Highlights
-
•
H reflexes can be obtained from many upper and lower limb muscles (e.g., tibialis anterior, the thenar muscles and forearm extensors) during a voluntary contraction of the test muscle.
-
•
The contraction raises motoneuron excitability and decreases the Ib limitation on the reflex.
-
•
H reflexes recorded at rest from muscles for which no reflex can normally be demonstrated is evidence of hyperreflexia, raising suspicion of ALS if there is denervation in that muscle.
Keywords: H reflex, Motoneuron excitability, EPSP, Plexus and nerve root lesions, Hyperreflexia, ALS
Abstract
H reflexes can be recorded from virtually all muscles that have muscle spindles, but reflex reinforcement may be required for the reflex response to be demonstrable. This can allow conduction across proximal nerve segments and most nerve root segments commonly involved by pathology. Stimulus rate is critical in subjects who are at rest. However the reflex attenuation with higher rates is greatly reduced during a background contraction of the test muscle, with only minor changes in latency if any. In addition the contraction ensures that the reflex response occurs in the desired muscle. Reflex latencies should be corrected for height (or limb length) and age. Because the reflex discharge requires a synchronised volley in group Ia afferents, large increases in reflex latency occur rarely with purely sensory lesions. If the H reflex of soleus, quadriceps femoris or flexor carpi radialis is absent at rest but appears during a voluntary contraction at near-normal latency, there is either low central excitability or a predominantly sensory abnormality. With the former H reflexes will be difficult to elicit throughout the body. If H reflexes can be recorded at rest from muscles for which no reflex can normally be demonstrated, there is good evidence for hyperreflexia. In the context of possible ALS, this is an important finding when there is EMG evidence of chronic partial denervation in that muscle.
The H reflex was first described in 1910 and 1918 by Hoffmann, 1910, Hoffmann, 1918 (hence the name), its basis was investigated in a series of papers by Magladery and colleagues (e.g., Magladery and McDougal, 1950, Magladery et al., 1951), and it was introduced into motor control studies by Paillard in 1955. It has been the subject of numerous reviews since then (e.g., Hugon, 1973, Schiepatti, 1987, Burke et al., 1999, Pierrot-Deseilligny and Mazevet, 2000, Fisher, 1992, Fisher, 2002, Zehr, 2002, Misiaszek, 2003, Knikou, 2008, Pierrot-Deseilligny and Burke, 2012).
The H reflex is perhaps one of the simplest reflexes that can be recorded in human subjects, and it has become a standard tool in motor control studies. However, the results of some of these studies do not survive critical analysis because they are based on overly simple views of the underlying mechanisms. This review will highlight some aspects of the underlying physiology and a number of misconceptions that are still taught and continue to plague the literature. In motor control research, the H reflex has been used too much without a full appreciation that it is actually not the simple “two-neurone reflex” envisaged by Magladery et al. (1951). On the other hand, in clinical practice the H reflex has been under-utilised, based on the misconception that it can only be recorded reliably for soleus.
Perhaps the major use for H reflex studies is that they allow the clinician to study conduction across proximal segments (i.e., plexuses and nerve roots) of the peripheral nerve and, in the author’s experience, they do so better than alternative techniques. Somatosensory evoked potentials (SEPs) allow sensory axons to be studied. F waves and spinal/nerve root stimulation allow motor axons to be studied. These tests provide different information (purely sensory, purely motor, sensory and motor), and they are therefore not equally sensitive in different pathologies. Other considerations affect the choice of which test should be used in a given situation.
To obtain a reflex response, the afferent volley must be synchronised when it reaches the motoneuron pool, and it must produce a compound excitatory postsynaptic potential (EPSP) sufficient to discharge the motoneurons of lowest threshold. Pathological dispersion of the afferent volley will delay a reflex response and reduce its size. When extreme, dispersion could abolish the reflex response completely even if all afferent axons were capable of conducting. Loss of conducting axons without slowing conduction can also cause a small conduction delay because threshold for the motoneuron discharge is reached later on the compound EPSP. As a result, the reflex response is sensitive to subtle changes, and may be absent when there is no clinically detectable deficit.
1. Underlying physiology
1.1. The H reflex may depend on monosynaptic excitation but it is not an exclusively monosynaptic reflex
Group Ia afferents from the primary endings of muscle spindles form the afferent limb for the H reflex. Ia afferents have a strong monosynaptic excitatory connection with motoneurons of the homonymous pool, but there are also weaker monosynaptic projections to heteronymous (synergistic) pools and there are probably oligosynatic projections to homonymous and synergistic motoneurons (see Pierrot-Deseilligny and Burke, 2012).
It is not possible to stimulate Ia afferents in isolation. Group Ib afferents from Golgi tendon organs are of the same size and, even with weak stimuli, they will be activated equally effectively. Assuming that conduction across the Ib inhibitory interneuron (now termed the “non-reciprocal group I inhibitory interneuron”) is intact, group Ib inhibition will begin in the motoneuron pool 0.5–1.0 ms after the onset of group Ia excitation, this delay being that required to cross an interneuron. This would not matter if the group Ia excitation was brief but, based on probable conduction velocities (Macefield et al., 1989, Pierrot-Deseilligny and Burke, 2012), the slowest group Ia afferents from soleus would reach soleus motoneurons 5–7.5 ms after the fastest (Pierrot-Deseilligny and Burke, 2012). There is therefore ample opportunity for Ib inhibition to truncate the Ia excitation and limit the size of the reflex response. There is now evidence that this does occur (see below).
That an inhibitory volley that reaches the motoneuron 1 ms after the onset of excitation will truncate the excitation is well established in the cat (Araki et al., 1960). There is evidence for this in human subjects. Although the Ia excitatory input lasts some 5–7.5 ms, the rising phase of the compound group I EPSP in human soleus motoneurons is remarkably brief: estimated to be only 1–2 ms (Birnbaum and Ashby, 1982, Burke et al., 1983, Burke et al., 1984). Group Ib inhibition due to medial gastrocnemius Ib afferents begins only 1 ms after the onset of monosynaptic excitation in soleus motoneurons (Pierrot-Deseilligny et al., 1981), so that the net effect on soleus motoneurons will be the sum of opposite Ia and Ib effects. It has been shown for quadriceps femoris that changes in transmission of the Ib component of the group I volley across the Ib inhibitory interneuron can change the size of the H reflex of quadriceps (Marchand-Pauvert et al., 2002). Accordingly, while the excitation underlying the H reflex may be largely monosynaptic, the reflex discharge will be determined by the balance between Ia excitation and Ib inhibition and will vary with the excitability of the Ib inhibitory interneuron.
1.2. The H reflex and the tendon jerk
The H reflex is generally considered the electrical equivalent of the tendon jerk, differing only in that the H reflex bypasses muscle spindle mechanisms. This belief has led to comparisons of the two reflexes as a means of assessing the sensitivity of muscle spindle endings and, by inference, the level of fusimotor drive. The assumption that the only significant difference between the reflexes involves muscle spindle mechanisms ignores other well-established differences (Table 1), and has led to many invalid conclusions in the literature (see Burke, 1983, Pierrot-Deseilligny and Burke, 2012).
Table 1.
Differences between the H reflex and the tendon jerk.
|
The excitatory pathway underlying the H reflex and tendon jerk may be the same, but the impulse traffic over that pathway differs. However, this does not invalidate the usefulness of timed tendon jerk studies in clinical neurophysiological studies, particularly when studying muscle groups for which it is technically difficult to record the H reflex.
1.3. Factors limiting the size of the H reflex
Each Ia afferent has an excitatory projection to almost every motoneuron in the homonymous pool (Mendell and Henneman, 1971). However, it is impossible for the soleus H reflex to involve all motoneurons in the motoneuron pool (Fig. 1). There are many reasons for this (Table 2). First, although the mean threshold for group Ia afferents is much lower (∼0.6×) than the threshold for the most excitable soleus motor axon (=“MT”), the individual thresholds vary greatly, and it is not possible to recruit all Ia afferents unless the stimulus approaches 4× MT (Gracies et al., 1994). Second, pre-motoneuronal gating of the Ia afferent volley may preclude activation of some motoneurons. This can be due to classical “presynaptic inhibition” associated with depolarisation of the primary afferent terminals at GABAA-mediated synapses or depletion of transmitter in Ia terminals when they are activated repetitively. The latter causes post-activation depression (also termed “homosynaptic” depression) of the H reflex. This phenomenon is important in clinical practice: it underlies the need for low stimulus rates when subjects are at rest (as discussed in the next section). Third, as discussed above, the monosynaptic excitation is truncated by Ib inhibition (and possibly by other oligosynaptic pathways). Fourth, as the M wave grows, any further growth of the H reflex will be prevented by the collision between the orthodromic reflex discharge and the antidromic volley in motor axons (Fig. 2, Fig. 4). This is largely the reason why H reflexes cannot be identified in thenar muscles when the stimulus is strong (see below). Finally, if the excitability of the motoneuron pool is too low, even a large synchronised excitatory input may be unable to discharge any motoneurons in the pool. This latter possibility can be eliminated if the H reflex is tested while the subject performs a voluntary contraction (see later).
Fig. 1.

The H reflex of soleus. A. The top trace shows the steady increase in the stimulus current from below motor threshold to the maximal M wave (Mmax), producing the H reflexes and M waves shown in the lower panel in A. Note that the current needed to produce a liminal H reflex was less than that for a liminal M wave, i.e., that the H reflex had a lower threshold than the M wave. B. 3 traces of raw data obtained at the letters indicated in A: a, an H reflex but no M wave; b, a larger H reflex and M wave; c, a larger M wave and smaller H reflex. The Hmax:Mmax ratio is 60%. Reflex latency should be measured to the first deflection from baseline (indicated by the vertical arrows in Ba and b, not to the onset of negativity (indicated by the star in b). Also note the slightly shorter latency of the H reflex in b than in a (as indicated by the vertical arrows). In A, F waves prevent the “H” wave measurement from reaching zero (the interrupted horizontal line is at 10% Mmax). In healthy subjects the soleus F wave deflection is a compound response consisting of the superimposed responses of a number of motoneurons that did not discharge reflexly (see also Fig. 3).
From McNulty et al. (2008), Fig. 3, with permission.
Table 2.
What limits the size of the H reflex in subjects at rest?
|
Fig. 2.
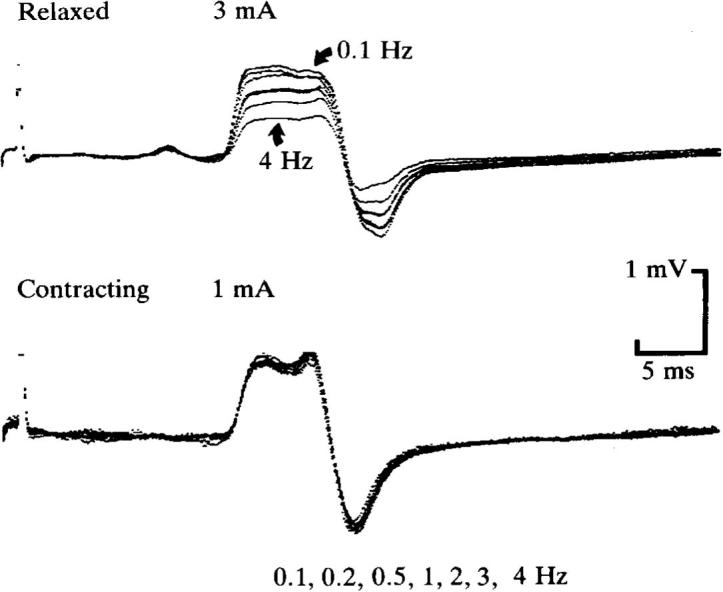
H reflex of flexor carpi radialis at rest (upper traces) and during weak voluntary wrist flexion (lower traces). The reflex responses evoked by stimuli delivered at seven different rates (0.1, 0.2, 0.5, 1, 2, 3 and 4 Hz) are superimposed. There is prominent rate-dependent depression of the H reflex when the subject was relaxed but no significant change when contracting the target muscle. Note that to produce reflex potentials of comparable amplitude, the stimulus intensities were 3 mA when relaxed (producing a liminal M wave) and 1 mA when contracting (producing no M wave). Each trace is the average of 16–32 responses.
From Burke et al. (1989), Fig. 2, with permission.
Fig. 4.
H reflexes recorded during voluntary abduction of the thumb against resistance and F waves of thenar muscles in the same subject. A. H reflex recorded using unrectified EMG (4 averages, each of 32 sweeps), as used to calculate H-reflex latencies. B. H reflex recorded using rectified EMG (3 averages, each of 32 sweeps). C. F waves of APB (3 averages of rectified EMG, each of 32 sweeps). Note that the large H reflex is associated with a liminal M wave, and that its latency is slightly longer than the latency of the fastest F wave (by approximately ×1.1 in healthy subjects).
From Espiritu et al. (2003), Fig. 3, with permission.
1.4. Effects of stimulus rate
All reflexes habituate when elicited repetitively (upper traces in Fig. 2), unless the stimulus rate is low. For the H reflex, this appears to be due to depletion of transmitter stored presynaptically in Ia afferent terminals (Curtis and Eccles, 1960, Hultborn et al., 1996, Hultborn and Nielsen, 1998). Transmitter turnover and release appears to be rate dependent (Lüscher et al., 1983): decreasing the faster the rate until very fast rates are reached (>10 Hz) when it is enhanced – a phenomenon not unlike the rate dependence of the endplate potential at the neuromuscular junction. However for the Ia/motoneuron synapse, the reflex depression (post-activation depression or “homosynaptic” depression) does have some features that are, as yet, incompletely understood: first, the reflex depression with a higher stimulus rate is greatly attenuated during a voluntary contraction (and this is clinically useful, see below; lower traces in Fig. 3), second, the reflex depression with a higher rate is reduced in patients with spasticity (and this abnormality correlates with the degree of spasticity), and third, the reflex depression may be altered by training – i.e., it undergoes usage-dependent plasticity (Meunier et al., 2007).
Fig. 3.
The reflex discharge of low-threshold motoneurons cannot produce an identifiable H reflex if the stimulus is strong. In human subjects, the supramaximal stimulus necessary for F wave studies will produce an intense afferent volley. If motoneurons are activated by this afferent input, the reflex discharge will collide with the antidromic volley in motor axons, thus preventing the reflex discharge from reaching the muscle. (Note that the collision will also prevent antidromic invasion of the motoneurons that were activated reflexly, and that F waves will occur only for higher-threshold motoneurons that have a smaller compound EPSP and do not discharge reflexly in response to the afferent volley.)
From Burke, D., in press. Motor control: spinal and cortical mechanisms. In: Mills, K.R. (ed.) Oxford Textbook of Clinical Neurophysiology, with permission.
The classical feature of post-activation depression is its long duration: it subsides over 10 s or more, much longer than any inhibitory process: 200–300 ms for presynaptic inhibition, perhaps 35 ms for recurrent inhibition, 5–10 ms for Ib and reciprocal Ia inhibitions (Pierrot-Deseilligny and Burke, 2012). In addition the depression is confined to the active reflex arc (hence “homosynaptic”).
1.5. Effects of voluntary contraction
Perhaps the best form of reflex reinforcement is to test the reflex during a voluntary contraction of the target muscle. Clinicians avoid this when testing the tendon jerk (in favour of the remote contractions of the “Jendrassik manoeuvre”) simply because subjects have difficulty maintaining the optimal contraction strength: a contraction that is too strong will attenuate the percussion wave and suppress the stimulus reaching spindle endings. This problem does not arise with H reflexes, which are consistently potentiated by a weak voluntary contraction (Hagbarth, 1962, Garcia et al., 1979, Burke et al., 1989, White, 1991).
The loss of post-activation depression during a voluntary contraction is not the only reason for the reflex potentiation. The contraction increases the excitability of the motoneuron pool, depolarising at least some motoneurons to threshold. It suppresses transmission across the Ib inhibitory interneuron, decreasing the Ib limitation on reflex size. Interestingly the decrease in Ib inhibition is sustained during tonic contractions, probably because afferent feedback from the contracting muscle prolongs the suppression due to the descending command (Pierrot-Deseilligny and Burke, 2012).
As a result of these changes, weak afferent inputs are more capable of producing a reflex response. H reflexes become obtainable where motoneuron excitability to afferent inputs is low (e.g., tibialis anterior) and when the afferent input is weak, either deliberately weak so that there is only a small M wave (e.g., biceps brachii) or in pathology (e.g., a sensory root disturbance). In addition, the contraction focuses the reflex on the contracting muscle rather than inactive neighbours, a feature that is advantageous with surface recordings, and it allows weak heteronymous reflexes to become demonstrable (e.g., median to biceps [Miller et al., 1995a]; femoral to soleus and TA [Meunier et al., 1996]).
The contraction does not alter the latency of the H reflex significantly though it does significantly decrease the latency of the tendon jerk (Uysal et al., 1999). Perhaps the only downsides are that reflex responses need to be averaged to define latency accurately, and that the subjects need to be capable of generating a tonic contraction.
2. Technical issues
2.1. Caveats
The following comments reflect the personal practice of the author, with experience using reflex testing in diagnostic practice for over >30 years. The methodology espoused below was developed in recognition that clinical practice often requires some compromises between perfection and what can be done to patients within reasonable time constraints. When clinically appropriate, the author studies routinely those reflexes listed in Table 3, often bilaterally, particularly when complaints are unilateral.
Table 3.
Reflexes in routine diagnostic practice.
| Nerve | Segment | |
|---|---|---|
| Reflexes present at rest | ||
|
Tibial | S1 |
|
Femoral | L(2)3,4 |
|
Median | C6/7 |
| ||
| Reflexes normally requiring voluntary contraction | ||
|
Musculocutaneous | C5/6 |
|
Radial | C6 |
|
Median | C8/T1 |
|
Peroneal | L4/5 |
| Technically difficult reflexes [not always obtainable in healthy subjects] | ||
|
Posterior tibial | S1 |
|
Ulnar | C8/T1 |
|
Radial | C5/6 |
|
Radial | C7/8 |
2.2. Unconventional stimulation sites
Soleus H reflexes have been recorded stimulating the sciatic nerve at the gluteal fold using a “Teflon-coated monopolar needle cathode inserted at the gluteal fold … advanced until the threshold current for evoking a soleus CMAP or an H reflex was 2–10 mA” (Sachs and Logigian, 1996). They may also be recorded to direct stimulation of the sciatic nerve or S1 root (Zhu et al., 1998, Zhu et al., 2013, Alfonsi et al., 2003, Jin et al., 2010), using either high-voltage electrical or magnetic stimulation. With these forms of stimulation, the effective stimulus waveform results in a current that decays rapidly, unlike the 1-ms wide “square-wave” stimulus generally recommended for H reflex studies (Hugon, 1973, Pierrot-Deseilligny and Burke, 2012). With S1 root stimulation, the H reflex can be recorded easily despite the unfavorable stimulus waveform because the shorter afferent conduction time to the motoneuron pool results in an EPSP of higher amplitude due to less dispersion of the group Ia component of the afferent volley. An additional “benefit” is that the Ib component of the afferent volley would be less able to curtail the reflex response (Burke et al., 1984, Marchand-Pauvert et al., 2002). For the H reflex of soleus, proximal stimulation may have advantages over stimulation at the popliteal fossa, particularly when the pathology is focal.
2.3. Posture
The ideal situation for the H reflex of soleus is a seated subject with the hip and knee flexed and the ankle fixed in 30° plantar flexion (Hugon, 1973). This takes stretch off the bi-articular gastrocnemius muscles. While appropriate for research studies, this situation is less convenient for diagnostic studies, and examining the patient lying on a bed is more convenient (prone for soleus and supine for tibialis anterior).
2.4. Stimulating electrodes
For all muscles, the cathode should be over the appropriate nerve, but the optimal site for the anode differs. For soleus it is ideally on the opposite side of the leg, over the knee-cap, so that current flows directly through the nerve (Hugon, 1973). This may be less convenient in diagnostic studies, and I use a bipolar configuration with the anode medial in the popliteal fossa at the same level as the cathode, minimising current spread to the peroneal nerve. For the femoral nerve, the anode should be placed on the buttocks, opposite the cathode, so that again current flows through the nerve. For the median nerve at the wrist, an anode over the radius is convenient because it minimises spread to the ulnar. For the median nerve at the elbow, the electrodes should theoretically be placed with cathode proximal in order to avoid anodal block. However, with the submaximal stimuli used in these studies, this risk is low, and in practice a proximal anode works well. For the radial nerve, bipolar electrodes should be avoided because of the depth of the nerve: the cathode should be over the spiral groove and the anode placed over biceps.
2.5. Stimulus width
In all studies (except perhaps biceps brachii, see below), the stimulus width should be 1 ms because this maximises the difference in the strength-duration properties of sensory and motor axons (Panizza et al., 1994, Mogyoros et al., 1996, Lin et al., 2002). It is worth noting that these differences, not size, are the main reason why sensory axons can be stimulated at lower threshold than motor axons. As previously mentioned, discernible group Ia effects on the soleus motoneuron pool occur with stimuli that are 0.6× MT. This requires activation of a population of afferents. By contrast, activation of a single motor axon is all that is required to produce a M wave.
2.6. Stimulus rate
When subjects are at rest, post-activation depression can be avoided only if the stimulus rate is very low – once every 10 s (upper traces in Fig. 2). This rate is tediously slow in diagnostic practice, and a reasonable compromise is once every 3–5 s. However, when subjects perform a steady voluntary contraction of the test muscle, post-activation depression is much less (Rothwell et al., 1986, Burke et al., 1989, Stein et al., 2007), and higher rates can be used (lower traces in Fig. 2). With the usual stimulus intensities, 3 Hz is tolerable. Multiple sweeps should be averaged and multiple averages should be superimposed to define the onset latency accurately. I find it convenient to record 3–5 averages each containing ∼32 trials – this takes <1 min. There are three situations when potential discomfort dictates that 3 Hz should not be used, even during a voluntary contraction: the musculocutaneous nerve at Erb’s point (biceps brachii), the radial nerve in the spiral groove (forearm extensors) and the femoral nerve in groin (quadriceps femoris). Here it is prudent not to exceed 1 Hz.
2.7. Measured parameters
Latency should be measured from the beginning of the 1-ms stimulus to the take-off of the H wave from baseline, not to the onset of negativity (as shown in Fig.1Bb – measure at the vertical arrow, not the star). This is important because the recording electrodes over soleus are not over the motor point. The initial positivity of the H wave reflects conduction along muscle fibres towards the recording electrode, i.e., after neuromuscular transmission has occurred. Reflex latency varies with limb length (or height) and age. While there are many normative studies in the literature (e.g., Guiheneuc and Bathien, 1976, Lachman et al., 1980, Sabbahi and Khalil, 1990; and particularly Buschbacher, 1999), the normative values and nomograms of Schimsheimer et al. (1987) are convenient (Table 4). A number of factors determine why latency varies with age. Leaving aside subclinical pathology, a major factor is probably that H reflexes tend to be smaller with age, and the latency of the response depends on reflex size. This is largely because the smaller the reflex response the more it is determined by low-threshold motoneurons which have more slowly conducting motor axons (compare vertical arrows in Fig.2Ba and b).
Table 4.
Normal values (from Schimsheimer et al., 1987).
| Soleus H reflex |
|
| FCR H reflex |
|
The amplitude of the reflex response is too variable between subjects to be a reliable criterion, but is a useful measure in side-to-side comparisons with unilateral pathology. Because recording electrode positions will vary from side to side, it is important to normalise the amplitude of the largest recorded reflex response to the size of the maximal M wave (so called H:M ratio). Here I use peak-to-peak amplitude for both measures. The threshold of the reflex response relative to the M wave is also worth noting as an indication of the ease of recording the reflex response (and this is so whether the reflex is recorded at rest or requires a voluntary contraction).
2.8. Reflexes normally recordable at rest
H reflexes should be recordable from soleus, quadriceps femoris and flexor carpi radialis in healthy subjects at rest. If the conditions are optimal, inability to do so normally reflects pathology within the reflex arc, though it could also be due to low central excitability. Low central excitability is a diffuse phenomenon, not confined to a single reflex arc, and there should be similar difficulty in recording reflex responses for other muscles (e.g., those in the upper limb when the clinical problem is possible S1 radiculopathy). Low central excitability can be eliminated by repeating the test while the subject maintains a steady weak contraction of the target muscle.
-
•
Start at 1 Hz, define H reflex or M wave, then drop the stimulus rate to once every 3–5 s.
-
•
Increase the stimulus slowly defining the maximal H wave and the maximal M wave (Fig. 1).
-
•
Note the threshold for the H reflex relative to the M wave (qualitatively: if reflex threshold is below M threshold, the reflex response is likely to be within normal limits).
-
•
Measure the latency and amplitude of the maximal H and M waves (“Hmax” and “Mmax”), and compare latencies with normal values using nomograms including height/limb length and age (Table 4).
-
•
Repeat the studies on the contralateral limb (if indicated).
-
•
If the H wave cannot be defined, return to M wave threshold, increase stimulus rate to 2–3 Hz for soleus and flexor carpi radialis, but keep it at 1 Hz for quadriceps (lest discomfort terminates testing). Ask the patient to perform a steady voluntary contraction of the target muscle. Superimpose repeated stimulus-triggered averages of unrectified EMG.
-
•
Vary the strength of the voluntary contraction and stimulus strength in different averages.
2.9. Reflexes normally recordable only during a voluntary contraction
For some muscle groups (extensor carpi radialis, tibialis anterior, thenar muscles), a reflex response cannot be identified with certainty in subjects at rest (Table 3). To be able to do so suggests hyperreflexia. This could be physiological (e.g., Miller et al. (1995b) could record the H reflex of ECR at rest in 8% of healthy subjects) or pathological. If the H reflex can be defined for one of these muscles, EMG evidence of denervation in that same muscle suggests upper and lower motor neurone lesions involving that muscle. This should raise suspicion of a degenerative disease, such as ALS.
H reflexes can be recorded for biceps brachii at rest, but it is more convenient to test biceps brachii during a voluntary contraction (i) because doing so allows a liminal stimulus to be used and this means that any preceding M wave is small and does not obscure the H wave, and (ii) because subjects are less likely to tense the shoulder and displace the cathode.
That the H reflex cannot be defined at rest does not mean that there was no reflex discharge. As shown in Fig. 3, there may be a reflex discharge in low-threshold motoneurons in response to an intense volley in median nerve afferents, but the reflex discharge does not appear in the EMG because of collision with the antidromic volley in motor axons. As a result, the H reflex of the thenar muscles cannot normally be identified in surface recordings. However, using single fibre EMG, Trontelj (1973) has demonstrated that H reflexes can occur in single motor units of abductor pollicis brevis when the subject is at rest. The H reflex becomes overt when the thenar muscles are contracting (Burke et al., 1989; see Fig. 4).
2.9.1. For thenar muscles, tibialis anterior and extensor carpi radialis
-
•
Start at 1 Hz, define a liminal M wave, then drop the stimulus rate to once every 3–5 s and determine whether the H reflex can be recorded at rest (if so, consider hyperreflexia).
-
•
Assuming that the H wave cannot be defined at rest, return to M wave threshold, increase stimulus rate to 2–3 Hz and ask the patient to perform a steady voluntary contraction of the target muscle. Collect stimulus-triggered averages of the unrectified EMG, as many sweeps as required (×32–64) for each average, repeating the average 3–5 times to ensure reproducibility (Fig. 4).
[Note that the reflex response is well seen in averages of raw EMG and that the latency of the thenar H reflex is slightly longer than the minimal F wave latency, usually by about ×1.1 in healthy subjects.]
-
•
Vary the strength of the voluntary contraction and stimulus strength in different averages.
-
•
Sum the averages and superimpose them to define onset latency. Also note the threshold for the reflex relative to that for the M wave (as an indication of ease of recording – e.g., in the recordings of Fig. 4 there is only a miniscule M wave). It is prudent to note the amplitude of the reflex relative to maximal M wave (though pathology cannot be defined on the basis of this measurement).
2.9.2. For biceps brachii
-
•
Here a stimulus duration of 0.5 ms may be preferable to 1.0 ms because then threshold currents are higher, and fine adjustments of stimulus intensity are easier.
-
•
Place the cathode at Erb’s point and anode over the vertebra prominens (C7). With the subject at rest, stimulate at 1 Hz, increasing the intensity slowly until a liminal H wave (at 9–11 ms) or the M wave (at 4–5 ms) appears.
-
•
Then ask the subject to perform a weak contraction of biceps brachii, averaging ∼8 sweeps, followed by relaxation, during which a further 8 sweeps are averaged, followed by further sequences of contraction and relaxation.
-
•
The reflex response can be identified as the component enhanced by contraction with a latency of 9–11 ms (Fig. 5).
-
•
These contractions should be weak and the shoulder and forearm should be restrained because the cathode is easily displaced. This is identifiable by a change in the M wave. Do not use stimuli >1.2–1.3× MT (or stimuli over 1 Hz) if you wish to retain patient cooperation.
Fig. 5.
H reflex of biceps brachii to stimulation at Erb’s point. A. Superimposed averages when relaxed and when contracting biceps, using three different stimulus intensities, sufficient to produce, in the upper two sets of averages, a small M wave. The H reflex appears or is potentiated during the contraction (middle and upper sets of averages respectively). C. Sixteen successive averages of biceps EMG using a constant stimulus intensity with the subject alternately relaxed or contracting biceps. B. Grand averages of the traces in C and the difference between the two grand averages.
From Miller et al. (1995a), Fig. 3, with permission.
2.10. Technically difficult reflexes
Apart from the facial muscles and the digastric, all skeletal muscles have muscle spindles, and theoretically H reflexes should be recordable for them. Increases in discharge probability at H reflex latencies have been defined in post-stimulus time histograms of the discharge of low-threshold single motor units from these muscles (see Pierrot-Deseilligny and Burke, 2012). However, using the techniques described above, it has proved difficult to obtain convincing reflex responses during voluntary contractions of some muscles in all healthy subjects (Table 3), and this limits their diagnostic value.
On the other hand, if one wishes to exclude abnormality, it can be very reassuring to define the H reflex for abductor digiti minimi (in, e.g., a possible thoracic outlet syndrome, though here the author relies more on the H reflex of the thenar muscles) or abductor hallucis/flexor hallucis brevis (in a patient with unexplained pain in the feet).
3. Usage and tips on interpretation
H reflexes are of value with proximal lesions and are advocated in patients with lumbosacral and cervical nerve root lesions, particularly involving S1 (e.g., Braddom and Johnson, 1974; Aiello et al., 1981, Nishida et al., 1996, Albeck et al., 2000), C6/7 (e.g., Schimsheimer et al., 1985, Schimsheimer et al., 1987, Schimsheimer et al., 1988, Sabbahi and Khalil, 1990, White, 1991, Eliaspour et al., 2009) or C7 (Zheng et al., 2014). Where there is unilateral pathology, the sensitivity of testing and the value of amplitude measurements (H:M ratio) is increased (Jankus et al., 1994; Nishida et al., 1996). It is then important to have normal values for side-to-side differences (as in Table 4; Schimsheimer et al., 1985, Schimsheimer et al., 1987; Jankus et al., 1994; Buschbacher, 1999). Some studies have used contraction-enhanced H reflexes to study segmental levels other than C6/7 and S1 (e.g., various upper and lower limb segments: White, 1991; various upper limb segments: Miller et al., 1999; tibialis anterior: Pradhan, 1993). H reflex studies are also of value in patients with plexus lesions (Ongerboer de Visser et al., 1984), and here again asymmetry of pathology results in a higher test sensitivity because side-to-side differences can be used, much as in putative root lesions.
In patients with polyneuropathy, H reflex studies allow conduction across proximal segments to be contrasted with conduction across the more distal segments studied in routine nerve conduction studies (e.g., Guiheneuc and Bathien, 1976, Panayiotopoulos and Lagos, 1980, Lachman et al., 1980, Ackil et al., 1981). Reflex studies may be particularly useful in acute inflammatory demyelinating polyradiculoneuropathy (Vucic et al., 2004).
In interpreting the findings, some issues should be kept in mind.
-
•
All comparisons with normal values require that the patient is studied under identical conditions to the healthy subjects. This is often not the case.
-
•
As mentioned above, smaller reflexes have a longer latency (as expected from size principle), and a reflex that is difficult to define may have a slightly slow latency merely because it is smaller than the reflexes of control subjects.
-
•
Purely sensory abnormalities produce mild latency prolongations of only a few milliseconds. If the reflex latency is very prolonged, there is likely to be slowing in motor axons because a purely afferent abnormality would disperse the volley too much, and would thereby abolish the reflex response.
-
•
It is not uncommon to see a broad long-latency deflection (at a latency >45 ms) when searching for the H reflex of thenar or hypothenar muscles. This long-latency activity could traverse a transcortical pathway (Deuschl et al., 1985, Noth et al., 1985, Pierrot-Deseilligny and Burke, 2012), and is not a delayed H reflex. It is usually better defined in averages of rectified EMG (though it is not apparent in Fig. 5).
-
•
Some subjects have difficulty in abducting the thumb forcefully without also contracting forearm muscles that cross or act on the wrist. When this occurs, the cathode can be displaced. This issue is often less of a problem if subjects are asked to oppose the thumb against digits 4 and 5 rather than abduct it.
-
•
Voluntary contraction does not affect latency greatly (Uysal et al., 1999) but can allow a weak afferent volley to produce a reflex response. Often the soleus H reflex is not obtainable at rest (despite good technique) but appears at (near-) normal latency during a voluntary contraction. In such instances a sensory disturbance is likely. The alternative of low central excitability can be excluded if H reflexes can be recorded contralaterally or from upper and lower limbs.
-
•
Hyperreflexia is likely if H reflexes are recordable with the subject at rest from muscles from which they are not normally obtainable at rest. The question then is whether the briskness of the reflexes represents a physiological extreme or a pathological change. Hyperreflexia could represent evidence of an UMN lesion, and this is particularly relevant if there are also fasciculation and EMG changes in that muscle.
Conflict of interest
The author has no conflicts of interest to report.
References
- Ackil A.A., Shahani B.T., Young R.R., Rubin N.E. Late response and sural conduction studies. Usefulness in patients with chronic renal failure. Arch. Neurol. 1981;38:482–485. doi: 10.1001/archneur.1981.00510080044004. [DOI] [PubMed] [Google Scholar]
- Aiello I., Rosati G., Serra G., Manca M. The diagnostic value of H-index in S1 root compression. J. Neurol. Neurosurg. Psychiatry. 1981;44:171–172. doi: 10.1136/jnnp.44.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck M.J., Taher G., Lauritzen M., Trojaborg W. Diagnostic value of electrophysiological tests in patients with sciatica. Acta Neurol. Scand. 2000;101:249–254. doi: 10.1034/j.1600-0404.2000.101004249.x. [DOI] [PubMed] [Google Scholar]
- Alfonsi E., Merlo I.M., Clerici A.M., Candeloro E., Marchioni E., Moglia A. Proximal nerve conduction by high-voltage electrical stimulation in S1 radiculopathies and acquired demyelinating neuropathies. Clin. Neurophysiol. 2003;114:239–247. doi: 10.1016/s1388-2457(02)00331-0. [DOI] [PubMed] [Google Scholar]
- Araki T., Eccles J.C., Ito M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J. Physiol. 1960;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum A., Ashby P. Postsynaptic potentials in individual soleus motoneurons in man produced by achilles tendon taps and electrical stimulation of tibial nerve. Electroencephalogr. Clin. Neurophysiol. 1982;54:469–471. doi: 10.1016/0013-4694(82)90211-5. [DOI] [PubMed] [Google Scholar]
- Braddom R.I., Johnson E.W. Standardization of H reflex and diagnostic use in Sl radiculopathy. Arch. Phys. Med. Rehabil. 1974;55:161–166. [PubMed] [Google Scholar]
- Burke D. Critical examination of the case for or against fusimotor involvement in disorders of muscle tone. In: Desmedt J.E., editor. vol. 39. Raven Press; New York: 1983. pp. 133–150. (Motor Control Mechanisms in Health and Disease, Advances in Neurology). [PubMed] [Google Scholar]
- Burke D., Adams R.W., Skuse N.F. The effects of voluntary contraction on the H reflex of human limb muscles. Brain. 1989;112:417–433. doi: 10.1093/brain/112.2.417. [DOI] [PubMed] [Google Scholar]
- Burke D., Gandevia S.C., McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J. Physiol. 1983;339:535–552. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Gandevia S.C., McKeon B. Monosynaptic and oligosynaptic contributions to the human ankle jerk and H reflex. J. Neurophysiol. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D., Hallett M., Fuhr P., Pierrot-Deseilligny E. H reflexes from the tibial and median nerves. In: Deuschl G., Eisen A., editors. Recommendations for the Practice of Clinical Neurophysiology. Elsevier; Amsterdam: 1999. pp. 259–262. [PubMed] [Google Scholar]
- Buschbacher R.M. Normal range for H-reflex recording from the calf muscles. Am. J. Phys. Med. Rehabil. 1999;8(6 Suppl):S75–S79. doi: 10.1097/00002060-199911001-00014. [DOI] [PubMed] [Google Scholar]
- Curtis D.R., Eccles J.C. Synaptic action during and after repetitive stimulation. J. Physiol. 1960;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G., Schenck E., Lücking C.H. Long-latency responses in human thenar muscles mediated by fast conducting muscle and cutaneous afferents. Neurosci. Lett. 1985;55:361–366. doi: 10.1016/0304-3940(85)90462-8. [DOI] [PubMed] [Google Scholar]
- Eliaspour D., Sanati E., Hedayati Moqadam M.R., Rayegani S.M., Bahrami M.H. Utility of flexor carpi radialis H-reflex in diagnosis of cervical radiculopathy. J. Clin. Neurophysiol. 2009;26:458–460. doi: 10.1097/WNP.0b013e3181c2bb00. [DOI] [PubMed] [Google Scholar]
- Espiritu M.G., Lin C.S.-Y., Burke D. Motoneuron excitability and the F wave. Muscle Nerve. 2003;27:720–727. doi: 10.1002/mus.10388. [DOI] [PubMed] [Google Scholar]
- Fisher M.A. AAEM Minimonograph #13: H reflexes and F waves: physiology and clinical indications. Muscle Nerve. 1992;15:1223–1233. doi: 10.1002/mus.880151102. [DOI] [PubMed] [Google Scholar]
- Fisher M.A. H reflexes and F waves. Fundamentals, normal and abnormal patterns. Neurol. Clin. N. Am. 2002;20:339–360. doi: 10.1016/s0733-8619(01)00004-4. [DOI] [PubMed] [Google Scholar]
- Garcia H.A., Fisher M.A., Gilai A. H reflex analysis of segmental reflex excitability in flexor and extensor muscles. Neurology. 1979;29:984–991. doi: 10.1212/wnl.29.7.984. [DOI] [PubMed] [Google Scholar]
- Gracies J.M., Pierrot-Deseilligny E., Robain G. Evidence for further recruitment of group I fibres with high stimulus intensities when using surface electrodes in man. Electroencephalogr. Clin. Neurophysiol. 1994;93:353–357. doi: 10.1016/0168-5597(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Guiheneuc P., Bathien N. Two patterns of results in polyneuropathies investigated with the H reflex. Correlation between proximal and distal conduction velocities. J. Neurol. Sci. 1976;30:83–94. doi: 10.1016/0022-510x(76)90257-4. [DOI] [PubMed] [Google Scholar]
- Hagbarth K.-E. Post-tetanic potentiation of myotatic reflexes in man. J. Neurol. Neurosurg. Psychiatry. 1962;25:1–10. doi: 10.1136/jnnp.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. Beitrag zur Kenntnis der menschlichen Reflexe mit besonderer Berucksichtigung der elektrischen Erscheinungen. Arch. Anat. Physiol. 1910;1:223–246. [Google Scholar]
- Hoffmann P. Über die Beziehungen der Sehnenreflexe zur willkürlichen Bewegung und zum Tonus. Z. Biol. 1918;68:351–370. [Google Scholar]
- Hugon M. Methodology of the Hoffmann reflex in man. In: Desmedt J.E., editor. vol. 3. Karger; Basel: 1973. (New Developments in Electromyography and Clinical Neurophysiology). [Google Scholar]
- Hultborn H., Illert M., Nielsen J., Paul A., Ballegaard M., Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp. Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Nielsen J.B. Modulation of transmitter release from Ia afferents by their preceding activity – a “Postactivation Depression”. In: Rudomin P., Romo R., Mendell L., editors. Oxford; New York: 1998. pp. 178–191. (Presynaptic Inhibition and Neural Control). [Google Scholar]
- Jankus W.R., Robinson L.R., Little J.W. Normal limits of side-to-side H-reflex amplitude variability. Arch. Phys. Med. Rehabil. 1994;75:3–7. [PubMed] [Google Scholar]
- Jin X., Zhu Y., Lu F.Z., Wu X.D., Zhu D.Q., Weber R., Dunn B., Jiang J.Y. H-reflex to S1-root stimulation improves utility for diagnosing S1 radiculopathy. Clin. Neurophysiol. 2010;121:1329–1335. doi: 10.1016/j.clinph.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Knikou M. The H-reflex as a probe: pathways and pitfalls. J. Neurosci. Methods. 2008;171:1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Lachman T., Shahani B.T., Young R.R. Late responses as aids to diagnosis in peripheral neuropathy. J. Neurol. Neurosurg. Psychiatry. 1980;43:156–162. doi: 10.1136/jnnp.43.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.S.-Y., Chan J.H.L., Pierrot-Deseilligny E., Burke D. Excitability of human muscle afferents studied using threshold tracking of the H reflex. J. Physiol. 2002;545:661–669. doi: 10.1113/jphysiol.2002.026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher H., Ruenzel P.W., Henneman E. Effects of impulse frequency, PTP, and temperature on responses elicited in large populations of motoneurons by impulses in single Ia-fibers. J. Neurophysiol. 1983;50:1045–1058. doi: 10.1152/jn.1983.50.5.1045. [DOI] [PubMed] [Google Scholar]
- Macefield G., Gandevia S.C., Burke D. Conduction velocities of muscle and cutaneous afferents in the upper and lower limbs of human subjects. Brain. 1989;112:1519–1532. doi: 10.1093/brain/112.6.1519. [DOI] [PubMed] [Google Scholar]
- Magladery J.W., McDougal D.B., Jr. Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull. Johns Hopkins Hosp. 1950;86:265–290. [PubMed] [Google Scholar]
- Magladery J.W., Porter W.E., Park A.M., Teasdall R.D. Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull. Johns Hopkins Hosp. 1951;88:499–519. [PubMed] [Google Scholar]
- Marchand-Pauvert V., Nicolas G., Burke D., Pierrot-Deseilligny E. Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley. J. Physiol. 2002;542:963–976. doi: 10.1113/jphysiol.2002.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty P.A., Jankelowitz S.K., Wiendels T.M., Burke D. Postactivation depression of the soleus H reflex measured using threshold tracking. J. Neurophysiol. 2008;100:3275–3284. doi: 10.1152/jn.90435.2008. [DOI] [PubMed] [Google Scholar]
- Meunier S., Mogyoros I., Kiernan M.C., Burke D. Effects of femoral nerve stimulation on the electromyogram and reflex excitability of tibialis anterior and soleus. Muscle Nerve. 1996;19:1110–1115. doi: 10.1002/(SICI)1097-4598(199609)19:9<1110::AID-MUS5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Meunier S., Kwon J., Russmann H., Ravindran S., Mazzocchio R., Cohen L. Spinal use-dependent plasticity of synaptic transmission in humans after a single cycling session. J. Physiol. 2007;579:375–388. doi: 10.1113/jphysiol.2006.122911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L.M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J. Neurophysiol. 1971;34:171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Miller T.A., Mogyoros I., Burke D. Homonymous and heteronymous monosynaptic reflexes in biceps brachii. Muscle Nerve. 1995;18:585–592. doi: 10.1002/mus.880180604. [DOI] [PubMed] [Google Scholar]
- Miller T.A., Newall A.R., Jackson D.A. H-reflexes in the upper extremity and the effects of voluntary contraction. Electromyogr. Clin. Neurophysiol. 1995;35:121–128. [PubMed] [Google Scholar]
- Miller T.A., Pardo R., Yaworski R. Clinical utility of reflex studies in assessing cervical radiculopathy. Muscle Nerve. 1999;22:1075–1079. doi: 10.1002/(sici)1097-4598(199908)22:8<1075::aid-mus11>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Misiaszek J.E. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve. 2003;28:144–160. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- Mogyoros I., Kiernan M.C., Burke D. Strength-duration properties of human peripheral nerve. Brain. 1996;119:439–447. doi: 10.1093/brain/119.2.439. [DOI] [PubMed] [Google Scholar]
- Nishida T., Kompoliti A., Janssen I., Levin K. H reflex in S-1 radiculopathy: latency versus amplitude controversy revisited. Muscle Nerve. 1996;19:915–917. doi: 10.1002/(SICI)1097-4598(199607)19:7<915::AID-MUS19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Noth J., Podoll K., Friedemann H.H. Long-loop reflexes in small hand muscles studied in normal subjects and in patients with Huntington’s disease. Brain. 1985;108:65–80. doi: 10.1093/brain/108.1.65. [DOI] [PubMed] [Google Scholar]
- Ongerboer de Visser B.W., Schimsheimer R.J., Hart A.A.M. The H reflex of the flexor carpi radialis muscle; a study in controls and radiation-induced brachial plexus lesions. J. Neurol. Neurosurg. Psychiatry. 1984;47:1098–1101. doi: 10.1136/jnnp.47.10.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard J. Librairie Arnette; Paris: 1955. Réflexes et régulations d’origine proprioceptive chez l’homme: étude neurophysiologique et psychophysiologique. (Thèse de Doctorat ès Sciences) [Google Scholar]
- Panayiotopoulos C.P., Lagos G. Tibial nerve H-reflex and F-wave studies in patients with uremic neuropathy. Muscle Nerve. 1980;3:423–426. doi: 10.1002/mus.880030507. [DOI] [PubMed] [Google Scholar]
- Panizza M., Nilsson J., Roth B.J., Rothwell J., Hallett M. The time constants of motor and sensory peripheral nerve fibers measured with the method of latent addition. Electroencephalogr. Clin. Neurophysiol. 1994;93:147–154. doi: 10.1016/0168-5597(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Burke D. Cambridge University Press; New York: 2012. The Circuitry of the Human Spinal Cord: Spinal and Corticospinal Mechanisms of Movement. 606p. [Google Scholar]
- Pierrot-Deseilligny E., Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol. Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp. Brain Res. 1981;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Pradhan S. Tibialis anterior R-1 response: physiological behaviour, normative data and clinical utility in L4–L5 radicular compression. Electroencephalogr. Clin. Neurophysiol. 1993;89:10–21. doi: 10.1016/0168-5597(93)90079-5. [DOI] [PubMed] [Google Scholar]
- Rothwell J.C., Day B.L., Berardelli A., Marsden C.D. Habituation and conditioning of the human long latency stretch reflex. Exp. Brain Res. 1986;63:197–204. doi: 10.1007/BF00235664. [DOI] [PubMed] [Google Scholar]
- Sabbahi M.A., Khalil M. Segmental H-reflex studies in upper and lower limbs of patients with radiculopathy. Arch. Phys. Med. Rehabil. 1990;71:223–227. [PubMed] [Google Scholar]
- Sachs G.M., Logigian E.L. Proximally evoked soleus H reflexes in the evaluation of axonal neuropathy. J. Neurol. Sci. 1996;138:88–92. doi: 10.1016/0022-510x(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Schiepatti M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog. Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Schimsheimer R.J., Ongerboer de Visser B.W., Kemp B. The flexor carpi radialis H-reflex in lesions of the sixth and seventh cervical nerve roots. J. Neurol. Neurosurg. Psychiatry. 1985;48:445–449. doi: 10.1136/jnnp.48.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimsheimer R.J., Ongerboer de Visser B.W., Kemp B. Digital nerve somatosensory evoked potentials and flexor carpi radialis H reflexes in cervical disc protrusion and involvement of the sixth or seventh cervical root: relations to clinical and myelographic findings. Electroencephalogr. Clin. Neurophysiol. 1988;70:313–324. doi: 10.1016/0013-4694(88)90050-8. [DOI] [PubMed] [Google Scholar]
- Schimsheimer R.J., Ongerboer de Visser B.W., Kemp B., Bour L.J. The flexor carpi radialis H-reflex in polyneuropathy: relations to conduction velocities of the median nerve and the soleus H-reflex latency. J. Neurol. Neurosurg. Psychiatry. 1987;50:447–452. doi: 10.1136/jnnp.50.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R.B., Estabrooks K.L., McGie S., Roth M.J., Jones K.E. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp. Brain Res. 2007;182:309–319. doi: 10.1007/s00221-007-0989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trontelj J.V. A study of the F response by single fibre electromyography. In: Desmedt J.E., editor. vol. 3. Karger; Basel: 1973. pp. 318–322. (New Developments in Electromyography and Clinical Neurophysiology). [Google Scholar]
- Uysal H., Mogyoros I., Burke D. Reproducibility of tendon jerk reflexes during a voluntary contraction. Clin. Neurophysiol. 1999;110:1481–1487. doi: 10.1016/s1388-2457(99)00082-6. [DOI] [PubMed] [Google Scholar]
- Vucic S., Cairns K.D., Black K.R., Chong P.S., Cros D. Neurophysiologic findings in early acute inflammatory demyelinating polyradiculoneuropathy. Clin. Neurophysiol. 2004;115:2329–2335. doi: 10.1016/j.clinph.2004.05.009. [DOI] [PubMed] [Google Scholar]
- White J.C. The ubiquity of contraction enhanced H reflex: normative data and use in the diagnosis of radiculopathies. Electroencephalogr. Clin. Neurophysiol. 1991;81:433–442. doi: 10.1016/0013-4694(91)90005-o. [DOI] [PubMed] [Google Scholar]
- Zehr E.P. Considerations for use of the Hoffmann reflex in exercise studies. Eur. J. Appl. Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- Zheng C., Zhu Y., Lv F., Ma X., Xia X., Wang L., Jin X., Weber R., Jiang J., Anuvat K. Abnormal flexor carpi radialis H-reflex as a specific indicator of C7 as compared with C6 radiculopathy. J. Clin. Neurophysiol. 2014;31:529–534. doi: 10.1097/WNP.0000000000000104. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Starr A., Haldeman S., Chu J.K., Sugerman R.A. Soleus H-reflex to S1 nerve root stimulation. Electroencephalogr. Clin. Neurophysiol. 1998;109:10–14. doi: 10.1016/s0924-980x(97)00058-1. [DOI] [PubMed] [Google Scholar]
- Zhu D.Q., Zhu Y., Qiao K., Zheng C.J., Bradley S., Weber R., Chen X.J. Proximally evoked soleus H-reflex to S1 nerve root stimulation in sensory neuronopathies (ganglionopathies) Muscle Nerve. 2013;48:814–816. doi: 10.1002/mus.23975. [DOI] [PubMed] [Google Scholar]





