Abstract
Background
Periostin is a matricellular protein that is expressed in bone and joint tissues. To determine the expression of periostin in primary bone tumours and to assess whether it plays a role in tumour progression, we carried out immunohistochemistry and ELISA for periostin in a range of neoplastic and non-neoplastic bone and joint lesions.
Methods
140 formalin-fixed paraffin-embedded sections of bone tumours and tumour-like lesions were stained by an indirect immunoperoxidase technique with a polyclonal anti-periostin antibody. Periostin expression was also assessed in rheumatoid arthritis (RA) and non-inflammatory osteoarthritis (OA) synovium and synovial fluid immunohistochemistry and ELISA respectively.
Results
Periostin was most strongly expressed in osteoid/woven bone of neoplastic and non-neoplastic bone-forming lesions, including osteoblastoma, osteosarcoma, fibrous dysplasia, osteofibrous dysplasia, fracture callus and myositis ossificans, and mineralised chondroid matrix/woven bone in chondroblastoma and clear cell chondrosarcoma. Reactive host bone at the edge of growing tumours, particularly in areas of increased vascularity and fibrosis, also stained strongly for periostin. Vascular elements in RA synovium strongly expressed periostin, and synovial fluid levels of periostin were higher in RA than OA.
Conclusions
In keeping with its known role in modulating the synthesis of collagen and other extracellular matrix proteins in bone, strong periostin expression was noted in benign and malignant lesions forming an osteoid or osteoid-like matrix. Periostin was also noted in other bone tumours and was found in areas of reactive bone and increased vascularity at the edge of growing tumours, consistent with its involvement in tissue remodelling and angiogenesis associated with tumour progression.
Keywords: Periostin expression, Bone tumours, Tumour progression
Background
Periostin, a secreted extracellular matrix protein that belongs to the fasciclin family, was originally characterised in osteoblasts and first termed osteoblast-specific factor 2 [1, 2]. Periostin is a matricellular protein that does not have a specific structural role but rather interacts with cell surface receptors, proteases and other molecules that modulate cell adhesion/migration and the fibrillogenesis of collagen and other extracellular matrix (ECM) proteins [3–6]. Periostin has a multi-domain structure in which particular domains bind to many proteins and enzymes that promote ECM protein crosslinking. Periostin is involved in the formation and maintenance of normal bone and teeth tissues and is highly expressed in tissue components that are subject to mechanical stress, such as the periosteum and the periodontal ligament. It has also been observed in other organs and tissues including heart, breast, lung, thyroid, skin, placenta and ovary [3–6].
Periostin is expressed at sites of injury/repair and inflammation [3, 6, 7]. It has been identified in rheumatoid arthritis (RA) and osteoarthritis (OA) joints [8, 9] with a recent study identifying periostin as a key regulator in RA synoviocyte migration/invasion associated with pannus formation [10]. Periostin is also expressed in a number of cancers where it is thought, by various mechanisms, to play a role in tumour progression [3, 6, 11, 12]. Periostin has been identified in a few bone tumours, including fibrous dysplasia and osteosarcoma [13–15], but its expression in other bone neoplasms has not been fully investigated.
In this study we investigated immunophenotypic expression of periostin in a wide range of primary tumours and tumour-like lesions of bone as well as in bone secondaries and metastatic osteosarcomas. Our aims were two-fold: first, to determine whether periostin expression is increased in specific bone tumour types; and second, to examine whether periostin plays a role in tumour progression.
Methods
Neoplastic and non-neoplastic tissue samples analysed
Tissue samples from 140 biopsies or surgical resections of bone tumours and tumour-like lesions, were retrieved from the files of the Nuffield Orthopaedic Centre, Histopathology Department, Oxford (Table 1). Criteria for the histological diagnosis of bone and joint lesions investigated in this study were those of the 2013 WHO Classification of Tumours of Soft Tissue and Bone [16]. The tissues were fixed in 10% buffered formalin and, where necessary, decalcified in 5% nitric acid or EDTA. In addition, formalin-fixed paraffin-embedded sections of synovial tissue derived from patients with RA (n = 21) and OA (n = 19) were examined. Samples of normal bone and joint tissues from amputation specimens of individuals with no history or evidence of joint disease or neoplasia were used as controls. Synovial fluid (SF) was also aspirated from the knee joint of nine patients with OA and nine patients with RA. Ethics approval was obtained from the National Research Ethical Committee, and patient consent was acquired prior to the collection of samples.
Table 1.
Bone tumours/tumour-like lesions analysed in this study
| Tissue type | Number analysed |
|---|---|
| Osteoma | 2 |
| Osteoid osteoma | 4 |
| Osteoblastoma | 6 |
| Osteosarcoma, conventional | 20 |
| Osteosarcoma, telangiectatic | 2 |
| Osteosarcoma, small cell | 2 |
| Osteosarcoma, parosteal | 2 |
| Fibrous dysplasia | 10 |
| Osteofibrous dysplasia | 2 |
| Fracture | 2 |
| Myositis ossificans | 2 |
| Enchondroma | 3 |
| Chondroblastoma | 9 |
| Chondromyxoid fibroma | 3 |
| Chondrosarcoma, conventional | 20 |
| Chondrosarcoma, clear cell | 5 |
| Giant cell tumour of bone | 8 |
| Aneurysmal bone cyst | 4 |
| Solitary bone cyst | 2 |
| Non-ossifying fibroma | 3 |
| Undifferentiated pleomorphic sarcoma | 3 |
| Ewing sarcoma | 6 |
| Adamantinoma of long bone | 1 |
| Chordoma | 2 |
| Plasma cell myeloma | 2 |
| Lymphoma | 2 |
| Langerhans cell histiocytoma | 2 |
| Metastatic breast carcinoma | 3 |
| Metastatic melanoma | 1 |
Immunohistochemistry for periostin
Immunohistochemical staining for periostin was carried out by an indirect immunoperoxidase technique (without any antigen retrieval procedure) using a polyclonal rabbit antiserum against human periostin raised by using peptide DNLDSDIRRGLESNVN (representing aminoacids 143–158 of human periostin) as an immunogen [13, 17]. As in previous studies [17], the antibody dilution was 1:250 and sections of normal skin were employed as a positive control.
Periostin expression in OA and RA synovial fluid
A quantitative measure of the level of periostin in knee joint RA and OA synovial fluid was determined by ELISA (enzyme-linked immunosorbent assay). The periostin concentration was assessed using a Human periostin/OSF-2 ELISA kit (Adiopo bioscience, Santa Clara, CA, USA). Statistical evaluation was performed using the Mann–Whitney U test with p values less than 0.05 considered as statistically significant.
Results
Periostin expression in normal bone and joint tissues
In normal bone there was strong expression of periostin in collagenous fibrous tissue of the periosteum. At points of tendon or ligament insertion into bone, strong periostin staining was also seen within collagenous tissue. There was no staining for periostin in normal lamellar cortical and cancellous bone, and osteocytes, bone lining cells, osteoblasts and osteoclasts did not express periostin. Fatty and hematopoietic marrow was generally negative for periostin but, in some specimens, staining for periostin was noted in small sinusoidal vascular channels within the marrow. In normal joints, the synovium and hyaline articular cartilage did not stain for periostin.
Periostin expression in tumours and tumour-like lesions of bone and joint
Strong staining for periostin was seen in the osteoid matrix formed by osteoblastic cells in neoplastic and non-neoplastic bone-forming lesions. Periostin staining was noted in the osteoid matrix covering organized reactive bone in fracture callus and myositis ossificans. In osteoblastoma, newly formed osteoid stained strongly for periostin (Fig. 1a); staining for periostin was less pronounced in woven bone and was absent in lamellar bone surrounding the lesion. In osteosarcoma, there was strong staining for periostin in the osteoid matrix formed by malignant cells (Fig. 1b); staining for periostin was seen in all osteosarcomas but the extent of expression was variable with osteoid-rich tumours showing the strongest and most diffuse staining (Fig. 1c). A similar pattern of staining for periostin was noted in lung nodules of metastatic osteosarcoma (Fig. 1d). In small cell osteosarcoma, periostin was expressed in the matrix between tumor cells (Fig. 1e). In chondroblastic and telangiectatic osteosarcomas, cartilage and giant cell components of the tumor were negative. In parosteal osteosarcoma, periostin expression was noted focally in the matrix in areas of tumor cell proliferation. There was strong staining for periostin in fibrous dysplasia and osteofibrous dysplasia, predominantly in the cellular fibrous stroma between bone trabeculae (Fig. 1f).
Fig. 1.
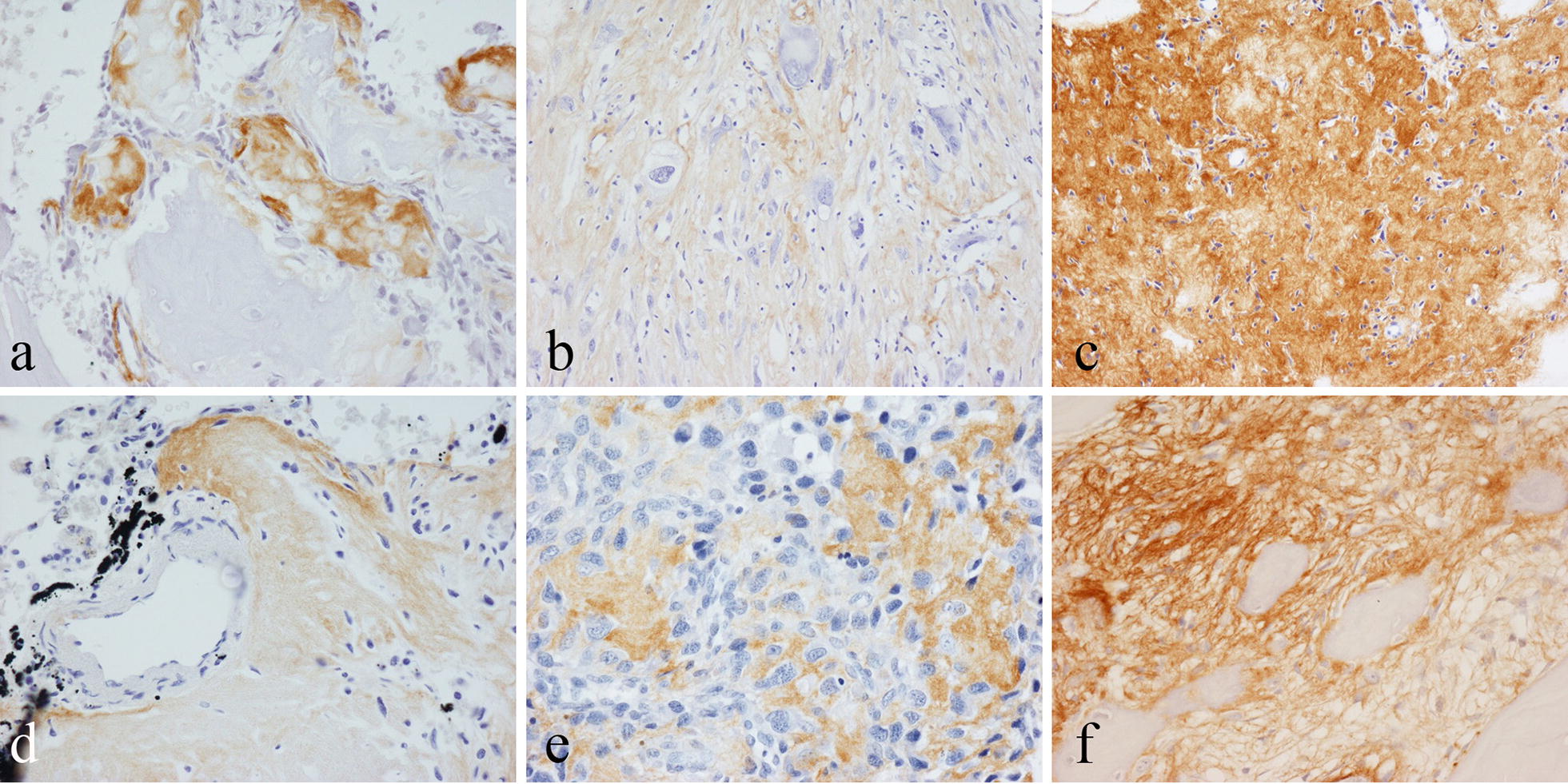
Immunohistochemical staining for periostin in osteoid/newly formed bone of: a osteoblastoma; b high-grade osteoblastic osteosarcoma; c osteoid-rich area of high-grade osteosarcoma; d small cell osteosarcoma; e osteosarcoma metastasis in lung; f fibrous dysplasia
There was no specific staining of the chondroid matrix or cartilage cells for periostin in enchondroma, osteochondroma, or low/high-grade conventional chondrosarcoma. The dense fibrous perichondrium covering osteochondromas (which is continuous with the periosteum) stained strongly for periostin (Fig. 2a). At the base of growing osteochondromas, there was focal staining of the matrix and thin-walled vessels in areas of enchondral ossification and remodeling of newly formed bone. In chondroblastoma, areas of chondroid matrix, some of which showed evidence of mineralisation, stained focally for periostin (Fig. 2b). There was also focal staining of the matrix around chondroblasts. There was no staining for periostin in chondromyxoid fibroma. In clear cell chondrosarcoma, periostin staining was seen in the mineralized osteoid-like matrix covering woven bone and cartilage formed by vacuolated tumor cells.
Fig. 2.
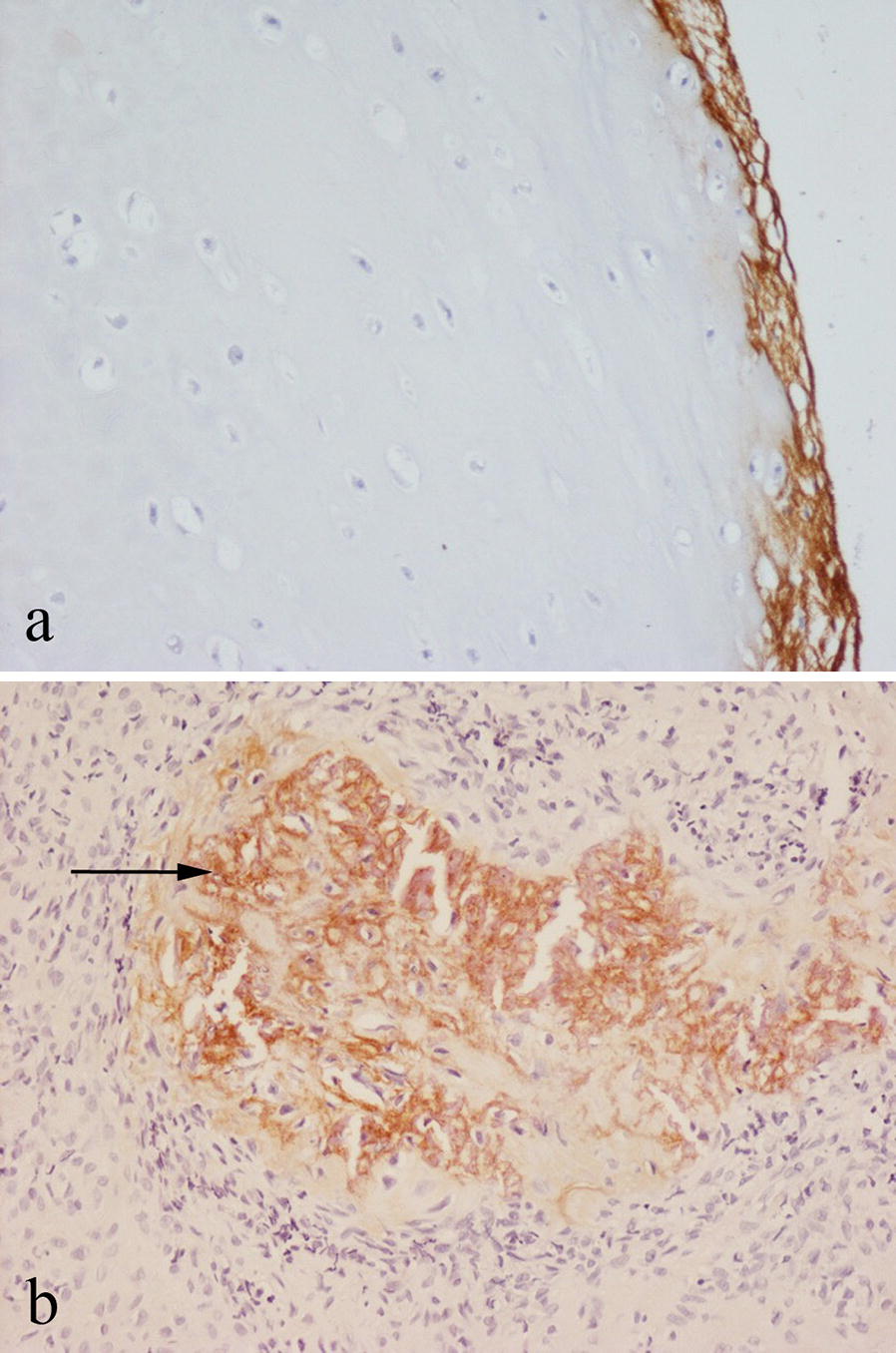
Immunohistochemical staining for periostin in: a benign osteochondroma showing staining of the perichondrium covering the (unstained) cartilage; b chondroblastoma showing staining in areas containing mineralised chondroid (arrowed)
In aneurysmal bone cyst (ABC) and simple bone cyst, the fibrous stroma and reactive bone within the cyst wall was positive for periostin (Fig. 3a). In giant cell tumour of bone (GCTB), there was focal, occasionally strong staining for periostin in the collagenous connective tissue matrix around mononuclear cells (Fig. 3b). Giant cells in GCTB, chondroblastoma and ABC were negative for periostin. Variable focal staining for periostin was seen in cellular and collagenous connective tissue of other bone tumours, including non-ossifying fibroma and undifferentiated pleomorphic sarcoma. No specific staining for periostin was noted in Langerhans cell histiocytosis, Ewing sarcoma, lymphoma, myeloma, chordoma or adamantinoma.
Fig. 3.
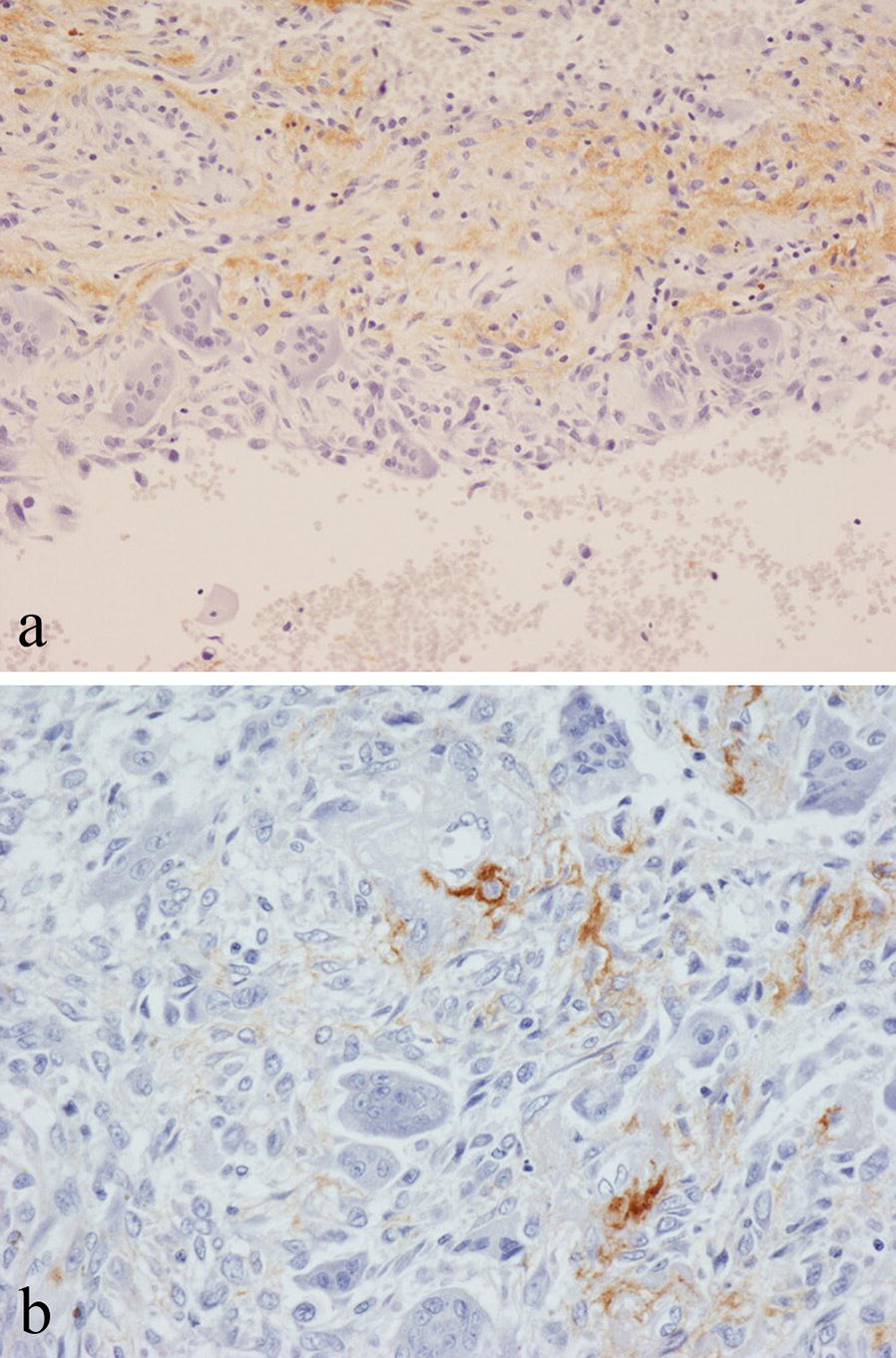
Immunohistochemical staining for periostin in: a aneurysmal bone cyst showing matrix staining in fibrous tissue of the cyst wall; b giant cell tumour of bone showing matrix staining around mononuclear cells
Staining for periostin was noted in non-neoplastic bone at the edge of growing benign and malignant primary bone tumours (Fig. 4a); this was in areas of fatty marrow in which there was fibrosis, increased vascularity and reactive bone formation with prominent staining often noted in the smooth muscle wall of small blood vessels with lining endothelial cells unstained. Infiltrating secondary carcinomas and melanomas that had metastasised to bone showed a similar pattern of staining for periostin in surrounding non-neoplastic bone. Strong periostin staining of vessels was also seen within metastatic tumours (Fig. 4b).
Fig. 4.
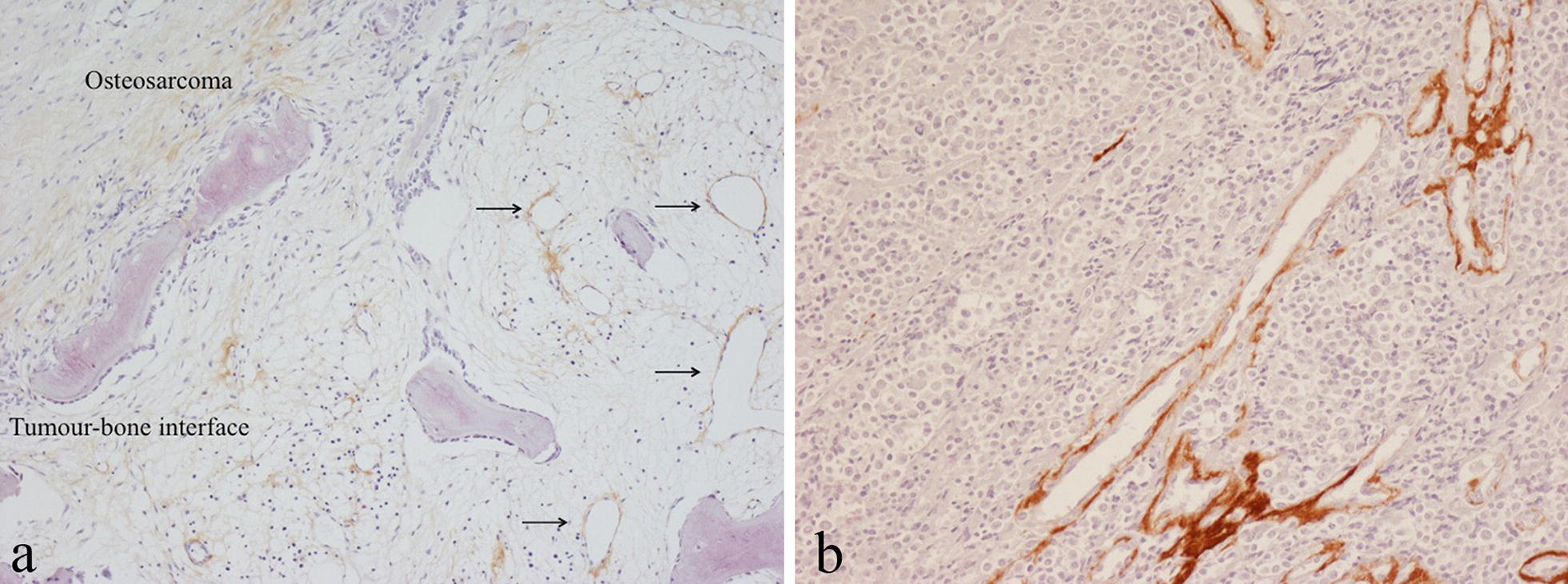
Immunohistochemical staining for periostin in: a osteosarcoma showing staining of vessels and matrix in fatty marrow in non-neoplastic bone at the tumour margin (arrowed); b metastatic melanoma showing prominent staining of vessels within the tumour
Periostin expression in OA and RA
Immunohistochemistry showed no staining for periostin of synovial lining cells in OA or RA synovium. Strong staining for periostin was noted in RA in the superficial subintima where there was staining of the fibrous tissue matrix and the smooth muscle wall of small blood vessels in areas of increased vascularity (Fig. 5a); in contrast, non-inflammatory OA synovium showed no subintimal periostin staining; (Fig. 5b). Periostin was also expressed in the matrix around large vessels in the deep subintima and capsule of RA joints. ELISA studies showed that the average human periostin concentration in synovial fluid was 107.4 ng/ml and 67.1 ng/ml in RA patients and OA patients respectively (Fig. 6). This was not of statistical significance (p = 0.29), but higher periostin levels were more frequently seen in RA than OA synovial fluid.
Fig. 5.
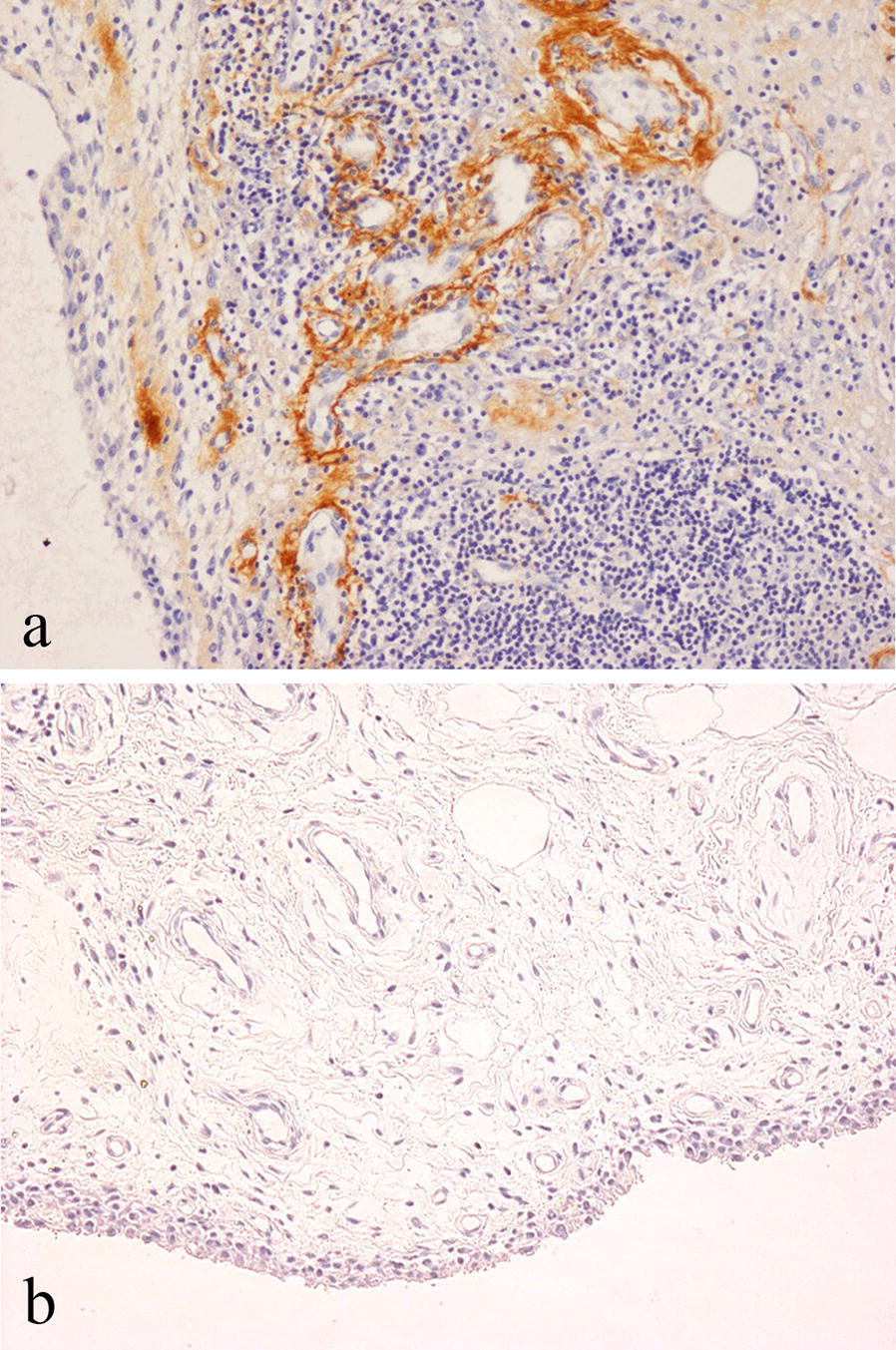
Immunohistochemical staining for periostin in: a RA synovium showing prominent staining of vessels in the subintima; b OA synovium
Fig. 6.
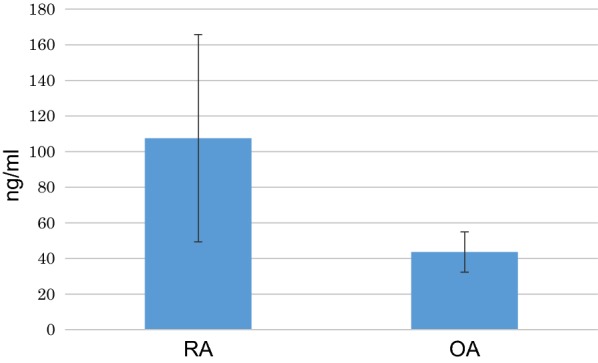
Amount of periostin in synovial fluid samples from RA and OA patients (n = 9), quantified using ELISA
Discussion
In this study, we have characterised periostin expression in neoplastic and non-neoplastic lesions of bone and joint. In keeping with its role in bone matrix formation, periostin was found to be strongly expressed in osteoid/bone-forming lesions; it was also noted in the mineralised chondroid/osteoid matrix of chondroblastomas and clear cell chondrosarcomas and in the connective tissue matrix of other primary bone tumours. Periostin expression was also prominent in areas of reactive host bone around infiltrating primary and secondary bone tumours, suggesting a role for this matricellular protein in tumour progression.
Periostin is a 90-kDa secreted protein which binds to type I collagen and other ECM proteins including fibronectin, Notch1, tenascin-C and BMP-1 [3–6, 18–20]; periostin acts to increase osteoblast proliferation, differentiation, adhesion and survival, and plays a key role in bone matrix formation. Periostin plays a role in bone remodelling by regulating collagen cross-linking and fibrillogenesis. In periostin-null mice, collagen fibrillogenesis is disrupted in the periosteum and mechanical loading results in a disorganised matrix formation. In addition, periostin expression is associated with reduced sclerostin and preservation of bone mass. Periostin increases ECM production by fibroblasts/myofibroblasts and promotes mesenchymal stem cell differentiation into osteoblasts, resulting in the formation of bone matrix. Periostin is known to function as a signalling molecule through integrin receptors and WNT-β-catenin pathways whereby it stimulates osteoblast function and bone formation.
Periostin was originally called osteoblast specific factor-2 and in this study we have shown that periostin is highly expressed in reparative lesions associated with osteoid/woven bone formation, such as fracture callus and myositis ossificans as well as in benign and malignant bone-forming tumours. Expression of periostin in osteosarcoma has previously been reported with high expression being correlated with tumour angiogenesis and poor prognosis [14, 15]. We noted periostin expression in both low-grade parosteal osteosarcomas and high-grade conventional osteosarcomas as well as in lung metastases of osteosarcoma with the extent of periostin expression appearing to be more related to formation of an osteoid matrix than histological parameters of tumour grade. Strong periostin expression was consistently noted in fibrous dysplasia and osteofibrous dysplasia, fibro-osseous bone tumours in which there is formation of woven bone with intramembranous ossification similar to that which occurs beneath the periosteum. Periostin was also seen in cellular fibrous tissue and areas of reactive osteoid/bone formation in other bone lesions, including simple bone cyst, ABC, fracture callus and myositis ossificans. It was also noted in the connective tissue matrix around mononuclear cells in giant cell tumour of bone; these cells are known to exhibit several osteoblast markers including alkaline phosphatase, RUNX2, osterix and RANKL [21]. Focal but less prominent staining for periostin was also noted in cellular and collagenous fibrous tissue of other primary bone tumours including non-ossifying fibroma and undifferentiated pleomorphic sarcoma.
Periostin was strongly expressed in the perichondrium covering osteochondromas and in areas of endochondral ossification at the base of growing osteochondromas but there was no staining in cells or matrix of the cartilage cap. Periostin expression was absent in other cartilage tumours including enchondroma and low/high-grade conventional chondrosarcoma. Our findings contrast with those of Lai and Chen [22], who identified periostin in chondrosarcoma and enchondroma using a commercial mouse monoclonal antibody TA804575 (OriGene Technologies, Inc., Rockville, MD, USA); in our study we employed a rabbit polyclonal antibody that had been characterised in previous investigations [13, 17]. In both chondroblastoma and clear cell chondrosarcoma, strong expression of periostin was noted in areas of chondroid matrix formation. In chondroblastoma, the chondroid matrix has been described as “chondrosteoid” by some observers [23]; it has been shown that this matrix contains dentine-matrix protein-1 and sclerostin, proteins found in newly formed osteoid [24, 25]. Clear cell chondrosarcoma, which is considered by some observers to be a malignant form of chondroblastoma [23], also showed expression of periostin in areas of woven bone formed within the clear cell cartilaginous stroma.
We consistently noted increased expression of periostin in non-lesional bone at the edge of growing benign and malignant bone tumours. It has been shown that periostin plays a role in the progression of inflammatory and neoplastic lesions [6, 11, 12, 23]. Periostin is known to be expressed by fibroblasts in RA, carcinomas and other malignant tumours [26–32]. Periostin is known to stimulate cell/matrix adhesion and migration of the endothelial cells through interaction with αVβ3 [33]. Endothelial cells strongly express αVβ3 when stimulated by growth factors produced in inflammation, wound healing and tumours. We noted that periostin was frequently expressed in the smooth muscle wall of small blood vessels within non-lesional bone around growing tumours, both benign and malignant. Interactions between periostin and vascular endothelial growth factor (VEGF) and its receptors are thought to play a key role in physiological and pathological angiogenesis [6, 12, 29, 34–36]. Periostin is strongly expressed by vascular smooth muscle cells, particularly those which are activated and migrating from the media to the intima or proliferating and synthesising matrix proteins. It has been shown that in breast carcinoma, squamous cell carcinoma and other tumours, blood vessel density in periostin-positive tumours is higher than in periostin-negative tumours with increased tumour invasion and metastasis being reported in these periostin over-expressing tumours [28, 37–40]. We noted prominent staining of the smooth muscle wall of small blood vessels in malignant tumours that had metastasised to bone.
There were similarities in the pattern of periostin expression in inflamed RA synovium and growing bone tumours. Periostin is known to be involved in the migration of synovial fibroblasts associated with RA pannus formation and joint destruction [4, 10]. We noted strong expression of periostin in the subintimal connective tissue matrix and smooth muscle wall of small blood vessels in RA synovium, indicating that periostin-associated angiogenesis may play a role in RA disease progression. We also noted higher levels of periostin in RA compared with OA synovial fluid and little staining for periostin in OA synovium. Increased levels of periostin have been associated with tumour angiogenesis, metastatic potential and poor prognosis in osteosarcoma patients [14, 15]. It has been shown that small interfering RNA against periostin significantly reduces the migration of fibroblast-like cells in RA [10]. Analogously, inhibition of periostin gene expression suppresses the proliferation and invasion of U2OS osteosarcoma cells [41] Our immunohistochemical finding of increased expression of periostin at the edge of growing bone tumours is in keeping with a role for periostin in tumour growth and, taken with the results of previous studies, suggests that periostin could represent a potential therapeutic target to control the growth of osteosarcoma and other bone tumours.
Conclusions
In keeping with its known role in modulating the synthesis of collagen and other extracellular matrix proteins in bone, strong periostin expression was noted in benign and malignant lesions forming an osteoid or osteoid-like matrix. Periostin was also noted in other bone tumours and was found in areas of reactive bone and increased vascularity at the edge of growing tumours, consistent with its involvement in tissue remodelling and angiogenesis associated with tumour progression.
Authors’ contributions
AK, AS, JMB, OU and NAA were the major contributors in writing the manuscript. NAA made the final editing of the manuscript. JMB, AM, ABH assisted in carrying out of the study, the data collection and the preparation of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Sarah Turton for typing the manuscript and David Mahoney and Takeshi Kashima for help with the ELISA studies.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent to publish
Not applicable.
Ethics approval and consent to participate
This study was approved by the Central Oxford Research Ethics Committee (C01.070 and C01.071). Informed consent was obtained from the use of tissue in this study.
Funding
This study was supported by the Sasakawa Foundation and the European Union through funding of the EuroBoNet and EuroSarc consortiums. The funders played no role in the collection of data, interpretation of results or writing of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ABC
aneurysmal bone cyst
- BMP-1
bone morphogenetic protein-1
- ECM
extracellular matrix
- ELISA
enzyme linked immunosorbent assay
- GCTB
giant cell tumour of bone
- OA
osteoarthritis
- RA
rheumatoid arthritis
- RANKL
receptor activator of nuclear factor kappa-B ligand
- RUNX2
Runt-related transcription factor 2
- SF
synovial Fluid
- VEGF
vascular endothelial growth factor
Contributor Information
Jennifer M. Brown, Email: jennifer.brown@ndcls.ox.ac.uk
Akiro Mantoku, Email: mantoku.a.aa@m.titech.ac.jp.
Afsie Sabokbar, Email: afsie.sabokbar@ndorms.ox.ac.uk.
Udo Oppermann, Email: udo.oppermann@sgc.ox.ac.uk.
A. Bass Hassan, Email: bass.hassan@path.ox.ac.uk.
Akiro Kudo, Email: akudo@jcom.zaq.ne.jp.
Nick Athanasou, Phone: +44 1865 (7) 38136, Email: nick.athanasou@ndorms.ox.ac.uk.
References
- 1.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 3.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68:3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merie B, Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos Int. 2012;23:1199–1212. doi: 10.1007/s00198-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 5.Cobo T, Voloria CG, Solares L, Fontanil T, Gonzales-Chanorro E, De Carlos F, Cobo JM, Cal S, Obaya AJ. Role of periostin in adhesion and migration of bone remodelling cells. PLoS ONE. 2016;11:e0147837. doi: 10.1371/journal.pone.0147837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, Arron JR, Holeweg CT, Kudo A. The role of periostin in tissue remodelling across health and disease. Cell Mol Life Sci. 2014;71:1279–1288. doi: 10.1007/s00018-013-1494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimazaki M, Nakamura K, Kii I, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasperkovitz PV, Timmer TC, Smeets TJ, et al. Fibroblast-like synoviocytes derived from patients with rheumatoid arthritis show the imprint of synovial tissue heterogeneity: evidence of a link between an increased myofibroblast-like phenotype and high-inflammation synovitis. Arthritis Rheum. 2005;52:430–441. doi: 10.1002/art.20811. [DOI] [PubMed] [Google Scholar]
- 9.Geyer M, Grassel S, Straub RH, et al. Differential transcriptome analysis of intra-articular lesional vs intact cartilage reveals new candidate genes in osteoarthritis pathophysiology. Osteoarthritis Cartilage. 2009;17:328–335. doi: 10.1016/j.joca.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 10.You S, Yoo SA, Choi S, et al. Identification of key regulators for the migration and invasion of rheumatoid synoviocytes through a systems approach. Proc Natl Acad Sci USA. 2014;111(550–555):11. doi: 10.1073/pnas.1311239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye D, Shen ZS, Qiu SJ, Li Q, Wang GL. Role and underlying mechanisms of the interstitial protein periostin in the diagnosis and treatment of malignant tumours. Oncol Lett. 2017;14:5099–5106. doi: 10.3892/ol.2017.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratajczak-Wielgomas K, Dziegiel P. The role of periostin in neoplatic processes. Folia hitiochem Cytobiol. 2015;53:120–132. doi: 10.5603/FHC.a2015.0014. [DOI] [PubMed] [Google Scholar]
- 13.Kashima TG, Nishiyama T, Shimazu K, et al. Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol. 2009;40:226–237. doi: 10.1016/j.humpath.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Hu F, Wang W, Zhou HC, Shang XF. High expression of periostin is dramatically associated with metastatic potential and poor prognosis of patients with osteosarcoma. World J Surg Oncol. 2014;12:287. doi: 10.1186/1477-7819-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu F, Shang XF, Wang W, Jiang W, Fang C, Tan D, Zhou HC. High expression of periostin is significantly correlated with tumour angiogenesis and poor prognosis in osteosarcoma. Int J Exp Pathol. 2016;97(1):86–92. doi: 10.1111/iep.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO classification of tumours of soft tissue and bone. 4. IARC: Lyon; 2013. [Google Scholar]
- 17.Kikuchi Y, Kashima TG, Nishiyama T, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 2008;56:753–764. doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruhasi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysl oxidase. J Biol Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kii I, Nishiyama T, Li M, Matumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ, Ferrari SL. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem. 2009;284:35939–35950. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Athanasou NA, Bansai M, Forsyth R, Reid RP, Sapi Z. Giant cell tumour of bone. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO classification of tumours of soft tissue and bone. 4. Lyon: IARC; 2013. pp. 321–324. [Google Scholar]
- 22.Lai X, Chen S. Identification of novel biomarker candidates for immunohistochemical diagnosis to distinguish low-grade chondrosarcoma from enchondroma. Proteomics. 2015;15:2358–2368. doi: 10.1002/pmic.201400528. [DOI] [PubMed] [Google Scholar]
- 23.Mirra JM. Bone tumors: clinical radiological and pathological correlation. Philadelphia: Lee and Febiger; 1989. [Google Scholar]
- 24.Kashima TG, Dongre A, Oppermann U, Athanasou NA. Dentine matrix protein (DMP-1) is a marker of bone-forming tumours. Virchows Arch. 2013;462(5):583–591. doi: 10.1007/s00428-013-1399-z. [DOI] [PubMed] [Google Scholar]
- 25.Inagaki Y, Hookway ES, Kashima TG, Munemoto M, Tanaka Y, Hassan AB, Oppermann U, Athanasou NA. Sclerostin expression in bone tumours and tumour-like lesion. Histopathology. 2016;69:470–478. doi: 10.1111/his.12953. [DOI] [PubMed] [Google Scholar]
- 26.Izuhara K, Nunomura S, Nanri Y, Ono J, Mitamura Y, Yoshihara T. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017;74:4293–4303. doi: 10.1007/s00018-017-2648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puglisi F, Puppin C, Pegolo E, Andreetta C, Pascoletti G, D’Aurizio F, Pandolfi M, Fasola G, Piga A, Damamte G, Di Loreto C. Expression of periostin in human breast cancer. J Clin Pathol. 2008;61:494–498. doi: 10.1136/jcp.2007.052506. [DOI] [PubMed] [Google Scholar]
- 28.Oh H, Bae JM, Wen XY, Chon Y, Kin JJ, Kang GH. Overexpression of periostin in tumour stroma is a poor prognostic indicator of colorectal cancer. J Pathol Trans Med. 2017;51:306–313. doi: 10.4132/jptm.2017.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Aquired expression of periostin by human breast cancer angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992–4003. doi: 10.1128/MCB.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukushima N, Kikuchi Y, Nishiyama T, et al. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol. 2008;21:1044–1053. doi: 10.1038/modpathol.2008.77. [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi Y, Kunita A, Iwata C, et al. The niche component periostin is produced by cancer-associated fibroblasts, supporting the growth of gastric cancer through ERK activation. Am J Pathol. 2014;184:859–870. doi: 10.1016/j.ajpath.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Tilman G, Mattiussi M, Brasseur F, van Baren N, Decottignies A. Human periostin gene expression in normal tissues, tumours and melanoma: evidence for periostin production by both stromal and melanoma cells. Mol Cancer. 2007;17(6):80. doi: 10.1186/1476-4598-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillian L, Matei D, Fisherman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha (V) beta (3) and alpha (V) beta (5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- 34.Litvin J, Chen X, Keleman S, Zhu S, Autleri M. Expression and function of periostin-like factor in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C1672–C1680. doi: 10.1152/ajpcell.00153.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kim BR, Kwon YW, Park GT, Choi EJ, Seo JK, Jang IH, Kim SC, Ko HC, Lee SC, Kim JH. Identification of a novel angiogenic peptide from periostin. PLoS ONE. 2017;12(11):e0187464. doi: 10.1371/journal.pone.0187464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu YI, Wang W, Jia WD, et al. High-level expression of periostin is closely related to metastatic potential and poor prognosis of hepatocellular carcinoma. Med Oncol. 2013;30:385. doi: 10.1007/s12032-012-0385-7. [DOI] [PubMed] [Google Scholar]
- 37.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- 38.Kudo Y, Ogawa I, Kitajima S, et al. Periostin, a stroma-associated protein, correlates with tumour invasiveness and progression in nasopharyngeal carcinoma. Clin Exp Metastasis. 2012;29:865–877. doi: 10.1007/s10585-012-9465-5. [DOI] [PubMed] [Google Scholar]
- 39.Contie S, Voorzanger-Rousselot N, Litvin J, Clezardin P, Garnero P. Increased expression and serum levels of the stromal cell protein periostin in breast cancer bone metastasis. Int J Cancer. 2011;128:352–360. doi: 10.1002/ijc.25591. [DOI] [PubMed] [Google Scholar]
- 40.Bao S, Ouyang G, Bai X, et al. Perisotin potently promotes metastatic growth of colon cancer by augmenting cell survival via the AkT/PKB pathway. Cancer Cell. 2004;5:329–539. doi: 10.1016/S1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Huang SJ, Qin ZL. Inhibition of periostin gene expression via RNA suppressed the proliferation, apoptosis and invasion in U2OS cells. Chin Med J. 2010;123:3677–3683. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


