Abstract
Castor oil (CO) is an inedible vegetable oil (VO) that has been employed extensively as a bioresource material for the synthesis of biodegradable polymers, cosmetics, lubricants, biofuels, coatings and adhesives. It is used in medicine, pharmaceuticals and biorefineries, due to its versatile chemistry. However, there has been less focus on CO as an alternative to toxic and expensive solvents, and capping/stabilizing agents routinely used in nanoparticle syntheses. It provides a richer chemistry than edible VOs as a solvent for green syntheses of nanoparticles. CO, being the only rich source of ricinoleic acid (RA), has been used as a solvent, co-solvent, stabilizing agent and polyol for the formation of polymer–nanoparticle composites. RA is a suitable alternative to oleic acid used as a capping and/or stabilizing agent. Unlike oleic acid, it provides a facile route to the functionalization of surfaces of nanoparticles and the coating of nanoparticles with polymers. For applications requiring more polar organic solvents, RA is more preferred than oleic acid. In this review, we discuss the production, chemical and physical properties, triglyceride and fatty acid (FA) compositions and applications of CO, focusing on the use of CO and RA as well as other VOs and FAs in syntheses of nanoparticles and surface functionalization.
Keywords: nanoparticles, vegetable oil, castor oil, ricinoleic acid
1. Introduction
The impact of nanotechnology on society is enormous. However, the use of expensive and toxic materials for the syntheses of nanoparticles is becoming a critical concern. Many researchers have resorted to employing environmentally friendly renewable bioresource materials such as vegetable oils (VOs), carbohydrates and plant extracts in the syntheses of nanoparticles [1–15]. Castor oil (CO) has been viewed as one of the most likely sources of plant oils suitable as solvents and/or capping agents for syntheses of metal and metal chalcogenide nanoparticles [14,16].
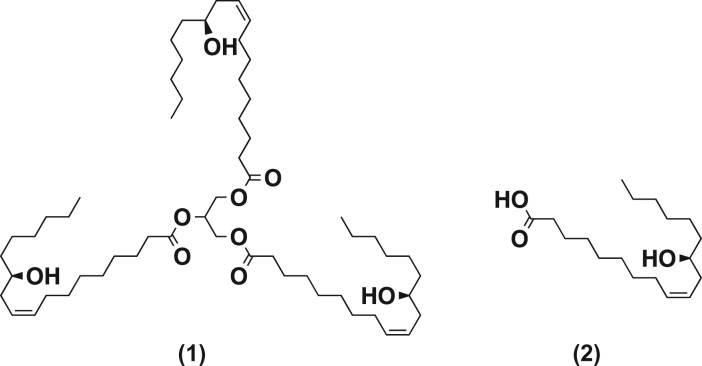
Oleum Palmae Christi (or CO) is a hydroxylated lipid obtained from the seed of the castor plant, Ricinus communis L. of the family Euphorbiaceae and native to tropical Asia and Africa [16–18]. It is considered a very important bioresource material for a wide variety of applications [19]. The triglycerides of CO consist of fatty acids (FAs), of which approximately 90% is ricinoleic acid (RA), a hydroxylated monounsaturated 18-carbon carboxylic acid (figure 1). It is the only pure source of RA [2,20,21].
Figure 1.
Chemical structures of triricinolein (1) and ricinoleic acid (2).
RA is a multifunctional compound, possessing a carboxylic acid, a double bond (between C9 and C10) and a secondary alcohol or hydroxyl (at C12) functional group. The hydroxyl group is beta to the double bond and protects that double bond from peroxide formation [22]. These functional groups make CO (and its derivatives) completely soluble in all alcohols and present viscosities that are up to approximately sevenfold higher than the viscosities of other VOs [23]. The triricinolein (in CO, figure 1) undergoes amidation, saponification, reduction, esterification, alcoholysis, hydrolysis, dehydration, caustic fusion, sulfonation, pyrolysis, oxidation, polymerization, halogenation, epoxidation, hydrogenation and olefin metathesis reactions [18,19,24,25]. Thus, CO is applicable as a valuable raw material in industries for production of coatings, biopolymers, paints, adhesives, cosmetics, lubricants, hydraulic fluids, inks, linoleum and chemicals including sebacic acid and undecylenic acid used in the production of plasticizers and nylon [23–26]. The properties of CO that make it appropriate as a solvent for nanoparticle syntheses are: (i) non-toxicity, (ii) high boiling temperature of 313°C, (iii) colourless to pale yellow liquid, (iv) mild or no odour or taste and (v) a clear liquid at room temperature and showing no solid fat at 0°C [27,28]. The alkyl groups impose a steric effect that controls the growth, crystal structure, morphology and surface characteristics of the nanoparticles [29,30].
Carboxylic acids such as oleic acid and stearic acid have been employed extensively as ligands or capping agents [31,32]. RA also represents a valuable alternative capping agent. The presence of the hydroxyl group attached to RA-capped nanoparticles allows them to be functionalized easily with other groups to enhance their dispersion in different solvent media specific to a particular application. However, the capping activities of CO and RA have not been extensively explored. CO has been reviewed as a vital bioresource of industrial raw material for production of different functional materials, and a majority of the reviews on it are centred on developing biodegradable polymers [19,33,34]. It remains as the most promising source of building blocks for the synthesis of polyurethane and polyesters [25,35–37]. CO has also been reviewed as a source of biobased chemicals and biodiesel [16,38,39]. Although CO (and RA) has a probable significant impact on nanoparticle syntheses and applications, it has been less emphasized, and there is little information regarding its innovative development in nanotechnology. Thus, this review examines CO (and RA) as valuable bioresource material for nanoparticle syntheses and functionalization.
This review is split into four main sections: (i) facts about CO (brief history on castor cultivation, production and physico-chemical properties), (ii) composition and structure of CO and isolation of RA, (iii) application of CO in biomedicine, biopolymers, biochemicals, bioenergy, lubricants and coatings and (iv) utilization of CO (as well as other VOs) and RA (as well as other FAs) as capping ligands or solvents for nanoparticle syntheses and functionalization. The review is then concluded by highlighting the areas in nanoparticle syntheses where CO and RA can be used.
2. Facts about castor oil
2.1. Cultivation of castor oil seed
Ethiopia (east Africa) is believed to be the most likely origin of castor in addition to places such as northwest and southwest Asia, the Arabian Peninsula and the subcontinent of India and China [40]. The history of the castor plant cultivation is well reported by Anjani [40]. The castor plant is cultivated for its seed oil, which is the only commercial source of RA for the chemical and pharmaceutical industries [41]. The CO plant is also used as an ornamental and in insect traps [40,42]. The castor plant is a cross-pollinated diploid (2n = 2x = 20) species within the family of Euphorbiaceae and the genus Ricinus. The castor plant is a coarse perennial crop that grows to approximately 10 ft in the tropics and has a stem diameter of 7.5–15 cm. In the temperate regions, the castor plant behaves as an annual crop with succulent stems and usually herbaceous [43]. Pictures of the castor plant, seeds and oil are shown in figure 2. CO possesses nauseating properties, and the seed kernel contains a poisonous glycoprotein called ricin. Nonetheless, the oil itself is not toxic, and the seed cake is usually detoxified using Ca(OH)2, NaOH or NaOCl to remove the toxins [16,38,44]. Detailed information about castor plant cultivation and CO extraction can be found in www.castoroil.in [43].
Figure 2.
Castor plant: (a) matured castor plant, (b) bunch of castor seeds and (c) dried castor seed pod, seed and oil.
2.2. Production of castor oil seed
The oil content of the castor seed is approximately 45–50%, with a yield of 470 kg of oil per hectare [28,45]. The average annual world production of CO seed from 2009 to 2013 was approximately 1.99 × 106 t [46]. The major producers of CO seed in Africa and other parts of the world are presented in table 1 and figure 3. India is the leading producer of castor seed oil. India produces approximately 83% of the world's annual production. Africa produces 90.3 × 103 t of castor seeds annually, representing approximately 4.54% of the world's production. Mozambique produces approximately 3.01% of the world's annual production and is the leading producer of CO seed in Africa. Ethiopia, South Africa, Angola, Tanzania and Kenya are African countries also involved in castor production though their production figures are low. China, Brazil, Paraguay and Thailand are also noted for castor production [46].
Table 1.
Major producers of CO seed (103 t) in the world (FAOSTAT [46]).
| country | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|
| India | 1009.0 | 1350.0 | 2295.0 | 1964.0 | 1644.0 |
| China | 170.0 | 150.0 | 180.0 | 120.0 | 60.0 |
| Brazil | 91.1 | 95.2 | 120.2 | 26.0 | 12.0 |
| Mozambique | 57.0 | 60.0 | 60.0 | 62.0 | 60.0 |
| Paraguay | 13.0 | 6.0 | 6.0 | 10.0 | 11.0 |
| Thailand | 11.7 | 12.2 | 11.3 | 11.0 | 12.0 |
| Ethiopia | 7.0 | 7.0 | 8.0 | 11.0 | 13.0 |
| Vietnam | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| South Africa | 5.6 | 6.2 | 5.7 | 6.0 | 6.2 |
| Angola | 3.4 | 4.0 | 4.0 | 4.0 | 4.0 |
| World | 1395.7 | 1720.6 | 2721.5 | 2244.9 | 1854.8 |
Figure 3.
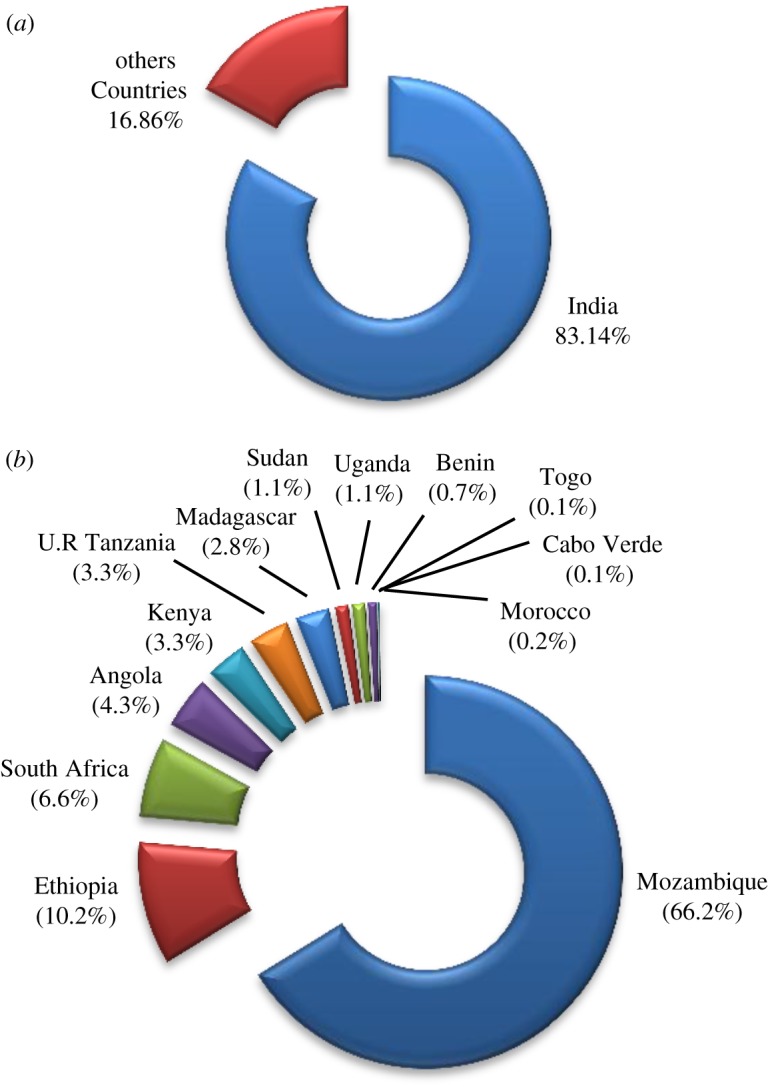
Charts showing production of CO seeds: (a) in the world and (b) in Africa, considering production figures from 2009 to 2013 obtained from FAOSTAT [46].
2.3. Physico-chemical properties of castor oil
The presence of the hydroxyl group on RA has a drastic effect on the viscosity, pour point, melting point, heat of fusion, solubility, crystal structure and polymorphism of CO [47]. The hydroxyl functionality induces the formation of hydrogen bonding between the hydroxylated triglyceride molecules that increases the steric hindrance of the oil, leading to the formation of weakly bound dimers and trimers of the original triglycerides; hence, CO has a high viscosity of 260.4 cSt at 40°C [48]. Molecular weights (MWs) of oils have an effect on oil viscosity. Da Silva et al. [49] analysed the MW of CO by vapour pressure osmometry and gas chromatography and found it to be 927.88 g mol−1 and 928.31 g mol−1, respectively. Salimon et al. [27] also reported the average MW of CO as 937.7 g mol−1. Compared with olive oil, CO has a higher MW and thus has a higher viscosity. The density of CO is also reported to be 961 kg m−3 [28]. CO is known as a non-drying oil because it has only one double bond (low iodine value) in each FA chain and does not harden when exposed to air [16,43]. The physico-chemical properties of CO reported by different research groups are presented in table 2.
Table 2.
Physico-chemical properties of CO.
| parameters | units | values | references |
|---|---|---|---|
| oil content | % | 43.3–56.2 | [27,50,51] |
| density | kg m−3 | 946–961 | [28,50,52] |
| moisture content | % | 0.2–3.9 | [27,50,52] |
| iodine value | mg g−1 | 84.5–85.5 | [27,53] |
| acid value | mg KOH g−1 | 0.03–4.9 | [27,50–52] |
| free FA | % | 0.06–3.4 | [27,50,53] |
| hydroxyl value | mg KOH g−1 | 164.5 | [52] |
| peroxide value | meq kg−1 | 10.2 | [27] |
| saponification value | mg g−1 | 182.9 | [27] |
| unsaponifiable matter | 3.4 | [27] | |
| kinematic viscosity at 40°C | cSt | 260.4 | [48] |
| refractive index | — | 1.47 | [27] |
3. Chemical composition and structure of castor oil
3.1. Triglycerides
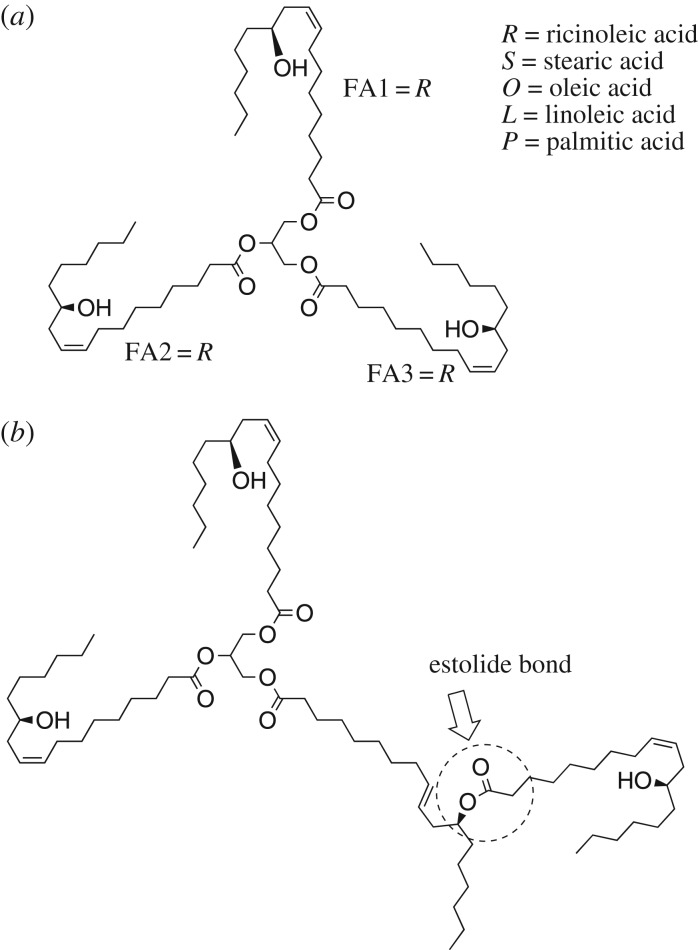
Most of the triglyceride molecules in CO consist of three molecules of RA connected to a glycerol moiety [55]. Salimon et al. [27] identified five major triacylglycerides in CO, which are triricinolein (RRR) (84.1%), diricinoleoystearoylglycerol (RRS) (8.2%), diricino-leoyloleoylglycerol (RRO) (5.6%), diricinoleoyllinoleoylglycerol (RRL) (1.2%) and diricinoleoylpalmitoyl-glycerol (RRP) (0.9%). Table 3 gives the triglyceride composition of CO as reported by Ndiaye et al. [54]; Plante et al. [55] and Lin [47], however, reported the RRR content to be 63% and 70%, respectively. Lin [47] found four new diacylglycerols and eight new triacylglycerols in castor (figure 4). Again, Lin & Chen [56] found 40 new molecular species of acylglycerols in CO that are less polar than RRR (the most abundant triglyceride). The chain lengths of those acylglycerols were C16, C18, C20, C22 and C23. The number of double bonds ranged from 0 to 3, and the number of hydroxyl groups 0–3. Additionally, some estolides and tetraacylglycerols reported in CO are (12-ricinoleoylricinoleoyl)-ricinoleoyl-linoleoyl-glycerol (RRRL), (12-ricinoleoylricinoleoyl)-ricinoleoyl-oleoyl-glycerol (RRRO), (12-ricinoleoylricinoleoyl)-ricinoleoyl-palmitoyl-glycerol (RRRP), (12-ricinoleoylricinoleoyl)-ricinoleoyl-stearoyl-glycerol (RRRS) and (12-ricinoleoylricinoleoyl)-ricinoleoyl-linolenoyl-glycerol (RRRLn) [56]. These acylglycerols were analysed using high-performance liquid chromatography and electrospray ionization mass spectrometry, and the level of the total acylglycerols containing polyhydroxy FAs was only 3% of the CO, while the individual molecular species of acylglycerols containing polyhydroxy FAs were approximately 0.5% or less of CO [56]. Estolides are identified by the secondary ester linkage of one FA molecule to the alkyl backbone of another FA fragment. Estolides can be in the form of free acids, esters or could be found within the structure of triglycerides. Estolides usually form in most hydroxylated oils such as castor and Lesquerella oils [48].
Table 3.
Triglyceride composition of CO [54].
| triglyceride | % composition |
|---|---|
| triestearin | 0.9 |
| tripalmitin | 1.4 |
| triolein | 3.5 |
| trilinolein | 4.9 |
| trilinolenin | 0.3 |
| triricinolein | 88.9 |
Figure 4.
(a) Triglycerides found in CO (FA1, FA2 and FA3 represent FAs attached to the glycerol, when FA1 = R, FA2 = R, FA3 = R, i.e. triricinolein (RRR), diricinoleoystearoylglycerol (FA1 = FA2 = R, FA3 = S, i.e. RRS), diricino-leoyloleoylglycerol (FA1 = FA2 = R, FA3 = O, i.e. RRO), diricinoleoyllinoleoylglycerol (FA1 = FA2 = R, FA3 = L, i.e. RRL) and diricinoleoylpalmitoyl-glycerol (FA1 = FA2 = R, FA3 = P, i.e. RRP) and (b) estolide formation between a triglyceride molecule and a free FA (source: Isbell [48]).
3.2. Fatty acids
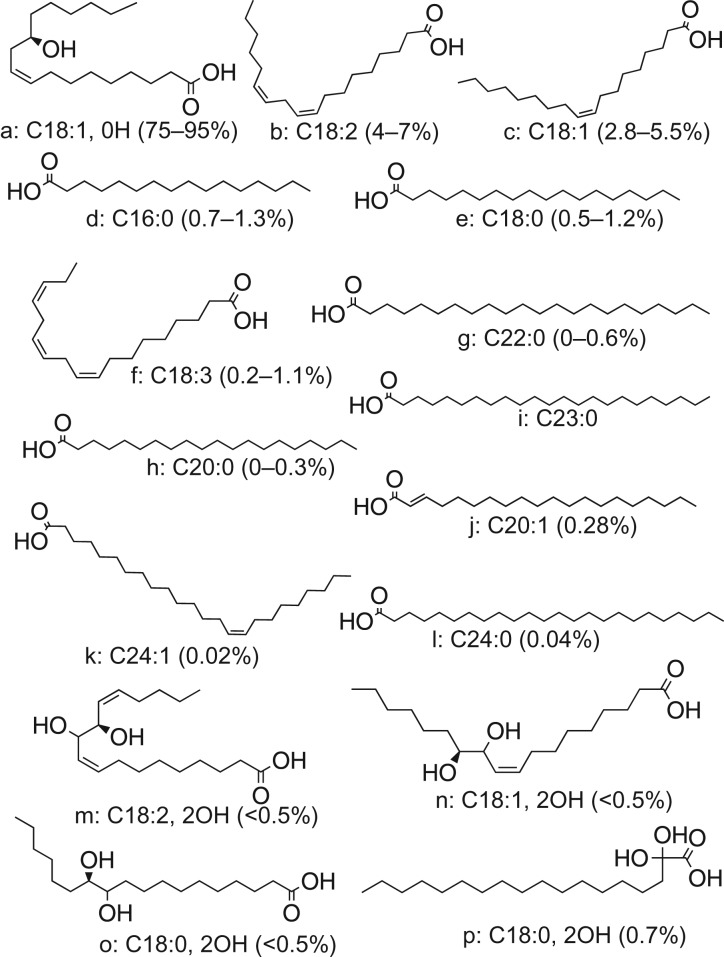
The uniqueness of CO compared with other VOs lies in its FA composition. Numerous groups have reported on CO FA composition from different countries (table 4). The FAs found to be present in CO are RA, linoleic acid, oleic acid, stearic acid and linolenic acid. Approximately 84–90% of the FAs is RA and 10–16% consists of the other FAs (table 4) [22,27,28,57,58]. The FA compositions varied only slightly from different countries. Da Silva et al. [49] also showed the CO composition as 89.5% RA, 3.7% linoleic acid, 3.0% oleic acid, 1.6% palmitic acid, 0.9% stearic acid, 0.6% behenic acid, 0.4% linolenic acid and 0.3% arachinic acid.
Table 4.
FA composition of CO from different countries.
Although CO is known to contain RA, which is a monohydroxy FA, Lin [47] has identified three new dihydroxy FAs in CO which they propose to be 11,12-dihydroxy-9-octadecenoic acid, 11,12-dihydroxy-9,13-octadecadienoic acid and 11,12-dihydroxyoctadecanoic acid (figure 5). Additionally, tricosanoic acid, which is an odd-numbered long FA (C23:0), has been identified to be present in CO by the same group [56]. Remarkably, Hosamani et al. [59] also reported Alternanthera triandra, Lam Syn. Alternanthera sessilis (L.) R. Br. seed oil as another source of RA (contains approx. 22.1% of RA). However, despite the possibility that other seed oils may contain RA, CO remains the only reported rich source of RA to date.
Figure 5.
Chemical structures of FAs (percentage composition in brackets) found to be present in CO (a: RA, b: linoleic acid, c: oleic acid, d: palmitic acid, e: stearic acid, f: linolenic acid, g: behenic acid, h: arachinic acid (or eicosanoic acid), i: tricosanoic acid, j: eicosenoic acid, k: nervonic acid, l: lignoceric acid, m: 11,12-dihydroxy-9,13-octadecadienoic acid, n: 11,12-dihydroxy-9-octadecenoic acid, o: 11,12-dihydroxyoctadecanoic acid, p: dihydroxystearic acid) (source: Lin [47]).
3.3. Isolation of ricinoleic acid from castor oil
Several methods, including chemical and biochemical pathways, have been used to isolate RA from CO. The isolation occurs by hydroxylation of the ester linkages in the triglyceride molecules to yield RA and glycerol. The salt solubility-based fractionation method reported by Vaisman et al. [22] is an example of a chemical method for isolating RA from CO. In this method, CO is hydrolysed by refluxing with an ethanol solution of KOH for 1 h, and the ethanol is evaporated to yield the potassium salt of the FA. The FAs are liberated by dissolving in deionized water and acidifying with concentrated HCl. The FAs are then extracted with ethyl acetate and dried over MgSO4. Clarification of the FAs is done by mixing with n-hexane (1 : 5 w/v) and keeping at −4°C for 72 h in darkness. Chromatographic analysis of the resultant FAs revealed the purity to be within 87.50–88.10% of RA and 12.5–11.9% of palmitic acid, stearic acid, oleic acid, vaccenic acid, linoleic acid and linolenic acid. Solid residues found after clarification were identified to be 9-,10-dihydroxystearic acid [22].
Biocatalysts such as lipase (triacylglycerol acylhydrolase, EC 3.1.1.3) enzymes have been used to isolate RA from CO. Foglia et al. [60] employed Candida rugosa, Pseudomonas cepacia and Geotrichum candidum lipases for hydrolysis of CO. In a typical reaction, tubes containing 100 mg of oil, 0.6 ml of 0.5 M phosphate buffer (pH 7) and approximately 2–5 mg of free lipase were stirred at 500 r.p.m. at 30°C for 1–4 h. The extent of hydrolysis was determined by titrating the hydrolysis mixture (in 20 ml of diethyl ether/ethanol/water (3 : 3 : 2)) to pH 12 with 0.1 N NaOH solution. The P. cepacia lipase was found to be effective in hydrolysing CO to RA to the tune of 27% compared with 13% recorded for C. rugosa and G. candidum. Ozcan & Sagiroglu [61] also employed immobilized C. rugosa, porcine pancreatic and castor bean lipases for lipolysis of CO and obtained a yield of RA within 20–40%, considering a number of parameters such as pH, temperature, amount of substrate and enzyme. Interestingly, Piazza & Farrell [18] used lipase from ground oats (Avena sativa L.) to hydrolyse CO and obtained approximately 90% yield of RA.
An eco-friendly approach by the use of microwave-assisted extraction of RA from CO has also been reported by Karpakavalli et al. [62]. In their approach, a 250 ml beaker containing 5 g of CO and a solution of ethanoic KOH with a few pieces of ice was covered and kept in a household microwave oven. The microwave oven was modified to contain a magnetic stirrer and a water condenser. Heating the reaction system continuously with the microwave oven at 160 W intensity for 19 min, 89% yield of RA was obtained. This microwave-assisted technique was found to be efficient compared to conventional heating of the hydrolysis system. All the above approaches for isolation of RA from CO show a good yield but differ only in the use of different catalysts and sources of heat.
4. Applications of castor oil
CO has received much attention as a valuable commercial feedstock for production of a variety of products in a wide range of industries spanning pharmaceuticals to lubricants. Figure 6 shows a chart illustrating the different areas of CO application reported in the literature.
Figure 6.
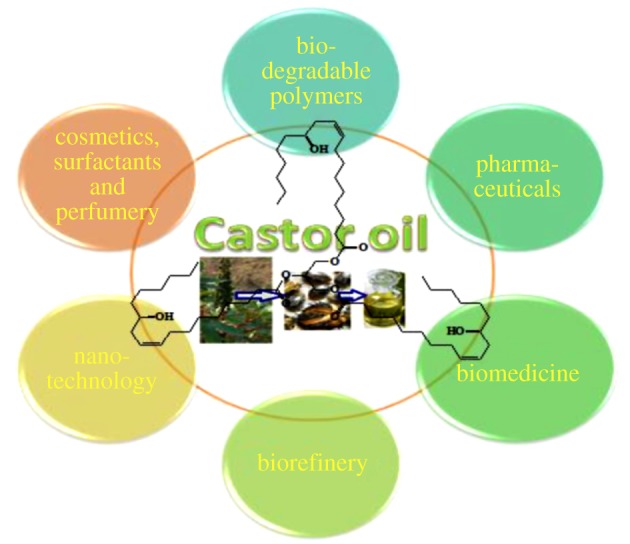
Application of CO in different fields.
4.1. Biomedicine and pharmaceuticals
Historically, CO has been known as a medicinal oil and primarily used as a purgative or laxative to ease constipation [16]. As far back as 500 BC, the Egyptians used CO for purging purposes [40]. According to Anjani [40], an ancient Egyptian treatise, Ebers Papyrus, in 1552 BC later described CO as a purgative. CO is also a known cathartic agent used to induce labour in females. Tunaru et al. [17] found that CO induces laxation and uterus contraction as the RA released from the CO by intestinal lipases activates the prostaglandin EP3 receptors.
Additionally, eye drops containing approximately 1.25% of homogenized CO are reported for the treatment of lipid-deficiency dry eye (i.e. meibomian gland dysfunction). The role of CO in treating dry eye is that it serves as a hydrophilic lipid that spreads over the human tear aqueous layer to correct the deficiency [63].
Katzer et al. [64] have also reported that RA has some potential anti-inflammatory properties. Undecylenic acid (a chemical derived from CO) is also reported to be an antifungal and antiviral and has been used as a chemical building block for vital compounds that possess mosquito repellent, cytotoxic and antibiotic activity [38].
4.2. Polymer synthesis
The use of CO as a raw material in the synthesis of polymeric materials is very well established. The hydroxyl functionality is more suitable for isocyanate reactions, yielding polyurethane, while the double bond is dehydrated to obtain dehydrated CO, which is applied in producing paints, enamels, lacquers and varnishes [80]. Polymers of CO are applied in various fields such as wound dressing, drug delivery, bone tissue engineering and membranes for fuel cell fabrication. Yari et al. [81] reported on a novel antibacterial and cytocompatible polyurethane membrane based on CO for wound dressing. The synthesis of this polymer employed the hydroxyl functional groups on the CO molecule as the anchoring groups to hold the antibacterial agent. The RA-based polyanhydrides are also reported to possess the desired physico-chemical and mechanical properties for use as drug carriers, and in vitro studies showed that these biopolymers degrade rapidly via hydrolysis after 10 days, releasing RA and its counterparts [82]. Table 5 shows the role of CO in different applications or products.
Table 5.
The role of CO in different applications or products.
| description | application/product | role of CO | references |
|---|---|---|---|
| coatings | polyamidoamine toughened epoxy coating | curing agent | [65] |
| polyurethane | polyurethane films for protein adsorption | cross-linking agent | [66] |
| biofuels | biodiesel | triglyceride | [67] |
| membranes | polyurethane/polyaniline membranes for electrodialysis and fuel cells | polyol | [68] |
| polyurethane | microcellular polyurethane | polyol | [69] |
| interpenetrating polymer | hydrogenated CO uralkyd resin/poly(butyl acrylate) blend | polyol | [70] |
| methoxycinnamic oil | sunscreen active ingredient | hydroxy oil | [71] |
| polymer composite | dehydrated CO epoxy/poly(methacrylic acid) blend | additive | [72] |
| thermosetting resins | FA-based co-monomer for styrene replacement | plasticizer | [73] |
| hyper-branched polyester | polyester | methyl 10-undecenoate | [74] |
| polyester | CO-based organogels | methyl ricinoleate | [75] |
| biochemicals | γ-decalactone | ricinoleic acid | [76] |
| biosurfactants | sophorolipids | non-conventional carbon source/substrate | [77] |
| organic–inorganic hybrid films | epoxidized CO/-3-aminopropyltriethoxysilane | alkene | [78] |
| non-ionic surfactants | polyethylene oxide monomethyl ether-based CO | alkene | [79] |
4.3. Cosmetics, perfumery, surfactants and biofuels
Very important industrial chemicals such as γ-decalactone, sophorolipids, undecylenic acid, linoleic acid, sebacic acid, capryl alcohol, heptaldehyde, zinc ricinoleate, glyceryl ricinoleate and lithium 12-hydroxystearate are produced from CO [76,77]. These chemicals have important roles in cosmetics, perfumery and surfactants, and even in polymer synthesis. Interestingly, Compton et al. [71] synthesized a novel sunscreen active ingredient, methoxycinnamic oil (MCO), using CO as the hydroxy oil. CO was reacted with 4-methoxycinnamic acid to yield MCO, which possessed broad UV absorbance from 250 to 345 nm, with the maximum at 305 nm. Another area where CO is massively used is in the production of biofuels. In 4000 BC, CO was being used as a fuel in wick lamps for lighting in ancient Egyptian tombs [43]. Biodiesel has been produced by transesterification of CO [67].
5. Syntheses and surface functionalization of nanoparticles
Green syntheses of nanoparticles are strongly advocated worldwide because of the disadvantages of the use of toxic solvents and chemicals, especially the effects on human health and the environment. Green chemistry principles embody the (i) design of less hazardous chemical syntheses, (ii) use of safer chemicals and solvents, (iii) use of renewable feedstocks and (iv) design of degradation [83]. Thus, renewable bioresource materials are currently the choice of raw materials for most nanochemistry researchers. VOs and FAs are used in green nanochemistry syntheses because they are:
(i) environmentally benign and inexpensive;
(ii) suitable alternatives to some toxic and expensive solvents or ligands traditionally used in nanoparticle syntheses;
(iii) renewable source of raw material;
(iv) biodegradable and provide versatile chemistry-based opportunities;
(v) a source of carboxylic acids suitable as ligands/capping agents or for synthesizing safe chemical precursors for metal oxide and sulfide nanoparticle syntheses; and
(vi) biocompatible, ensuring dispersion of nanoparticles in non-polar solvents.
For biomedical applications (e.g. staining of proteins), nanoparticles should be: (i) biocompatible, (ii) water soluble and (iii) easily functionalized or chemically modified at the surface to tailor the interaction of the nanoparticles with target biomolecules [30,83,84]. Fundamentally, ligands used for nanoparticle surface functionalization must: (i) have minimal cytotoxicity and (ii) be specific to the targeted biomolecule. VOs and FAs meet these requirements perfectly [30]. Thus, CO, olive oil, sunflower oil, almond oil, rapeseed oil, corn oil, palm oil and coconut oil have all been applied for syntheses of metal, metal chalcogenide and up-conversion of nanoparticles as well as biodegradable nanocomposites (table 6). Oleic acid and stearic acid have traditionally been massively employed in nanoparticle syntheses as both capping agents and solvents. RA has also recently received attention as a suitable alternative to oleic acid. CO together with RA has extra advantages that are not common to the advantages reported for edible oils:
(i) CO is inedible and obviates possible competition as raw material for the food industry;
(ii) CO is a natural source of polyol and presents a simple avenue for versatile chemical reactions;
(iii) CO is the only rich source of RA that has been used as a building block for synthesis of several biochemicals;
(iv) RA due to the presence of the hydroxyl functional group on its hydrocarbon chain provides a facile route for chemical functionalization and manipulation of nanoparticle surfaces to tailor it to a specific application;
(v) CO and RA are more suitable for applications requiring highly polar organic solvents; and
CO and RA possess antimicrobial properties.
Table 6.
Different VOs and FAs employed in nanoparticle syntheses.
| solvent/capping agent | nanoparticles | method | morphology | size (nm) | references |
|---|---|---|---|---|---|
| CO | CdS | colloidal thermolysis | spherical | 4.64 | [14] |
| CO | Ag | laser ablation | spherical | 5 | [86] |
| CO | Au | sputtering | spherical | 2.4–3.8 | [87] |
| CO | Au | wet chemical synthesis (i) NaBH4, (ii) citrate, (iii) KOH |
quasi-spherical | (i) 9 (ii) 66 (iii) 13 |
[2] |
| other VOs (edible) | |||||
| rapeseed oil | Fe | sonochemical synthesis | spherical | 20–30 | [85] |
| corn oil | Fe | sonochemical synthesis | spherical | 10–15 | [85] |
| palm oil | Ag | laser ablation | spherical | 2–2.5 | [88] |
| coconut oil | Au | wet chemical synthesis | triangular to nearly spherical | 38–49 | [15] |
| coconut oil | Ag | wet chemical synthesis | triangular to nearly spherical | 21 | [15] |
| sunflower oil | ZnO | colloidal thermolysis | spheroidal | 3 | [89] |
| sunflower oil | Fe2O3/Fe3O4 | colloidal thermolysis | spheroidal | 7 | [89] |
| almond oil | Mn3O4 | sonochemical synthesis | spherical | 7 | [90] |
| olive oil | Mn3O4 | sonochemical synthesis | spherical | 7 | [90] |
| olive oil | ZnS | colloidal thermolysis | dots to flower-like | 4–7 | [91] |
| olive oil | CdSe | colloidal thermolysis | dots | 6 | [92] |
| olive oil | PbS | colloidal thermolysis | cubic | 18.74 | [93] |
| olive oil | CdS | colloidal thermolysis | spherical | 4.75 | [93] |
| olive oil | Fe2O3/Fe3O4 | co-precipitation | spherical | 19.2 | [94] |
| olive oil | CdSe | colloidal thermolysis | spherical | 2.3–6 | [95] |
| olive oil | PbS | colloidal thermolysis | — | 3.4–4.7 | [96] |
| CO-based FA | |||||
| RA | CdS | colloidal thermolysis | spherical | 5.56 | [14] |
| RA | Fe3O4 | co-precipitation | Janus-type | — | [97] |
| RA | Fe2O3/Fe3O4 | Co-precipitation | — | 11.1 | [98] |
| RA | CoFe2O4 | co-precipitation | spherical | 15 | [99] |
| RA | NaYF4:Yb/Er | solvothermal | spherical | 20 | [100] |
| other FAs | |||||
| stearic acid | CdSe | colloidal thermolysis | dots | 4–25 | [32] |
| oleic acid | Fe2O3/Fe3O4 | co-precipitation | — | 10.4 | [98] |
| oleic acid | PbS | chemical co-deposition | — | 8 | [101] |
5.1. Metal nanoparticles
Metal nanoparticles are synthesized either by wet chemical, laser ablation, sputtering deposition or sonochemical methods. Diphenylmethane is a common solvent used in sonochemical reactions. Diphenylmethane is, however, reported to decompose to toxic by-products [85]. In addition, diphenylmethane is an expensive solvent and Koltypin et al. [85] have demonstrated that VOs are the best alternatives. Edible VOs such as rapeseed, corn, coconut and palm oils have been used as cheap and environmentally friendly solvents for the synthesis of silver, gold and iron nanoparticles (table 6) [2,15,85–88]. Thermodynamically, nanoparticles are bent on agglomeration to form larger particles. VOs and FAs are amphiphilic molecules that are used to effectively control nanoparticle agglomeration. The polar or hydrophilic end (carboxylic group) of the FAs interacts with the nanoparticles while the non-polar aliphatic tails or hydrophobic end disperses the particles by a steric effect [86]. However, the use of edible oils as solvents in an advancing nanotechnology industry presents an intrinsic problem that may relate to cost and competition for raw materials.
As previously stated, CO is inedible and the seed oil is high yielding compared with most edible oils, and is found to be the best and inexpensive alternative to edible oils for metal nanoparticle syntheses [87]. In addition, the high viscosity, high polarity, low vapour pressure and relative optical activity of CO makes it a more suitable stabilizer compared to other VOs [86,87]. In a sustainable approach, Zamiri et al. [86] combined laser ablation (considered a green method) and CO (as solvent) to provide a more sustainable green synthetic route for silver nanoparticles. Likewise, Da Silva et al. [2] made colloidal solutions of gold nanoparticles using CO as a non-toxic organic dispersant and/or a stabilizing agent and proved that CO nanoparticle colloids remain completely stable even after 3 months. The stability of the CO colloid was assumed to be a result of hydrogen bonding between the hydroxyl group on CO and the oxygenated negatively charged surface of the gold nanoparticle (produced via decomposition of HAuCl4 in KOH solution), because several attempts to synthesize similar stable colloids with soya bean or cottonseed oils failed [2].
Interestingly, antimicrobial paints based on VOs and silver nanoparticles have been developed via a simple method centred on free radicals generated in situ by autoxidation of the drying oil [102]. Drying oils (hardening on exposure to air) are preferred to non-drying oils in the making of paints [102]. However, CO is a non-drying oil, but can be dehydrated to obtain semi-drying or drying oil useful for developing such antimicrobial coatings [16].
5.2. Metal chalcogenide semiconductor nanoparticles
Metal chalcogenide semiconductor nanoparticles are a useful class of inorganic materials that have received tremendous applications in solar cells and biomedical labelling [103]. Some key chemicals used in the traditional syntheses of magnetic and luminescent nanoparticles (such as TOPO and TOP) are extremely toxic, pyrophoric, explosive and/or expensive, and therefore have a serious negative environmental impact [32]. In this regard, many phosphine-free synthetic methods have been proposed by different research groups to avoid the use of these toxic solvents. One common green route often reported is the use of VOs and FAs as coordinating solvents for synthesis of metal chalcogenide nanoparticles. Examples of VOs and FAs that have been used are olive oil, sunflower oil, almond oil, CO, oleic acid, stearic acid and RA [14,32,89–93,98,99]. Metal oxides, sulfides and selenides have been prepared using VOs and FAs as: (i) both solvent and capping agent; (ii) co-solvent (mixture of solvents); (iii) capping agent or ligand; and (iv) metal FA salts.
5.2.1. Vegetable oil as both solvent and capping agent
Xiao et al. [91] demonstrated the shape evolution of ZnS nanoparticles in a green chemistry approach by employing olive oil as both solvent and capping agent. Specifically, a solution (made of 0.2 mmol of sulfur powder and 1 ml of olive oil, prepared at 100°C) was swiftly injected into a hot ZnO powder solution (consisting of 5 ml of olive oil and 0.4 mmol ZnO powder) at 330°C (Schlenk line with nitrogen gas). Dot- and flower-like morphologies of ZnS were obtained when there was sufficient ligand protection and limited ligand protection, respectively. Ligand protection is associated with the passivation of the surface of the nanoparticles by the ligand. Similarly, Bera et al. [104] prepared high-quality CdSe nanocrystals using olive oil. Their procedure consisted of three steps: (i) selenium powder (0.25 mM) was dissolved in 5 ml of olive oil at 220°C, (ii) CdO powder (0.5 mM) was dissolved in 25 ml of olive oil at 300°C, and (iii) the selenium solution was injected into the Cd, keeping the CdSe growth at 300°C. The TEM micrograph and electron diffraction pattern of the olive oil-capped CdSe showed that they were spherical and had an average size of 6 nm, zinc blende in nature. Mondal et al. [92] and Hardman et al. [96] replicated similar procedures to synthesize CdSe and PbS nanoparticles using olive oil.
Though olive oil was reported to be a suitable green solvent, Hardman et al. [96] noted that the hydrophobic groups were highly insulating and limited the as-prepared olive oil-capped nanoparticles for applications requiring charge transport to and from the nanoparticles. Thus, ligand-exchange processes are always required to tailor the nanoparticles to a particular application.
5.2.2. Vegetable oils and fatty acids as co-solvent
Solvents such as octadecene and TOPO are often used as co-solvents with VOs and FAs. The reasons for the co-solvent addition are to: (i) decrease the viscosity of the oil to ensure uniform nucleation of the nanoparticles and (ii) reduce the strong binding of the FAs to the nanoparticles or reduce the extent of inhibition of the nanoparticle growth [29,105]. Akhtar et al. [105] synthesized high-quality PbS nanoparticles at 60°C in an olive oil/oleic acid/octadecene system (12.5 ml of olive oil, 1 ml of oleic acid and 1 ml of octadecene). The reasons stated for the co-solvent were for the oleic acid to dissolve the PbO and the octadecene to reduce the viscosity of the olive oil. The PbS nanoparticles were spherical and had sizes within 2.79–5.22 nm.
Nyamen et al. [93] thermolysed heterocyclic dithiocarbamate single-source precursors of Pb and Cd in an olive oil/TOPO solvent system and obtained an average size of 18.47 nm and 4.75 nm of PbS and CdS nanoparticles, respectively. The use of the single-source precursors makes the process greener and obviates problems related to stoichiometry. Qu et al. [32] synthesized high-quality wurtzite CdSe nanocrystals using stearic acid as the solvent and TOP or tributylphosphine as the injection solvent. The reason for adding the co-solvents (with relatively slow solidification rates compared to stearic acid) was to ensure ease in taking sample aliquots. Dickerson et al. [106] also, applying a similar system (5% of TOPO and 95% stearic acid), added TOPO as a co-solvent to ensure adequate coordination of ligands.
Qu et al. [32] revealed that FAs–co-solvent systems are: (i) versatile and more reproducible than TOPO alone; (ii) not recommended for synthesis of very small nanocrystals because of the fast growth rate of nanoparticles in such systems; and (iii) suitable for broad nanocrystals with a size distribution (2–25 nm), compared with purely phosphonic acid/TOPO systems that are more suited for synthesizing monodispersed (6–8 nm) strong confinement size regimes of nanoparticles. Dickerson et al. [106] found the activation energy of nanoparticle growth rate to be dependent on the MW of the solvent. The average activation energies were determined for CdSe nanocrystal growth in stearic acid (with MW of 284.47 g mol−1) and in TOPO (with molecular weight of 386.65 g mol−1) to be 0.56 and 0.95 eV molecule−1, respectively [106]. Thus, the addition of TOPO as a co-solvent helps to reduce the fast growth rate of nanoparticles in FAs, thereby producing high-quality nanoparticles. Though extensive work has been reported on the kinetic study of nanoparticle growth in FAs, less or no focus has been paid to kinetics related to the growth of nanoparticles in VOs (with molecular weights between 800 and 950 g mol−1, higher than the molecular weight of TOPO).
5.2.3. Fatty acids as capping agents
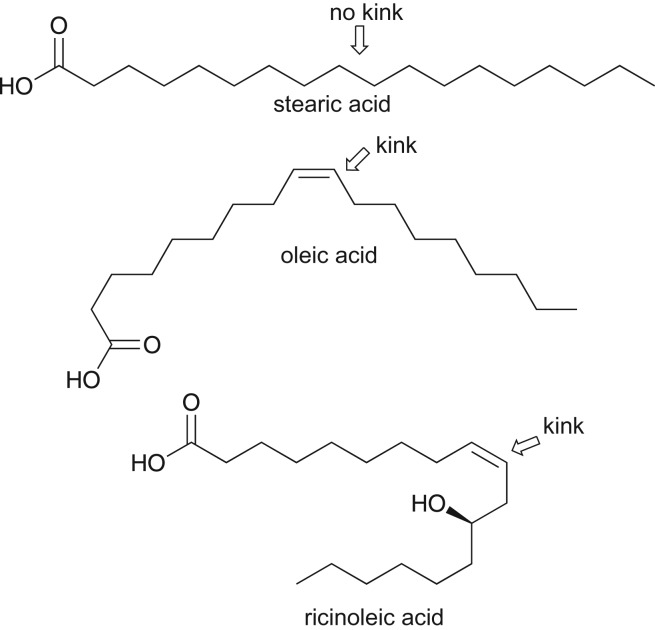
FAs are Lewis acids and have been extensively applied as capping agents and surfactants in the syntheses of nanoparticles. Oleic acid is known as a standard FA; the double bond and alkyl chain forming a ‘kink’ imparts colloidal stability [107]. Oleic acid is found to be more efficient in stabilizing magnetic nanoparticles than stearic acid, which has a no ‘kink’ in its chemical structure (figure 7) [29]. A more closely related FA to oleic acid is RA. RA (derived from CO) is isostructural with oleic acid and has also received considerable attention for stabilizing and capping of magnetic and luminescent nanoparticles [14,97–99]. Table 7 gives the distinction between oleic acid and RA (related to their nanoparticle surface passivation, stabilization and application). One intriguing difference between RA and oleic acid is that the former has an OH in its carbon chain that is susceptible to reactions such as acetylation and polymerization, providing an avenue for small RA-capped nanoparticles to be coated with polymers for specific applications such as drug delivery. RA also provides an avenue for further chemical functionalization or modification of nanoparticles to improve on their dispersion or solubility in varying solvent media. In addition, for applications requiring highly polar organic solvents, RA is preferred over oleic acid. Unfortunately, due to the possibility of oxidation of the OH functional group to ketones, RA is less preferred than oleic acid in high-temperature organometallic synthesis of monodispersed nanoparticles [98]. However, oleic acid can be replaced with RA in a ligand-exchange process [107].
Figure 7.
Chemical structures of stearic acid, oleic acid and ricinoleic acid. The kink in the structures is shown by an arrow [98].
Table 7.
Difference between oleic acid and ricinoleic acid [98].
| oleic acid | ricinoleic acid |
|---|---|
| 1. Is a C18 FA with one double bond between C9 and C10 | Is isostructural with oleic acid but has a hydroxyl group at C12 in the C18 tail |
| 2. The double bond in the middle of its carbon chain forms a ‘kink’ believed to be effective at nanoparticle stabilization | Has a similar ‘kink’ for nanoparticle stabilization. Provides a functional group (OH) in addition to the steric repulsion: (i) the OH can be acetylated under mild conditions, (ii) the OH has low affinity for iron oxide surfaces, and (iii) the OH could initiate ring opening polymerization reactions |
| 3. Only colloidal suspensions in non-polar organic media can be prepared (oleic acid-coated nanoparticles cannot be dispersed in organic media with a dielectric constant larger than 5) | Colloidal suspensions in more polar organic media can be prepared |
| 4. Suitable for capping of monodispersed nanoparticles synthesized at very high temperatures | Not suitable for high-temperature synthesis due to possible oxidation of the OH groups to ketones |
5.2.4. Metal fatty acid salt
Metal FA salts (MFASs) are polyvalent metal soaps, prepared by: (i) metathesis of a sodium or potassium FA salt with metal salts in aqueous or polar solvents, (ii) dissolution or fusion of metal oxides (or hydroxides, oxy-hydroxides, hydrocarbonates and carbonates) in hot FAs, or (iii) direct reaction of metal with hot FAs [31]. MFASs have become attractive as precursors for large-scale synthesis of metal oxide and metal chalcogenide nanoparticles because they: (i) are environmentally benign and (ii) yield reproducible results. Pereira et al. [89] combined MFAS (iron and zinc oleates) single-source precursors and sunflower oil (as solvent) for the synthesis of iron oxide and zinc oxide nanoparticles (at 200–250°C). Iron oxide and zinc oxide nanoparticles with average diameters of 7 and 3 nm and spheroidal in shape were obtained. While this route is considered green, the sunflower oil was prone to autoxidation via the double bonds in the oil at elevated temperatures (such as 310°C). Decomposition products such as ketones, esters, aldehydes, carbonates and carboxylic acids were identified. Factors such as temperature, UV light and metal ion complexes (iron and tin) were suggested to have accelerated the autoxidation process. Thus, choosing the right organic solvent (with a suitable boiling point) to decompose MFASs is crucial to obtaining monodispersed nanoparticles.
Chen et al. [31] decomposed an iron–oleate complex in five different organic solvents (oleylalcohol, benzyl ether, octadecene or trioctylamine)/oleic acid mixtures. Typically, the MFASs and the solvent mixture were placed in a flask and refluxed at the boiling point of the respective organic solvent for an hour to decompose the precursor. The outcome was monodispersed spherical iron oxide nanoparticles with sizes between 4.5 and 20.4 nm. The sizes were found to be dependent on both the boiling point of the organic solvent and the amount of oleic acid. The oleic acid was found to control the decomposition of the MFASs and the growth of the nanoparticles. By contrast, Cha et al. [108] decomposed iron–oleate complexes in the absence of an organic solvent (i.e. a solvent-less method) and obtained similar monodispersed iron oxide nanoparticles. By varying the annealing time and vacuum pressure, different shapes (spherical, regular triangular, short rod, diamond and long rod shapes) of iron oxide were produced with a mean size of 10.6 nm. The decomposition of MFASs follows equations (5.1) and (5.2):
| 5.1 |
MFASs decompose thermally through the formation of free radicals that combine, disintegrate into smaller molecules or react with other metal carboxylates to propagate to decompose metal carboxylates in MFASs [108].
Choi et al. [109] synthesized Cu2S, MnS, PbS, CdS and ZnS nanocrystals by the solution-phase thermolysis of metal–oleate complexes in alkane thiol. This method was considered simple and general for the synthesis of metal chalcogenides. Specifically, the metal–oleate precursors were dissolved in solvent mixtures of oleylamine and dodecanethiol. The resultant mixtures were then heated to the required temperatures and maintained for a period. The reaction temperature, time and the molar ratio of the two solvents were varied to tune the sizes of the nanoparticles. The nanoparticle sizes were uniform and had average particle sizes of 18, 11, 47, 10 and 10 nm for Cu2S, MnS, PbS, CdS and ZnS nanocrystals, respectively. Similarly, Patel et al. [110] obtained uniform-sized CdS, ZnS and PbS nanoparticles by sulfurization of metal–oleate precursors with thioacetamide at 140°C. Thermogravimetric and infrared studies indicated that the FAs were strongly coordinated or attached (symmetrically) to the surface of the nanocrystals via the carboxylate functional group. Metal–oleate complexes have been extensively explored for synthesis of metal chalcogenide nanocrystals. However, metal–ricinoleate complexes (prepared by reacting RA with metal salts) have not been explored, though RA is isostructural with oleic acid.
5.3. Up-conversion nanoparticles
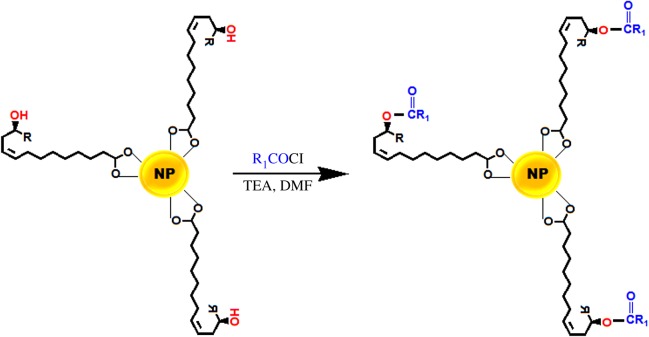
Up-conversion nanoparticles (UCNps) have received considerable attention as fluorophores in bioimaging over organic fluorophores and semiconductor quantum dots. UCNps have high quantum yields, high photostability and narrow emission peaks. However, to efficiently use UCNps in bioimaging, the UCNps must be rendered water-dispersible and their surfaces functionalized [100]. To render UCNps water-dispersible, hydrophobic UCNps are first prepared using oleic acid (or oleylamine) and then a ligand exchange, ligand attraction, silica coating, ligand oxidation or epoxidation process is performed. These surface modification routes are limited due to (i) the inability of the chemical reagents to get to the double bond in the oleic acid (as a surface ligand) because of high steric hindrance, (ii) complexity of the phase-transfer processes, (iii) long reaction time, and (iv) increase in the mean size of nanoparticles after ligand exchange [100,111]. In an attempt to overcome these shortfalls, RA is suggested by Meesaragandla et al. [111] as an excellent ligand alternative to oleic acid in making nanocrystals water-dispersible because the hydroxyl group (at C12) in the RA facilitates diffusion of chemical reagents for hydroxylation of the nearby double bond (between C9 and C10). To demonstrate the reactivity of the hydroxyl groups, He et al. [100] reacted dodecanoyl chloride with RA-capped NaYF4:Yb/Er and obtained NaYF4:Yb/Er-C12 (figure 8). Interestingly, RA-capped UCNps retained their average size and shape and even exhibited strong up-conversion properties in different protic and aprotic solvents after hydroxylation of the double bond [111]. However, there are only a few studies reported on RA-capped UCNps.
Figure 8.
Reaction scheme showing the surface functionalization of ricinoleic acid-capped nanoparticles with dodecanoyl chloride. R = CH3(CH2)5, R1 = CH3(CH2)10 (adapted from He et al. [100]).
5.4. Nanocomposite materials
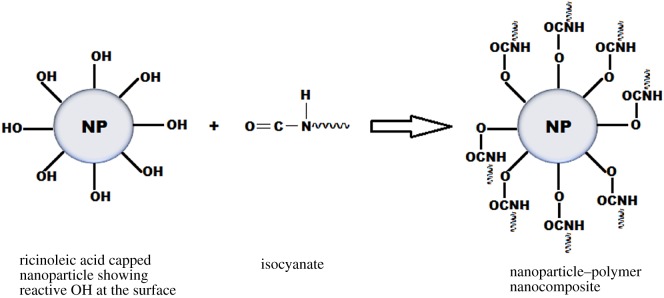
The coating of surfaces of very small size monodispersed nanoparticles with polymers for specific application in biomedicine is a major challenge. Nanoparticle surfaces can be polymerized in various solvents with the appropriate polymer for a specific purpose if ligands on the surfaces are suitable for such polymerization reactions. RA (and CO) in this regard stands out as the most suitable ligand (because of its freely available hydroxyl functional group) compared to oleic acid. In an attempt to develop polymer-coated monodispersed Fe3O4 nanoparticles, Lattuada & Hatton [107] first (i) performed a ligand exchange to replace oleic acid on the surface of the nanoparticles with RA and then (ii) exploited the hydroxyl group (on RA) to initiate ring opening polymerization of polylactic acid, which grew on the surface of the nanoparticles. This facile flexible method of functionalization is suggested to be efficient in tailoring nanoparticle solubility in a variety of solvents for different useful applications.
Similarly, RA stabilized Fe3O4 nanoparticles composited with poly(lactic-co-glycolic) acid was reported by Furlan et al. [97] as a magnetically responsive drug delivery system. The Fe3O4 nanoparticles were synthesized using the Massart co-precipitation method. The RA played its role as a biocompatible ligand rendering the nanoparticles hydrophobic, which ensured their dispersibility in apolar and mildly polar organic solvents such as dichloromethane. Additionally, a wound healing bio-nanocomposite based on CO and chitosan-modified ZnO nanoparticles has also been reported [112]. The CO was used as the matrix material. The composites were made by mixing CO, chitosan-modified ZnO and hexamethylene diisocyanate in the presence of stannous octanoate as catalyst and glutaraldehyde as the cross-linking agent. The CO served as a polyol that reacted with the diisocyanate to form the polymer composite (figure 9). The polymer composites were said to be biocompatible and biodegradable. Xia & Larock [113] also prepared CO-based polyurethane (PU)–silica nanoparticle nanocomposites with an increased cross-link density that resulted in improved thermal stability and mechanical properties of the composite. The CO–PU was identified to be chemically bonded to the silica nanoparticles. The interface interaction in such organic–inorganic composites determines the properties of the composite. Thus, the availability of reactive OH groups on CO and RA guaranteed the strong chemical interaction between the composite materials.
Figure 9.
Isocyanate reaction with hydroxyl groups on RA-capped nanoparticle to form a nanoparticle–polymer nanocomposite [112].
6. Conclusion
CO has a useful versatile chemistry and has been reviewed as a valuable bioresource material for green syntheses of nanoparticles. It is used as a biocompatible solvent, co-solvent, and a capping and stabilizing agent in the syntheses of metal and metal chalcogenide nanoparticles, as well as a source of polyol for forming chemically bonded polymer–nanoparticle composites that are biodegradable. CO is distinct from other VOs and contains huge amounts of ricinoleic acid, which is isostructural to the traditional oleic acid for capping magnetic and luminescent nanocrystals [14,16,97,98,100,112].
Ricinoleic acid is a more suitable stabilizing and/or capping agent for applications requiring more polar organic solvents (e.g. in lubricants) [98]. It provides a facile route for surface functionalization of nanoparticles with different functional molecules to tailor their dispersion/solubility in different solvent media [107]. Functionalization is extremely necessary for various important applications, such as drug delivery, where nanoparticles are used. The free reactive hydroxyl group on ricinoleic acid enhances chemical interaction with different molecular species (such as polymers, isocyanates, acids and dodecanoyl chloride) [112]. Additionally, ricinoleic acid is a natural source of carboxylic acid and alcohol, and could be exploited for synthesis of (single-source) precursors for preparation of metal oxide and chalcogenide nanoparticles.
Though the utilization of CO and ricinoleic acid as solvent/capping/stabilizing agents for nanoparticle syntheses has substantial potential in expanding the spectrum of nanoparticle applications, it is somewhat limited. Ricinoleic acid is more suited for low-temperature organometallic synthesis because of the possible oxidation of the hydroxyl group on its hydrocarbon chain [98]. However, the various ways (shown in this review) that ricinoleic acid and CO can be used in nanoparticle syntheses have not been thoroughly explored.
Acknowledgements
Our appreciation is extended to all others who helped in the preparation of the manuscript, including Pan African Chemistry Network (PACN), INASP's AuthorAid and the American Journal Experts (AJE).
Data accessibility
This article has no additional data.
Authors' contributions
M.B.M. designed the study, obtained literature and drafted the manuscript. P.O. and J.A.M.A. revised the manuscript critically for important intellectual content and gave their approval for submission.
Competing interests
The authors declare no competing interests.
Funding
This work is funded by the Royal Society – Department for International Development (RS-DFID) Africa Capacity Building Initiative (ACBI), UK.
References
- 1.Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. 2009. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surfaces A Physicochem. Eng. Asp. 339, 134–139. ( 10.1016/j.colsurfa.2009.02.008) [DOI] [Google Scholar]
- 2.Da Silva EC, Da Silva MGA, Meneghetti SMP, Machado G, Alencar MARC, Hickmann JM, Meneghetti MR. 2008. Synthesis of colloids based on gold nanoparticles dispersed in castor oil. J. Nanopart. Res. 10, 201–208. ( 10.1007/s11051-008-9483-z) [DOI] [Google Scholar]
- 3.Mubayi A, Chatterji S, Rai PM, Watal G. 2012. Evidence based green synthesis of nanoparticles. Adv. Mat. Lett. 3, 519–525. ( 10.5185/amlett.2012.icnano.353) [DOI] [Google Scholar]
- 4.Nadagouda MN, Varma RS. 2008. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 10, 859–862. ( 10.1021/ar1001457) [DOI] [Google Scholar]
- 5.Sharma VK, Yngard RA, Lin Y. 2009. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 145, 83–96. ( 10.1016/j.cis.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 6.Ambika, Singh PP. 2018. Nanotechnology: greener approach for sustainable environment. In Nanotechnology in environmental science, 1st edn (eds Hussain CM, Mishra AK), pp. 805–824. Wiley-VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- 7.Baruwati B, Polshettiwar V, Varma RS. 2009. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 11, 926–930. ( 10.1039/b902184a) [DOI] [Google Scholar]
- 8.Kou J, Varma RS. 2012. Beet juice utilization: expeditious green synthesis of noble metal nanoparticles (Ag, Au, Pt, and Pd) using microwaves. RSC Adv. 2, 10 283–10 290. ( 10.1039/c2ra21908e) [DOI] [Google Scholar]
- 9.Hebbalalu D, Lalley J, Nadagouda MN, Varma RS. 2013. Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers and microwaves. ACS Sustain. Chem. Eng. 1, 703–712. ( 10.1021/sc4000362) [DOI] [Google Scholar]
- 10.Kou J, Varma RS. 2012. Beet juice-induced green fabrication of plasmonic AgCl/Ag nanoparticles. ChemSusChem 1–8. ( 10.1002/cssc.201200477) [DOI] [PubMed] [Google Scholar]
- 11.Nadagouda MN, Varma RS. 2006. Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: density-assisted self-assembly of nanospheres, wires and rods. Green Chem. 8, 516–518. ( 10.1039/b601271j) [DOI] [Google Scholar]
- 12.Nadagouda MN, Speth TF, Varma RS. 2011. Microwave-assisted green synthesis of silver nanoparticles. Acc. Chem. Res. 44, 469–478. [DOI] [PubMed] [Google Scholar]
- 13.Nadagouda MN, Iyanna N, Lalley J, Han C, Dionysiou DD, Varma RS. 2014. Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate and turmeric extracts. ACS Sustain. Chem. Eng. 2, 1717–1723. ( 10.1021/sc500237k) [DOI] [Google Scholar]
- 14.Kyobe JW, Mubofu EB, Makame YMM, Mlowe S, Revaprasadu N. 2016. Cadmium sulfide quantum dots stabilized by castor oil and ricinoleic acid. Phys. E Low-Dimensional Syst. Nanostruct. 76, 95–102. ( 10.1007/s40089-016-0189-4) [DOI] [Google Scholar]
- 15.Kumari MM, Philip D. 2013. Facile one-pot synthesis of gold and silver nanocatalysts using edible coconut oil. Spectrochim. Acta A Mol. Biomol. Spectrosc. 111, 154–160. ( 10.1016/j.saa.2013.03.076) [DOI] [PubMed] [Google Scholar]
- 16.Mutlu H, Meier MAR. 2010. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 112, 10–30. ( 10.1002/ejlt.200900138) [DOI] [Google Scholar]
- 17.Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. 2012. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc. Natl Acad. Sci. USA 2, 9179–9184. ( 10.1073/pnas.1201627109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piazza GJ, Farrell HM. 1991. Generation of ricinoleic acid from castor oil using the lipase from ground oat (Avena sativa L.) seeds as a catalyst. Biotechnol. Lett. 13, 179–184. ( 10.1007/BF01025814) [DOI] [Google Scholar]
- 19.Mubofu EB. 2016. Castor oil as a potential renewable resource for the production of functional materials. Sustain Chem. Process. 4, 1–12. ( 10.1186/s40508-016-0055-8) [DOI] [Google Scholar]
- 20.Concei MM, Fernandes VJ, Bezerra AF, Silva MCD, Santos IÃMG, Silva FC, Souza AG. 2007. Dynamic kinetic calculation of castor oil biodiesel. J. Therm. Anal. Cal. 87, 865–869. ( 10.1007/s10973-006-8194-x) [DOI] [Google Scholar]
- 21.Ogunniyi DS. 2006. Castor oil: a vital industrial raw material. Bioresour. Technol. 97, 1086–1091. ( 10.1016/j.biortech.2005.03.028) [DOI] [PubMed] [Google Scholar]
- 22.Vaisman B, Shikanov A, Domb AJ. 2008. The isolation of ricinoleic acid from castor oil by salt-solubility-based fractionation for the biopharmaceutical applications. J. Am. Oil Chem. Soc. 85, 169–184. ( 10.1007/s11746-007-1172-z) [DOI] [Google Scholar]
- 23.Meneghetti SMP, Meneghetti MR, Wolf CR, Silva EC, Lima GES, Silva LDL, Serra TM, Cauduro F. 2006. Biodiesel from castor oil: a comparison of ethanolysis versus methanolysis. Energy Fuels 20, 2262–2265. ( 10.1021/ef060118m) [DOI] [Google Scholar]
- 24.Kulkarni MG, Sawant SB. 2003. Kinetics of the catalytic esterification of castor oil with lauric acid using n-butyl benzene as a water entrainer. JAOCS 80, 1033–1038. ( 10.1007/s11746-003-0816-8) [DOI] [Google Scholar]
- 25.Xia Y, Larock RC. 2010. Vegetable oil-based polymeric materials: synthesis, properties, and applications. Green Chem. 12, 1893–1909. ( 10.1039/c0gc00264j) [DOI] [Google Scholar]
- 26.Hablot E, Zheng D, Bouquey M, Ave L. 2008. Polyurethanes based on castor oil: kinetics, chemical, mechanical and thermal properties. Macromol. Mater. Eng. 293, 922–929. ( 10.1002/mame.200800185) [DOI] [Google Scholar]
- 27.Salimon J, Noor DAM, Nazrizawati AT, Firdaus MYM, Noraishah A. 2010. Fatty acid composition and physicochemical properties of Malaysian castor bean Ricinus communis L. seed oil. Sains Malaysiana 39, 761–764. [Google Scholar]
- 28.Chakrabarti MH, Ahmad R. 2008. Transesterification studies on castor oil as a first step towards its use in biodiesel production. Pak. J. Bot. 40, 1153–1157. [Google Scholar]
- 29.Green M. 2010. The nature of quantum dot capping ligands. J. Mater. Chem. 20, 5797–5809. ( 10.1039/C0JM00007H) [DOI] [Google Scholar]
- 30.Thanh NTK, Green LAW. 2010. Functionalisation of nanoparticles for biomedical applications. Nano Today 5, 213–230. ( 10.1016/j.nantod.2010.05.003) [DOI] [Google Scholar]
- 31.Chen CJ, Lai HY, Lin CC, Wang JS, Chiang RK. 2009. Preparation of monodisperse iron oxide nanoparticles via the synthesis and decomposition of iron fatty acid complexes. Nanoscale Res. Lett. 4, 1343–1350. ( 10.1007/s11671-009-9403-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qu L, Peng Z, Peng X. 2001. Alternative routes toward high quality CdSe nanocrystals. Nano Lett. 1, 333–337. ( 10.1021/nl0155532) [DOI] [Google Scholar]
- 33.Meier MAR, Metzger JO, Schubert US. 2007. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 36, 1788–1802. ( 10.1039/b703294c) [DOI] [PubMed] [Google Scholar]
- 34.Nayak P, Mishra DK, Sahoo KC, Pati NC, Jena PK, Lenka S, Nayak PL. 2001. Polymers from renewable resources. XIII. Interpenetrating polymer networks derived from castor oil–hexamethylene diisocyanate and polymethacrylamide. J. Polym. Sci. 80, 1349–1353. [Google Scholar]
- 35.Shirke A, Dholakiya B, Kuperkar K. 2015. Novel applications of castor oil based polyurethanes: a short review. Polym. Sci. Ser. B 57, 292–297. ( 10.1134/S1560090415040132) [DOI] [Google Scholar]
- 36.Kunduru KR, Basu A, Haim Zada M, Domb AJ. 2015. Castor oil-based biodegradable polyesters. Biomacromolecules 16, 2572–2587. ( 10.1021/acs.biomac.5b00923) [DOI] [PubMed] [Google Scholar]
- 37.Vilela C, Sousa AF, Fonseca AC, Serra AC, Coelho JFJ, Freirea CSR, Silvestrea AJD. 2014. The quest for sustainable polyesters: insights into the future. Polym. Chem. 5, 3119–3141. ( 10.1039/c3py01213a) [DOI] [Google Scholar]
- 38.Van Der Steen M, Stevens CV. 2009. Undecylenic acid: a valuable and physiologically active renewable building block from castor oil. ChemSusChem 2, 692–713. ( 10.1002/cssc.200900075) [DOI] [PubMed] [Google Scholar]
- 39.Shrirame HY. 2011. Bio diesel from castor oil–a green energy option. Low Carbon Econ. 2, 1–6. ( 10.4236/lce.2011.21001) [DOI] [Google Scholar]
- 40.Anjani K. 2012. Castor genetic resources: a primary gene pool for exploitation. Ind. Crop. Prod. 35, 1–14. ( 10.1016/j.indcrop.2011.06.011) [DOI] [Google Scholar]
- 41.Golakia PR, Poshiya VK, Monpara BA. 2015. Identification of superior donor parents for earliness through combining ability in castor. Int. J. Res. Plant Sci. 5, 26–31. [Google Scholar]
- 42.Milani M, Nobrega MBD. 2013. Castor breeding. INTECH 239–254. ( 10.5772/56216) [DOI] [Google Scholar]
- 43.Castor cultivation. 2015. Castor oil industry reference and resources. www.castoroil.in.
- 44.Sule MI, Sani UM. 2008. Isolation of ricinine from methanol extracts of three different seed varieties of Ricinus communis Linn (Euphorbiaceae). Nig. Journ. Pharm. Sci. 7, 114–118. [Google Scholar]
- 45.Santana GCS, Martins PF, de Lima da Silva N, Batistella CB, Filho RM, Maciel MRW. 2010. Simulation and cost estimate for biodiesel production using castor oil. Chem. Eng. Res. Des. 88, 626–632. ( 10.1016/j.cherd.2009.09.015) [DOI] [Google Scholar]
- 46.Castor Oil Seed Production. 2016. FAOSTAT. See www.fao.org/faostat/en/#data.
- 47.Lin J. 2009. Ratios of regioisomers of triacylglycerols containing dihydroxy fatty acids in castor oil by mass spectrometry. J. Am. Oil Chem. Soc. 86, 1031–1035. ( 10.1007/s11746-009-1472-6) [DOI] [Google Scholar]
- 48.Isbell BTA. 2011. Chemistry and physical properties of estolides. Grasas y Aceites 62, 8–20. ( 10.3989/gya/010810) [DOI] [Google Scholar]
- 49.Da Silva NDL, Batistella C, Filho RM, Maciel MRW. 2011. Determination of castor oil molecular weight by vapour pressure osmometry technique. Chem. Eng. Trans. 24, 601–606. ( 10.3303/CET1124101) [DOI] [Google Scholar]
- 50.Perdomo FA, Acosta-Osorio AA, Herrera G, Vasco-Leal JF, Mosquera-Artamonov JD, Millan-Malo B, Rodriguez-Garcia ME. 2013. Physicochemical characterization of seven Mexican Ricinus communis L. seeds and oil contents. Biomass Bioenergy 48, 17–24. ( 10.1016/j.biombioe.2012.10.020) [DOI] [Google Scholar]
- 51.Prasad L, Das LM, Naik SN. 2012. Effect of castor oil, methyl and ethyl esters as lubricity enhancer for low lubricity diesel fuel (LLDF). Energy Fuels 26, 5307–5315. ( 10.1021/ef300845v) [DOI] [Google Scholar]
- 52.Saied MA, Mansour SH, Eweis M, El-sabee MZ, Saad ALG, Nour KNA. 2008. Some biophysical properties of castor oil esterified with some acid anhydrides. Eur. J. Lipid Sci. Technol. 110, 926–934. ( 10.1002/ejlt.200800001) [DOI] [Google Scholar]
- 53.Pena R, Romero R, Martı SL, Ramos MJ, Martinez A, Natividad R. 2009. Transesterification of castor oil: effect of catalyst and co-solvent. Ind. Eng. Chem. Res 48, 1186–1189. ( 10.1021/ie8005929) [DOI] [Google Scholar]
- 54.Ndiaye PM, Tavares FW, Dalmolin I, Oliveira JV. 2005. Vapor pressure data of soybean oil, castor oil, and their fatty acid ethyl ester derivatives. J. Chem. Eng. Data 50, 330–333. ( 10.1021/je049898o) [DOI] [Google Scholar]
- 55.Plante M, Crafts C, Bailey B, Acworth I. 2011. Characterization of castor oil by HPLC and charged aerosol detection. DIONEX 1–5. [Google Scholar]
- 56.Lin J-T, Chen GQ. 2012. Ratios of regioisomers of minor acylglycerols less polar than triricinolein in castor oil estimated by mass spectrometry. J. Am. Oil Chem. Soc. 89, 1785–1792. ( 10.1007/s11746-012-2083-1) [DOI] [Google Scholar]
- 57.Jafari M, Kadivar M, Goli SAH. 2013. Response surface-optimized synthesis of cis-9, trans-11-octadecadienoic acid through dehydration of castor oil. Int. Food Res. 20, 1997–2003. [Google Scholar]
- 58.Schneider RCDS, Baldissarelli VZ, Trombetta F, Martinelli M, Caramao BE. 2004. Optimization of gas chromatographic–mass spectrometric analysis for fatty acids in hydrogenated castor oil obtained by catalytic transfer hydrogenation. Anal. Chim. Acta 505, 223–226. ( 10.1016/j.aca.2003.10.070) [DOI] [Google Scholar]
- 59.Hosamani KM, Ganjihal SS, Chavadi DV. 2004. Alternanthera triandra seed oil: a moderate source of ricinoleic acid and its possible industrial utilisation. Ind. Crops Prod. 19, 133–136. ( 10.1016/j.indcrop.2003.07.009) [DOI] [Google Scholar]
- 60.Foglia TA, Jones KC, Sonnet PE. 2000. Selectivity of lipases: isolation of fatty acids from castor, coriander, and meadowfoam oils. Eur. J. Lipid Sci. Technol. 102, 612–617. [Google Scholar]
- 61.Ozcan A, Sagiroglu HM. 2009. Production of ricinoleic acid from castor oil by immobilised lipases. Prep. Biochem. Biotechnol. 39, 170–182. ( 10.1080/10826060902800841) [DOI] [PubMed] [Google Scholar]
- 62.Karpakavalli M, Arthi I, Seena KX. 2012. Microwave assisted isolation of hesperidin, ricinoleic acid and piperic acid. J. Sci. Res. Pharm. 1, 76–79. [Google Scholar]
- 63.Goto E, Shimazaki J, Monden Y, Takano Y, Yagi Y, Shimmura S, Tsubota K. 2002. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology 109, 2030–2035. ( 10.1016/S0161-6420(02)01262-9) [DOI] [PubMed] [Google Scholar]
- 64.Katzer T, Chaves P, Bernardi A, Pohlmann AR, Guterres SS, Beck RCR. 2014. Castor oil and mineral oil nanoemulsion: development and compatibility with a soft contact lens. Pharm. Dev. Technol. 19, 232–237. ( 10.3109/10837450.2013.769569) [DOI] [PubMed] [Google Scholar]
- 65.Abbasi E, Vatankhah M, Hosseini Y, Ariana MA, Ayazi M. 2013. Synthesis, structure, and mechanical properties of castor oil-based polyamidoamines toughened epoxy coatings. J. Appl. Polym. Sci. 128, 4023–4030. ( 10.1002/app.38583) [DOI] [Google Scholar]
- 66.Akkas FS, Citak T, Sirkecioglu C, Guner A. 2012. Which is more effective for protein adsorption: surface roughness, surface wettability or swelling? Case study of polyurethane films prepared from castor oil and poly(ethylene glycol). Polym. Int. 62, 1202–1209. ( 10.1002/pi.4408) [DOI] [Google Scholar]
- 67.Albuquerque MCG, Cavalcante CL, Torres AEB, Azevedo DCS, Parente EJS. 2009. Transesterification of castor oil using ethanol: effect of water removal by adsorption onto zeolite 3A. Energy Fuels 1, 1136–1138. ( 10.1021/ef8009684) [DOI] [Google Scholar]
- 68.Amado FDR, Forte MMC, Ferreira CA. 2006. Properties evaluation of the membranes synthesized with castor oil polyurethane and polyaniline. Polym. Eng. Sci. 46, 1485–1489. ( 10.1002/pen.20602) [DOI] [Google Scholar]
- 69.Aranguren MI, Racz I, Marcovich NE. 2007. Microfoams based on castor oil polyurethanes and vegetable fibers. J. Appl. Polym. Sci. 105, 2791–2800. ( 10.1002/app.26526) [DOI] [Google Scholar]
- 70.Athawale D, Pillay S. 2002. Interpenetrating polymer networks based on hydrogenated castor oil–isophorone diisocyanate and poly (butyl methacrylate). Bull. Chem. Soc. Jpn 75, 369–373. ( 10.1246/bcsj.75.369) [DOI] [Google Scholar]
- 71.Compton DL, Laszlo JA, Isbell TA. 2004. Cinnamoyl esters of lesquerella and castor oil: novel sunscreen active ingredients. JAOCS 81, 945–951. ( 10.1007/s11746-004-1006-4) [DOI] [Google Scholar]
- 72.Ashraf SM, Ahmad S, Riaz U, Alam M, Sharma HO. 2005. Compatibility studies on dehydrated castor oil epoxy blend with poly(methacrylic acid). J. Macromol. Sci. A 42, 1409–1421. ( 10.1080/10601320500206903) [DOI] [Google Scholar]
- 73.Campanella A, La Scala JJ, Wool RP. 2010. Fatty acid-based comonomers as styrene replacements in soybean and castor oil-based thermosetting polymers. J. Appl. Polym. Sci. 119, 1000–1010. ( 10.1002/app.32810) [DOI] [Google Scholar]
- 74.Bao Y, He J, Li Y. 2013. Facile and efficient synthesis of hyperbranched polyesters based on renewable castor oil. Polym. Int. 62, 1457–1464. ( 10.1002/pi.4440) [DOI] [Google Scholar]
- 75.El-ghazawy RAM, Farag RK, Elsaeed SM, Abde-Halim E-DA, Yossef MA, Toyor WE. 2014. Castor oil based organogels: I. Synthesis, swelling, and network parameters. J. Dispers. Sci. Technol. 35, 350–357. [Google Scholar]
- 76.Braga A, Gomes N, Belo I. 2012. Lipase induction in Yarrowia lipolytica for castor oil hydrolysis and its effect on c-decalactone production. J. Am. Oil Chem. Soc. 89, 1041–1047. ( 10.1007/s11746-011-1987-5) [DOI] [Google Scholar]
- 77.Bhangale A, Wadekar S, Kale S, Bhowmick D, Pratap A. 2014. Production of sophorolipids synthesized on castor oil with glucose and glycerol by using Starmerella bombicola. Eur. J. Lipid Sci. Technol. 116, 336–343. ( 10.1002/ejlt.201300236) [DOI] [Google Scholar]
- 78.Becchi DM, De Luca MA, Martinelli M, Mitidieri S. 2011. Organic–inorganic coatings based on epoxidised castor oil/APTES/TEOS. J. Am. Oil Chem. Soc. 88, 101–109. ( 10.1007/s11746-010-1651-5) [DOI] [Google Scholar]
- 79.El-Azab WIM, Aiad I, Azzam EMS, Gad EAM. 2010. Molecular, surface, and thermodynamic properties of nonionic surfactants based on castor oil. J. Dispers. Sci. Technol. 31, 1150–1156. [Google Scholar]
- 80.Hirayama K, Irie T, Teramoto N, Shibata M. 2009. High-performance bio-based thermosetting resins composed of dehydrated castor oil and bismaleimide. J. Appl. Polym. Sci. 114, 1033–1039. ( 10.1002/app.30562) [DOI] [Google Scholar]
- 81.Yari A, Yeganeh H, Bakhshi H, Gharibi R. 2014. Preparation and characterization of novel antibacterial castor oil-based polyurethane membranes for wound dressing application. J. Biomed. Mater. Res. A 102A, 84–96. ( 10.1002/jbm.a.34672) [DOI] [PubMed] [Google Scholar]
- 82.Teomim D, Nyska A, Domb AJ. 1999. Ricinoleic acid-based biopolymers. J. Biomed. Mater. Res. 45, 258–267. ( 10.1002/(SICI)1097-4636(19990605)45:3%3C258::AID-JBM14%3E3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 83.Alam MN, Roy N, Mandal D, Begum NA. 2013. Green chemistry for nanochemistry: exploring medicinal plants for the biogenic synthesis of metal NPs. RSC Adv. 3, 11 935–11 956. ( 10.1039/c3ra23133j) [DOI] [Google Scholar]
- 84.Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. 2008. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 5, 763–775. ( 10.1038/nmeth.1248) [DOI] [PubMed] [Google Scholar]
- 85.Koltypin Y, Perkas N, Gedanken A. 2004. Commercial edible oils as new solvents for ultrasonic synthesis of nanoparticles: the preparation of air stable nanocrystalline iron particles. J. Mater. Chem. 14, 2975–2977. ( 10.1039/b411983e) [DOI] [Google Scholar]
- 86.Zamiri R, Zakaria A, Abbastabar H, Darroudi M, Husin MS, Mahdi MA. 2011. Laser-fabricated castor oil-capped silver nanoparticles. Int. J. Nanomed. 6, 565–568. ( 10.2147/IJN.S16384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wender H, de Oliveira LF, Feil AF, Lissner E, Migowski P, Meneghetti MR, Teixeira SR, Dupont J. 2010. ‘Synthesis of gold nanoparticles in a biocompatible fluid from sputtering deposition onto castor oil. Chem. Commun. 46, 7019–7021. ( 10.1039/c0cc01353f) [DOI] [PubMed] [Google Scholar]
- 88.Zamiri R, Zakaria A, Ahangar HA, Sadrolhosseini AR, Mahdi MA. 2010. Fabrication of silver nanoparticles dispersed in palm oil using laser ablation. Int. J. Mol. Sci. 11, 4764–4770. ( 10.3390/ijms11114764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pereira AS, Silva NJO, Trindade T, Pereira S. 2012. A single-source route for the synthesis of metal oxide nanoparticles using vegetable oil solvents. J. Nanosci. Nanotechnol. 12, 8963–8968. ( 10.1166/jnn.2012.6700) [DOI] [PubMed] [Google Scholar]
- 90.Bastami TR, Entezari MH. 2010. Sono-synthesis of Mn3O4 nanoparticles in different media without additives. Chem. Eng. J. 164, 261–266. ( 10.1016/j.cej.2010.08.030) [DOI] [Google Scholar]
- 91.Xiao N, Dai Q, Wang Y, Ning J, Liu B, Zou G, Zou B. 2012. ZnS nanocrystals and nanoflowers synthesized by a green chemistry approach: rare excitonic photoluminescence achieved by the tunable molar ratio of precursors. J. Hazard. Mater. 211–212, 62–67. ( 10.1016/j.jhazmat.2011.11.020) [DOI] [PubMed] [Google Scholar]
- 92.Mondal SP, Bera S, Narender G, Ray SK. 2012. CdSe quantum dots-poly(3-hexylthiophene) nanocomposite sensors for selective chloroform vapor detection at room temperature. Appl. Phys. Lett. 101, 2–6. ( 10.1063/1.4762861) [DOI] [Google Scholar]
- 93.Nyamen LD, Revaprasadu N, Ndifon PT. 2014. Low temperature synthesis of PbS and CdS nanoparticles in olive oil. Mater. Sci. Semicond. Process. 27, 191–196. ( 10.1016/j.mssp.2014.06.010) [DOI] [Google Scholar]
- 94.Palanisamy KL, Devabharathi V, Sundaram NM. 2013. Antibacterial study of olive oil stabilized superparamagnetic iron oxide nanoparticles. Nano Vis. 3, 145–152. [Google Scholar]
- 95.Sapra S, Rogach AL, Feldmann J. 2006. Phosphine-free synthesis of monodisperse CdSe nanocrystals in olive oil. J. Mater. Chem. 16, 3391–3395. ( 10.1039/b607022a) [DOI] [Google Scholar]
- 96.Hardman SJO, et al. 2011. Electronic and surface properties of PbS nanoparticles exhibiting efficient multiple exciton generation. Phys. Chem. Chem. Phys. 13, 20 275–20 283. ( 10.1039/c1cp22330e) [DOI] [PubMed] [Google Scholar]
- 97.Furlan M, Kluge J, Mazzotti M, Lattuada M. 2010. Preparation of biocompatible magnetite–PLGA composite nanoparticles using supercritical fluid extraction of emulsions. J. Supercrit. Fluids 54, 348–356. ( 10.1016/j.supflu.2010.05.010) [DOI] [Google Scholar]
- 98.Gyergyek S, Makovec D, Drofenik M. 2011. Colloidal stability of oleic- and ricinoleic-acid-coated magnetic nanoparticles in organic solvents. J. Colloid Interface Sci. 354, 498–505. ( 10.1016/j.jcis.2010.11.043) [DOI] [PubMed] [Google Scholar]
- 99.Jacintho GVM, Kosaka PM, Petri DFS, Suarez PAZ, Rubim JC. 2009. Synthesis and characterization of magnetic composites based on cis-polyisoprene and CoFe2O4 nanoparticles. J. Nanosci. Nanotechnol. 9, 3617–3621. ( 10.1166/jnn.2009.NS39) [DOI] [PubMed] [Google Scholar]
- 100.He B, Zhou L, Huang J. 2014. One-step synthesis of water-dispersible hydroxyl-functionalized NaYF4:Yb/Er upconversion nanoparticles. Mater. Lett. 117, 142–145. ( 10.1016/j.matlet.2013.11.105) [DOI] [Google Scholar]
- 101.Chen S, Liu W. 2006. Oleic acid capped PbS nanoparticles: synthesis, characterization and tribological properties. Mater. Chem. Phys. 98, 183–189. ( 10.1016/j.matchemphys.2005.09.043) [DOI] [Google Scholar]
- 102.Kumar A, Vemula PK, Ajayan PM, John G. 2008. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 7, 236–241. ( 10.1038/nmat2099) [DOI] [PubMed] [Google Scholar]
- 103.Jiang P, Zhu D-L, Zhu C-N, Zhang Z-L, Zhang G-J, Pang D-W. 2015. Highly reactive chalcogenide precursor for the synthesis of metal chalcogenide quantum dots. Nanoscale 7, 19 310–19 316. ( 10.1039/C5NR05747G) [DOI] [PubMed] [Google Scholar]
- 104.Bera S, Singh SB, Ray SK. 2012. Green route synthesis of high quality CdSe quantum dots for applications in light emitting devices. J. Solid State Chem. 189, 75–79. ( 10.1016/j.jssc.2011.11.048) [DOI] [Google Scholar]
- 105.Akhtar J, et al. 2010. A greener route to photoelectrochemically active PbS nanoparticles. J. Mater. Chem. 20, 2336–2344. ( 10.1039/b924436k) [DOI] [Google Scholar]
- 106.Dickerson BD, Irving DM, Herz E, Claus RO, Spillman WBJ. 2005. Synthesis kinetics of CdSe quantum dots in trioctylphosphine oxide and in stearic acid. Appl. Phys. Lett. 86, 171915 ( 10.1063/1.1921347) [DOI] [Google Scholar]
- 107.Lattuada M, Hatton TA. 2007. Functionalization of monodisperse magnetic nanoparticles. Langmuir 23, 2158–2168. ( 10.1021/la062092x) [DOI] [PubMed] [Google Scholar]
- 108.Cha HG, Lee DK, Kim YH, Kim CW, Lee CS, Kang YS. 2007. Solventless nanoparticles synthesis under low pressure. Inorg. Chem. 47, 121–127. ( 10.1021/ic701570z) [DOI] [PubMed] [Google Scholar]
- 109.Choi S-H, An K, Kim E-G, Yu JH, Kim JH, Hyeon T. 2009. Simple and generalized synthesis of semiconducting metal sulfide nanocrystals. Adv. Funct. Mater. 19, 1645–1649. ( 10.1002/adfm.200800832) [DOI] [Google Scholar]
- 110.Patel JD, Mighri F, Ajji A. 2012. Generalized chemical route to develop fatty acid capped highly dispersed semiconducting metal sulphide nanocrystals. Mater. Res. Bull. 47, 2016–2021. ( 10.1016/j.materresbull.2012.04.007) [DOI] [Google Scholar]
- 111.Meesaragandla B, Sarkar S, Hazra C, Mahalingam V. 2013. Ricinoleic acid-capped upconverting nanocrystals: an ideal capping ligand to render nanocrystals water dispersible. ChemPlusChem 78, 1338–1342. ( 10.1002/cplu.201300205) [DOI] [PubMed] [Google Scholar]
- 112.Diez-Pascual AM, Diez-Vicente AL. 2015. Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. ACS Biomacromol. 16, 2631–2644. ( 10.1021/acs.biomac.5b00447) [DOI] [PubMed] [Google Scholar]
- 113.Xia Y, Larock RC. 2011. Preparation and properties of aqueous castor oil-based polyurethane-silica nanocomposite dispersions through a sol-gel process. Macromol. Rapid Commun. 32, 1331–1337. ( 10.1002/marc.201100203) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.