Abstract
Invasive species continue to severely impact biodiversity, yet predicting the success or failure of introduced species has remained elusive. In particular, the relationship between community invasibility and native species diversity remains obscure. Here, we apply two traditional ecological concepts that inform prey population stability and hence invasibility. We first show that the native predatory crustacean Gammarus duebeni celticus exhibited similar type II (destabilizing) functional responses (FRs) towards native mayfly prey and invasive amphipod prey, when these prey species were presented separately. However, when the two prey species were presented simultaneously, the predator did not exhibit prey switching, instead consuming disproportionately more native prey than expected from the relative abundance of native and invasive species. These consumptive propensities foster reductions of native prey, while simultaneously limiting biotic resistance against the invasive species by the native predator. Since our theoretical considerations and laboratory results match known field invasion patterns, we advocate the increased consideration of FR and prey switching studies to understand and predict the success of invasive species.
Keywords: invasive species, success, prey switching, functional response, predation, biotic resistance
1. Introduction
Invasive alien species present a continuing global threat to biodiversity, with the rate of invasions continuing to increase [1]. Debate continues surrounding the relationship between community invasibility and levels of ‘biotic resistance’ provided by community diversity [2], with a distinct lack of methodologies that can predict the success or failure of invasions. Here, we consider invasion success as the ability of an invader to establish, spread and reproduce in a novel environment (see [3]). Indeed, invasion science has been slow to develop truly predictive methods for invasion success and has often neglected to incorporate traditional ecological concepts [4]. Unifying such concepts across taxa and trophic groups and developing methods to quantify and better understand invasive species success are thus central to protection of biodiversity and ecosystem structure and function [5]. In particular, predicting the strength of biotic resistance by recipient communities to new invaders might inform strategies to mitigate invasion impacts.
Traditionally, ecologists have used the ‘functional response’ (FR; relationship between resource supply and resource use) to quantify interaction strengths, such as between predator and prey [6]. While this has seen some success in explaining invasions, there has been no incorporation of prey switching, also known as frequency-dependent predation [7], into predictive methods for invasion success. This is critical, as patterns of prey switching and prey preferences by predators have implications for the stability of prey populations and hence the degree of biotic resistance that community members exert on invasive species. That is, by regulating abundant prey while providing refuge for rare prey at low prey densities, prey switching may contribute to type III population stabilizing FRs, thus facilitating prey persistence [8]. Alternatively, where there are strong prey preferences and lack of prey switching behaviour, predators may severely lower the abundance of one prey species while facilitating expansion of another. In the context of invasion ecology, then, the latter scenarios could help predict if an invasive species would encounter high biotic resistance, and hence fail, or low biotic resistance, and hence invade successfully.
In this study, a common field pattern is examined, where an invasive amphipod, Crangonyx pseudogracilis, invades species-rich freshwater habitats, but faces predation by resident native amphipods [9,10]. A pattern of invasion success is theoretically likely, and hence predictable, if the invader suffers low biotic resistance from the native predator due to lack of prey switching and high preference for native prey, thus facilitating the invader. Here, we thus compare the FRs, prey switching and prey preferences of the native predatory amphipod Gammarus duebeni celticus to larvae of the native mayfly Baetis rhodani and the invasive gammarid C. pseudogracilis.
2. Material and methods
In November 2014, unparasitized male native river shrimp G. d. celticus (1.5–2 cm body length) were collected from Glen Road stream, County Down, UK (54.508° N, 5.9708° W). Their commonly consumed prey [11], native mayfly B. rhodani (0.7–0.8 cm) were collected from Dunore stream, County Antrim (54.680° N, 6.225° W), and the invasive amphipod C. pseudogracilis (0.7–0.9 cm) from ponds in Clandeboye Estate, County Down (54.641° N, 5.7139° W). Each species was transported to the Queen's University Belfast laboratory in source water and maintained separately in continuously aerated source water with stream flora and fauna supplied ad libitum at 12°C (±2°C) and a 12 L : 12 D regime.
Gammarus duebeni celticus were then selected haphazardly and starved individually for 24 h in cylindrical arenas of 8 cm diameter with 50 ml of filtered source water. In Experiment 1, for the two prey species separately, five prey densities (2, 4, 8, 16 and 32; n = 3 replicates per density) were introduced into arenas as above containing 150 ml of water and allowed to settle for 30 min prior to the addition of individual predators which were allowed to feed for 24 h; live and eaten prey were then counted. In Experiment 2, the two prey species combined were presented to individual predators for 3 h at seven prey species ratios (2 : 28, 4 : 26, 8 : 22, 15 : 15, 22 : 8, 26 : 4, 28 : 2; n = 6 replicates per ratio). Prey were replaced as they were consumed to maintain nominal prey species ratios. Controls for both experiments were one replicate of each experimental group with the predator absent (that is, 167 B. rhodani and 167 C. pseudogracilis).
Data analyses were undertaken in ‘R’. The package ‘frair’ was used for FR analyses [12]. In Experiment 1, logistic regression was used to infer FR forms, whereby a type II response is indicated by a significantly negative first-order term. To account for prey depletion, we fitted Rogers' random predator equation [13]:
| 2.1 |
where Ne is the number of prey eaten, N0 is the initial density of prey, a is the attack constant, h is the handling time and T is the total experimental period. The Lambert W function was used for model fitting [14]. Data were non-parametrically bootstrapped (n = 2000) to generate 95% confidence intervals. In Experiment 2, Chesson's selectivity index [15,16] assuming prey replacement was used to determine preferences between prey across the varying prey proportions:
| 2.2 |
where αi is Chesson's selectivity index for prey type i, ni is the number of prey type i available at the start of the experiment, ri is the number of prey type i consumed, m the number of prey types, rj is the number of prey type j consumed and nj the number of prey type j available at the start of the experiment. The value of αi ranges from 0 to 1, with 0 indicating complete avoidance and 1 indicating complete preference. In the two prey systems, values of 0.5 are indicative of no selectivity. Chesson's indices were transformed to reduce extremes (0s, 1s):
| 2.3 |
where αt is the transformed output and n is the sample size. Beta-regression using the ‘betareg’ package [17] in ‘R’ was used to compare αt values between ‘prey’ and ‘proportion’, and their interaction.
3. Results
Survival in controls was 100% in both experiments. Thus, prey mortality was attributed entirely to predation, which was also observed directly. In Experiment 1, destabilizing type II FRs were detected for predation on each prey species (B. rhodani, first-order term = −0.07, p < 0.001; C. pseudogracilis, first-order term = −0.05, p < 0.001). Functional response magnitude (curve asymptote) trended towards being higher upon the native B. rhodani; however, confidence intervals overlapped across all prey densities, and thus, similarities for prey in attack rate and handling time parameters can be deduced (figure 1). In Experiment 2, however, significantly disproportionately more B. rhodani were consumed by G. d. celticus at all prey proportions, indicating a lack of switching between prey and consistent preferential predation of the native B. rhodani over the invasive C. pseudogracilis (figure 2). Chesson's indices indicated a significant preference for B. rhodani over C. pseudogracilis overall (χ2 = 59.29, d.f. = 1, p < 0.001). Prey preferences were significantly affected by proportions available (χ2 = 64.50, d.f. = 6, p < 0.001), and there was a significant ‘prey × proportion’ interaction (χ2 = 20.20, d.f. = 6, p = 0.003) reflecting increases in preference towards B. rhodani at intermediate prey proportions (figure 2).
Figure 1.
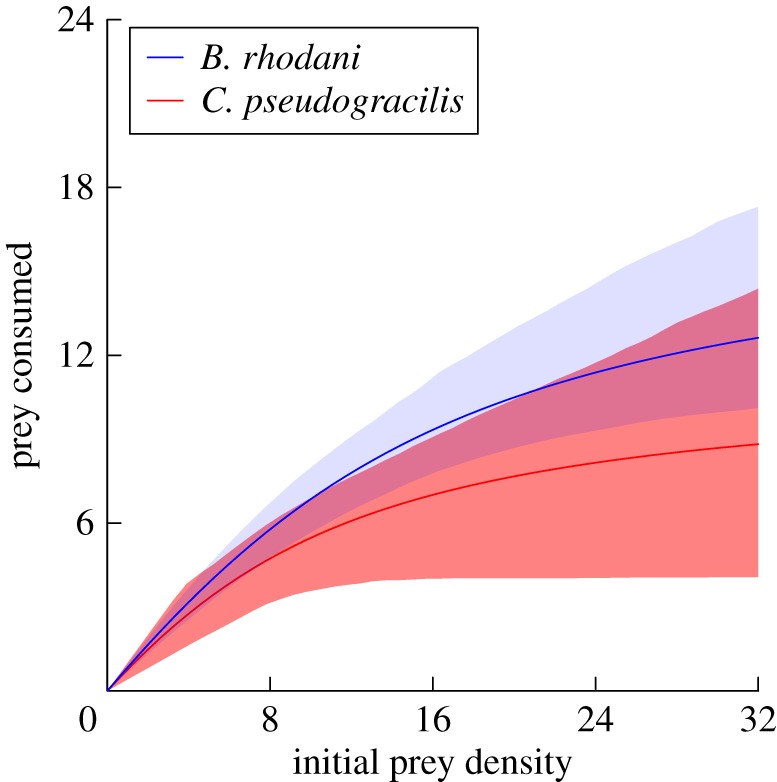
FRs of the native predator G. d. celticus towards native B. rhodani and invasive C. pseudogracilis prey with bootstrapped (n = 2000) 95% CIs.
Figure 2.
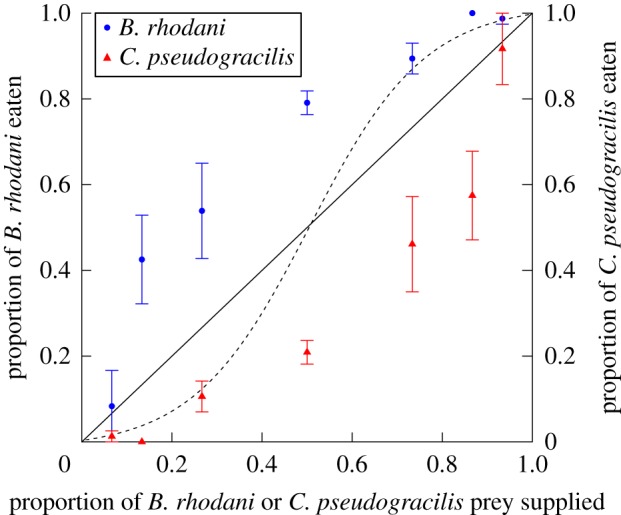
Proportion of either B. rhodani or C. pseudogracilis in the diet of G. d. celticus as a function of the proportion of each prey species supplied. The solid line indicates the expected values if there was no preference between the two prey types. The dashed sigmoid line represents a hypothetical prey switching pattern. Means are ±s.e. (n = 6).
4. Discussion
The impacts of invasive species on native species populations can be severe and are continuing, and thus, the development of predictive methodologies is crucial to effectively understand and forecast invader success or failure in terms of establishment and spread. Furthermore, we require elucidation of why there are no consistent patterns of invasibility with respect to community diversity. The use of FRs has provided meaningful insights in the context of invader impact [5], particularly for predators, but examining just single-prey species obscures effects on prey populations mediated through prey switching, or lack thereof [7]. Furthermore, while there are other biotic interactions, such as parasitism [18] and competition [19], that can have profound effects on community interaction outcomes, FRs are also able to quantify such outcomes [4,5]. Here, G. d. celticus exhibited potentially population destabilizing type II FRs towards two prey species, with similarities in consumption (attack rates and handling times) of both prey species when presented separately. However, where the two prey species were supplied to the predator simultaneously, no prey switching occurred across the prey ratio spectrum, with G. d. celticus consistently showing a significant preference for the native B. rhodani over the invasive C. pseudogracilis. The biotic resistance hypothesis posits that non-native species can be prevented from establishing and spreading, and hence exerting negative ecological impact, due to resident predators and competitors in the recipient area, with higher species richness conducive to higher resistance [20]. However, we demonstrate that C. pseudogracilis populations may be alleviated of resistance by predators which disproportionately consume higher levels of native prey across relative prey availabilities. This, coupled with the type II FR demonstrated, may drive localized reductions/extinctions of native species and hence facilitation of invasive species. Indeed, mayfly populations are known to be seriously depleted in the face of predation by Gammarus species [11,21] and C. pseudogracilis invades species-rich aquatic systems [9,10]. Our current study thus corroborates theory with field patterns and hence shows predictability of invasion success where native prey items are also present.
In summary, the application of FRs, prey switching and prey preferences to invasion ecology can foster a new framework to better understand biotic resistance and invader success. While the comparative FR method has been shown repeatedly to be a robust predictor of invader impact [5], shortcomings exist with regard to its use in testing biotic resistance in single-prey studies. We propose that the study of prey switching and preference alongside FRs under different environmental contexts is crucial to derive a more holistic account of the success of invaders and hence impacts on native prey populations, alongside the capacity for biotic resistance by recipient communities.
Supplementary Material
Supplementary Material
Acknowledgements
We extend thanks to Gillian Riddell and Elisabeth Baas for assistance with field sampling and experimental set-up.
Ethics
We complied with all necessary ethical protocols sought from the School of Biological Sciences ethics committee, Queen's University Belfast.
Data accessibility
Raw data can be found in the electronic supplementary material.
Authors' contributions
J.T.A.D., C.M. and C.L. conceived the study, performed the experiments and produced initial results. R.N.C. conducted statistical analyses. R.N.C., J.W.E.D. and J.T.A.D. prepared the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
J.T.A.D., R.N.C., C.M. and C.L. received funding from the Department for the Economy, Northern Ireland. J.T.A.D. and J.W.E.D. obtained funding from Queen's University Belfast and Inland Fisheries Ireland. We additionally acknowledge funding received from the Natural Environment Research Council.
References
- 1.Seebens H, et al. 2017. No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 14435 ( 10.1038/ncomms14435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howeth JG. 2017. Native species dispersal reduces invasibility by increasing species richness and biotic resistance. J. Anim. Ecol. 86, 1380–1393. ( 10.1111/1365-2656.12733) [DOI] [PubMed] [Google Scholar]
- 3.Ricciardi A, Cohen J. 2007. The invasiveness of an introduced species does not predict its impact. Biol. Invasions 9, 305–315. ( 10.1007/s10530-006-9034-4) [DOI] [Google Scholar]
- 4.Dick JTA, et al. 2017. Invader Relative Impact Potential: a new metric to understand and predict the ecological impacts of existing, emerging and future invasive alien species. J. Appl. Ecol. 54, 1259–1267. ( 10.1111/1365-2664.12849) [DOI] [Google Scholar]
- 5.Dick JTA, et al. 2017. Functional responses can unify invasion ecology. Biol. Invasions 19, 1667–1672. ( 10.1007/s10530-016-1355-3) [DOI] [Google Scholar]
- 6.Dick JTA, et al. 2014. Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol. Invasions 16, 735–753. ( 10.1007/s10530-013-0550-8) [DOI] [Google Scholar]
- 7.Murdoch WW. 1969. Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol. Monogr. 39, 335–354. ( 10.2307/1942352) [DOI] [Google Scholar]
- 8.Hughes RN, Croy MI. 1993. An experimental analysis of frequency-dependent predation (switching) in the 15-spined stickleback, Spinachia spinachia . J. Anim. Ecol. 62, 341–352. ( 10.2307/5365) [DOI] [Google Scholar]
- 9.Dick JTA. 1996. Post-invasion amphipod communities of Lough Neagh, N. Ireland: influences of habitat selection and mutual predation. J. Anim. Ecol. 65, 756–767. ( 10.2307/5674) [DOI] [Google Scholar]
- 10.Dick JTA, Nelson N, Bishop JDD. 1997. Introduction experiments with Gammarus spp. (Crustacea: Amphipoda) in the Isle of Man (British Isles), 1949–1995. J. Zool. 242, 209–216. ( 10.1111/j.1469-7998.1997.tb05797.x) [DOI] [Google Scholar]
- 11.Kelly DW, Dick JTA, Montgomery WI. 2002. Predation on mayfly nymph, Baetis rhodani, by native and introduced Gammarus: direct effects and the facilitation of salmonid predation. Freshw. Biol. 47, 1257–1268. ( 10.1046/j.1365-2427.2002.00864.x) [DOI] [Google Scholar]
- 12.Pritchard DW, Paterson R, Bovy HC, Barrios-O'Neill D. 2017. Frair: an R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 8, 1528–1534. ( 10.1111/2041-210X.12784) [DOI] [Google Scholar]
- 13.Juliano SA. 2001. Nonlinear curve fitting: predation and functional response curves. In Design and analysis of ecological experiments (eds Scheiner SM, Gurevitch J), pp. 178–196. Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Bolker BM. 2008. Emdbook: ecological models and data in R. Princeton, NJ: Princeton University Press. [Google Scholar]
- 15.Chesson J. 1978. Measuring preference in selective predation. Ecology 59, 211–215. ( 10.2307/1936364) [DOI] [Google Scholar]
- 16.Chesson J. 1983. The estimation and analysis of preference and its relationship to foraging models. Ecology 64, 1297–1304. ( 10.2307/1937838) [DOI] [Google Scholar]
- 17.Cribari-Neto F, Zeileis A. 2010. Beta regression in R. J. Stat. Softw. 34, 1–24. (doi:10.18637/jss.v034.i02) [Google Scholar]
- 18.Bunke M, Alexander ME, Dick JTA, Hatcher MJ, Paterson R, Dunn AM. 2015. Eaten alive: cannibalism is enhanced by parasites. R. Soc. Open Sci. 2, 140369 ( 10.1098/rsos.140369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branch GM, Odendaal F, Robinson TB. 2010. Competition and facilitation between the alien mussel Mytilus galloprovincialis and indigenous species: moderation by wave action. J. Exp. Mar. Biol. Ecol. 383, 65–78. ( 10.1016/j.jembe.2009.10.007) [DOI] [Google Scholar]
- 20.Elton CS. 1958. The ecology of invasions by animals and plants. Chicago: University of Chicago Press. [Google Scholar]
- 21.Kelly DW, Bailey RJE, MacNeil C, Dick JTA, McDonald RA. 2006. Invasion by the amphipod Gammarus pulex alters community composition of native freshwater macroinvertebrates. Divers. Distrib. 12, 525–534. ( 10.1111/j.1366-9516.2006.00275.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data can be found in the electronic supplementary material.


