Highlights
-
•
We analyzed 200 patients undergoing ACDF with multi-channel MEP monitoring.
-
•
Multi-channel MEP monitoring showed a higher diagnostic accuracy for long tract and segmental injury.
-
•
Multi-channel MEP monitoring could be useful for single- and multi-level ACDF.
Keywords: Disc disease, Somatosensory evoked potentials, Intraoperative neurophysiological monitoring, Motor evoked potentials, Anterior cervical discectomy and fusion
Abstract
Objectives
Anterior cervical discectomy and fusion (ACDF) surgery is the most common surgical procedure for the cervical spine with low complication rate. Despite the potential prognostic benefit, intraoperative neurophysiological monitoring (IONM), a method for detecting impending neurological compromise, is not routinely used in ACDF surgery. The present study aimed to identify the potential benefits of monitoring multi-channel motor evoked potentials (MEPs) during ACDF surgery.
Methods
We retrospectively reviewed 200 consecutive patients who received IONM with multi-channel MEPs and somatosensory evoked potentials (SSEPs). On average, 9.2 muscles per patient were evaluated under MEP monitoring.
Results
The rate of MEP change during surgery in the multi-level ACDF group was significantly higher than the single-level group. Two patients from the single-level ACDF group (1.7%) and four patients from the multi-level ACDF group (4.9%) experienced post-operative motor deficits. Multi-channel MEPs monitoring during single and multi-level ACDF surgery demonstrated higher sensitivity, specificity, positive predictive and negative predictive value than SSEP monitoring.
Conclusions
Multi-channel MEP monitoring might be beneficial for the detection of segmental injury as well as long tract injury during single- and multi-level ACDF surgery.
Significance
This is first large scale study to identify the usefulness of multi-channel MEPs in monitoring ACDF surgery.
1. Introduction
Anterior cervical discectomy and fusion (ACDF) is one of the most common surgical procedures for decompression of spinal cord and roots of the cervical spine with low complication rate (Marawar et al., 2010). Intraoperative neurophysiological monitoring (IONM) is a method employed to determine the likelihood of post-operative motor deficits (Macdonald et al., 2013). However, the rate of IONM usage during ACDF surgery in the United States is as low as 6.9%, possibly due to the limited risk of the surgery (Ney et al., 2015).
Relatively few studies have investigated the influence of IONM on ACDF prognosis. Studies employing the use of IONM techniques, such as somatosensory evoked potentials (SSEPs) have reported no significant correlation with postoperative motor deficits or prognostic outcome (Taunt et al., 2005, Khan et al., 2006, Smith et al., 2007, Cole et al., 2014). Notwithstanding unfavorable results of intraoperative SSEP monitoring, a national study demonstrated that the use of IONM produced a better clinical outcome in low risk spinal procedures that included ACDF surgery (Ney et al., 2015).
Supporting this, a previous study recorded SSEPs and motor evoked potentials (MEPs) from the abductor halluces, tibialis anterior, and abductor pollicis brevis during ACDF surgery, and demonstrated sensitivity and specificity in the detection of postoperative motor deficits (Xu et al., 2011). Recent studies have provided additional evidence for the usefulness of Multi-channel MEPs (at least 8 muscles) during spinal surgery (Ito et al., 2013), and other studies suggested that MEPs demonstrated greater sensitivity during ACDF surgery than SSEPs (Lee et al., 2006, Xu et al., 2011). However, since no previous studies have investigated multi-channel MEPs during ACDF surgery, the current study aims to evaluate the usefulness of multi-channel MEPs monitoring on post-operative motor deficits of ACDF surgery.
2. Methods
2.1. Patients
A consecutive series of 200 ACDF surgeries conducted at Seoul National University Bundang Hospital between June 2012 and October 2015 was retrospectively analyzed. The mean subject age of 200 patients (Male, 123; female, 77) was 53.7 ± 12.6 years. Patients presented with foraminal stenosis (n = 175; 87.5%), and severe central canal stenosis (n = 116; 58%) (Kang et al., 2011). Almost all patients (n = 198, 99%) featured herniated intervertebral discs, while 21% of patients (n = 42) demonstrated the combined ossification of posterior longitudinal ligaments. Of these, 59% of patients (n = 118) had undergone single level ACDF surgery, while 33.5% (n = 67) had received 2-level ACDF surgery. The remaining 15 patients had undergone 3-level ACDF surgery (Table 1). The present study was approved by the Institutional Review Board at Seoul National University Bundang Hospital.
Table 1.
Demographics and radiologic findings of the patients.
| Overall ACDF | Single-level ACDF | Multi-level ACDF | p-Value | |
|---|---|---|---|---|
| Number | 200 | 118 | 82 | |
| Sex (M:F) | 123:77 | 81:37 | 42:40 | <0.05 |
| Age (years ± SD) | 53.7 ± 12.6 | 53.8 ± 12.6 | 53.4 ± 12.7 | 0.76 |
| Extent of surgery | ||||
| Single level | 118 | 118 | ||
| Two level | 67 | 67 | ||
| Three level | 15 | 15 | ||
| Radiological finding | ||||
| Foraminal stenosis | 175 | 100 | 75 | 0.2 |
| Central canal stenosis | 195 | 115 | 80 | 0.67 |
| Grade 0 (no stenosis) | 5 | 3 | 2 | |
| Grade 1 | 21 | 14 | 7 | |
| Grade 2 | 58 | 31 | 27 | |
| Grade 3 (severe stenosis) | 116 | 70 | 46 | |
| HIVD | 198 | 116 | 82 | 0.51 |
| OPLL | 42 | 20 | 22 | 0.11 |
*p-Value between single-level ACDF and multi-level ACDF surgeries.
ACDF = anterior cervical discectomy and fusion; HIVD = herniated intervertebral disc; OPLL = ossifications of posterior longitudinal ligament.
2.2. Anesthesia
To avoid confounding effects in MEP monitoring, a neuromuscular blocker (rocuronium 0.5–1.0 mg/kg) was applied prior to intubation. Pre-medication was 2 mg of midazolam, and intravenous lidocaine (0.3–0.5 mg/kg) was administered for the induction of anesthesia. Total intravenous anesthesia was achieved using propofol (3.0–4.0 μg/mL) and remifentanil (1.5–4.0 μg/mL) was used to maintain anesthesia. The anesthesiologist maintained end-tidal CO2 in the normal range throughout surgery.
2.3. Surgical procedures
ACDF surgery involved regions C3-4 to the C7-T1 intervertebral disc. The C5-C6 intervertebral disc was the most common site (n = 117) for both single-level (n = 51) and multi-level ACDF surgery (n = 66). Accordingly, C7-T1 was the least involved site (n = 9) for both single-level (n = 2) and multi-level ACDF (n = 7; Table 1).
2.4. Intraoperative neurophysiological monitoring
2.4.1. Transcranial electrical stimulation
Transcranial electrical stimulation was delivered using needle electrodes inserted at C3 and C4 according to the international 10–20 electrode placement system. The C3 anode and C4 cathode pair was used to stimulate the left hemisphere, and the reverse arrangement was used to stimulate the right hemisphere. Multi-pulse transcranial electrical stimulation was performed using a commercially available IONM electrical stimulator (Xltek protektor 32 IOM system; Natus Medical Inc., Oakville, Canada). Five square-wave stimuli were delivered, with individual pulse durations of 0.05 ms, interstimulus intervals of 1–2 ms, intensity of 250–500 V, 10–1000 Hz filter, and a time base of 100 ms. MEPs were recorded approximately every 10 min in all patients. We checked MEPs before and after discectomy and foraminotomy as well as when surgeons requested.
2.4.2. Multi-channel recording for MEPs
On average, MEPs were recorded in 9.2 muscles per patient in the upper and lower extremities using subdermal needle electrodes. Electrodes were placed in the trapezius (to represent the C4 spinal nerve root), the deltoid and/or biceps brachii (C5 and C6), the triceps brachii (C7), and the abductor pollicis brevis (C8) for the upper extremities. In the lower extremities, MEPs were recorded from the tibialis anterior (TA) and abductor hallucis (AH) muscles. Several muscles were selected in the upper extremity to identify segmental injury as well as long tract injury. The lower extremity muscles (TA and AH) were used to identify corticospinal tract (long tract) injury.
2.4.3. Somatosensory evoked potentials
In order to obtain SSEPs, square-wave electrical pulses of 0.3 ms duration and approximately 10-20 mA intensity were used for the upper extremities at a frequency of 2.31 Hz. Pulses of approximately 20–30 mA intensity were used for the lower extremities at a frequency of 2.31 Hz. Stimulating needle electrodes were placed at the wrist and ankle for the evaluation of the median and tibial nerves, respectively. SSEPs were recorded using scalp electrodes for recording at C3′ (2 cm posterior to C3), C4′ (2 cm posterior to C4), and Cz′ (2 cm posterior to Cz) against a reference electrode at the Fpz. The low pass cut-off filter was 30 Hz, and the high pass filter was 1000 Hz. SSEPs were recorded every 60 s in all patients.
2.5. Alarm criteria
For the MEP, we defined the alarm point as a reduction in the amplitude of the compound muscle action potential by 80% or more. The alarm point criterion was applied to all muscles recorded and was used as an indicator of postoperative neurological deficit. MEPs were recorded at least two times in order to reduce inter-trial variability of MEPs. During SSEPs recording, we defined the alarm point as a decrease of 50% or more in amplitude, and/or a 10% delay in latency. Two experienced electrophysiologists, blind to the patients’ clinical information reviewed the intraoperative monitoring data.
2.6. Evaluation of postoperative motor deficits
The motor function of each limb was assessed preoperatively and immediately after surgery. A decrease of more than one point in the Medical Research Council (MRC) motor grade score, compared to the preoperative score, was defined as a postoperative motor deficit. We reviewed key joint movements of the upper extremity such as shoulder abduction (deltoid), elbow flexion (biceps brachii) elbow extension (triceps brachii) and thumb abduction (abductor pollicis brevis) for the identification of postoperative segmental injury. We also reviewed key joint movements of lower extremity such as knee extension (Vastus latealis), ankle dorsiflexion (Tibialis anterior) and ankle plantar flexion (gastrocnemius) for the identification of long tract injury.
2.7. Analysis of intraoperative neurophysiological monitoring data
IONM data was analyzed for each patient. Monitoring results were classified into five categories as previously described (true-positive, false-positive, true-negative, false-negative and indeterminate cases) (Kim et al., 2007). True-positive cases were defined as patients with a persistent decrease in potentials at the end of the operation, and the immediate appearance of postoperative motor deficits. False-positive cases had a persistent decrease in potentials, but without postoperative motor deficits. True-negative cases did not breach the alarm criteria during surgery, and did not have immediate postoperative motor deficits. False-negative cases did not present any alarm during surgery, but had immediate postoperative motor deficits. Indeterminate cases were defined as patients that met the alarm criteria during operation, but recovered by the end of the operation, and did not present any postoperative deficits. We analyzed MEP and SSEP findings and cross-referenced these with the surgery outcomes.
2.8. Statistical analysis
A Student’s t-test and Chi-square test were used for the comparison of demographic, clinical characteristics and IONM results between single-level and multi-level ACDF surgeries. SPSS v22.0 was used for data analysis, and the significance level was set at p < 0.05.
3. Results
The mean duration of spinal surgery was 162.5 ± 60 min. Multi-level ACDF surgery featured a significantly longer surgery duration compared with single-level ACDF (207.1 ± 61.4 vs. 131.4 ± 33.8 min, p < 0.05) The mean estimated blood loss for overall ACDF surgeries was 205 ± 200 ml. There was greater blood loss during multi-level ACDF than single-level ACDF (258.2 ± 223 vs 169.1 ± 174 ml, p < 0.05). Twenty-five patients (12.5%) were treated with steroids during the surgery, and similarly, a greater number of multi-level ACDF patients were treated with steroid compared with single-level ACDF surgery. Only three patients (1.5%) suffered a mean blood pressure lower than 60 mmHg and required blood transfusion.
Of the 200 consecutive patients, baseline MEPs and SSEPs were successfully recorded in 177 (88.5%) and 196 (98%) patients, respectively. In twelve patients out of 177 (6.8%) undergoing MEPs monitoring there was decrement of the MEPs during surgery. A significantly higher rate of MEP change was observed during surgery in the multi-level ACDF group compared with the single-level ACDF group (p < 0.05). Nine patients (5.1%) demonstrated persistent MEPs abnormalities at the end of surgery. Two patients out of 196 (1%) who underwent SSEPs monitoring had changes in the SSEPs at the end of surgery. Six patients (3%) experienced post-operative motor weakness and neurological deficit. Of these, two patients underwent hematoma revision surgery (Table 2).
Table 2.
Intraoperative and postoperative findings of the patients.
| Overall ACDF | Single level ACDF | Multi-level ACDF | p-Value* | |
|---|---|---|---|---|
| Number | 200 | 118 | 82 | |
| Findings during operation | ||||
| Duration of surgery (min ± SD) | 162.5 ± 60 | 131.4 ± 33.8 | 207.1 ± 61.4 | <0.05 |
| Estimated blood loss (mL ± SD) | 205.6 ± 200 | 169.1 ± 174 | 258.2 ± 223 | <0.05 |
| Steroid Treatment during surgery | 25/200 | 7 | 18 | <0.05 |
| MBP < 60 mmHg | 3/200 | 0 | 3 | 0.07 |
| Transfusion during surgery | 3/200 | 1 | 2 | 0.57 |
| Findings during IONM | ||||
| Monitorability of MEPs on baseline | 177/200 | 103/118 | 74/82 | 0.65 |
| MEPs change during surgery | 12/177 | 3 | 9 | <0.05 |
| MEPs change at the end of surgery | 9/177 | 3 | 6 | 0.17 |
| Monitorability of SSEPs on baseline | 196/200 | 115/118 | 81/82 | 0.65 |
| SSEPs change during surgery | 2/196 | 0 | 2 | 0.17 |
| SSEPs change at the end of surgery | 2/196 | 0 | 2 | 0.17 |
| Postoperative findings | ||||
| Postoperative weakness | 6/200 | 2 | 4 | 0.23 |
| Revision due to hematoma | 2/200 | 1 | 1 | 0.65 |
ACDF = anterior cervical discectomy and fusion; MBP = mean blood pressure; IONM = intraoperative neurophysiologic monitoring; MEPs = motor evoked potentials; SSEPs = somatosensory evoked potentials.
p-Value between single-level ACDF and multi-level ACDF surgeries.
Table 3 displays the clinical and IONM profiles of the six patients who experienced post-operative motor deficits. Two patients underwent single-level ACDF surgeries (2/118, 1.7%) while four patients received multiple level ACDF surgeries (4/82, 4.9%). Three patients demonstrated post-operative motor deficits below the site of surgical lesion (long tract injury), while the remaining three patients displayed focal motor deficits (segmental injury). The long tract injuries in two patients were later linked to compression by hematoma. Among them, one patient (No. 2) demonstrated changes in MEPs during surgery while the other patient (No. 3) did not. In patient No. 6, MEPs were not recorded before positioning. Despite the absence of motor weakness prior to surgery, baseline MEPs could not be measured immediately after the positioning. The patient had post-operative motor deficits, suggesting nerve damage by positioning.
Table 3.
Clinical and intraoperative monitoring profiles of the cases with post-operative motor deficit.
| Pt No. | Age | Sex | ACDF level | Disease | Stenosis |
IONM change |
Motor weakness |
Post-op imaging study | Treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cent | Fora | MEP | SSEP | Pre-Op | Post-Op | |||||||
| 1 | 52 | M | C6-7 | HIVD | G1 | Yes | APB loss | No | No | Hand grip | Well decompression | Rehabilitation |
| 2 | 58 | M | C5-6 | HIVD OPLL | G3 | Yes | APB, TA, AH loss | No | No | Below lesion | Hematoma | Hematoma revision |
| 3 | 47 | F | C5-6, C6-7 | HIVD | G3 | No | No | No | No | Below lesion | Hematoma | Hematoma revision |
| 4 | 70 | F | C4-5, C5-6 | HIVD OPLL | G3 | Yes | Deltoid loss | No | No | Shoulder abduction | Well decompression | Rehabilitation |
| 5 | 73 | M | C4-5, C5-6 | HIVD | G3 | Yes | Deltoid loss | No | No | Shoulder abduction | Well decompression | Rehabilitation |
| 6 | 64 | M | C4-5, C6-7 | HIVD OPLL | G3 | Yes | Unobtainable from baseline | No | No | Below lesion | Well decompression + Myelopathy on C4-5 level | Rehabilitation |
Pt No. = patient number; ACDF = anterior cervical discectomy and fusion; Cent = central canal; Fora = foramen; Op = operation; F = female, M = male; HIVD = herniated intervertebral disc; OPLL = ossification of posterior longitudinal ligament; APB = abductor pollicis brevis, TA = tibialis anterior, AH = abductor halluces.
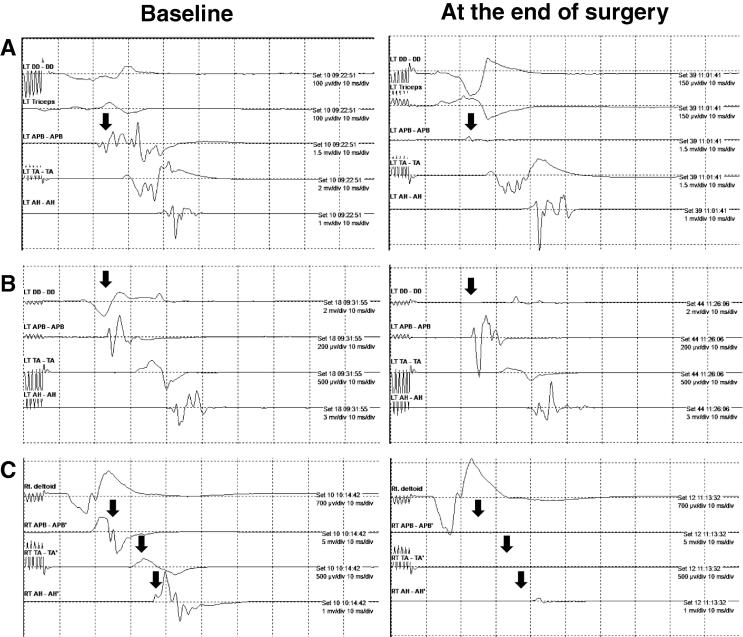
The three patients demonstrating segmental injury showed a strong correlation between focal MEP loss and the extent of post-operative motor deficits. By comparison, no SSEP abnormalities were observed in the six patients experiencing post-operative motor deficits. Fig. 1 demonstrates typical changes in MEPs of segmental injury (Cases 1 and 4, Table 3) and long tract injury (Case 2, Table 3). Segmental injury showed MEPs changes and post-operative weakness at the surgery level, while long tract injury showed MEP change and post-operative weakness below the lesion.
Fig. 1.
MEP monitoring samples depicting typical findings. A: Example of segmental injury detected by MEP (case 1, Table 3). MEP recorded from APB muscle was nearly lost. B: Example of segmental injury detected in MEP (case 4, Table 3). MEP recorded from DD muscle was nearly lost. C: Example of long tract injury detected in MEP (case 2, Table 3). MEPs recorded from the APB and TA muscles were lost. MEP recorded from the AH muscle was nearly lost.
We analyzed the IONM data for patients displaying post-operative motor deficits. Multi-channel MEP monitoring had higher sensitivity, positive predictive value and negative predictive value than SSEP monitoring. Despite this, specificity was slightly greater for SSEP monitoring. When contrasting surgical levels, the results of MEPs monitoring in single-level ACDF surgery displayed higher sensitivity, specificity, positive predictive and negative predictive value than multi-level ACDF surgery. Table 4 displays the results of MEP and SSEP monitoring during ACDF surgery.
Table 4.
Analysis of monitoring data on motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs).
| MEP |
SSEP | |||
|---|---|---|---|---|
| Overall ACDF | Single level ACDF | Multi-level ACDF | Overall ACDF | |
| Total (n) | 177 | 103 | 74 | 196 |
| True positive (n) | 4 | 2 | 2 | 0 |
| False positive (n) | 5 | 1 | 4 | 2 |
| True negative (n) | 164 | 100 | 64 | 188 |
| False negative (n) | 1* | 0 | 1* | 6 |
| Indeterminate (n) | 3 | 0 | 3 | 0 |
| Sensitivity (%) | 80 | 100 | 66.7 | 0 |
| Specificity (%) | 97 | 99 | 94.1 | 98.9 |
| Positive predictive value (%) | 44.4 | 66.7 | 33.3 | 0 |
| Negative predictive value (%) | 99.4 | 100 | 98.5 | 96.9 |
False negative case on MEP monitoring: weakness due to hematoma (Case 3, Table 3).
One false negative case was observed during MEP monitoring in the present study. The patient in question subsequently underwent revision surgery for a hematoma (Case 3, Table 3).
4. Discussion
The current study has demonstrated the usefulness of multi-channel MEPs monitoring during ACDF surgery. Multi-channel MEP monitoring for single-level and multi-level ACDF surgery had higher sensitivity, specificity, positive predictive and negative predictive value in the detection of postoperative motor deficits.
Because of the one false negative case, the sensitivity of MEP monitoring for total ACDF and multi-level ACDF surgery was 80% and 66.7%, rather than 100% and 100%, respectively. MEPs and SSEPs were monitored continuously between skin incision and muscle closure in this patient, and the absence of intraoperative IONM change was therefore likely due to a hematoma. Since the hematoma would have only expanded to compress the corticospinal tract or dorsal column after recovery from the anesthesia, this might suitably explain the absence of IONM alteration. In order to avoid this, it might be beneficial to prolong IONM until skin closure to minimize the occurrence of false-negative cases due to compression by a hematoma.
A few indeterminate cases arose during MEP monitoring (n = 3, 1.7%), none of which fell within the single-level ACDF group. The relatively low occurrence of indeterminate cases in the single-level group could be explained in that ACDF surgery is performed in the narrow cervical intervertebral space. As a function of this, neural damage acquired during ACDF surgery is unlikely to recover spontaneously. Secondly, the time duration of ACDF surgery is relatively short compared with other spinal surgery procedures. Since single-level ACDF involves a shorter surgery duration than multi-level ACDF surgery (Table 2), it is possible that there was insufficient time for neurological damage to recover during the surgery. Supporting this, more false positive cases emerged than indeterminate cases for ACDF surgery overall (n = 5 vs. n = 3).
Of the 23 cases that were unable to be monitored with MEPs, one patient displayed post-operative motor deficit. Preoperatively, the patient did not present any detectable motor weakness (Case 6, Table 3), and magnetic resonance imaging revealed severe central canal stenosis. Positioning was reported as one of the etiological factors that can causes nerve compression or injury during spinal surgery (Anderson et al., 2001, Jones et al., 2004, Ofiram et al., 2006, Anastasian et al., 2009, Raynor et al., 2013). A recent study suggested that the use of IONM prior to positioning could reduce the likelihood of neural injury during positioning (Plata Bello et al., 2015). For patients with severe canal stenosis, the use of MEP and SSEP monitoring prior to positioning might reduce the likelihood of neurological injury or nerve compression.
No true-positive cases were detected by SSEP monitoring in the current study. While SSEPs traverse the dorsal columns, ACDF surgery is performed predominantly on the ventral part of the spinal cord. Therefore, neural injury during ACDF surgery would be less likely to induce changes in SSEPs. MEPs would be sensitive than SSEPs due to the ventral location of corticospinal tract, thereby providing a more accurate representation of neural prognosis and function. Therefore, in the event of ACDF surgery, the use of MEPs might be more beneficial than traditional SSEP methods, since alterations in MEPs correlate better with postoperative neurological outcomes. Moreover, previous study showed higher false negative rate (72%) of SSEP on definite cervical radiculopathy (Schmid et al., 1988).
However, MEP were more often unrecordable than SSEPs (n = 23, n = 4), and SSEP monitoring might therefore be useful in cases of unobtainable MEPs.
Intraoperative steroid therapy was used on several occasions: first, when MEPs and/or SSEPs met the alarm criteria during the surgery, and second, when there were requests from the surgeon during decompression of severe foraminal/central stenosis, massive hemorrhage, etc. However, we could not identify a relationship between steroid use and surgical outcome in this study. A recent study showed no relationship between steroid use and motor recovery in acute spinal cord injury (Evaniew et al., 2015).
The present study has several limitations. Firstly, the longitudinal effects of motor weakness were not assessed in patients. The persistence of a motor deficit is an important issue for the patient’s quality of life. Secondly, we did not analyze the preoperative motor weakness of patients with unobtainable baseline MEPs. Indeed, a previous study indicated that successful MEP recordings were possible in only 27.5% patients with an MRC grade 0–3 (Rajshekhar et al., 2011).
5. Conclusions
This is first large scale study to identify usefulness of multi-channel MEPs on ACDF surgery. The Multi-channel MEPs monitoring might be beneficial for the detection of segmental injury as well as long tract injury during single- and multi-level ACDF surgery.
Disclosure
Authors report no disclosures.
Study funding
None.
Acknowledgments
None.
References
- Anastasian Z.H., Ramnath B., Komotar R.J., Bruce J.N., Sisti M.B., Gallo E.J. Evoked potential monitoring identifies possible neurological injury during positioning for craniotomy. Anesth. Analg. 2009;109(3):817–821. doi: 10.1213/ane.0b013e3181b086bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.C., Emerson R.G., Dowling K.C., Feldstein N.A. Attenuation of somatosensory evoked potentials during positioning in a patient undergoing suboccipital craniectomy for Chiari I malformation with syringomyelia. J. Child Neurol. 2001;16(12):936–939. doi: 10.1177/088307380101601214. [DOI] [PubMed] [Google Scholar]
- Cole T., Veeravagu A., Zhang M., Li A., Ratliff J.K. Intraoperative neuromonitoring in single-level spinal procedures: a retrospective propensity score-matched analysis in a national longitudinal database. Spine (Phila Pa 1976) 2014;39(23):1950–1959. doi: 10.1097/BRS.0000000000000593. [DOI] [PubMed] [Google Scholar]
- Evaniew N., Noonan V.K., Fallah N., Kwon B.K., Rivers C.S. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a canadian multi-center spinal cord injury registry. J. Neurotrauma. 2015;32(21):1674–1683. doi: 10.1089/neu.2015.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Z., Matsuyama Y., Shinomiya K., Ando M., Kawabata S., Kanchiku T. Usefulness of multi-channels in intraoperative spinal cord monitoring: multi-center study by the Monitoring Committee of the Japanese Society for Spine Surgery and Related Research. Eur. Spine J. 2013;22(8):1891–1896. doi: 10.1007/s00586-013-2722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.C., Fernau R., Woeltjen B.L. Use of somatosensory evoked potentials to detect peripheral ischemia and potential injury resulting from positioning of the surgical patient: case reports and discussion. Spine J. 2004;4(3):360–362. doi: 10.1016/j.spinee.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Kang Y., Lee J.W., Koh Y.H., Hur S., Kim S.J., Chai J.W. New MRI grading system for the cervical canal stenosis. AJR Am. J. Roentgenol. 2011;197(1):W134–W140. doi: 10.2214/AJR.10.5560. [DOI] [PubMed] [Google Scholar]
- Khan M.H., Smith P.N., Balzer J.R., Crammond D., Welch W.C., Gerszten P. Intraoperative somatosensory evoked potential monitoring during cervical spine corpectomy surgery: experience with 508 cases. Spine (Phila Pa 1976) 2006;31(4):E105–E113. doi: 10.1097/01.brs.0000200163.71909.1f. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Zaremski J., Kwon B., Jenis L., Woodard E., Bode R. Risk factors for false positive transcranial motor evoked potential monitoring alerts during surgical treatment of cervical myelopathy. Spine (Phila Pa 1976) 2007;32(26):3041–3046. doi: 10.1097/BRS.0b013e31815d0072. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Hilibrand A.S., Lim M.R., Zavatsky J., Zeiller S., Schwartz D.M. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine (Phila Pa 1976) 2006;31(17):1916–1922. doi: 10.1097/01.brs.0000228724.01795.a2. [DOI] [PubMed] [Google Scholar]
- Macdonald D.B., Skinner S., Shils J., Yingling C. Intraoperative motor evoked potential monitoring – a position statement by the American Society of Neurophysiological Monitoring. Clin. Neurophysiol. 2013;124(12):2291–2316. doi: 10.1016/j.clinph.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Marawar S., Girardi F.P., Sama A.A., Ma Y., Gaber-Baylis L.K., Besculides M.C. National trends in anterior cervical fusion procedures. Spine (Phila Pa 1976) 2010;35(15):1454–1459. doi: 10.1097/BRS.0b013e3181bef3cb. [DOI] [PubMed] [Google Scholar]
- Ney J.P., van der Goes D.N., Nuwer M.R. Does intraoperative neurophysiologic monitoring matter in noncomplex spine surgeries? Neurology. 2015;85(24):2151–2158. doi: 10.1212/WNL.0000000000002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofiram E., Lonstein J.E., Skinner S., Perra J.H. “The disappearing evoked potentials”: a special problem of positioning patients with skeletal dysplasia: case report. Spine (Phila Pa 1976) 2006;31(14):E464–E470. doi: 10.1097/01.brs.0000222122.37415.4d. [DOI] [PubMed] [Google Scholar]
- Plata Bello J., Perez-Lorensu P.J., Roldan-Delgado H., Brage L., Rocha V., Hernandez-Hernandez V. Role of multimodal intraoperative neurophysiological monitoring during positioning of patient prior to cervical spine surgery. Clin. Neurophysiol. 2015;126(6):1264–1270. doi: 10.1016/j.clinph.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Rajshekhar V., Velayutham P., Joseph M., Babu K.S. Factors predicting the feasibility of monitoring lower-limb muscle motor evoked potentials in patients undergoing excision of spinal cord tumors. J. Neurosurg. Spine. 2011;14(6):748–753. doi: 10.3171/2011.1.SPINE10310. [DOI] [PubMed] [Google Scholar]
- Raynor B.L., Bright J.D., Lenke L.G., Rahman R.K., Bridwell K.H., Riew K.D. Significant change or loss of intraoperative monitoring data: a 25-year experience in 12,375 spinal surgeries. Spine (Phila Pa 1976) 2013;38(2):E101–E108. doi: 10.1097/BRS.0b013e31827aafb9. [DOI] [PubMed] [Google Scholar]
- Schmid U.D., Hess C.W., Ludin H.P. Somatosensory evoked potentials following nerve and segmental stimulation do not confirm cervical radiculopathy with sensory deficit. J. Neurol. Neurosurg. Psychiatry. 1988;51(2):182–187. doi: 10.1136/jnnp.51.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.N., Balzer J.R., Khan M.H., Davis R.A., Crammond D., Welch W.C. Intraoperative somatosensory evoked potential monitoring during anterior cervical discectomy and fusion in nonmyelopathic patients–a review of 1,039 cases. Spine J. 2007;7(1):83–87. doi: 10.1016/j.spinee.2006.04.008. Epub 2006 Nov 28. [DOI] [PubMed] [Google Scholar]
- Taunt C.J., Jr., Sidhu K.S., Andrew S.A. Somatosensory evoked potential monitoring during anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2005;30(17):1970–1972. doi: 10.1097/01.brs.0000176321.02963.72. [DOI] [PubMed] [Google Scholar]
- Xu R., Ritzl E.K., Sait M., Sciubba D.M., Wolinsky J.P., Witham T.F. A role for motor and somatosensory evoked potentials during anterior cervical discectomy and fusion for patients without myelopathy: analysis of 57 consecutive cases. Surg. Neurol. Int. 2011;2:133. doi: 10.4103/2152-7806.85606. [DOI] [PMC free article] [PubMed] [Google Scholar]