Summary
Circular RNAs constitute a unique class of RNAs whose precise functions remain to be elucidated. In particular, cancer-associated chromosomal translocations can give rise to fusion circular RNAs that play a role in leukemia progression. However, how and when fusion circular RNAs are formed and whether they are being selected in cancer cells remains unknown. Here, we used CRISPR/Cas9 to generate physiological translocation models of NPM1-ALK fusion gene. We showed that, in addition to generating fusion proteins and activating specific oncogenic pathways, chromosomal translocation induced by CRISPR/Cas9 led to the formation of de novo fusion circular RNAs. Specifically, we could recover different classes of circular RNAs composed of different circularization junctions, mainly back-spliced species. In addition, we identified fusion circular RNAs identical to those found in related patient tumor cells providing evidence that fusion circular RNAs arise early after chromosomal formation and are not just a consequence of the oncogenesis process.
Subject Areas: Molecular Biology, Molecular Genetics, Cancer
Graphical Abstract
Highlights
-
•
CRISPR/Cas9 model of ALCL translocation leads to oncogene pathway activation
-
•
CRISPR/Cas9 translocations generate de novo fusion circular RNAs
-
•
Shared fusion circular RNAs are found in CRISPR/Cas9 models and ALCL tumor cells
Molecular Biology; Molecular Genetics; Cancer
Introduction
Circular RNAs (circRNAs) were discovered in eukaryotic cells more than 30 years ago (Hsu and Coca-Prados, 1979) but were first classified as splice errors (Cocquerelle et al., 1993). Only recently new reports showing the emerging role of circRNAs in multiple biological functions drew renewed attention to this specific repertoire of non-coding RNAs (Hansen et al., 2013, Jeck et al., 2013). In particular, recent advances in high throughput sequencing showed high abundance and conservation of circRNAs among species. Biogenesis of circRNAs remains unclear, but different mechanisms have been described that are associated with canonical and non-canonical splicing, and with the presence of repeat sequences in the introns surrounding the junction of circularization (Conn et al., 2015, Errichelli et al., 2017, Ivanov et al., 2015, Zhang et al., 2013, Zhang et al., 2016). CircRNAs can act as transcription regulators (Li et al., 2015), microRNA sponges (Hansen et al., 2013, Memczak et al., 2013, Chen et al., 2017), or platforms for RNA binding proteins (Ashwal-Fluss et al., 2014). In addition, a small subset of circRNAs is translated (Pamudurti et al., 2017). Recently, circRNAs have been shown to be implicated in cell differentiation (Legnini et al., 2017) and to be associated with human diseases (Chen et al., 2017, Hsiao et al., 2017, Shao and Chen, 2016) and could be used as diagnostic biomarkers (Lu et al., 2017). A study from Pandolfi's laboratory demonstrated that chromosomal translocations lead to the expression of fusion-specific circRNAs in various types of tumor cells (Guarnerio et al., 2016).
Recurrent cancer translocations disrupt tumor suppressors, activate oncogenes, or generate aberrant fusion proteins. Expression of the resulting fusion cDNA has been the main strategy to study translocation-driven oncogenesis. Although this strategy provides valuable insights and understanding regarding the role of several specific translocations, the outcome does not always phenocopy the respective disease and may not accurately recapitulate the multistep process of translocation-driven oncogenesis. For instance, TEL-AML1 knock-in mice remain without evidence of disease, and treatment of these animals with mutagenic compounds mainly induces T cell malignancies without characteristics of B-ALL as observed in humans (Schindler et al., 2009). Particularly, overexpression of fusion cDNA hardly reproduces physiological expression of the fusion gene (dosage effects) and does not take into account other potential oncogenic consequences of translocations including reciprocal fusion gene formation, disruption of endogenous fused genes, or transcriptional and epigenetic changes resulting from repositioning of translocated chromosomes in the nucleus.
The consistent advances in programmable nuclease technology, including ZFNs (zinc-finger nucleases), TALENs (transcription activator-like effector nucleases), and CRISPR/Cas9 RNA-guided nucleases, facilitates easier and more precise genome editing. Particularly, we and others have shown that introduction of DNA double-strand breaks (DSBs) at patient translocation breakpoints enables specific oncogenic translocation formation (Brunet et al., 2009, Piganeau et al., 2013, Choi and Meyerson, 2014, Ghezraoui et al., 2014, Torres et al., 2014).
In this study, we used CRISPR/Cas9 technology to induce specific chromosomal translocations. We managed to assess immediate cellular consequences, including newly expressed fusion circular RNAs (f-circRNAs). Focusing on NPM1-ALK fusion found in anaplastic large cell lymphoma (ALCL) (Morris et al., 1994), we confirmed that CRISPR-induced NPM1-ALK translocation was not only sufficient to trigger murine Ba/F3 pro-B cell line transformation but also induced the activation of STAT3, MEK/ERK, and AKT pathways, which are upregulated in ALCL tumors. We demonstrated that newly formed translocations lead to direct expression of specific f-circRNAs transcribed from the NPM1-ALK breakpoint junction. Sequencing of f-circRNAs reveals different types of circularization junctions. Strikingly, f-circRNAs found in tumor cell lines of patients with ALCL were also identified in our different translocation models. Thus, our study provides strong evidences that different f-circRNAs, including specific f-circRNAs, found in patient tumor cells are expressed directly after de novo translocation induction. These results further support the use of CRISPR/Cas9 to induce in situ translocations to reach more relevant cancer models including expression of de novo f-circRNAs.
Results
CRISPR/Cas9-Induced NPM1-ALK Fusion Leads to STAT3, AKT, and ERK Pathway Activation in Mouse Cells
NPM1-ALK is an oncogenic fusion protein that is capable of transforming multiple rodent cell lines (Bai et al., 1998, Fujimoto et al., 1996). Particularly, human NPM1-ALK overexpression has been shown to confer interleukin (IL)-3-independent survival and proliferation of Ba/F3 murine pro-B lymphocytes (Bai et al., 1998). In the mouse genome, Npm1 and Alk genes are located on chromosomes 11 and 17, respectively. NPM1-ALK is expressed from the derivative chromosome 17 (Der17) (Figure 1A). We designed single guide (sgRNAs) targeting murine Npm1 intron 4 and Alk intron 19 to induce concomitant DSBs at loci found as breakpoints in human ALCL. Transient co-expression of Cas9 with sgRNANpm1 and sgRNAAlk led to the formation of the two derivative chromosomes (Der11 and Der17). In contrast, a single break on Npm1 or Alk was not sufficient to induce translocation (Figure 1A). The frequency of translocation at day 5 after transfection was ≥2.5 × 10−4 (Figure S1A) (as calculated in [Renouf et al., 2014]). However, the translocation frequency of cells growing in the presence of IL-3 dropped to 6.25 × 10−5 at day 15 after transfection, indicating that Npm1-Alk does not provide a growth advantage in these conditions (Figure 1B). To investigate the ability of CRISPR/Cas9-induced Npm1-Alk translocation to transform Ba/F3 cells, we removed IL-3 from the medium of transfected cells. IL-3 withdrawal led to major growth arrest and cell death when cells were treated with a single sgRNA. In contrast, after 6 days without IL-3, we observed proliferation of cellular clones from the pool treated with both sgRNANpm1 and sgRNAAlk. After 20 days, we were able to recover multiple translocation junctions. We assessed translocation frequency in proliferating cells without IL-3 and detected Der17 junctions starting from as little as 0.8 ng of DNA, suggesting that most cells carried translocation (Figure 1B). Thus, we confirmed that CRISPR/Cas9-induced mouse Npm1-Alk translocation leads to the transformation of Ba/F3 cells as recently shown (van de Krogt et al., 2017). In addition, to validate that IL-3-independent proliferation was driven by constitutive activation of NPM1-ALK, we treated the cells with crizotinib, an ALK phosphorylation inhibitor. We found that crizotinib led to complete proliferation arrest of selected cells in the absence of IL-3, indicative of active NPM1-ALK fusion protein in the whole population (Figure S1B).
Figure 1.
NPM1-ALK Translocation Induces Ba/F3 Cells Transformation and Leads to f-circRNA Formation
(A) In mouse cells, Npm1 and Alk genes are located on chromosomes 11 and 17, respectively. To induce t(11; 17) translocation, CRISPR/Cas9 system is used to create specific Npm1 (using sgRNANpm1) and Alk (using sgRNAAlk) DSBs. The derivative chromosome 17 (Der17) will lead to Npm1-Alk fusion gene expression. Both derivative chromosomes, Der11 and Der17, are recovered only when Npm1 and Alk DSBs are concomitantly induced (detected by nested PCR).
(B) Proliferation curve of CRISPR/Cas9-treated cells after IL-3 removal. Left panel: cytokine-independent growth was observed only from cells originating from the pool treated with sgRNANpm1 and sgRNAAlk (mean of three experiments ± SD). Right panels: estimate of translocation frequency using PCR on serial dilutions of genomic DNA from Ba/F3 cells. The number of times the PCR was positive for each dilution is indicated (amplicons) (from four independent experiments). At day 15 after transfection (upper panel), translocation junction from cells cultured with IL-3 was detected in two wells of 50 ng DNA, reflecting a frequency around 6.25 × 10−5. Instead, serial dilutions of DNA from cells growing in the absence of IL-3 (lower panel) showed PCR amplicons for all DNA dilutions (the gel showing all the dilutions is the result of the association of two consecutive gel lines).
(C) PCR amplification of Der17 from seven clones growing without IL-3.
(D) Western Blot analysis of NPM1-ALK expression. Each of the seven clones expressed NPM1-ALK fusion protein.
(E) Western Blot detection of STAT3, AKT, and ERK1/2 phosphorylation for clones 1, 4, and 6. STAT3, AKT, and ERK1/2 activation was detected in all clones.
(F) PCR strategy to detect fusion circular RNAs (f-circRNAs) using divergent primers (DivF and R). Convergent primers (cDNA-F and R) are used to detect linear fusion mRNA (Npm1 exons in blue and Alk exons in red).
(G) PCR-amplified products obtained using divergent primers to detect f-circRNAs corresponding to Npm1-Alk gene in Ba/F3 cells (wild-type cells and seven translocated clones). Representative gel from three independent experiments. Schemes of f-circRNA-mA and f-circRNA-mB (right panel) detected in the clones. f-circRNA-mA and f-circRNA-mB were amplified with specific primers.
(H) Number of circular junctions sequenced for each different type of f-circRNA (back-spliced, non-canonical back-spliced, and mixed). Sequencing was done from three independent experiments.
We then isolated seven random Ba/F3 clones from cells growing without IL-3. We could detect Der17 in all of them, further confirming the observation that translocations had occurred in IL-3-independent cells (Figure 1C). Importantly, NPM1-ALK fusion protein was expressed in all the clones (Figure 1D). Insertions and deletions were detected at the translocation junctions but restricted to the intronic region between Npm1 and Alk genes (Figure S1C). We further analyzed three clones (clones 1, 4, and 6). Each of them showed clear co-localization between one Npm1 and one Alk signal as observed by fluorescence in situ hybridization (FISH) (Figure S1D). In vitro and in vivo studies have shown that NPM1-ALK kinase promotes cell growth, cell cycle progression, and survival through transphosphorylation of cytosolic targets, including effector proteins of STAT3, MEK/ERK, and AKT pathways (Marzec et al., 2007a, Marzec et al., 2007b, Zamo et al., 2002, Zhang et al., 2002). We therefore wondered if de novo mouse t(11; 17) translocation induced by CRISPR activates the same signaling cascades. We showed that STAT3 was expressed both in surviving clones and wild-type Ba/F3 cells. However, phosphorylated STAT3 was exclusively detected in the three NPM1-ALK-positive clones, indicating that newly formed NPM1-ALK activates the STAT3 pathway. Similarly, the phosphorylation of ERK1/2 protein was substantially enhanced in the three translocated clones compared with wild-type cells and we could observe greater phosphorylation of specific phospho-sites of AKT (Ser473 and Thr308) (Figure 1E). Our results clearly demonstrate that CRISPR-induced Npm1-Alk translocation is sufficient to trigger transformation of murine Ba/F3 pro-B cell line through the activation of ALCL-specific oncogenic pathways.
De Novo Npm1-Alk Translocation Leads to Expression of Circular RNAs Specific to Breakpoint Junctions (f-circRNAs) in Mouse Cells
Recently, f-circRNAs expressed from translocation junctions have been identified in various tumor cells associated with PML-RARα, MLL-AF9, EWSR1-FLI1, or EML4-ALK1 translocation (Guarnerio et al., 2016). This work demonstrated that the presence of MLL-AF9-specific f-circRNAs contributes to cell survival upon therapy and plays an active role in leukemia progression in vivo. Thus, we wondered if de novo translocation formation leads to the expression of f-circRNAs specific to the breakpoint junction. We amplified f-circRNAs using outward-facing PCR primers around the Npm1-Alk translocation junction, after RnaseR treatment of our samples to decrease the quantity of linear RNAs as previously described (Guarnerio et al., 2016) (Figures 1F and 1G). Importantly, no f-circRNAs could be amplified in wild-type Ba/F3 cells. We investigated the formation of f-circRNAs in the Ba/F3 clones growing in the absence of IL-3, positive for NPM1-ALK translocation. Several PCR amplicons could be detected in each of the seven clones, indicating that f-circRNAs are specific to de novo translocation (Figures 1G and 1H). To validate that our amplicons result from f-circRNA formation in cells and are not amplified from the linear Npm1-Alk counterpart, we transcribed in vitro the NPM1-ALK cDNA from human ALCL cells (homology between mouse and human of NPM1-ALK exons is >80%), and in parallel, an identified f-circRNA containing six exons as positive control (f-circRNA-hF described in the following section) (Figure S1E). After RT-PCR and PCR amplification using similar conditions as for f-circRNA amplification (in the presence of 1 μg RNA from wild-type cells and with RnaseR treatment), we could detect some PCR amplicons using divergent primers and starting from as less as 1 ng of the linear NPM1-ALK in vitro transcript. Sequencing revealed that these unspecific amplicons (18 sequences) corresponded exclusively to sequences containing short direct repeats located at an intra-exonic junction between NPM1 and ALK (Table S1). These unspecific products have been considered as PCR artifacts and have subsequently been excluded from our datasets. More precisely, we excluded every sequence with at least 1 bp found in common at the breakpoint between NPM1 and ALK intra-exonic sequences.
We sequenced four to seven random PCR products from Npm1-Alk clones 1 to 7. After excluding PCR artifacts, we defined three different groups of f-circRNAs: (1) back-spliced f-circRNAs reflecting proper back-spliced events (circularized from two canonical splice sites), (2) non-canonical back-spliced f-circRNAs (circularized within two exons that probably arise from alternative splice sites), and (3) mixed f-circRNAs (circularized from one canonical splice site to a site located within an exon) (Figure 1H and Table S1). Of note, some f-circRNAs showed exon skipping (for example, f-circRNA-mA loses the exon 21, Figure 1G). This phenomenon could be similar to circRNAs described by Zhao's team and was called alternative splicing events (Gao et al., 2016). We could identify back-spliced f-circRNAs in every clone (three to six for each clone), and importantly, sequencing revealed that some back-spliced f-circRNAs could be found in several clones (Table S1). We could detect f-circRNA-mA (joining exon 2 of Npm1 to exon 22 of Alk) and f-circRNA-mB (joining exon 2 of Npm1 to exon 20 of Alk) in each of our seven clones by using specific PCR amplification (Figure 1G). In conclusion, Npm1-Alk-translocated mouse clones expressed different types of f-circRNAs. Two of them are commonly expressed in all clones, indicating a potential selection process of specific f-circRNAs upon transformation of Ba/F3 cells.
CRISPR/Cas9-Induced NPM1-ALK Fusion Leads to STAT3 Pathway Activation, Growth Advantage in Low-Serum Medium, and Expression of Specific f-circRNAs in Human Cells
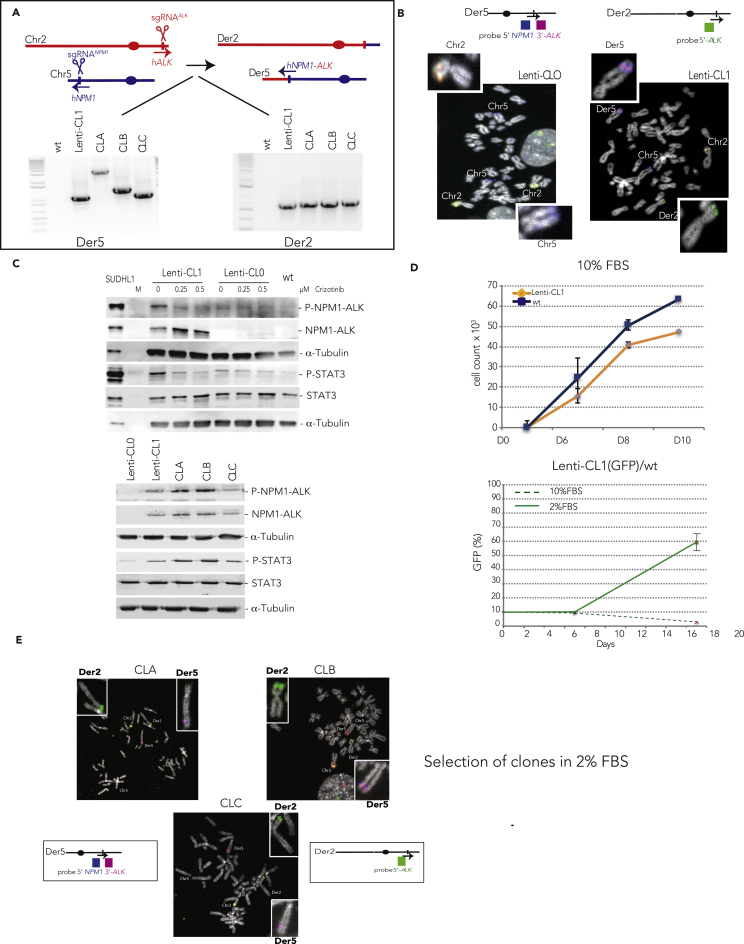
We further decided to confirm the formation of f-circRNAs in a human cell line after NPM1-ALK formation. We first induced t(2; 5) (p23; q35) translocation in the human RPE-1 hTERT-immortalized retinal pigment epithelial cell line, which has a near-diploid karyotype, making it relevant to analyze chromosomal rearrangements. We previously set up the CRISPR/Cas9 transfection system to efficiently induce t(2; 5) (p23; q35) in RPE-1 cells using sgRNAs targeting NPM1 intron 4 and ALK intron 19 (Ghezraoui et al., 2014, Renouf et al., 2014). However, in these studies, we failed to efficiently recover translocated clones. Here we adapted a lentiviral strategy (Heckl et al., 2014) to stably integrate the Cas9 nuclease and the two sgRNAs targeting NPM1 and ALK (Figure S2A). After transduction and fluorescence-activated cell sorting (FACS) sorting of transduced cells, we isolated one NPM1-ALK positive clone (Lenti-CL1) and one NPM1-ALK-negative clone (Lenti-CL0) confirmed by FISH analysis (Figures 2A and 2B). Sequencing of derivative chromosome junctions confirmed the formation of fusion junctions in Lenti-CL1 (Figure S2A). NPM1-ALK fusion protein was stably expressed and active as shown by its auto-phosphorylation (Figure 2C). Importantly, the phosphorylation dropped back to background level upon crizotinib treatment. Similarly, STAT3 showed enhanced phosphorylation in Lenti-CL1 clone compared with negative Lenti-CL0 cells and phosphorylation went back to a basal level with crizotinib treatment, indicating a reliance on NPM1-ALK activity. Of note, RPE-1 cells have high endogenous ERK1/2 and AKT phosphorylation, preventing the assessment of NPM1-ALK-driven phosphorylation for those pathways. Intriguingly, crizotinib treatment had no discernable effect on cell growth or survival of Lenti-CL1 using normal culture conditions (data not shown). Instead, wild-type cells grew faster than Lenti-CL1 cells in 10% of fetal bovine serum (FBS) (Figure 2D), possibly explaining the difficulties we previously faced in isolating translocated cells by traditional cloning method. Thus, as for Ba/F3 cells, we also observed a dramatic decrease in the frequency of translocated cells over time for cells transfected with sgRNANPM1 and sgRNAALK. This phenomenon was independent of the initial frequency of translocation (5 × 10−4 [high] or 1.25 × 10−4 [low] [Figure S2B]). These results prompted us to test the growth of NPM1-ALK clone in the presence of low serum concentration (2% FBS). In this condition, Lenti-CL1 (GFP-positive cells) showed then a strong proliferative advantage over wild-type cells (2% FBS) (Figure 2D) and the translocation frequency increased up to 16-fold after 16 days (Figure S2B). These experiments strongly indicate that NPM1-ALK translocation led to a marked growth advantage in culture conditions containing lower growth factor concentrations. Thus we envisioned that the recovery of translocated clones would be enhanced by culturing sgRNANPM1- and sgRNAALK-transfected cells in 2% FBS medium. As expected, we easily isolated three additional NPM1-ALK-positive clones by simple cloning using low-serum medium (CLA, CLB, and CLC; Figures 2A and S2A). These clones were translocated as shown by FISH (Figure 2E) and expressed an active form of NPM1-ALK, leading to STAT3 phosphorylation (Figure 2C).
Figure 2.
NPM1-ALK Activates STAT3 Pathway and Increases Cell Growth in Low-Serum Culture
(A) In human cells, NPM1 and ALK genes are located on chromosomes 5 and 2, respectively. To induce t(2; 5) translocation, CRISPR/Cas9 system is used to induce specific NPM1 (using sgRNANPM1) and ALK (using sgRNAALK) DSBs. The derivative chromosome (Der5) will lead to NPM1-ALK fusion gene expression. Der5 and Der2 junctions were detected in the four clones Lenti-CL1 (obtained using a lentivirus vector), CLA, CLB, and CLC isolated from sgRNANPM1- and sgRNAALK-treated cells.
(B) FISH for NPM1 and ALK detection in Lenti-CL0 (control clone) and Lenti-CL1 metaphases. ALK locus is detected with a break-apart probe (green + purple). Blue signal corresponds to the 5′ end NPM1 locus: Der5 and Der2 formation leads to split purple and green signals. Colocalization of purple and blue signals reveals Der5 formation.
(C) Western blot detection of NPM1-ALK and STAT3 activation in the translocated clones. SUDHL1 is an ALK-positive ALCL cell line. Lenti-CL0 and Lenti-CL1 were also treated with different amounts of crizotinib.
(D) Upper panel: growth curve of wild-type RPE-1 cells and Lenti-CL1 in 10% FBS. Lower panel: competitive proliferation assay between wild-type RPE-1 cells and Lenti-CL1 in the presence of 10% or 2% FBS. Lenti-CL1 stably expressed GFP (mean of three experiments ± SD).
(E) FISH analysis of RPE-1 clones growing in 2% FBS: CLA, CLB, and CLC. A break-apart probe (purple and green) was used for ALK and a BAC probe (blue) was used to probe NPM1. Der5 and Der2 formation leads to split purple and green signals. Colocalization of purple and blue signals reveals Der5 formation.
See also Figure S2.
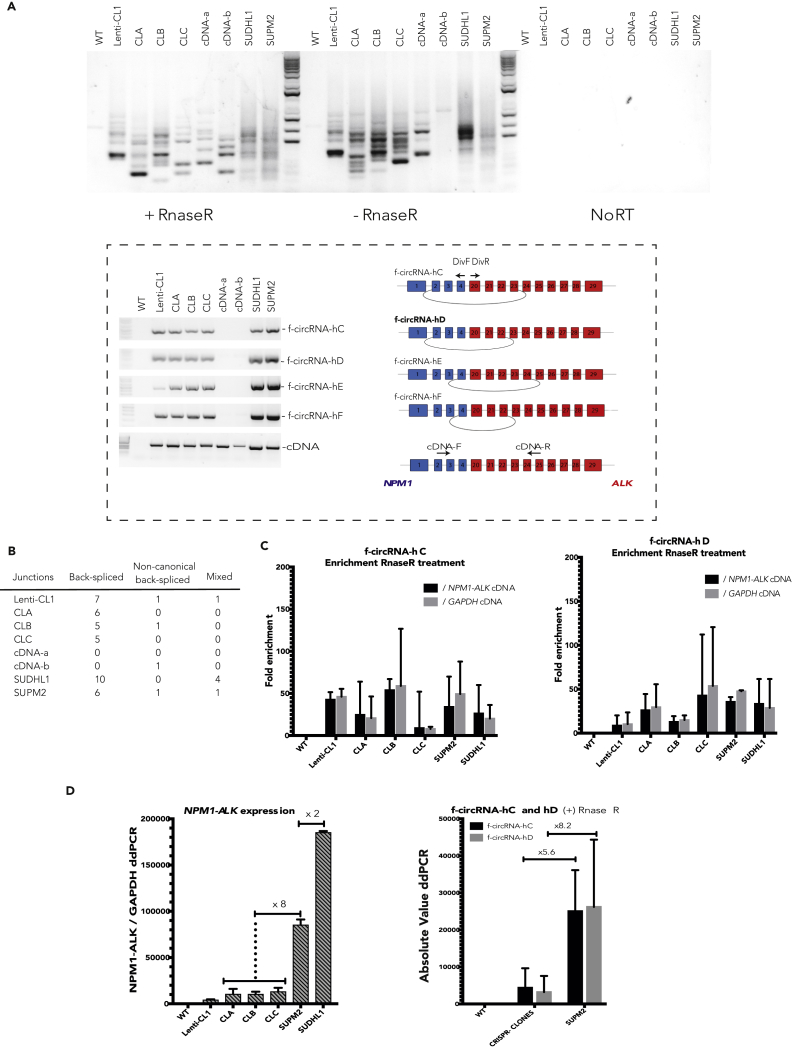
We next looked for the formation of f-circRNAs in our different human RPE-1 clones carrying NPM1-ALK translocation (Figure 3A). As for mouse cells, no f-circRNA could be amplified from wild-type cells. In contrast, several PCR products were detected and sequenced from all four NPM1-ALK clones (Figures 3A and 3B and Table S2). NPM1-ALK translocation induced in human cells also led to the expression of the three different types of f-circRNAs. Importantly, four back-spliced f-circRNAs were found common in all clones (Figure 3A and Table S2). Here we also confirmed that our treatment with RnaseR specifically diminished the quantity of linear RNAs compared with the identified f-circRNAs (from 8- to 50-fold as quantified in Figure 3C), decreasing the potential background of PCR due to unspecific amplification from linear cDNA (described in the previous section).
Figure 3.
NPM1-ALK Induces the Expression of ALCL-Specific f-circRNAs
(A) PCR-amplified products obtained using divergent primers to detect f-circRNAs corresponding to NPM1-ALK gene in RPE-1 human cells: from wild-type cells, CRISPR/Cas9-translocated clones, cells overexpressing NPM1-ALK (labeled cDNA-a and cDNA-b), and SUDHL1 and SUPM2 ALCL cells. Representative gel from three independent experiments. Schemes of f-circRNA-hC, f-circRNA-hD, f-circRNA-hE, and f-circRNA-hF (middle panel) shared by Lenti-CL1, CLA, CLB, CLC, SUDHL1, and SUPM2 cells. The PCR product corresponding to f-circRNA-hD (bold) has similar circularization junction as f-circRNA-mA in Ba/F3 cells (Figure 1A). f-circRNA-hC, f-circRNA-hD, f-circRNA-hE, and f-circRNA-hF were also amplified with specific primers. Detection of NPM1-ALK fusion gene (cDNA) was done using the convergent primers.
(B) Number of circular junctions sequenced for each type of NPM1-ALK f-circRNA in RPE-1 and ALCL cells (back-spliced, non-canonical back-spliced, and mixed). Sequencing was done from three independent experiments.
(C) Fold enrichment for f-circRNA-hC/ f-circRNA-hD after RnaseR treatment normalized by NPM1-ALK linear cDNA and GAPDH cDNA (mean of three experiments ± SEM).
(D) Left panel: NPM1-ALK linear cDNA expression level in CRISPR/Cas9 clones and SUPM2 and SUDHL1 cells. Right panel: f-circRNA-hC or f-circRNA-hD expression level in CRISPR clones compared with SUPM2 cells (average from all the clones) (mean of three experiments ± SEM).
Furthermore, we generated RPE-1 clones stably expressing human NPM1-ALK cDNA (named cDNA-a and cDNA-b). NPM1-ALK expression was confirmed by PCR amplification of fusion cDNA (Figure 3A). In our conditions of amplification and after excluding PCR artifacts, only one non-canonical back-spliced f-circRNA has been detected in clones expressing NPM1-ALK cDNA (Figure 3B and Table S2), in contrast to cells carrying translocated chromosomes, which expressed more f-circRNAs and mostly canonical back-spliced f-circRNAs.
One particular f-circRNA from MLL-AF9 fusion was extensively studied by Guarnerio et al. (2016) and found as tumor specific. Thus, to further investigate the circRNA repertoire formed after CRISPR-induced translocation, we designed a translocation system for Mll-Af9 fusion (Drynan et al., 2005). We could reproduce the translocation in a mouse pro-B cell line and obtained three translocated clones for Mll-Af9 genes as confirmed by PCR amplification of the derivative chromosomes, Mll-Af9 cDNA amplification, and FISH analysis (Figures S3A and S3B). We could readily detect different types of f-circRNAs, and some back-spliced f-circRNAs were prevalent in all clones, as observed for NPM1-ALK model (Figure S3C). For instance, back-spliced f-circRNA-mG and f-circRNA-mH were found in each of the three clones (Figure S3C and Table S3). These preliminary results validate that common f-circRNAs are generated by the formation of endogenous Mll-Af9 translocation, as observed for the NPM1-ALK model. Further validation by RNA-seq in human CRISPR/Cas9 clones would be needed.
In conclusion, the formation of derivative chromosomes using CRISPR/Cas9 technology leads to a specific landscape of f-circRNAs.
De Novo NPM1-ALK Translocation Leads to the Expression of f-circRNAs Specific to Human ALCL Tumor Cells
To gain insight into the relevance of the repertoire of f-circRNAs formed in our clones, we compared them with the ones found in ALCL tumor cells, SUDHL1 and SUPM2 cell lines. As for the RPE-1 clones, we recovered all types of f-circRNAs in the two ALCL cell lines. Notably, the four back-spliced f-circRNAs shared by all NPM1-ALK RPE-1 clones induced by CRISPR/Cas9 were also expressed in SUDHL1 and in SUPM2 patient cells: f-circRNA-hC joining exon 2 to exon 23, f-circRNA-hD joining exon 2 to exon 22, f-circRNA-hE joining exon 4 to exon 24, and f-circRNA-hF joining exon 4 to exon 22 (Figure 3A and Table S2). Remarkably, f-circRNA-hD, which circularized the exon 2 of NPM1 to the exon 22 of ALK, was also expressed in all the mouse Npm1-Alk-positive clones (corresponding to f-circRNA-mA, Figure 1G). We then estimate the relative expression level of two of the four common f-circRNAs. The level of f-circRNA-hC and f-circRNA-hD in CRISPR/Cas9 clones was found to be 5- to 8-fold lower than in SUPM2 tumor cells. This level directly correlates to the difference of expression of NPM1-ALK cDNA between the clones and the tumor cells (of note SUDHL1 tumor cells contain two copies of the translocation, leading to 2-fold higher expression of NPM1-ALK compared with SUPM2 cells) (Figure 3D).
Thereby CRISPR/Cas9 approach to induce translocation leads to modifications of the non-coding RNA repertoire by expressing tumor-related f-circRNAs.
Discussion
Here, we successfully used CRISPR/Cas9 nucleases to induce de novo chromosomal translocations leading to NPM1-ALK fusion oncogene expression in mouse and human cell lines. We confirmed that endogenous mouse NPM1-ALK expression is sufficient to confer cytokine-independent growth of mouse cells as recently shown by the group of Wlodarska (van de Krogt et al., 2017). Furthermore, we demonstrated that the cell survival depended on NPM1-ALK activation and was inhibited by crizotinib treatment. CRISPR/Cas9-mediated rearranged mouse and human cell lines displayed STAT3 (for our human cells), AKT, and ERK1/2 phosphorylation depending on NPM1-ALK kinase expression and activity. Activation of these three proteins was found essential for cell transformation and tumor maintenance in cancer cells (Marzec et al., 2007b, Zamo et al., 2002, Zhang et al., 2002). In addition, low concentrations of growth factors favored proliferation of translocated clones over wild-type cells. This observation is consistent with the fact that NPM1-ALK fusion protein expressed at physiological levels can substitute cytokine stimulation. In conditions in which wild-type cells grow easily (10% serum), translocated cells are probably diluted before acquiring any advantage phenotype.
Our results demonstrated that de novo translocation formation promptly leads to the expression of de novo circRNAs specific to the fusion breakpoint. Interestingly, f-circRNAs found in cancer-associated chromosomal translocation have already been shown to participate in cellular transformation (Guarnerio et al., 2016). In this study, expression of a specific f-circRNA in hematopoietic stem cells (containing the MLL-AF9 fusion) led to a proliferative advantage and favored the progression of leukemia in vivo, demonstrating a physiological role for f-circRNAs. To gain further insight into f-circRNA formation, we generated CRISPR-mediated translocations. We observed the expression of several f-circRNAs upon translocation formation encompassing from two to nine exons (with a length up to 980 bp). The fact that several different f-circRNAs were expressed from the same fusion gene is quite surprising, and whether or not they all have a physiological role remains to be determined. The presence of specific exons may also have a particular biological relevance. Remarkably, we identified specific f-circRNAs expressed in translocated clones perfectly matching those found in tumor-related cells. We also recovered one specific f-circRNA (f-circRNA-mA and f-circRNA-hD) in our mouse and human NPM1-ALK-positive clones, respectively, that was also expressed in two ALCL cell lines, supporting the idea that circRNAs are well conserved between human and mouse cells (Legnini et al., 2017, Rybak-Wolf et al., 2015).
Several types of f-circRNAs were identified in this study. Importantly, CRISPR/Cas9 NPM1-ALK-translocated clones showed a different f-circRNA repertoire compared with clones that expressed ectopically NPM1-ALK cDNA. Several back-spliced species were expressed in CRISPR/Cas9 clones. This class of f-circRNAs properly joins a 5′ splice site with a 3′ splice site of an upstream intron. These events are thought to arise from the annealing of inverted repeat sequences inside flanking exons, enhancing the juxtaposition of the splice sites leading to back-splicing (Jeck et al., 2013). The f-circRNAs that were found common to several clones and tumor cells (but not in cells ectopically expressing NPM1-ALK cDNA) were exclusively canonical back-spliced f-circRNAs, in agreement with their identification in earlier RNA-seq studies.
Surprisingly, in vitro transcription revealed that unspecific PCR amplicons can be generated when amplifying linear cDNA using classical divergent PCR designed for circRNAs. All these events exhibit short direct repeats for at least 1 bp located at the circularized junction between NPM1 and ALK. They are probably produced after PCR template switching, although we can wonder if 1 bp is enough to lead to efficient polymerase template switch. Efficient treatment with RnaseR is then essential for decreasing false-positives from these types of experiments. Instead, we kept mixed events where no repeat could be found, indicating that these types of PCR products were not found as amplified from linear cDNA. The relevance of these forms of f-circRNA (which probably reflect the presence of non-canonical back-spliced sites) remains to be elucidated in further RNA sequencing (RNA-seq) studies. In conclusion, the CRISPR/Cas9 strategy to induce chromosomal translocation provides experimental settings where de novo f-circRNAs are formed: this method will certainly help to precisely dissect the biogenesis and the functional roles of circRNAs since de novo circRNAs can be easily tracked.
To conclude, our study provides evidence that f-circRNAs found in tumor cells are produced directly from newly rearranged chromosomes. Therefore, precise genome rearrangements obtained with CRIPSR/Cas9 provide faithful models phenocopying chromosomal translocation-associated diseases including activation of oncogenic pathways and also expression of tumor-specific f-circRNAs by bypassing limitations of models expressing the fusion gene ectopically. Identification of specific f-circRNAs could provide new diagnostic markers related to cancer aggressiveness and might become therapeutic targets in the near future.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are very grateful to Dr. S. Kabir for commenting on the manuscript, to Dr. J-P de Villartay/P. Revy lab and to Dr. A. De Cian for scientific feedback, to Dr. F. Megetto for providing Ba/F3 cells, and to Dr. C. Lescale and J. Bianchi for providing pro-B cell lines. This research work is supported by a joint grant (E.B., T.M., and L.D.) from the Institut National du Cancer and the Cancéropôle IdF (2016-1-PLBIO-07-INSERM11-1). E.B. research was supported by ANR-12-JSV6-0005. E.B.'s group and T.M.'s team belong to two “La ligue contre le Cancer” teams. M.P. was supported by fellowships from Le Cancéropole IdF and La Ligue Contre le Cancer, and C.T., by a fellowship from la Fondation de France. L.D.'s research is supported by the Institut Pasteur as well as by the European Research Council under the ERC starting grant agreement # 310917, and T.M.'s research, by SIRIC SOCRATE and an Emergence grant from the Canceropole IdF.
Author Contributions
E.C.B conceived the initial study. E.C.B, L.B., and M.P. designed research experiments with the help of L.D., T.M., and C.G. M.P. and L.B. performed most of the experiments, B.R. designed and tested the CRISPR/Cas9 system for NPM1-ALK, and K.L. provided technical support for FISH and protein analysis and for f-circRNA detection. C.T. established the lentiviral transduction and FACS sorting in RPE-1 cells. E.B., L.B., and M.P. wrote the manuscript with valuable feedback from all the authors.
Declaration of Interests
None declared.
Published: July 27, 2018
Footnotes
Supplemental Information includes Transparent Methods, three figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.06.007.
Data and Software Availability
Original imaging data have been deposited to Mendeley Data and are available at https://doi.org/10.17632/22mbdtz59s.1 (https://doi.org/10.17632/22mbdtz59s.1).
Supplemental Information
References
- Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Bai R.Y., Dieter P., Peschel C., Morris S.W., Duyster J. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-gamma to mediate its mitogenicity. Mol. Cell. Biol. 1998;18:6951–6961. doi: 10.1128/mcb.18.12.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet E., Simsek D., Tomishima M., DeKelver R., Choi V.M., Gregory P., Urnov F., Weinstock D.M., Jasin M. Chromosomal translocations induced at specified loci in human stem cells. Proc. Natl. Acad. Sci. USA. 2009;106:10620–10625. doi: 10.1073/pnas.0902076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhang S., Wu J., Cui J., Zhong L., Zeng L., Ge S. circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR-29 family. Oncogene. 2017;36:4551–4561. doi: 10.1038/onc.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Choi P.S., Meyerson M. Targeted genomic rearrangements using CRISPR/Cas technology. Nat. Commun. 2014;5:3728. doi: 10.1038/ncomms4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquerelle C., Mascrez B., Hetuin D., Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Drynan L.F., Pannell R., Forster A., Chan N.M., Cano F., Daser A., Rabbitts T.H. Mll fusions generated by Cre-loxP-mediated de novo translocations can induce lineage reassignment in tumorigenesis. EMBO J. 2005;24:3136–3146. doi: 10.1038/sj.emboj.7600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D., Rosa A., De Santis R., Scarfo R., Peruzzi G. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017;8:14741. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J., Shiota M., Iwahara T., Seki N., Satoh H., Mori S., Yamamoto T. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5) Proc. Natl. Acad. Sci. USA. 1996;93:4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Wang J., Zheng Y., Zhang J., Chen S., Zhao F. Comprehensive identification of internal structure and alternative splicing events in circular RNAs. Nat. Commun. 2016;7:12060. doi: 10.1038/ncomms12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezraoui H., Piganeau M., Renouf B., Renaud J.B., Sallmyr A., Ruis B., Oh S., Tomkinson A.E., Hendrickson E.A., Giovannangeli C. Chromosomal translocations in human cells are generated by canonical nonhomologous end-joining. Mol. Cell. 2014;55:829–842. doi: 10.1016/j.molcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnerio J., Bezzi M., Jeong J.C., Paffenholz S.V., Berry K., Naldini M.M., Lo-Coco F., Tay Y., Beck A.H., Pandolfi P.P. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- Heckl D., Kowalczyk M.S., Yudovich D., Belizaire R., Puram R.V., McConkey M.E., Thielke A., Aster J.C., Regev A., Ebert B.L. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol. 2014;32:941–946. doi: 10.1038/nbt.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K.Y., Lin Y.C., Gupta S.K., Chang N., Yen L., Sun H.S., Tsai S.J. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77:2339–2350. doi: 10.1158/0008-5472.CAN-16-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M.T., Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- Ivanov A., Memczak S., Wyler E., Torti F., Porath H.T., Orejuela M.R., Piechotta M., Levanon E.Y., Landthaler M., Dieterich C. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnini I., Di Timoteo G., Rossi F., Morlando M., Briganti F., Sthandier O., Fatica A., Santini T., Andronache A., Wade M. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell. 2017;66:22–37 e29. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Zhang L., Li W., Deng J., Zheng J., An M., Lu J., Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Sun J., Shi P., Kong W., Xu K., He B., Zhang S., Wang J. Identification of circular RNAs as a promising new class of diagnostic biomarkers for human breast cancer. Oncotarget. 2017;8:44096–44107. doi: 10.18632/oncotarget.17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M., Kasprzycka M., Liu X., El-Salem M., Halasa K., Raghunath P.N., Bucki R., Wlodarski P., Wasik M.A. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene. 2007;26:5606–5614. doi: 10.1038/sj.onc.1210346. [DOI] [PubMed] [Google Scholar]
- Marzec M., Kasprzycka M., Liu X., Raghunath P.N., Wlodarski P., Wasik M.A. Oncogenic tyrosine kinase NPM/ALK induces activation of the MEK/ERK signaling pathway independently of c-Raf. Oncogene. 2007;26:813–821. doi: 10.1038/sj.onc.1209843. [DOI] [PubMed] [Google Scholar]
- Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- Morris S.W., Kirstein M.N., Valentine M.B., Dittmer K.G., Shapiro D.N., Saltman D.L., Look A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L., Hanan M., Wyler E., Perez-Hernandez D., Ramberger E. Translation of CircRNAs. Mol. Cell. 2017;66:9–21 e27. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piganeau M., Ghezraoui H., De Cian A., Guittat L., Tomishima M., Perrouault L., Rene O., Katibah G.E., Zhang L., Holmes M.C. Cancer translocations in human cells induced by zinc finger and TALE nucleases. Genome Res. 2013;23:1182–1193. doi: 10.1101/gr.147314.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renouf B., Piganeau M., Ghezraoui H., Jasin M., Brunet E. Creating cancer translocations in human cells using Cas9 DSBs and nCas9 paired nicks. Methods Enzymol. 2014;546:251–271. doi: 10.1016/B978-0-12-801185-0.00012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak-Wolf A., Stottmeister C., Glazar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Schindler J.W., Van Buren D., Foudi A., Krejci O., Qin J., Orkin S.H., Hock H. TEL-AML1 corrupts hematopoietic stem cells to persist in the bone marrow and initiate leukemia. Cell Stem Cell. 2009;5:43–53. doi: 10.1016/j.stem.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Shao Y., Chen Y. Roles of circular RNAs in neurologic disease. Front. Mol. Neurosci. 2016;9:25. doi: 10.3389/fnmol.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R., Martin M.C., Garcia A., Cigudosa J.C., Ramirez J.C., Rodriguez-Perales S. Engineering human tumour-associated chromosomal translocations with the RNA-guided CRISPR-Cas9 system. Nat. Commun. 2014;5:3964. doi: 10.1038/ncomms4964. [DOI] [PubMed] [Google Scholar]
- van de Krogt J.A., Vanden Bempt M., Finalet Ferreiro J., Mentens N., Jacobs K., Pluys U., Doms K., Geerdens E., Uyttebroeck A., Pierre P. ALK-positive anaplastic large cell lymphoma with the variant RNF213-, ATIC- and TPM3-ALK fusions is characterized by copy number gain of the rearranged ALK gene. Haematologica. 2017;102:1605–1616. doi: 10.3324/haematol.2016.146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamo A., Chiarle R., Piva R., Howes J., Fan Y., Chilosi M., Levy D.E., Inghirami G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Raghunath P.N., Xue L., Majewski M., Carpentieri D.F., Odum N., Morris S., Skorski T., Wasik M.A. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J. Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- Zhang X.O., Dong R., Zhang Y., Zhang J.L., Luo Z., Zhang J., Chen L.L., Yang L. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26:1277–1287. doi: 10.1101/gr.202895.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.