Highlights
-
•
Warming the feet improved vibration perception thresholds in healthy subjects.
-
•
The sensitivity improvement was more marked with active warming (walking) than with passive warming (heat).
-
•
Patients with postural disturbances could benefit from increasing skin temperature in their feet.
Keywords: Plantar temperatures, Plantar sensitivity, Mechanical stimulation, VPTs
Abstract
Objective
Skin temperatures are known to increase cutaneous sensitivity. However, it is unclear whether the amount of improved sensitivity differs depending on the protocol of heat application. Therefore, this study aimed to investigate the effects of active (treadmill walking) and passive (infrared radiator) warming of the foot sole on vibration perception thresholds.
Methods
Sixty healthy and injury-free subjects voluntarily participated in this study. Vibration perception thresholds (200 Hz) and plantar temperatures were measured at the hallux and 1st metatarsal head. In experiment 1, warming and mechanically stimulating the skin was achieved by walking on a treadmill for 30 min. In a follow-up study (experiment 2), external plantar heat was administered via an infrared radiator (30 min).
Results
In both experiments, increasing temperatures led to increased plantar sensitivity. However, the amount of improved sensitivity was greater in experiment 1, although plantar temperature increases were lower compared to experiment 2.
Conclusions
Warming in conjunction with mechanical stimulation seems to have a greater potential to enhance plantar sensitivity compared to external heat supply only.
Significance
The possible influence of mechanical stimulation and warming towards superior plantar afferent feedback highlights its importance regarding human posture and fall prevention.
1. Introduction
Detailed knowledge of how humans maintain and regain balance in their daily lives is of fundamental importance to reduce the risk of falls or to simply improve quality of life. For example, in various diseases or with aging, there is a high susceptibility to falls (Rubenstein, 2006; Allan et al., 2009) and, consequently, to fall-related injuries. For those reasons, there is research focused on sensory systems which participate in balance control. It is known that visual, vestibular, proprioceptive, and also cutaneous inputs contribute to balance control (Horak et al., 1990, Kavounoudias et al., 1998). In particular, the influencing role of plantar cutaneous receptors on movement control and posture is well established (Chua et al., 2002, Kennedy and Inglis, 2002, Meyer et al., 2004). Various clinical studies show that in many diseases, e.g. peripheral neuropathy, patients exhibit deteriorated plantar sensitivity, which is associated with less stability, especially in dynamic balance conditions (Inglis et al., 1994). In neuropathic patients the loss of sensation and decline of afferent fibers is not limited to plantar aspects. Therefore, several studies focus on plantar afferent inputs (e.g. by reducing sensation using hypothermic procedures (Perry et al., 2000, Nurse and Nigg, 2001, Eils et al., 2002, McKeon and Hertel, 2007, Schlee et al., 2009b; Germano et al., 2016)) in order to examine their role on human balance. Such studies demonstrate that cooling the foot sole reduces plantar cutaneous afferent firing (Lowrey et al., 2013) and plantar vibration perception (Schlee et al., 2009b; Germano et al., 2016). In particular, Germano et al., (2016) state that plantar hypothermia induces both reduced plantar sensitivity and a threat towards balance, hence creating changes in balance responses to avoid loss of balance. They observed decreased electromyographic activity and center of pressure data, which was interpreted as a more cautious behavior to minimize the threat towards balance. Therefore, it seems that mechanisms which have the ability to improve plantar afferent feedback may also improve human posture. Schlee et al., (2009b) show that increasing plantar temperatures (5–6 °C) significantly improves plantar sensitivity. In their study, external heat was supplied to the skin using an infrared radiator (passive). In general, studies examining the effects of cooling on sensitivity and human posture are more common than examining the same effects caused by warming. On the other hand, devices like textured insoles are believed to enhance afferent feedback stemming from the foot sole through higher degrees of tactile stimulation (Palluel et al., 2008). Alfuth and Rosenbaum (2011) investigated the influence of different amounts of active mechanical stimulation induced by daily step activity on foot sole sensations and also found improvements. However, to the best of our knowledge, there are no other studies which examine whether there are differences in improving plantar sensitivity depending on how the foot sole was warmed: externally using infrared radiation or through mechanical stimulation. Therefore, this study aimed to compare the improvement of plantar sensitivity when warming foot soles externally using infrared radiation (passive) to warming through mechanical stimulation of the skin (active).
2. Methods
2.1. Subjects
60 healthy and injury free subjects of both genders voluntarily participated in this study (mean ± SD: 175.3 ± 8.0 cm, 69.1 ± 9.8 kg, 23.8 ± 2.4 yrs). Subjects did not exhibit lower extremity pain or lower leg injuries for at least six months before the measurements. Furthermore, subjects were free of neurological diseases, like neuropathies, diabetes mellitus, or Parkinson’s disease. Before the measurements, subjects were informed about the aims of this study and gave their written informed consent. Participants were free to withdraw from this study at any time, especially when they experienced any discomfort. All procedures were executed in accordance with the recommendations of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Faculty of Behavioural and Social Sciences of the corresponding university.
2.2. Main instrumentation
Vibration perception thresholds (VPTs) were determined using a Tira Vib vibration exciter (model TV51075, Schalkau, Germany), powered by a Voltcraft oscillator (model FG 506, Hirschau, Germany). The vertical movement of the contactor (diameter 7.8 mm; elevation of contactor tip 2 mm above surrounding surface level, see Nurse and Nigg, 1999, Schlee et al., 2009b of the vibration exciter was laser calibrated before the measurements to enable direct readings of the amplitude of the vibrating contactor into the skin. Plantar temperatures were collected using an infrared thermal camera (FLIR E40bx, Flir Systems Inc., Wilsonville, USA). Room temperature was controlled according to EN ISO/IEC 17025 (23 ± 2 °C) and was monitored using a digital C28 type K thermocouple (Comark Instruments, UK).
2.3. Testing procedure
Prior to the measurements, subjects had an equilibrium period of 10 min to adjust to the room temperature. In a first experiment (exp. 1), baseline VPTs and baseline plantar temperatures were measured on 30 healthy subjects (15 females, 15 males) at the hallux and first metatarsal head (Met I) of the dominant leg (barefoot). VPTs were measured at a frequency of 200 Hz, a frequency at which VPT is lowest, and known to be optimum for Vater-Pacini-corpuscles (Verillo, 1985, Gescheider et al., 1994). Subjects were in a seated position with the plantar force applied towards the contactor being monitored and controlled using a single axis force transducer within a range of ±0.5 N (however, without applying additional load towards the contactor). Subjects were instructed to verbally indicate when they perceived the vibrating contactor after increasing its amplitude beginning from zero (small amplitudes correspond to better vibration perception and vice versa). In order to avoid any distracting noises, subjects wore noise cancelling earphones (Bose QuietComfort 25 Acoutic Noise Cancelling, Bose Corp., Framingham, USA). Three consecutive VPT-values of one anatomical location were measured before going to the second location, and the order of locations measured was randomized. Subsequently, plantar temperatures were measured. Subjects were in a supine position, with their legs extended and an ankle angle of 90°. The infrared camera was positioned perpendicular to plantar aspects at a distance of 1 m and an infrared image was taken. After this, subjects walked in their own shoes on a treadmill (h/p/cosmos quasar 5.0, Sports&Medical GmbH, Nussdorf-Traunstein, Germany) for 30 min, representing the active group (AG). They were allowed to adjust their walking speed within a range of 4.3–4.7 km/h, since in a pilot test this was shown to be the most comfortable walking speed. Directly after the treadmill intervention, post plantar temperatures and post VPTs were quantified in the same way as the baseline measurements.
In a follow-up study (exp. 2), another 30 healthy subjects (13 females, 17 males) participated. Baseline measurements and post intervention measurements of plantar temperatures and VPTs were conducted in the same manner as in exp. 1. However, the intervention was different: In exp. 2, the warming of one foot (randomly assigned) was performed using an infrared radiating bulb (type R95E, Philips, Hamburg, Germany) positioned approx. 35 cm in front of the foot sole for the duration of 30 min (passive group, PG). The contra-lateral foot remained untreated for 30 min, representing the control group (CG).
2.4. Data analysis and statistics
Regarding VPTs, the median of the three individual trials at each location was calculated and used for further analysis. Means ± SD and medians were then calculated for all subjects and implemented in statistical analysis. Circles were superimposed on both anatomical locations (Met I, Hallux) to measure plantar temperatures. Using a special software (ThermaCAM™ Researcher Pro 2.8 SR-1, Flir-Systems), mean ± SD temperatures of the circles were calculated and used for further analysis. Data were tested for normal distribution using the Shapiro-Wilk test (α = 0,05). In the case of not normally distributed data, the Wilcoxon-test and Mann-Whitney-U-test were performed for dependent and independent samples, respectively. In the case of normal distribution, t-tests were performed. For all pairwise comparisons, the level of significance was adjusted due to the number of anatomical locations from α = 0.05 to α = 0.05/2 = 0.025. The statistical relevance of differences was assessed based on root mean square error (RMSE) calculations (Altman and Bland, 1996). Results were considered statistically relevant when the differences observed between the comparisons exceeded the RMSE value.
3. Results
There were no significant gender differences (p > 0.025) in temperature or VPT data for all pre and post measurements, for all three groups (CG, PG, AG), and for both anatomical locations (Met I, Hallux). Therefore, data of both genders were analyzed together.
3.1. Temperature data
Plantar temperatures (mean ± SD) for both anatomical locations are depicted in Table 1. Due to the active treadmill intervention in experiment 1 (AG), average plantar temperature increases were significant (p < 0.025) and showed changes of 8.2 °C (34.8%) and 8.3 °C (37.2%) for Met I and Hallux, respectively. For PG in experiment 2, externally applied heat also led to significant (p < 0.025) temperature increases of 10.3 °C (40.8%) and 12.0 °C (52.1%) for Met I and Hallux, respectively. All significant differences were shown to be relevant (RMSEMet I = 4.6 °C, RMSEHallux = 3.4 °C), since RMSE values were smaller than mean differences. For the control foot, representing the CG in experiment 2, no significant temperature changes (p > 0.025) were present for Met I (0.1 °C, 0.5%) when comparing pre versus post heat application. For Hallux, temperature changes were significant (1.3 °C, 5.3%, (p < 0.025)), however, not relevant.
Table 1.
Plantar temperatures (Mean ± SD) at the first metatarsal head (Met I) and Hallux before (pre) and after (post) the two interventions: Experiment 1: Walking on a treadmill, representing the active group (AG). Experiment 2: Externally warming one foot sole via an infrared radiator in the passive group (PG), whilst the other foot remained untreated, representing the control group (CG).
| Pre |
Post |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group |
Passive Group |
Active Group |
Control Group |
Passive Group |
Active Group |
|||||||
| Met I | Hallux | Met I | Hallux | Met I | Hallux | Met I | Hallux | Met I | Hallux | Met I | Hallux | |
| Temperature (°C) | 25.4a | 22.8I | 25.3II,b | 23.1III | 23.6IV,a,b | 22.4V | 25.5∗ | 24.1I,§ | 35.6II,∗ | 35.1III,§ | 31.8IV∗ | 30.7V,§ |
| ±2.5 | ±3.2 | ±2.3 | ±3.3 | ±2.6 | ±3.2 | ±2.6 | ±2.9 | ±2.3 | ±2.5 | ±2.2 | ±3.3 | |
Significant differences:
Pre versus Post: Ip = 0.012; IIp < 0.001; IIIp < 0.001; IVp < 0.001; Vp < 0.001.
Within Pre: ap < 0.001, bp = 0.001.
Within Post: *p < 0.001; §p < 0.001.
Within pre comparisons, temperature data of CG versus PG did not exhibit significant differences (p > 0.025) for either anatomical location. Comparing CG versus AG and PG versus AG, only Met I showed significant differences (p < 0.025). However, these differences were not relevant.
Within post comparisons, plantar temperature data of all group comparisons (CG versus PG, CG versus AG, PG versus AG) presented significant differences (p < 0.025). Furthermore, when comparing CG versus AG and PG, differences were shown to be relevant. Comparing PG versus AG, only Hallux exhibited relevant differences. In both anatomical locations, PG always presented higher temperatures compared to AG.
Comparing plantar temperatures of both anatomical locations, Met I always exhibited higher temperatures compared to Hallux.
3.2. Vibration perception
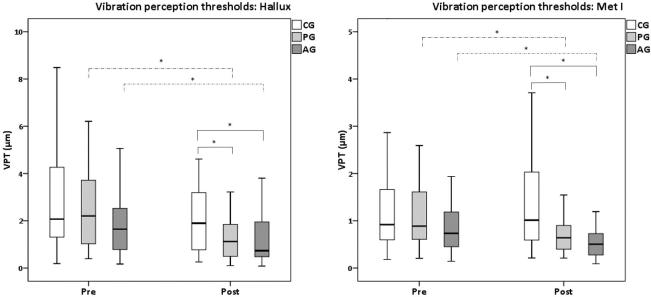
First, comparing VPTs of both anatomical locations, Met I always presented significantly lower thresholds (p < 0.025) compared to Hallux in all groups and both measurement times. For Hallux (Fig. 1, left), both interventions (infrared radiator → PG; treadmill → AG) led to significantly reduced VPTs (PG: 1.5 ± 1.5 μm; AG: 1.1 ± 1.0 μm) when compared to the pre measurements (PG: 2.5 ± 1.8 μm (p < 0.001); AG: 2.0 ± 1.6 μm (p < 0.001)). No significant differences were observed for CG when comparing pre to post measurements (p > 0.025). Based on median vibration thresholds for Hallux, significant differences due to the interventions correspond to 41.0% and 28.3% of VPT-reductions for AG and PG, respectively.
Fig. 1.
Vibration perception thresholds for hallux (left) and first metatarsal head (met I, right). Significant differences (P < 0.025) are marked with asterisks, whereas pre versus post comparisons are illustrated using dashed lines, and within pre and within post comparisons are depicted with solid lines.
Within each of the two measurement times (pre and post), significant VPT differences at the Hallux were only present for post conditions: Presenting 2.9 ± 2.9 μm, CG exhibited significantly larger (p < 0.025) VPTs compared to PG (1.5 ± 1.5 μm) and AG (1.1 ± 1.0 μm). For PG versus AG, no significant differences (p > 0.025) were observed.
Since mean differences were greater than calculated RMSE terms (Hallux: 0.407 μm), all significant differences mentioned above can be considered relevant.
For Met I (Fig. 1, right), both interventions (infrared radiator → PG; treadmill → AG) also led to significantly reduced VPTs (1.1 ± 1.5 μm (p < 0.001); 0.6 ± 0.5 μm (p < 0.001)) when compared to the pre measurements (1.3 ± 1.3 μm; 1.2 ± 1.3 μm; PG and AG, respectively). No significant differences (p > 0.025) were detected for CG. For Met I, subjects presented a median VPT reduction of 35.3% and 22.5% for AG and PG, respectively.
Similarly to Hallux, significant differences (p < 0.025) for Met I were only present within post conditions: The control group exhibited significantly larger (p < 0.025) VPTs (1.6 ± 1.5 μm, p < 0.025) compared to PG (1.1 ± 1.5 μm) and AG (0.6 ± 0.5 μm). No significant differences (p > 0.025) were found for PG versus AG. Again, based on RMSE calculations (Met I: 0.404 μm), all significant differences we found can be considered as relevant.
4. Discussion
The present study investigated the effects of plantar warming induced by treadmill walking and infrared radiation on vibration perception thresholds. Both interventions showed increasing plantar temperatures and, therefore, increased plantar sensitivity. However, the amount of improved sensitivity was greater in treadmill walking, although plantar temperature increases were lower compared to infrared radiation. Treadmill walking appears to have a greater potential to enhance plantar sensitivity compared to infrared radiation only.
4.1. Temperature data
The CG did not show relevant changes comparing pre vs. post measurements. The significant, but not relevant, temperature increases for Hallux may be a consequence of further acclimatization. Both interventions (warming via infrared radiator in PG, and warming via walking on a treadmill for AG) led to significant and relevant plantar temperature increases at both anatomical locations. The intervention-induced amount of plantar temperature increase was generally higher when the skin was warmed via the infrared radiator due to the fact that the distance of the radiator did not exceed 35 cm. This led to a high amount of heat radiation towards plantar skin areas in PG. Temperature increases were therefore higher compared to AG, in which physiologically mediated mechanisms (e.g. increased peripheral blood flow, muscular activity and resulting heat convection or friction occurrence during walking) exhibited lower temperature increases.
Intervention-induced temperature increases can affect plantar mechanoreceptors but also afferent nerve fibers. Since both interventions lasted relatively long (30 min), not only superficial skin layers were affected, but likely also deeper skin tissue, where Vater-Pacini-corpuscles are located (Halata, 1975). This is also supported by changes found for VPTs at 200 Hz in response to both interventions. Note, however, that we did not measure other frequencies. In addition, due to the active warming character of the treadmill intervention through muscular contractions and convection currents for AG, it seems that proximally located afferent nerve tissue might be more affected by increasing temperatures compared to PG. However, we did not measure proximal temperatures or temperatures surrounding the nerve tissue. It is also questionable whether those temperature differences -if present- would be sufficient to influence nerve conduction properties. In this context, Lowitzsch et al. (1977) demonstrated that tissue temperature changes of 10 °C (Q10: 35 vs. 25 °C and 25 vs. 15 °C) measured close to the ulnar sensory nerve fibers did change the nerve conduction velocity. Similar results were shown by Dioszeghy and Stålberg (1992), who found that sensory conduction velocities of the right median nerve increased comparing superficial skin temperatures of 25 and 35 °C. Again, it is unlikely that such temperature differences in tissue close to the proximal nerve innervating plantar aspects of the feet occurred in our protocol.
As further expected, relevant temperature increases for PG and AG due to the intervention and no changes for CG are also reflected in the findings of the within-post-comparisons. There were relevant differences for CG temperatures compared to PG and AG temperatures at all analyzed locations. No relevant differences were found for the within-pre-comparisons.
4.2. Vibration data
The finding that Met I consistently exhibited lower VPTs compared to Hallux is in line with previous literature (Hennig and Sterzing, 2009, Schlee et al., 2009a, Schlee et al., 2009b) and may be explained by the possibility of a higher density of plantar mechanoreceptors at this location.
Despite these differences in sensitivity, temperature changes had the same effect for both anatomical locations. For both groups (PG, AG), increasing plantar temperatures due to the interventions led to significantly and relevantly better plantar sensitivities. As expected, CG showed no differences. In the literature, the influencing effect of skin temperatures towards afferent firing responses (Lowrey et al. 2013) and vibration perception (Nurse and Nigg, 2001, Eils et al., 2002, Schlee et al., 2009b) has been well established.
The particular positive influence of skin warming towards better sensitivity as found in our study was not always confirmed in previous studies. For example, Gerr and Letz (1994) found only small positive effects of increasing skin temperatures on vibrotactile sensitivity (125 Hz) at the index finger and no effects at the great toe. Thyagarajan and Dyck (1994) conducted similar measurements (also at 125 Hz) associated with warming and cooling the skin and found no differences over a broad temperature range of 22 °C. In addition, contrary to our results, Meh and Denislic (1995) found significantly reduced sensitivities (at 100 Hz) after skin temperature increases.
Note that the above mentioned studies exhibit certain methodological aspects which make it difficult to directly compare their with our results. For example, it remains unclear whether Gerr and Letz (1994) measured dorsal or palmar/plantar aspects. Thyagarajan and Dyck (1994) tested only 11 subjects at the dorsum of the big toe. Dorsal compared to plantar sensitivity may differ due to a distinct distribution of mechanoreceptors. Also, since Vater-Pacini corpuscles exhibit an optimal frequency range at around 200–250 Hz (Verillo, 1985, Gescheider et al., 1994), results of Thyagarajan and Dyck, 1994, Gerr and Letz, 1994 and Meh and Denislic (1995) may not be directly comparable to ours, although it would be interesting to see if those changes are the same for other afferent types. Furthermore, in the study of Thyagarajan and Dyck (1994), cooling the skin and subsequent sensory testing were sometimes executed before warming the skin. This might have caused confounding effects, since Kunesch et al. (1987) demonstrated that impaired sensitivity persisted for a few minutes even after the initial skin temperature was regained. In the study of Meh and Denislic (1995) temperature increases were smaller than in our study. However, no information is provided whether or not skin properties were affected by water immersion (e.g. swelling or softening), causing the reduced sensitivity observed.
The findings of our study are consistent with Schlee et al. (2009b), who used a very similar protocol. They already found increased sensitivity after 5–6 °C of skin warming. Nevertheless, none of the above-mentioned studies reported relevance. In our study, all differences regarding VPTs were relevant, emphasizing the strong effects of skin warming towards skin sensitivity. A new finding of the present study was that the two interventions caused different amounts of enhanced plantar sensitivity. Although plantar temperature increases were greater for PG compared to AG, VPTs improved more in the active group compared to the passive group for both anatomical locations (Met I (AG: 35.3%, PG: 22.5%), Hallux (AG: 41.0%, PG: 28.3%), see Table 2. Apparently, warming by mechanical stimulation during the treadmill intervention leads to more than only temperature mediated mechanisms compared to warming only via infrared radiation. Those mechanisms seem to have the potential to improve plantar sensitivity to a greater extent. It was shown that increased plantar mechanical stimulation, for example by textured insoles, can possibly improve plantar afferent feedback (Palluel et al., 2009). One study investigating the effects of enhanced mechanical stimulation (textured insoles) on plantar sensitivity at four anatomical locations found no effects (Palluel et al., 2008). However, they did not measure plantar temperatures and used Semmes-Weinstein-Filaments (SWF) to assess plantar sensitivity. With respect to the application of SWF, however, there are some limitations. SWF were shown to be inaccurate measurement tools under various temperature and humidity conditions (Haloua et al., 2011), and not a very sensitive instrument in clinical settings (Pagel et al., 2002). Data derived from SWF should therefore be considered and analyzed carefully.
Table 2.
Temperature increases (°C) and sensitivity improvements (%) comparing the passive (PG) and active groups (AG) at the Hallux and first metatarsal head (Met I). Sensitivity improvements are based on calculating median improvements (%) over single subjects (n = 30).
| Hallux |
Met I |
|||
|---|---|---|---|---|
| PG | AG | PG | AG | |
| Temperature Increase (°C) | 12.0 | 8.3 | 10.3 | 8.2 |
| Sensitivity improvement (%) | 28.3 | 41.0 | 22.5 | 33.0 |
Interestingly, another study examined the influence of different amounts of active mechanical stimulation (by daily step activity) on foot sole sensations and found positive effects (Alfuth and Rosenbaum, 2011). Measurements with SWF were performed three times per day, whereas greater step activity was associated with measurements performed later in the day. Average plantar temperatures before and after measurements were within a range of 1 °C. Alfuth and Rosenbaum (2011) found significantly enhanced sensory feedback at the afternoon-measurements at two out of six anatomical locations (third metatarsal head, heel). However, no significant but “minor” effects were found for the remaining four anatomical locations (hallux, first and fifth metatarsal head and medial arch). The authors conclude that plantar sensitivity seems to increase with prolonged walking activity, whereas they discuss pressure-induced vasodilatation. Alternating pressure stimuli lead to the relaxation of small vascular muscles, hence leading to vasodilatation. Alfuth and Rosenbaum (2011) assume that there is only little vasodilatation at the medial arch due to low pressures. This would lead to less blood supply, possibly explaining no changes in sensitivity at this region (Alfuth and Rosenbaum, 2011). However, other anatomical locations with higher pressure levels did not show significant interactions. Still, significant findings from their study are in line with the findings of our study. In our study, the improved plantar sensitivity was already evident after 30 min of walking.
It should be acknowledged, however, that Alfuth and Rosenbaum (2011) did not explicitly verify the relevance of their results (although they doubt clinical relevance). SWF are discussed controversially with respect to what kind of mechanoreceptors are being measured: While Palluel et al. (2008) and Alfuth and Rosenbaum (2011) state that those are slowly adapting receptors of type 1 (Merkel) and type 2 (Ruffini), Cheng et al. (1999) state that those would be Merkel and Meissner corpuscles. However, a recent microneurographic study by Strzalkowski et al. (2015) demonstrated that SWF preferentially activate fast adapting afferents in the foot sole (type FAI and FAII). Most of the mentioned receptors have in common that they are more or less superficially located within the skin. In contrast, our protocol measured afferent responses stemming from Vater-Pacini corpuscles, which are located more deeply within the skin. This may also explain why the findings from our study are different. Cutaneous information stemming from the foot sole was shown to contribute to the evaluation of the support surface (Maurer et al., 2001). Slowly adapting cutaneous mechanoreceptors are believed to contribute to postural regulation, especially to changes in center of pressure parameters (Perry et al., 2000, Lowrey et al., 2013). In contrast, Lowrey et al. (2013) suggested that fast-adapting cutaneous mechanoreceptors (e.g. Vater-Pacini corpuscles) code for dynamic postural events. Since it has been shown that cooling plantar aspects affects all types of cutaneous mechanoreceptors (Lowrey et al., 2013), we can presume the same behavior in our study regarding warming the foot soles. Therefore, both measurement instruments (SWF, vibration exciter) can assess changes in sensitivity, although SWF exhibit certain limitations.
As mentioned above, plantar mechanoreceptors contribute to postural regulation. Our study found that the treadmill intervention (active mechanical and warming stimulation) improved plantar sensitivity to a greater extend compared to only external and passive warming (infrared radiator). However, it can only be speculated whether these effects are also reflected in postural parameters since we did not measure those variables. Therefore, this would be an interesting objective for future research.
Recent studies regarding increased sensitivity due to temperature increases do not mention explanations of such findings. We would have expected that viscosity properties would have an influence, since the outer core of the Vater-Pacini corpuscle is responsible for the transmission of mechanical stimuli towards the inner core. However, such an influence was shown to be unlikely (Ishiko and Loewenstein, 1961). There are other early studies examining possible explanations for the new finding of our study. For example, Ide and Saito (1980) found that only the inner core of the Vater-Pacini corpuscle is enzymatically active. They further state that cholinesterase activity, as observed in the inner core, might play an active role in the mechano-electric conversion at the nerve ending innervating the corpuscle (Ide and Saito, 1980). Since enzymatic activity is known to be temperature dependent, this could explain the improved vibratory perception in our study. Additionally, it was demonstrated that at lower temperatures (17 °C), receptor potentials are also of lower magnitude and reduced rate of development compared to higher temperatures (36 °C) (Inman and Peruzzi, 1961). Inman and Peruzzi (1961) further conclude that for higher temperatures, a certain mechanism is involved as to why receptor potential amplitudes increase and thresholds decrease: They assume that refractory periods decrease sufficiently to allow faster than usual re-excitements. Additionally, Ishiko and Loewenstein (1961) show that pure mechanical stretching potentially widens pores in the membrane of the Pacinian corpuscle, which facilitates ion diffusion along their gradient. Therefore, a mechanical stimulus causes an increase in the conductance of the receptor membrane, whereas conductance is strongly temperature dependent (Ishiko and Loewenstein, 1961). This could explain the finding of our study: Combining mechanical stimuli with temperature increases during the treadmill intervention seemed to have resulted in superimposing, synergistic effects exceeding those obtained by supplying heat only externally. It is important to note that the studies mentioned above objectively examined electrical potentials at the receptor/afferent nerve level. We examined the process of perception to vibratory stimuli as a subjective response, which also includes information processing at various levels within the central nervous system. Due to this, objective and subjective observations may not necessarily be directly comparable. Furthermore, and to the best of our knowledge, there is no study investigating central, gait-induced reweighing processes with respect to plantar cutaneous afferent inputs. This would represent a further explanation as to why we found the described changes in VPTs.
With respect to our study, we would like to mention a limitation. First, our subjects were all young and injury-free. Therefore, the mechanisms which led to improved plantar sensitivity may be different in older and/or patient populations. Consequently, our observations may not be directly extrapolated to other groups. Hence, further studies are planned to investigate these effects in other populations.
5. Conclusions
The present study showed that both interventions (treadmill walking, infrared radiation) led to relevantly improved plantar sensitivity due to increased temperatures. However, the amount of improved sensitivity was higher for the treadmill intervention (active mechanical and warming stimulation) compared to only external and passive warming (infrared radiator). This can be explained by various physiological mechanisms occurring, for example, at the mechanoreceptor level. These findings may have beneficial clinical implications, since such interventions are simple in their feasibility as well as inexpensive. Hence, further studies should investigate these aspects in patients, and also whether the consequences of such effects can be seen in postural control.
Conflict of interest
None of the authors had any financial or personal conflict of interest which influenced (biased) their work.
Acknowledgements
Special thanks to our technicians, and to the Sächsische Aufbaubank (SAB) for providing scholarship funding. The publication costs of this article were funded by the German Research Foundation/DFG and the Technische Universität Chemnitz in the funding programme Open Access Publishing.
References
- Alfuth M., Rosenbaum D. Are diurnal changes in foot sole sensation dependent on gait activity? Neurosci. Lett. 2011;504(3):247–251. doi: 10.1016/j.neulet.2011.09.037. [DOI] [PubMed] [Google Scholar]
- Allan L.M., Ballard C.G., Rowan E.N., Kenny R.A. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS ONE. 2009;4(5):e5521. doi: 10.1371/journal.pone.0005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D.G., Bland J.M. Statistics notes : measurement error. Br. Med. J. 1996;744:744. [Google Scholar]
- Cheng W.Y., Jiang Y.D., Chuang L.M., Huang C.N., Heng L.T., Wu H.P. Quantitative sensory testing and risk factors of diabetic sensory neuropathy. J. Neurol. 1999;246(5):394–398. doi: 10.1007/s004150050370. [DOI] [PubMed] [Google Scholar]
- Chua R., Inglis T., Kennedy P., Wells C. The role of cutaneous receptors in the foot. Adv. Exp. Med. Biol. 2002;508:111–117. doi: 10.1007/978-1-4615-0713-0_14. [DOI] [PubMed] [Google Scholar]
- Dioszeghy P., Stålberg E. Changes in motor and sensory nerve conduction parameters with temperature in normal and diseased nerve. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1992;85(4):229–235. doi: 10.1016/0168-5597(92)90110-w. [DOI] [PubMed] [Google Scholar]
- Eils E., Nolte S., Tewes M., Thorwesten L., Völker K., Rosenbaum D. Modified pressure distribution patterns in walking following reduction of plantar sensation. J. Biomech. 2002;35(10):1307–1313. doi: 10.1016/s0021-9290(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Germano A., Schmidt D., Milani T. Effects of hypothermically reduced plantar skin inputs on anticipatory and compensatory balance responses. BMC Neurosci. 2016;17:41. doi: 10.1186/s12868-016-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerr F., Letz R. Covariates of human peripheral-nerve function. 2. vibrotactile and thermal thresholds. Neurotoxicol. Teratol. 1994;16(1):105–112. doi: 10.1016/0892-0362(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Gescheider G.A., Bolanowski S.J., Hall K.L., Hoffman K.E., Verrillo R.T. The effects of aging on information-processing channels in the sense of touch: I absolute sensitivity. Somatosens. Mot. Res. 1994;11(4):345–357. doi: 10.3109/08990229409028878. [DOI] [PubMed] [Google Scholar]
- Halata Z. 50th ed. Springer-Verlag; Berlin Heidelberg New York: 1975. The Mechanoreceptors of the Mammalian Skin - Ultrastructure and Morphological Classification. [DOI] [PubMed] [Google Scholar]
- Haloua M.H., Sierevelt I., Theuvenet W.J. Semmes-weinstein monofilaments: influence of temperature, humidity, and age. J. Hand Surg. Am. 2011;36(7):1191–1196. doi: 10.1016/j.jhsa.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Hennig E.M., Sterzing T. Sensitivity mapping of the human foot: thresholds at 30 skin locations. Foot Ankle Int. 2009;30(10):986–991. doi: 10.3113/FAI.2009.0986. [DOI] [PubMed] [Google Scholar]
- Horak F.B., Nashner L.M., Diener H.C. Postural strategies associated with somatosensory and vestibular loss. Exp. Brain Res. 1990;82:167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- Ide C., Saito T. Electron microscopic histochemistry of cholinesterase activity of Vater-Pacini Corpuscle. Acta Histochem. Cytochem. 1980;13(3):298–305. [Google Scholar]
- Inglis J.T., Horak F.B., Shupert C.L., Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp. Brain Res. 1994;101(1):159–164. doi: 10.1007/BF00243226. [DOI] [PubMed] [Google Scholar]
- Inman D.R., Peruzzi P. The effects of temperature on the responses of Pacinian corpuscles. J. Physiol. 1961;155:280–301. doi: 10.1113/jphysiol.1961.sp006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiko N., Loewenstein W.R. Effects of temperature on the generator and action potentials of a sense organ. J. Gen. Physiol. 1961;45:105–124. doi: 10.1085/jgp.45.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavounoudias A., Roll R., Roll J.P. The plantar sole is a “dynamometric map” for human balance control. NeuroReport. 1998;9(14):3247–3252. doi: 10.1097/00001756-199810050-00021. [DOI] [PubMed] [Google Scholar]
- Kennedy P.M., Inglis J.T. Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J. Physiol. 2002;538(3):995–1002. doi: 10.1113/jphysiol.2001.013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunesch E., Schmidt R., Nordin M., Wallin U., Hagbarth K.-E. Peripheral neural correlates of cutaneous anaesthesia induced by skin cooling in man. Acta Physiol. Scand. 1987;129:247–257. doi: 10.1111/j.1748-1716.1987.tb08065.x. [DOI] [PubMed] [Google Scholar]
- Lowitzsch K., Hopf H.C., Galland J. Changes of sensory conduction velocity and refractory periods with decreasing tissue temperature in man. J. Neurol. 1977;216(3):181–188. doi: 10.1007/BF00313619. [DOI] [PubMed] [Google Scholar]
- Lowrey C.R., Strzalkowski N.D.J., Bent L.R. Cooling reduces the cutaneous afferent firing response to vibratory stimuli in glabrous skin of the human foot sole. J. Neurophysiol. 2013;109(3):839–850. doi: 10.1152/jn.00381.2012. [DOI] [PubMed] [Google Scholar]
- Maurer C., Mergner T., Bolha B., Hlavacka F. Human balance control during cutaneous stimulation of the plantar soles. Neurosci. Lett. 2001;302(1):45–48. doi: 10.1016/s0304-3940(01)01655-x. [DOI] [PubMed] [Google Scholar]
- McKeon P., Hertel J. Diminished plantar cutaneous sensation and postural control. Percept. Mot. Skills. 2007;104:56–66. doi: 10.2466/pms.104.1.56-66. [DOI] [PubMed] [Google Scholar]
- Meh D., Denislic M. Influence of age, temperature, sex, height and diazepam on vibration perception. J. Neurol. Sci. 1995;134(1–2):136–142. doi: 10.1016/0022-510x(95)00230-9. [DOI] [PubMed] [Google Scholar]
- Meyer P.F., Oddsson L.I.E., DeLuca C.J. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 2004;156:505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- Nurse M.A., Nigg B.M. The effect of changes in foot sensation on plantar pressure and muscle activity. Clin. Biomech. 2001;16(9):719–727. doi: 10.1016/s0268-0033(01)00090-0. [DOI] [PubMed] [Google Scholar]
- Nurse M.A., Nigg B.M. Quantifying a relationship between tactile and vibration sensitivity of the human foot with plantar pressure distributions during gait. Clin. Biomech. 1999;14(9):667–672. doi: 10.1016/s0268-0033(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Pagel K.J., Kaul M.P., Dryden J.D. Lack of utility of Semmes-Weinstein monofilament testing in suspected carpal tunnel syndrome. Am. J. Phys. Med. Rehabil. 2002;81(8):597–600. doi: 10.1097/00002060-200208000-00007. [DOI] [PubMed] [Google Scholar]
- Palluel E., Nougier V., Olivier I. Do spike insoles enhance postural stability and plantar-surface cutaneous sensitivity in the elderly? Age. 2008;30(1):53–61. doi: 10.1007/s11357-008-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palluel E., Olivier I., Nougier V. The lasting effects of spike insoles on postural control in the elderly. Behav. Neurosci. 2009;123(5):1141–1147. doi: 10.1037/a0017115. [DOI] [PubMed] [Google Scholar]
- Perry S.D., McIlroy W.E., Maki B.E. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 2000;877(2):401–406. doi: 10.1016/s0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]
- Rubenstein L.Z. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- Schlee G., Milani T.L., Sterzing T. Short-time lower Leg ischemia reduces plantar foot sensitivity. Neurosci. Lett. 2009;462:286–288. doi: 10.1016/j.neulet.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Schlee G., Sterzing T., Milani T.L. Foot sole skin temperature affects plantar foot sensitivity. Clin. Neurophysiol. 2009;120(8):1548–1551. doi: 10.1016/j.clinph.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Strzalkowski N., Mildren R., Bent L. Thresholds of cutaneous afferents related to perceptual threshold across the human foot sole. J. Neurophysiol. 2015;114:2144–2151. doi: 10.1152/jn.00524.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan D., Dyck P.J. Influence of local tissue temperature on vibration detection threshold. J. Neurol. Sci. 1994;126(2):149–152. doi: 10.1016/0022-510x(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Verillo R. Psychophysics of vibrotactile stimulation. J. Acoust. Soc. Am. 1985;77:225–232. doi: 10.1121/1.392263. [DOI] [PubMed] [Google Scholar]