Abstract
Aims
There are previous studies on quality of life (QoL) in cardiac resynchronization therapy (CRT) patients; however, there are no data with the short EuroQol-five dimensions (EQ-5D) questionnaire predicting outcomes. We aimed to assess the predictive role of baseline QoL and QoL change at 6 months after CRT with EQ-5D on 5-year mortality and response.
Methods and results
In our prospective follow-up study, 130 heart failure (HF) patients undergoing CRT were enrolled. Clinical evaluation, echocardiography, and EQ-5D were performed at baseline and at 6 months of follow–up, continued to 5 years. Primary endpoint was all-cause mortality at 5 years. Secondary endpoints were (i) clinical response with at least one class improvement in New York Heart Association without HF hospitalization and (ii) reverse remodelling with 15% reduction in left ventricular end-systolic volume at 6 months. Fifty-four (41.5%) patients died during 5 years, 85 (65.3%) clinical responders were identified, and 63 patients (48.5%) had reverse remodelling. Baseline issues with mobility were associated with lower response [odds ratio (OR) 0.36, 95% confidence interval (CI) 0.16–0.84; P = 0.018]. Lack of reverse remodelling correlated with self-care issues at baseline (OR 0.10, 95% CI 0.01–0.94; P = 0.04). Furthermore, self-care difficulties [hazard ratio (HR) 2.39, 95% CI 1.17–4.86; P = 0.01) or more anxiety (HR 1.51, 95% CI 1.00–2.26; P = 0.04) predicted worse long-term survival. At 6 months, mobility (HR 3.95, 95% CI 1.89–8.20; P < 0.001), self-care (HR 7.69, 95% CI 2.23–25.9; P = 0.001), or ≥ 10% visual analogue scale (VAS) (HR 2.24, 95% CI 1.27–3.94; P = 0.005) improvement anticipated better survival at 5 years.
Conclusion
EuroQol-five dimension is a simple method assessing QoL in CRT population. Mobility issues at baseline are associated with lower clinical response, whereas self-care issues predict lack of reverse remodelling. Problems with mobility or anxiety before CRT and persistent issues with mobility, self-care, and VAS scale at 6 months predict adverse outcome.
Keywords: Quality of life, EQ-5D, Responder, Reverse remodelling, Survival, Cardiac resynchronisation therapy
What’s new?
EuroQol-five dimensions (EQ-5D) questionnaire is a simple method to assess quality of life (QoL) in cardiac resynchronization therapy (CRT) patients.
The different domains of EQ-5D were able to predict long-term mortality, clinical response, and reverse remodelling in our CRT patient population.
Issues with mobility before CRT was associated with lower clinical response.
Baseline problems with self-care predicted lower rate of reverse remodelling.
In patients with CRT indication, self-care difficulties, and more anxiety/depression, lack of improvement in mobility, self-care, and visual analogue scale of current health status predicted mortality at 5 years.
Based on our results, it should be considered to integrate the use of EQ-5D questionnaire in the everyday clinical practice for better patient selection and follow-up care.
Introduction
Cardiac resynchronization therapy (CRT) is an effective therapeutic option for patients with worsening systolic heart failure (HF) and dys-synchrony, despite optimal medical treatment.1,2 Moreover, it can help improving functional status and provides a better quality of life (QoL) in more than 60% of CRT patients, responders to CRT therapy. After CRT implantation, the improvement in clinical status can be assessed by several diagnostic techniques, but the subjective QoL has superior importance.3 However, it is not in the routine use as it is believed to be a less objective marker.
Several specific questionnaires were established to measure the functional and mental status of patients. The EuroQoL five dimensions (EQ-5D) questionnaire is focused on assessing the general health status in a simple and easy to obtain way. This questionnaire is mainly used for assessing response to CRT therapy, but its prognostic significance in this patient population has not been studied yet.4 It is, furthermore, not known whether baseline QoL or changes in QoL at 6 months predict reverse remodelling, clinical response, or all-cause mortality.
This study aimed to (i) assess the predictive value of baseline QoL and changes in QoL at 6 months to predict all-cause mortality at 5 years, (ii) evaluate the value of baseline and 6 months EQ-5D to predict functional improvement, and (iii) elucidate baseline and 6 months QoL to predict reverse remodelling.
Methods
In our centre, a prospective, single-centre observational, clinical study was performed from September 2009 to December 2010. A total of 141 consecutive, chronic HF patients with CRT indication were enrolled, to identify predictors of CRT response and outcomes, as described elsewhere.5–7 According to the current guidelines in that period, the inclusion criteria were as follows: congestive, symptomatic HF [New York Heart Association (NYHA) II–IVa functional class] treated with maximal tolerated medical therapy for at least 3 months. Left ventricular ejection fraction (LVEF) below 35%, QRS duration broader than 120 ms, measured on surface electrocardiogram (ECG) using the widest QRS complex from II, V1, and V6 leads.8,9 Patients with known malignancies, diagnosed inflammatory diseases, and primary genetic cardiomyopathies were excluded, resulting in a total of four patient exclusions. All patients provided written informed consent. The study adhered to the Declaration of Helsinki and was approved by the local ethics committee of the Semmelweis University. The predictive role of laboratory biomarkers in the cohort was described previously.5–7
Study protocol
The study protocol consisted of baseline examination and follow-up at 6 months and 5 years. At baseline, prior to CRT implantation medical history was obtained, EQ-5D questionnaire completion, physical examination, ECG recording, and echocardiography were performed. Seven patients did not consent to complete QOL questionnaire or had incomplete data, thus altogether 130 patients were included in this analysis. Ischaemic aetiology was confirmed based on earlier documented history of myocardial infarction and/or coronary angiography performed <1 year before CRT. At the 6 months of follow-up, physical status was examined, EQ-5D was filled out, and ECG and device interrogation were performed.
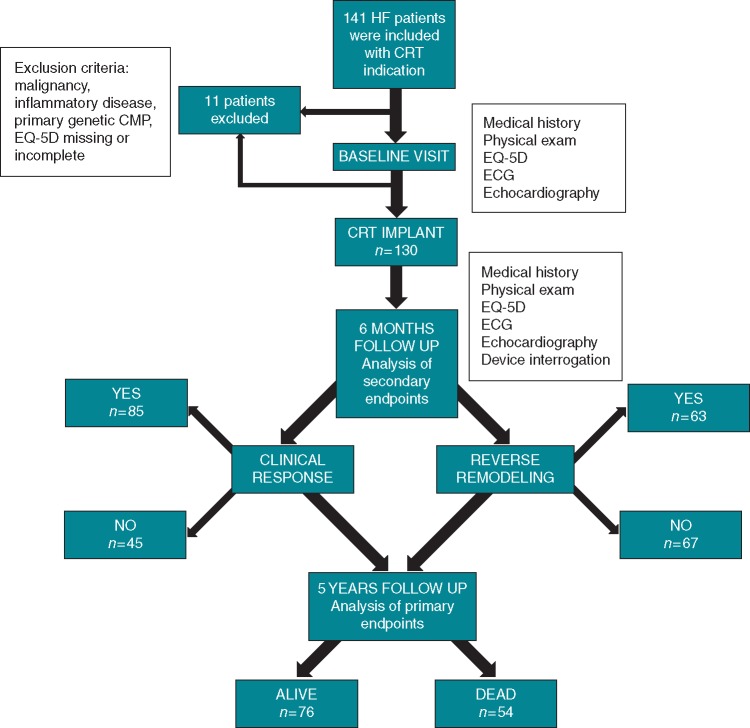
The primary endpoint was all-cause mortality at 5 years after CRT implantation. Secondary endpoints were reverse remodelling and clinical response to therapy. Reverse remodelling was defined as an absolute 15% reduction in left ventricular end-systolic volume (LVESV), whereas clinical response was determined with at least one class improvement in NYHA status and lack of HF hospitalization at 6 months following implantation. Figure 1 shows the flowchart of the study design and outcomes for the endpoints.
Figure 1.
Flowchart of the study design and outcomes regarding the primary and secondary endpoints.
EuroQoL five dimensions questionnaire
The official Hungarian version of the EQ-5D (www.euroqol.org) questionnaire was filled out before and 6 months after CRT implantation by the patients. The EQ-5D is a self-administered, generic, validated, multi-attribute preference-based utility instrument to measure QoL. It provides a simple descriptive profile for health status in wide range of diseases and treatments, also proved to be applicable in HF patients.10 The questionnaire consists of five measures: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.11 All domains have three levels: no, some, and extreme problems. Respondents were asked to rate the severity of their current issues (Level 1 = none, Level 2 = some/moderate, and Level 3 = severe/extreme). Based on that, patients could be classified into 243 health states and 2 additional states (unconscious and dead). It also includes a single-index visual analogue scale (VAS) for global assessment of health status, where patients can individually estimate their own health state between 0 (‘worst imaginable health status’) and 100 (‘best imaginable health status’).
Echocardiographic parameters
Standard transthoracic echocardiography was performed before and 6 months after CRT implantation with a cardiovascular ultrasound system (iE33, Philips Healthcare, Best, The Netherlands). For 2D imaging, S5-1 transducer (Philips Healthcare) was used. In left lateral supine position of the patient, standard parasternal short-, long-axis, and apical views were acquired. All parameters were measured three times in separate cardiac cycles with the averages used for further analysis. Left ventricular end-diastolic volume (LVEDV) and LVESV were determined, and LVEF was calculated with the modified Simpson’s biplane method.
Device implantation
Cardiac resynchronization therapy was implanted successfully in all patients without major complications. The right atrial (where appropriate) and right ventricular (RV) leads were positioned in the right appendage and mid-interventricular septum or in the RV apex, respectively. Coronary sinus venography was used for LV lead positioning. Lateral and posterolateral cardiac veins were preferred for LV lead implantation. Implanted devices were Contak Renewal (Guidant Corp., Arden Hills, MN, USA). Cognis (Boston Scientific, Marlborough, MA, USA), Stratos, Lumax (Biotronik, Berlin, Germany), and Insync III (Medtronic Inc., Minneapolis, MN, USA).
Statistical analysis
Categorical variables were expressed as absolute numbers and percentages, whereas continuous variables were presented as medians and interquartile ranges. The evaluation of EQ-5D questionnaire was simplified, meaning that the presence of some and extreme problem categories were not assessed separately regarding the five elements. Thus, two subgroups were created: (i) patients with no reported issues in each domain and (ii) patients with mild or severe reported issues in each domain, and the subgroups were compared using the χ2 test. In this study, the variables deviated from normal distribution (analysed by Shapiro–Wilk test). Therefore, continuous variables were compared using the Mann–Whitney’s non-parametric test.
We constructed a multivariable Cox model, and those variables that were statistically significant with univariate Cox analysis were included. Logarithmic transformation of continuous variables was used, ranked, and then standardized by 1 SD increase. In the multivariable Cox analyses, forward stepwise models were applied to find independent predictors of mortality. The proportional hazard assumption was evaluated using Schoenfeld residuals for continuous covariates and log minus log plots for categorical variables.12
Statistical significance was set at a two-tailed P-value <0.05. Data analysis was performed with Graphpad Prism 6.03 (GraphPad Softwares Inc., San Diego, CA, USA) and IBM SPSS 21 (Apache Software Foundation, Forest Hill, MD, USA).
Results
Patient population and clinical data
A total of 130 patients were included in this analysis. At 6 months following CRT, 63 (48.5%) patients had positive reverse remodelling with greater than 15% reduction in LVESV, and 85 (65.3%) were clinical responders with at least one grade improvement in NYHA class and lack of HF hospitalization. During the 5 years of follow-up, 54 (41.5%) patients died (Figure 1).
Baseline clinical and echocardiographic parameters are shown in Table 1. The median age was 67 years, and patients were predominantly male (80%). More than half of the population had ischaemic HF, with NYHA III–IV functional class (87%) at baseline. Median LVEF was 28% (23.0–33.0). Majority of the patients (83%) had left bundle branch block morphology (LBBB) on ECG, with a median QRS duration of 163 ms.
Table 1.
Baseline clinical characteristics of the population
| Total cohort (n = 130) | |
|---|---|
| Age (years) | 67.3 (60.3–73.3) |
| Gender (male) | 104 (80) |
| Ischaemic aetiology | 75 (58) |
| NYHA III–IV | 113 (87) |
| LBBB | 108 (83) |
| QRS-duration (ms) | 163.0 (140.5–184.1) |
| Persistent AF | 37 (28) |
| BMI (kg/m2) | 27.0 (24.0–30.0) |
| Diabetes | 48 (37) |
| Hypertension | 71 (55) |
| BB | 117 (90) |
| ACEi/ARB | 124 (95) |
| Diuretics | 106 (82) |
| Digitalis | 39 (30) |
| Amiodarone | 39 (30) |
| LVEF (%) | 27.5 (23.0–33.0) |
| LVEDV (mL) | 312.6 (250.6–361.2) |
| LVESV (mL) | 218.2 (153.6–276.3) |
| Issues identified on the EQ-5D questionnaire | |
| Mobility | 70 (54) |
| Self-care | 11 (8) |
| Usual activities | 62 (48) |
| Pain/discomfort | 86 (66) |
| Anxiety/depression | 50 (38) |
| VAS (%) | 50.0 (35.0–65.0) |
Data are expressed as median (interquartile range) for continuous variables and as n (%) for categorical variables.
ACEi/ARB, angiotensin convertase inhibitor/angiotensin receptor blocker; BB, beta-blocker; LBBB, left bundle branch block; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MI, mitral insufficiency; NYHA, New York Heart Association Classification; VAS, visual analogue scale.
Clinical predictors of response, reverse remodelling, and mortality
Before analysing QoL, we examined the predictive value of baseline clinical data in our population using univariate Cox analysis. When assessing clinical response, ischaemic HF [odds ratio (OR) 0.48, 95% confidence interval (CI) 0.23–1.04; P = 0.05] and decreased LVEF (OR0.66, 95% CI 0.45–0.97; P = 0.03) were associated with a higher risk of clinical non-response. Lack of reverse remodelling was predicted by increasing age (OR 0.56, 95% CI 0.37–0.85; P = 0.006), NYHA III–IV vs. I–II functional class (OR 0.19, 95% CI 0.05–0.72; P = 0.014), and an increased LVEDV (OR 1.55, 95% CI 1.06–2.27; P = 0.02) in univariate analysis.
Furthermore, the absence of LBBB ECG morphology [hazard ratio (HR) 0.39, 95% CI 0.21–0.971; P = 0.002), beta-blocker use (HR 0.36, 95% CI 0.18–0.75; P = 0.006), diuretics intake (HR 3.40, 95% CI 1.23–9.43; P = 0.02), and the presence of persistent atrial fibrillation (AF) (HR 1.90, 95% CI 1.11–3.25; P = 0.018) were associated with a higher risk of death.
Baseline quality of life and change at 6 months of follow-up
Before CRT implantation, 70 (54%) patients had mobility issues, 11 (8.5%) patients had self-care limitations, 62 (48%) patients reported difficulties with usual activities, 86 (66%) patients had pain or discomfort, and 50 (38%) patients had anxiety or depression (Table 1). The median general health status represented by the VAS scale was 50% (30.0–65.0). At 6 months of follow-up, there was a significant improvement in usual activities (P = 0.03) and pain/discomfort (P = 0.03). The overall health state based on the VAS scale also improved (P < 0.001).
Association between baseline quality of life with clinical response and reverse remodelling after cardiac resynchronization therapy
Univariate Cox regression analysis for clinical response showed that only problems with mobility before implantation were significantly associated with non-response (OR 0.44, 95% CI 0.21–0.94; P = 0.035). For patients with self-care problems at baseline, the rate of reverse remodelling was significantly less (OR 0.11, 95% CI 0.01–0.89; P = 0.039). Reverse remodelling also correlated with baseline mobility issues (OR 0.48, 95% CI 0.23–1.01; P = 0.05) (Table 2).
Table 2.
Issues identified at the different baseline QoL elements as univariate predictors of clinical response, reverse remodelling, and mortality following CRT
| EQ-5D baseline | OR | 95% CI | χ2 | P-value | |
|---|---|---|---|---|---|
| Clinical response | |||||
| Mobility | 0.44 | 0.21–0.94 | 34.46 | 0.03 | |
| Self-care | 0.61 | 0.17–2.11 | 0.61 | 0.43 | |
| Usual activities | 1.06 | 0.52–2.19 | 0.03 | 0.86 | |
| Pain | 0.71 | 0.32–1.54 | 0.75 | 0.38 | |
| Anxiety | 0.59 | 0.28–1.23 | 1.94 | 0.16 | |
| VAS scale | 0.97 | 0.67–1.40 | 0.02 | 0.87 | |
| Reverse remodelling | |||||
| Mobility | 0.48 | 0.23–1.01 | 3.71 | 0.05 | |
| Self-care | 0.11 | 0.01–0.89 | 4.26 | 0.03 | |
| Usual activities | 0.64 | 0.31–1.33 | 1.41 | 0.23 | |
| Pain | 0.82 | 0.38–1.75 | 0.26 | 0.61 | |
| Anxiety | 1.21 | 0.57–2.56 | 0.25 | 0.62 | |
| VAS scale | 1.02 | 0.71–1.47 | 0.02 | 0.89 | |
| Mortality at 5 years | HR | 95% CI | χ2 | P-value | |
| Mobility | 1.75 | 1.00–3.03 | 3.89 | 0.04 | |
| Self-care | 2.22 | 1.00–4.92 | 3.85 | 0.05 | |
| Usual activities | 1.13 | 0.66–1.93 | 0.21 | 0.65 | |
| Pain | 1.55 | 0.84–2.85 | 1.98 | 0.16 | |
| Anxiety | 1.92 | 1.12–3.27 | 5.67 | 0.01 | |
| VAS scale | 0.87 | 0.66–1.13 | 1.09 | 0.29 | |
Hazard ratios refer to 1 SD increase.
CI, confidence interval; HR, hazard ratio; OR, odds ratio; VAS, visual analogue scale; χ2, Wald chi square.
P value lower than 0.05 was considered statistically significant.
In multivariate Cox model adjusted for relevant clinical variables, such as reverse remodelling: age, NYHA class, and LVEDV and clinical response: LVEF and ischaemic aetiology, only baseline problems with self-care proved to be an independent predictor of reverse remodelling (OR 0.10, 95% CI 0.01–0.94; P = 0.04, Table 3). Regarding clinical response, problems with mobility before CRT had an independent predictive value in our patient population (OR 0.36, 95% CI 0.16–0.84; P = 0.018).
Table 3.
Multivariate analysis of issues identified at the different baseline QoL elements in the prediction of clinical response, reverse remodelling, and mortality following CRT
| OR | 95% CI | χ2 | P-value | |
|---|---|---|---|---|
| Clinical response | ||||
| Mobility | 0.36 | 0.16–0.84 | 5.62 | 0.01 |
| Echo responder | ||||
| Self-care | 0.10 | 0.01–0.94 | 4.06 | 0.04 |
| Mortality at 5 years | HR | 95% CI | χ2 | P-value |
| Self-care | 2.39 | 1.17–4.86 | 5.78 | 0.01 |
| Anxiety/depression | 1.51 | 1.00–2.26 | 3.93 | 0.04 |
Hazard ratios refer to 1 SD increase. Clinical responder model was adjusted for LVEF and ischaemic HF; echocardiographic responder model was adjusted for age, NYHA, and LVEDV; and mortality at 5 years was adjusted for LBBB, presence of AF, BB, and diuretics intake.
CI, confidence interval; HR, hazard ratio; OR, odds ratio; χ2: Wald chi square.
P value lower than 0.05 was considered statistically significant.
Baseline quality of life and quality of life change as predictors of mortality at 5 years
From baseline QoL elements, problems with mobility (HR 1.75, 95% CI 1.00–3.03; P = 0.04), self-care (HR 2.22, 95% CI 1.00–4.92; P = 0.05), and anxiety/depression (HR 1.92, 95% CI 1.12–3.27; P = 0.02) were significantly associated with mortality at 5 years (Table 2). Multivariate Cox analysis adjusted for LBBB morphology, BB intake, diuretics intake, and persistent AF showed self-care problems (HR 2.39, 95% CI 1.17–4.86; P = 0.01), and presence of anxiety/depression (HR 1.51, 95% CI 1.00–2.26; P = 0.04) to be significant predictors of mortality at 5 years. Moreover, those patients who reported improvement in mobility (HR 0.25, 95% CI 0.12–0.53; P < 0.001], self-care (HR 0.13, 95% CI 0.04–0.44; P = 0.0001), and at least 10% improvement in their VAS scale (HR 0.45, 95% CI 0.25–0.78; P = 0.0005) had significantly better survival at 5 years of follow-up (Table 4).
Table 4.
Changes in QoL elements and the VAS scale as univariate predictors of mortality following CRT
| Mortality at 5 years | HR | 95% CI | χ2 | P-value |
|---|---|---|---|---|
| Mobility | 0.25 | 0.12–0.53 | 13.53 | <0.0001 |
| Self-care | 0.13 | 0.04–0.44 | 10.82 | 0.001 |
| Usual activities | 0.53 | 0.24–1.16 | 2.51 | 0.11 |
| Pain/discomfort | 0.48 | 0.21–1.09 | 3.03 | 0.08 |
| Anxiety/depression | 0.91 | 0.38–2.18 | 0.04 | 0.83 |
| VAS 10% improvement | 0.45 | 0.25–0.78 | 7.77 | 0.005 |
Hazard ratios refer to 1 SD increase.
CI, confidence interval; HR, hazard ratio; VAS, visual analogue scale; QoL, change was defined as one point increase in QoL elements and 10% improvement in VAS scale at 6 months predictive of 5 years of mortality during univariant Cox regression analysis; χ2, Wald chi square.
P value lower than 0.05 was considered statistically significant.
During the Kaplan–Meier analysis, patients who had issues with mobility (P = 0.04), self-care (P = 0.04), and had more anxiety or depression (P = 0.01) before CRT implantation had significantly lower long-term survival rate (Figure 2A–C).
Figure 2.
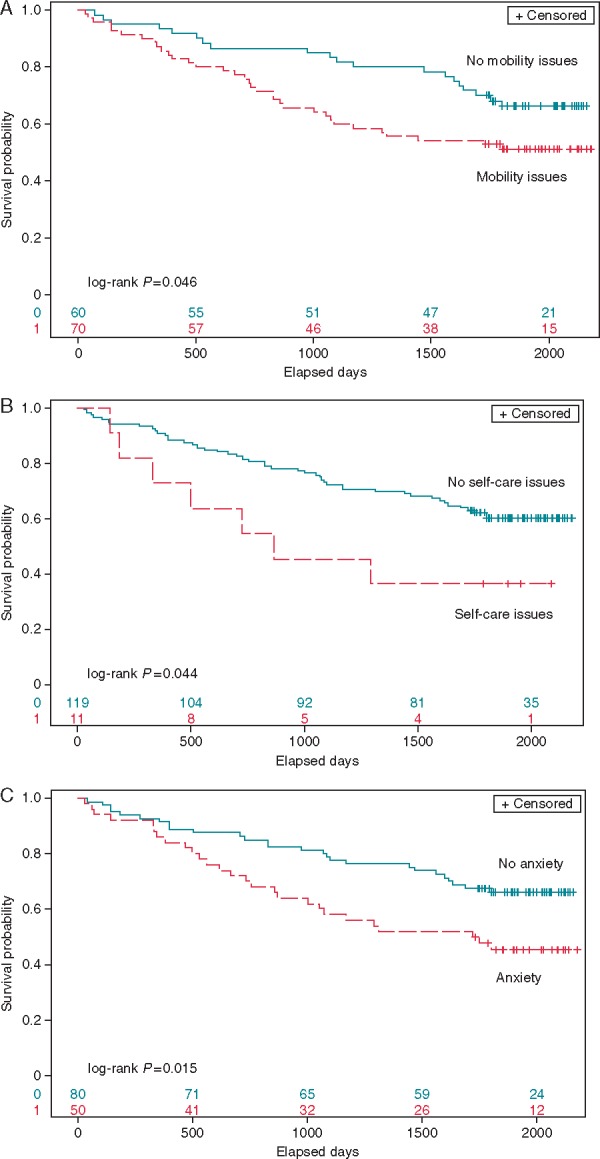
The Kaplan–Meier survival analyses of probability of freedom of long-term all-cause mortality after CRT according to baseline issues of (A) mobility, (B) self-care, and (C) anxiety/depression.
Discussion
Our investigation revealed that QoL in CRT candidates is a strong predictor of both clinical and echocardiographic response and long-term all-cause mortality. Therefore, the EQ-5D general health questionnaire could be an easy to obtain, simple parameter to predict outcomes in CRT patients.
There are several prior descriptive studies that reported on QoL and QoL change in HF patients implanted with CRT.13–16 In most of these investigations, HF-specific questionnaires were used in different HF populations with CRT indication. Turley et al.17 investigated the effect of CRT on survival and QoL in patients with end-stage HF. This was a systematic review of 356 papers, from which 9 papers, 4 randomized controlled trials (COMPANION, MUSTIC, CARE-HF, and MIRACLE), and 5 meta-analyses were selected to represent the best evidence. In all included investigations, CRT improved health-related QoL.17 Additionally, in the study of Becker et al.18, QoL and survival was assessed in 105 patients with CRT for severe HF and in 112 heart transplant (HTX) recipients using the Medical Outcome Short Form 36 (SF-36). They found no significant differences in the subjective measures of health-related QoL between patients on CRT and after HTX. They concluded that contemporary management of patients with advanced HF including CRT diminishes the difference between HTX and conservative HF treatment.18 On the other hand, patients with minimally symptomatic HF with CRT indication were investigated by Veazie et al. in a MADIT-CRT subanalysis. Quality of life was measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ).19 In this study, CRT was associated with improvement in HF specific QoL compared with the Implantable Cardioverter Defibrillator (ICD)-only group. However, due to the better baseline functional status of these patients, the improvement of QoL after CRT was not significant in the CRT group. These findings were statistically significant only in patients with LBBB. However, there were limited studies in advanced HF patients to assess the effects of baseline QoL and change in QoL to predict outcomes. Our study has important clinical implications suggesting that baseline QoL and changes in QoL predict response and outcomes and could be used as a surrogate marker for CRT benefit.
The effects of CRT on health-related QoL was also investigated in an elderly population of 21 HF patients by Hoth et al.20 Heart failure-specific QoL questionnaires were used before and 3 months after CRT implantation, when better physical status was observed. In this study, younger age was significantly associated with improvement in physical status.20 In a cost-effectiveness subanalysis of the MADIT-CRT trial, which evaluated 4-year cost-effectiveness of CRT-ICD compared with ICD alone, EQ-5D questionnaires were used to assess patient QoL. The KCCQ scores averaged similar for the CRT-ICD and ICD-only groups at baseline, with statistically significant improvement in the subsequent scores in each of the groups; however, the CRT group showed greater improvement in QoL.21
The appropriateness of EQ-5D questionnaire in HF patients has been shown earlier. Calvert et al.10 published a substudy of the CARE-HF trial, using EQ-5D and Minnesota Living with Heart Failure Questionnaires. They found that EQ-5D is an acceptable, valid measure in patients with HF.10 In a subanalysis of the same trial, Cleland et al.3 investigated the effects of CRT on long-term QoL, which was measured at baseline, 3 months, and 18 months post-implantation and at the study end using EQ-5D questionnaire. Based on the 5D and three levels, patients were classified into 243 (35) health states plus two further additional states (unconscious and dead). The EQ-5D health states were converted into an EQ-5D use score ranging from −0.594 to 1.0 using a set of weighted preferences produced from the UK population. At baseline, impaired EQ-5D scores were found, but at 18 months, QoL was significantly better in CRT patients primarily because of improved functional capacity.3
Despite of the large number of QoL investigations in HF patients, limited data are available on the predictive value of QoL in CRT implanted patients. Recently, Lenarczyk et al.22 published the association of QoL with response and outcome in CRT-implanted patients. Ninety-seven participants of the Triple-Site vs. Standard Cardiac Resynchronization Therapy Trial (TRUST CRT) were included. Minnesota-QoL questionnaires were completed prior to CRT implantation and 6 months after CRT. Data on major adverse cardiac events (death, HF hospitalization, and heart transplant) were collected during the next 2.5 years. Clinical response, but not echocardiographic response, was associated with improved QoL. Subjects without QoL improvement were significantly more prone to experience MACE (61% vs. 32%) and die (44% vs. 18%) within the follow-up. Unimproved QoL increased the probability of future Major Adverse Cardiovascular Event (MACE) by 2.7 times and death by 3.2 times independently from clinical and echocardiographic response.22 In our study, we showed similar outcome prediction with the simple and easy to obtain EQ-5D questionnaire in a larger group of traditional CRT patients. Patients in the TRUST CRT trial included a patient group with two LV electrodes and triple-site pacing; therefore, their findings cannot be externally validated to the CRT cohort. Another strength of our analysis is the ability to assess the predictive value of baseline QoL and changes in QoL during long-term 5 years of follow-up.
Limitations
Some limitations, however, exist regarding to the current investigation. This was a single-centre, observational study with relatively low sample size. All-cause mortality was the primary endpoint of the study, and no distinction was made between cardiovascular and non-cardiovascular death. Furthermore, there are more specific and complex tools for assessment of QoL in HF patients; however, we intended to use the simplest method to assess QoL for wider clinical application. This is a self-administered questionnaire, regarding the 5D the patients answer individually if they have problem and the seriousness of problem (none, some, and severe) regarding the different domains. Therefore we are lack of the ability to precisely determine what extent of a problem the patient filling out the form adjudicate as severe. Larger studies are needed to confirm our results, which might allow better understanding and identification of possible mechanisms or patients at risk.
Conclusions
We suggest that the EQ-5D health questionnaire is an easily obtainable method to assess QoL in severe HF patients undergoing CRT implantation. Our results show that issues with mobility and more anxiety before CRT, the lack of improvement in mobility, self-care, and VAS scale at 6 months predict long-term mortality. Furthermore, mobility issues at baseline are associated with lower clinical response, whereas self-care problems are linked with lower rate of reverse remodelling. Integrating the use of a simple measurement of EQ-5D could be considered for better patient selection and follow-up care.
Funding
This work was supported by the National Development Agency of Hungary (‘Semmelweis Egyetem Híd Projekt’ (TÁMOP-4.2.2-08/1/KMR-2008-0004), ‘Semmelweis Egyetem Magiszter Program’ (TÁMOP-4.2.2./B10/1.-210-0013), the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (G.S., L.G.), and the Hungarian Scientific Research Found (OTKA K 105555).
Conflict of interest: none declared.
References
- 1. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 2. Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S. et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002;40:111–8. [DOI] [PubMed] [Google Scholar]
- 3. Cleland JG, Calvert MJ, Verboven Y, Freemantle N.. Effects of cardiac resynchronization therapy on long-term quality of life: an analysis from the CArdiac Resynchronisation-Heart Failure (CARE-HF) study. Am Heart J 2009;157:457–66. [DOI] [PubMed] [Google Scholar]
- 4. Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ.. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010;8:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Széplaki G, Boros AM, Szilágyi S, Osztheimer I, Jenei Z, Kosztin A. et al. Complement C3a predicts outcome in cardiac resynchronization therapy of heart failure. Inflamm Res 2016;65:933–40. [DOI] [PubMed] [Google Scholar]
- 6. Boros AM, Perge P, Jenei Z, Karády J, Zima E, Molnár L. et al. Measurement of the red blood cell distribution width improves the risk prediction in cardiac resynchronization therapy. Dis Markers 2016;2016:7304538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boros AM, Széplaki G, Perge P, Jenei Z, Bagyura Z, Zima E. et al. The ratio of the neutrophil leucocytes to the lymphocytes predicts the outcome after cardiac resynchronization therapy. Europace 2016;18:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor J. Focused update of the ESC Guidelines on device therapy in heart failure. Eur Heart J 2010;31:2559–60. [DOI] [PubMed] [Google Scholar]
- 9. Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J. et al. 2010 focused update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur J Heart Fail 2010;12:1143–53. [DOI] [PubMed] [Google Scholar]
- 10. Calvert MJ, Freemantle N, Cleland JG.. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail 2005;7:243–51. [DOI] [PubMed] [Google Scholar]
- 11. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D. et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrell FE, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist. Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 13. De Marco T, Wolfel E, Feldman AM, Lowes B, Higginbotham MB, Ghali JK. et al. Impact of cardiac resynchronization therapy on exercise performance, functional capacity, and quality of life in systolic heart failure with QRS prolongation: COMPANION trial sub-study. J Card Fail 2008;14:9–18. [DOI] [PubMed] [Google Scholar]
- 14. Chen S, Yin Y, Krucoff MW.. Effect of cardiac resynchronization therapy and implantable cardioverter defibrillator on quality of life in patients with heart failure: a meta-analysis. Europace 2012;14:1602–7. [DOI] [PubMed] [Google Scholar]
- 15. Ford J, Sears S, Ramza B, Reynolds DW, Nguyen P, Fedewa M. et al. The Registry Evaluating Functional Outcomes of Resynchronization Management (REFORM): quality of life and psychological functioning in patients receiving cardiac resynchronization therapy. J Cardiovasc Electrophysiol 2014;25:43–51. [DOI] [PubMed] [Google Scholar]
- 16. Gilliam FR, Kaplan AJ, Black J, Chase KJ, Mullin CM.. Changes in heart rate variability, quality of life, and activity in cardiac resynchronization therapy patients: results of the HF-HRV registry. Pacing Clin Electrophysiol 2007;30:56–64. [DOI] [PubMed] [Google Scholar]
- 17. Turley AJ, Raja SG, Salhiyyah K, Nagarajan K.. Does cardiac resynchronisation therapy improve survival and quality of life in patients with end-stage heart failure? Interact Cardiovasc Thorac Surg 2008;7:1141–6. [DOI] [PubMed] [Google Scholar]
- 18. Becker M, Erdmann N, Stegemann E, Benke D, Schauerte PN, Schaefer WM. et al. Survival and quality of life in patients with cardiac resynchronization therapy for severe heart failure and in heart transplant recipients within a contemporary heart failure management program. J Heart Lung Transplant 2008;27:746–52. [DOI] [PubMed] [Google Scholar]
- 19. Veazie PJ, Noyes K, Li Q, Hall WJ, Buttaccio A, Thevenet-Morrison K. et al. Cardiac resynchronization and quality of life in patients with minimally symptomatic heart failure. J Am Coll Cardiol 2012;60:1940–4. [DOI] [PubMed] [Google Scholar]
- 20. Hoth KF, Nash J, Poppas A, Ellison KE, Paul RH, Cohen RA.. Effects of cardiac resynchronization therapy on health-related quality of life in older adults with heart failure. Cia 2008;3:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noyes K, Veazie P, Hall WJ, Zhao H, Buttaccio A, Thevenet-Morrison K. et al. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol 2013;24:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lenarczyk R, Jędrzejczyk-Patej E, Mazurek M, Szulik M, Kowalski O, Pruszkowska P. et al. Quality of life in cardiac resynchronization recipients: association with response and impact on outcome. Pacing Clin Electrophysiol 2015;38:8–17. [DOI] [PubMed] [Google Scholar]