. L: left. H: hemisphere. V: view. B: bottom. The color scale corresponds to t-values corrected for multiple testing by means of non-parametric randomization of the maximum-statistics. a) Theta band. b) Alpha1 band. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Effective connectivity based on iCoh
The strength of effective directional connectivity (i.e. of information flow), as a function of frequency, was assessed with the isolated effective coherence (iCoh), applied to estimated signals of cortical current density. A summarized description of some noteworthy features will be described below. Statistical significance is reported using corrected p-values based on non-parametric randomization for exceedance proportion tests (Friston et al., 1990, Friston et al., 1991).
3.2.1. Mental arithmetic task compared to resting state
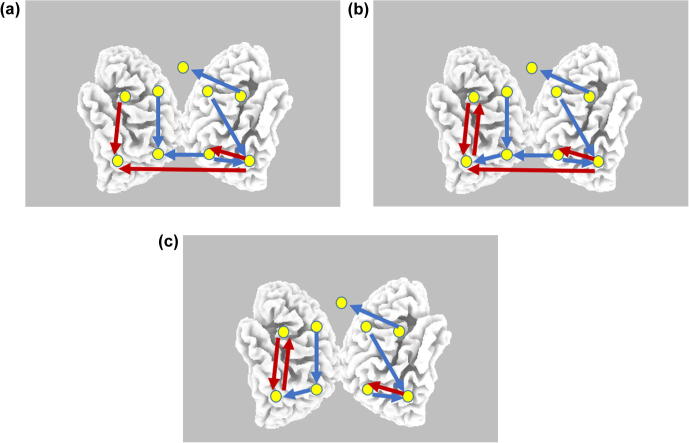
The statistical comparison of connections between task and resting states show a complex pattern, with many significant increases and decreases in task-related information flow (Fig. 3a, b and c). In Fig. 3, where only statistically significant connectivity changes are displayed, red arrows correspond to task-related connectivity increase (equivalent to a resting state connectivity decrease), and blue arrows correspond to task-related connectivity decrease (equivalent to a resting state connectivity increase). Referring to Fig. 3, a general observation is that there are more significant decreased connections (blue arrows) within the right hemisphere, as compared to the left hemisphere within which there are more significant increased connections (red arrows).
Fig. 3.
Statistical comparison of oscillatory information flow obtained with the isolated effective coherence (iCoh) measure: arithmetic task minus resting state. Red arrows correspond to task-related connectivity increase (equivalent to a resting state connectivity decrease), and blue arrows correspond to task-related connectivity decrease (equivalent to a resting state connectivity increase). All displayed arrows are significant at p < .05, with correction for multiple testing by means of non-parametric randomization of the exceedance proportion test. a) Theta band. b) Alpha1 band. c) Alpha2 band. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Some particular connectivity change of note during the arithmetic task are:
-
1.
Decreased flow from right IFG area to ACC for theta, and low and high alpha oscillations.
-
2.
Increased feedback (bidirectional) between left STG and left IFG regions.
4. Discussion
In terms of functional localization, oscillatory theta activity in the arithmetic task was significantly increased in medial prefrontal gyrus, including the ACC, which seems to correspond to the well documented FmT. FmT activity, consisting of frontal scalp electric potential waves in the 4–8 Hz frequency band, has been observed in healthy subjects during focused attention states (Ishihara and Yoshi, 1972, Mitchell et al., 2008). Based on MEG recordings, other studies have also found the sources of FmT during mental calculation tasks to be located in medial prefrontal cortex, including the ACC (Ishii et al., 2013, Ishii et al., 2014, Ishii et al., 1999). A previous LORETA study during sustained attention also found the theta oscillations to be generated in the ACC (Sauseng et al., 2007a).
In addition, our results show a task related left parietal decrease of theta band oscillations, and decrease of alpha1 band oscillations in bilateral parietal cortex. These areas are known to actively participate in calculation tasks (Rickard et al., 2000).
There is no clear interpretation for the decrease of theta oscillations in left parietal cortex. However, one plausible simple explanation would be to assume that a localized theta decrease indicates suppressed localized attention function, allowing for other non-attentional aspects of cognitive processing. This line of reasoning might find justification in the fact that the left angular gyrus plays an essential role in verbal number manipulations (Dehaene et al., 2003).
On the other hand, alpha1 activity decrease of the parietal cortex is most likely due to the active excitatory participation of these regions in the arithmetic task. This interpretation is in line with the inhibitory role of alpha oscillations (De Pesters et al., 2016, Klimesch et al., 2007, Pfurtscheller and Da Silva, 1999).
In terms of information flow between the regions of interest, a complex pattern of task related changes was observed. On a global level, considering intra-hemispheric connections, there were more significant decreased connections (blue arrows) within the right hemisphere, as compared to the left hemisphere within which there were more significant increased connections (red arrows) (Fig. 3). The increased connectivity within left cortical regions seems to be in agreement with the view that the numerical task requires the coordinated use of language areas, while the decreased connectivity within the right hemisphere might indicate that these regions need to be disengaged from one another in order to not interfere with the ongoing numerical processing. A very related, recent MEG paper also presents results that agree with our own regarding left hemisphere activation (Iijima and Nishitani, 2017).
One particular result was the observed task related decrease of input to the ACC from right IFG, for all frequency bands tested. It is well known that the ACC is involved in working memory tasks, which is an essential function in numerical tasks (Inzlicht et al., 2015, Lenartowicz and McIntosh, 2005). However, it has been shown that depending on the nature of the task, the connection between ACC and other regions change, i.e. some connections actually are decreased in one task relative to the other task (Lenartowicz and McIntosh, 2005).
The most notable particular connectivity result is the increased feedback between left STG and IFG regions. These two areas have multiple functions that integrate a diversity of cognitive tasks. In particular, they participate in networks that play essential roles in attention (Womelsdorf and Everling, 2015) and verbal processing (Skeide and Friederici, 2016). All these processes are active during the mental arithmetic task. For instance, as reviewed by Dehaene and Cohen (2007), these two tightly connected regions found in our study correspond to the two main regions labeled as essential for “language and calculation”. The increased feedback observed between IFG and STG regions is likely due to the functional interaction of Wernicke’s area which interprets and stores the numbers, with the IFG which produces the number as language.
According to the diagram proposed by Dehaene et al., their Fig. 1 therein, the interconnections within the left hemisphere are essential in numerical tasks (Dehaene et al., 2004). Our results are in partial agreement with this diagram. However, due to the detailed nature of our connectivity results, we have an additional detail to contribute to this diagram: not only is there a connection from left STG to left IFG area, but also a return connection from IFG to STG.
The flow from left STG area to left IFG area in alpha band is in the direction of posterior to anterior regions, which is a typical bottom-up flow (Buschman and Miller, 2007, Engel et al., 2001). The combined functional interactions between the left IFG area and left STG area are in correspondence with the attention network, as described in the fMRI resting state network literature (Vossel et al., 2014), which is ubiquitous in concentration and in performing cognitive task. Thus, the regions where we obtained significant results are well supported by fMRI studies.
Finally, it is worthy pointing out that the EEG-based connectivity methods used here not only compliment the connectivity methods typically used in fMRI, but actually provide additional spectral information.
This study has some limitations. First, the sample of participants is relatively small and biased, not being diverse, for instance, in terms of sex and age range.
With respect to vigilance control, we did not control and standardize the hours of sleep, bedtime and wake-up time of subjects whereas we standardize time for recording the EEG. In addition, most importantly, we could not control for how many times the subjects made subtractions in two minutes during the arithmetic task.
5. Conclusions
Our results show that frontal regions (especially the ACC), and the parietal lobe (particularly left IPL) are essential in this task, in agreement with previous fMRI studies. However, it remains unclear if this result is specific to mental arithmetic, or general to attentional/cognitive processing. In addition, the analysis of neurophysiological information flow demonstrated a disconnection of the ACC, while an increased bidirectional effective connectivity between left IFG and left STG was observed, thus establishing a feedback loop.
The roles of localized electric neuronal activity and the directional flow of information are important in describing and understanding the attentional state, which in this case was studied in a mental arithmetic task.
Conflicts of interest
None of the authors had any conflict of interest.
Acknowledgments
All authors contributed to and have approved the final manuscript. This project was supported by the Eli Lilly Japan K.K, and partially supported by The Center of Innovation (COI) Program of the Japan Science and Technology Agency (JST), Japan. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Blair R.C., Karniski W. Academic Press; San Diego: 1994. Distribution-Free Statistical Analyses of Surface and Volumetric Maps. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network. Ann. N. Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buschman T.J., Miller E.K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Wang X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012;35:203. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton M.S., Yeung N., Kadosh R.C. The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 2015;19(4):188–195. doi: 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Daszykowski M., Kaczmarek K., Vander Heyden Y., Walczak B. Robust statistics in data analysis–a review: basic concepts. Chemom. Intell. Lab. Syst. 2007;85(2):203–219. [Google Scholar]
- De Pesters A., Coon W., Brunner P., Gunduz A., Ritaccio A., Brunet N., de Weerd P., Roberts M., Oostenveld R., Fries P. Alpha power indexes task-related networks on large and small scales: a multimodal ECoG study in humans and a non-human primate. Neuroimage. 2016;134:122–131. doi: 10.1016/j.neuroimage.2016.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56(2):384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Molko N., Cohen L., Wilson A.J. Arithmetic and the brain. Curr. Opin. Neurobiol. 2004;14(2):218–224. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Piazza M., Pinel P., Cohen L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003;20(3–6):487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat. Rev. Neurosci. 2001;2(10):704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak R.S., Friston K.J., Frith C.D., Dolan R.J., Price C.J., Zeki S., Ashburner J.T., Penny W.D. Academic Press; USA: 2004. Human Brain Function. [Google Scholar]
- Frei E., Gamma A., Pascual-Marqui R., Lehmann D., Hell D., Vollenweider F.X. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA) Hum. Brain Mapp. 2001;14(3):152–165. doi: 10.1002/hbm.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K., Frith C., Liddle P., Dolan R., Lammertsma A., Frackowiak R. The relationship between global and local changes in PET scans. J. Cereb. Blood Flow Metab. 1990;10(4):458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C., Liddle P., Frackowiak R. Comparing functional (PET) images: the assessment of significant change. J. Cereb. Blood Flow Metab. 1991;11(4):690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Fuchs M., Kastner J., Wagner M., Hawes S., Ebersole J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002;113(5):702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt R.E., Ossadtchi A., Pflieger M.E. Local linear estimators for the bioelectromagnetic inverse problem. IEEE Trans. Signal Process. 2005;53(9):3403–3412. [Google Scholar]
- Herrmann W., Fichte K., Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatr. Neuropsychopharmakol. 1979;12(02):237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- Hinault T., Lemaire P. What does EEG tell us about arithmetic strategies? A review. Int. J. Psychophysiol. 2016;106:115–126. doi: 10.1016/j.ijpsycho.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Hsieh L.T., Ranganath C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage. 2014;85:721–729. doi: 10.1016/j.neuroimage.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M., Nishitani N. Cortical dynamics during simple calculation processes: a magnetoencephalography study. Clin. Neurophysiol. Pract. 2017;2:54–61. doi: 10.1016/j.cnp.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M., Bartholow B.D., Hirsh J.B. Emotional foundations of cognitive control. Trends Cogn. Sci. 2015;19(3):126–132. doi: 10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Yoshi N. Multivariate analytic study of EEG and mental activity in juvenile delinquents. Electroencephalogr. Clin. Neurophysiol. 1972;33(1):71–80. doi: 10.1016/0013-4694(72)90026-0. [DOI] [PubMed] [Google Scholar]
- Ishii R., Canuet L., Aoki Y., Ikeda S., Hata M., Iwase M., Takeda M. Non-parametric permutation thresholding for adaptive nonlinear beamformer analysis on MEG revealed oscillatory neuronal dynamics in human brain. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013;2013:4807–4810. doi: 10.1109/EMBC.2013.6610623. [DOI] [PubMed] [Google Scholar]
- Ishii R., Canuet L., Ishihara T., Aoki Y., Ikeda S., Hata M., Katsimichas T., Gunji A., Takahashi H., Nakahachi T. Frontal midline theta rhythm and gamma power changes during focused attention on mental calculation: an MEG beamformer analysis. Front. Hum. Neurosci. 2014;8:406. doi: 10.3389/fnhum.2014.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R., Shinosaki K., Ukai S., Inouye T., Ishihara T., Yoshimine T., Hirabuki N., Asada H., Kihara T., Robinson S.E., Takeda M. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10(4):675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Jann K., Dierks T., Boesch C., Kottlow M., Strik W., Koenig T. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. Neuroimage. 2009;45(3):903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Karniski W., Blair R.C., Snider A.D. An exact statistical method for comparing topographic maps, with any number of subjects and electrodes. Brain Topogr. 1994;6(3):203–210. doi: 10.1007/BF01187710. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29(2):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res. Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kubicki S., Herrmann W.M., Fichte K., Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatr. Neuropsychopharmakol. 1979;12(2):237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- Lehmann D., Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr. Clin. Neurophysiol. 1980;48(6):609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A., McIntosh A.R. The role of anterior cingulate cortex in working memory is shaped by functional connectivity. J. Cogn. Neurosci. 2005;17(7):1026–1042. doi: 10.1162/0898929054475127. [DOI] [PubMed] [Google Scholar]
- Lin F.H., Chu Y.H., Hsu Y.C., Lin J.F., Tsai K.W., Tsai S.Y., Kuo W.J. Significant feed-forward connectivity revealed by high frequency components of bold fMRI signals. Neuroimage. 2015;121:69–77. doi: 10.1016/j.neuroimage.2015.07.036. [DOI] [PubMed] [Google Scholar]
- Manning R.T. The serial sevens test. Arch. Intern. Med. 1982;142(6):1192. [PubMed] [Google Scholar]
- Mazziotta J., Toga A., Evans A., Fox P., Lancaster J., Zilles K., Woods R., Paus T., Simpson G., Pike B. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM) Philos. Trans. R Soc. Lond. B Biol. Sci. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milz P., Faber P.L., Lehmann D., Kochi K., Pascual-Marqui R.D. sLORETA intracortical lagged coherence during breath counting in meditation-naive participants. Front. Hum. Neurosci. 2014;8:303. doi: 10.3389/fnhum.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.J., McNaughton N., Flanagan D., Kirk I.J. Frontal-midline theta from the perspective of hippocampal theta. Prog. Neurobiol. 2008;86(3):156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Nichols T.E. Multiple testing corrections, nonparametric methods, and random field theory. Neuroimage. 2012;62(2):811–815. doi: 10.1016/j.neuroimage.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., Razavi N., Jann K., Yoshimura M., Dierks T., Kinoshita T., Koenig T. Integrating different aspects of resting brain activity: a review of electroencephalographic signatures in resting state networks derived from functional magnetic resonance imaging. Neuropsychobiology. 2015;71(1):6–16. doi: 10.1159/000363342. [DOI] [PubMed] [Google Scholar]
- Nolan H., Whelan R., Reilly R. FASTER: fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods. 2010;192(1):152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R., Sekihara K., Brandeis D., Michel C. Imaging the electrical neuronal generators of EEG/MEG. In: Michel C.M., Koenig T., Brandeis D., Gianotti L.R.R., Wackermann J., editors. Electrical Neuroimaging. Cambridge University Press; Cambridge: 2009. pp. 49–78. [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp. Clin. Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp. Clin. Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pascual-Marqui R.D., Biscay R.J., Bosch-Bayard J., Lehmann D., Kochi K., Kinoshita T., Yamada N., Sadato N. Assessing direct paths of intracortical causal information flow of oscillatory activity with the isolated effective coherence (iCoh) Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson K.M., Nichols T.E., Poline J.-B., Holmes A.P. Statistical limitations in functional neuroimaging II. Signal detection and statistical inference. Philos. Trans. R Soc. Lond. B Biol. Sci. 1999;354(1387):1261–1281. doi: 10.1098/rstb.1999.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G., Da Silva F.L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110(11):1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Rickard T.C., Romero S.G., Basso G., Wharton C., Flitman S., Grafman J. The calculating brain: an fMRI study. Neuropsychologia. 2000;38(3):325–335. doi: 10.1016/s0028-3932(99)00068-8. [DOI] [PubMed] [Google Scholar]
- Rueckert L., Lange N., Partiot A., Appollonio I., Litvan I., Le Bihan D., Grafman J. Visualizing cortical activation during mental calculation with functional MRI. Neuroimage. 1996;3(2):97–103. doi: 10.1006/nimg.1996.0011. [DOI] [PubMed] [Google Scholar]
- Sauseng P., Hoppe J., Klimesch W., Gerloff C., Hummel F. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur. J. Neurosci. 2007;25(2):587–593. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- Sekihara K., Sahani M., Nagarajan S.S. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. Neuroimage. 2005;25(4):1056–1067. doi: 10.1016/j.neuroimage.2004.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., McMenamin B.W., Maxwell J.S., Greischar L.L., Davidson R.J. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51(4):1319–1333. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeide M.A., Friederici A.D. The ontogeny of the cortical language network. Nat. Rev. Neurosci. 2016;17(5):323–332. doi: 10.1038/nrn.2016.23. [DOI] [PubMed] [Google Scholar]
- Smith A. The serial sevens subtraction test. Arch. Neurol. 1967;17(1):78–80. doi: 10.1001/archneur.1967.00470250082008. [DOI] [PubMed] [Google Scholar]
- von Stein A., Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T., Everling S. Long-Range attention networks: circuit motifs underlying endogenously controlled stimulus selection. Trends Neurosci. 2015;38(11):682–700. doi: 10.1016/j.tins.2015.08.009. [DOI] [PubMed] [Google Scholar]