Abstract
Physical activity is recommended in the management of individuals with metabolic syndrome (MetS), and recent studies have suggested whole-body vibration exercise (WBVe) for this population. The aim of this study was to evaluate the functionality through the Short Physical Performance Battery (SPPB) in individuals with MetS after WBVe. The SPPB evaluates the balance, the gait speed, and the lower limb strength (five-chair stand [5CS] test). Forty-four individuals with MetS were divided into WBVe (WBVeG) and control (CG) groups. The individuals of the WBVeG performed 10 sessions of WBVe in an oscillating/vibratory platform (OVP), barefoot, for 3 minutes at the peak-to-peak displacements of 2.5, 5.0, and 7.5 mm, with a resting period of 1 minute (total time: 18 minutes/session). The frequencies ranged from 5 up to 14 Hz. The individuals of the CG performed all the steps of the study, but the OVP was turned off. Before the first and after the tenth session, the individuals performed the SPPB. Significant responses were found in the WBVeG, analyzing the total score of the SPPB (P = .005), the balance test (P = .01), the gait speed (P = .006), and the 5CS test (P = .03), resulting in the improvement of the functionality of individuals with MetS.
Keywords: metabolic syndrome, short physical performance battery, gait, balance, five-chair stand test, whole-body vibration exercise
Introduction
Metabolic syndrome (MetS) is characterized by complex and interconnected physiological, biochemical, clinical, and metabolic factors that directly increase the risk of cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM).1,2 Insulin resistance, visceral adiposity, atherogenic dyslipidemia, endothelial dysfunction, genetic susceptibility, elevated blood pressure, hypercoagulable state, and chronic stress are clinical conditions related to MetS.2 Body mass index (BMI), waist circumference (WC), waist to hip ratio, and waist to height ratio are relevant predictors of MetS.3
According to Beavers et al,4 the presence of MetS is significantly associated with poorer physical performance in older adults. Moreover, other researchers5 reported that patients with chronic diseases, as MetS, generally have a poor functional capacity due to resistance to physical activity.
Recently, whole-body vibration exercise (WBVe) has been considered a type of physical activity to be used in the management of various populations,6,7 especially among individuals who have a resistance to perform exercise. Whole-body vibration exercise, produced throughout the exposition of individuals to mechanical vibration generated in an oscillating/vibratory platform (OVP), is a suitable and safe intervention. There are 2 main types of OVP, side alternating and vertical (synchronicus and triplanar).8 Whole-body vibration exercise is reported to be an efficient and conventional resistance intervention to improve strength and speed of movement in older women,9,10 which could be also important to the MetS population.
Usually the WBVe protocols are defined from parameters that establish greater control of their effects on the individual and make them possible to compare with other studies previously performed in the same population. For this, biomechanical parameters are determined as (1) frequency (f), measured in Hz; (2) amplitude (A)/ peak-to-peak displacement (PPD), measured in mm, and (3) acceleration peak (pacel), measured in ×g.
In addition, other adjustments should be considered such as (1) time of exposure (work) to the OVP, (2) resting time, (3) number of repetitions, (4) session numbers, (5) periodicity of sessions, (6) positioning of the individual on the base of the OVP, (7) association with other interventions/exercises, and (8) the use and type of footwear or barefoot individuals.
The WBVe effects have been evaluated after acute and chronic exposure using different protocols,10-12 with positive effects being found in individuals with Parkinson disease,13 osteoarthritis,14 and multiple sclerosis (MS).15 There are several studies that evaluate the effects of WBVe in individuals with clinical conditions related to MetS,6,7 but it is still difficult to define an ideal protocol for this population because different f, PPD, and consequently, varied Pacel have been used.
The WBVe effects also have been investigated in the biomarker responses, such as growth hormone (GH) concentration.16 In view of the already defined, direct relationship between GH deficiency and visceral obesity, which makes up the diagnosis of MetS, WBVe has been strongly considered as a proposal of intervention capable of reducing obesity, favoring the stimulation of GH and consequently preventing MetS and its complications.
As WBVe is a viable, easy, and safe exercise modality in the management of individuals suffering from several diseases,13,17-19 its potential application in patients with MetS could represent a promising challenge, as an aerobic exercise that can improve insulin sensitivity and functional parameters.
Considering functional impairments associated with MetS, such as low muscle strength and performance and loss of muscle mass,20 a growing scientific interest has been presented in the investigation of the effects of WBVe on these functional impairments in individuals with MetS.21,22 Carvalho-Lima et al21 found the improvement of the quality of life of individuals with MetS who have performed WBVe in a protocol with 10 sessions and with a progressive and increased frequency (5-14 Hz). This same protocol has been used by Sá-Caputo et al22 and found an increase in flexibility and a better functional performance of individuals with MetS after WBVe.
So far only these 2 studies have investigated some kind of effect involving MetS and WBVe.21,22 Strength and balance were not highlighted in these previous studies, but they were considered in the physical domain of a questionnaire of the quality of life of these individuals, contributing to a significant improvement.21
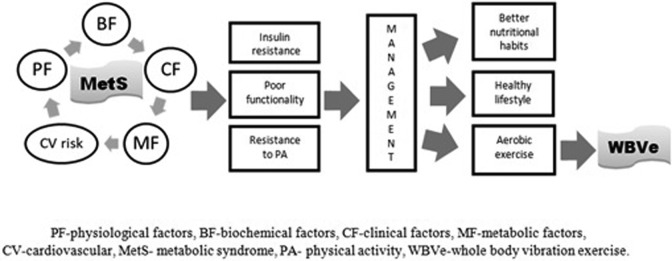
Beavers et al4 reported that MetS is predictive of lower physical performance. Figure 1 shows the interconnection of the factors causing the MetS.1,2,23 All these factors contribute to hamper the practice of physical activity, thus leading to a poor functionality.4 Whole-body vibration exercise could be considered a safe and suitable kind of aerobic exercise for this population21,22 jointly with a healthy lifestyle and a better nutritional habit of these individuals. Moreover, it can be suggested to prevent clinical conditions related to MetS such as obesity and T2DM.
Figure 1.
The interconnection of the factors that cause metabolic syndrome (MetS) with the whole-body vibration exercise (WBVe) as a suggestion of exercise modality for individuals with MetS.
Various tools can be used to evaluate the functionality in individuals with different diseases.24 An appropriate, simple discriminative tool for identifying risk for disability progression, particularly in routine clinical care,25 is the Short Physical Performance Battery (SPPB).
Short Physical Performance Battery has gained popularity in the past years since it is a standardized objective tool, rapid and simple to conduct, and scarcely influenced by cultural and educational backgrounds, compared with self-report measures.26,27 It can be used to measure lower extremity function28 and has high predictive ability in identifying those older adults at greater risk for hospitalization and incidence of disability.25,29,30 Due to the characteristics of the individuals with MetS, the SPPB would be also relevant to evaluate the functionality in this population.
The criteria for analyzing the functionality of this study resemble the components evaluated in the Health ABC Study4 (balance, gait, and lower limb strength) to determine the functionality in individuals with and without MetS.
Putting together all the previous considerations, the aim of this study was to evaluate the functionality through the SPPB in individuals with MetS after WBVe. The hypothesis of this work is that the overall functionality of individuals with MetS may show improvement after WBVe.
Materials and Methods
Design
This investigation is a pseudorandomized controlled trial study with alternate allocation in 2 subgroups: the WBVe group (WBVeG) and the control group (CG). The study protocol was approved by the Ethics Committee on Research with Humans of the Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro—HUPE/UERJ (CAAE 19826413.8.0000.5259). Trials registration: UTN: U1111-1181-1177; Brazilian Registry of Clinical Trials-ReBEC RBR-2bghmh.
The design of the current work was performed according to the STrengthening the Reporting of OBservational studies in Epidemiology criteria,31 as demonstrated in Figure 2.
Figure 2.
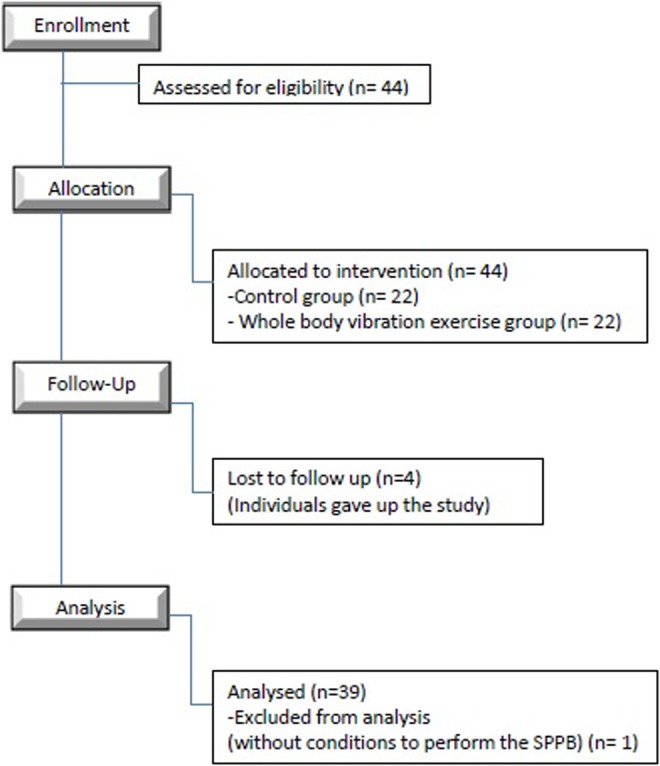
Flow diagram according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria.
Individuals
The population comprised 44 outpatients of the Serviço de Clínica Médica, HUPE/UERJ, aged 47 to 69 years, suffering from MetS according to the International Diabetes Federation (IDF), selected by a physician from September 2014 up to October 2016. The interventions were carried out in Laboratório de Vibrações Mecânicas e Práticas Integrativas, UERJ. The principles embodied in the Declaration of Helsinki were followed.
Inclusion and Exclusion Criteria
The inclusion criteria were (1) age over 40 years old and (2) previous clinical diagnosis of MetS, based on the criteria described by the IDF. The exclusion criteria were (1) very high blood pressure (≥180 × 110 mmHg); (2) coronary artery disease or stroke; (3) neurological, musculoskeletal, or rheumatologic diseases hampering to perform WBV exercises; and (4) refusal to sign the informed consent form for participation.
Sample Size
For a statistical power of 95% and significance level of 5%, a sample size of 13 patients was calculated.32
Measurements
Anthropometric characteristics
In an interview, the supervisor asked before the protocol to the individuals about smoking habit, diseases (T2DM and hypertension), and practice of physical activity. The answer was “yes” or “no.”
The abdominal obesity is defined by WC and it was introduced as a compulsory component of MetS by the IDF.33 Waist circumference was measured with a flexible plastic rule, using as reference the midpoint between the last rib and the iliac crest.34 This measurement was performed before and after the protocol (10 sessions).
Body mass and height were measured (before and after the protocol) on a digital balance (MIC 200 PPA; Micheletti, São Paulo, Brazil). The BMI was calculated in accordance with the National Health and Nutrition Examination Survey criteria, calculated with the body mass in kilogram (kg)/height in square meters (m 2).35
Evaluation of functionality throughout the SPPB
The SPPB comprises 3 tests: (1) the balance test, a hierarchical assessment of standing balance; (2) the gait speed test, a short walk at the usual pace; and (3) the five-chair stand (5CS) test, standing 5 times from a seated position in a chair.36
The total score of SPPB can be routinely used as primary outcome measures in randomized controlled trials,37 since it combines several outcomes useful to define physical impairment. In the current study, the individuals were subjected to the SPPB for functional assessment before the first and after the tenth session.
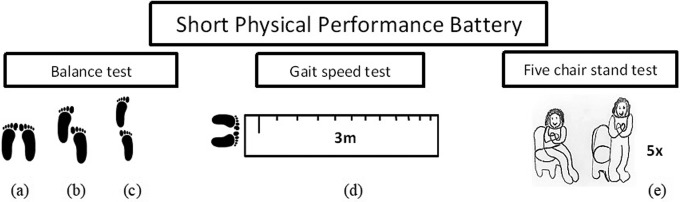
Figure 3 illustrates the 3 tests of the SPPB38 performed by the individuals. In the first test (balance test), individuals are instructed to perform 3 different positions: (1) side-by-side stand (Figure 3A), when the feet are together, for 10 seconds. The score is 0-1 depending on the time the patient is able to be in this position (0 = the individuals are unable to be in this position for 10 seconds and 1 = the individuals maintain this position for 10 seconds); (2) semitandem stand (Figure 3B), when the individual places one foot partially in front of another, touching the heel of the partially front foot with hallux from behind standing, also for 10 seconds. The score is 0-1 depending on the time the patient is able to stay in this position (0 = the individuals are unable to be this position for 10 seconds and 1 = the individuals maintain this position for 10 seconds); and (iii) tandem stand (Figure 3C), when the individual stands with one foot in front of the other one, touching the toes, also for 10 seconds. The score is 0-1-2 depending on the time the patient is able to be in this position (0 = the individuals stay less than 3 seconds; 1 = the individuals stay between 3 and 9.99 seconds and 2 = the individuals stay for 10 seconds). The total score range of this first test is comprised between 0 and 4.
Figure 3.
The three tests of the Short Physical Performance Battery. (a) side-by-side stand; (b) semi tandem stand; (c) tandem stand; (d) the gait speed test and (e) the five chair stand (5CS) test.
In the second test, the gait speed test (Figure 3D), individuals are instructed to walk a defined distance (3 m) with normal and usual walking speed from a specific point and to come back. A chronometer was used to measure accurately the gait time. This test was performed twice and the score considers the smallest time of the 2 attempts: 1, more than 6.52 seconds; 2, between 4.66 and 6.52 seconds; 3, between 3.62 and 4.65 seconds; and 4, less than 3.62 seconds. If the individual was unable to perform the walk, the score is 0.
In the third test, the five-chair stand (5CS) test (Figure 3E), the lower limb strength is analyzed and the individuals are instructed to stand up from a chair without the aid of their arms with 5 repetitions, without stopping. The score depends on the time of execution: 0, more than 60 seconds; 1, 16.70 seconds or more; 2, between 13.70 and 16.69 seconds; 3, between 11.20 and 13.69 seconds; and 4, 11.19 seconds or less.
A chronometer (Cronobio SW2018, Brazil[AW: Please provide city for “Cronobio SW2018.”]) was used evaluate the 3 tests. The results are evaluated in 4 steps: (1) analysis of total score of the SPPB, (2) the score of the balance test, (3) the score of the gait speed test and the time of execution of the gait, and (4) the score of the 5CS test and the analysis of the time to perform the 5CS test.
Interventions
The individuals of the WBVeG (n = 22) and CG (n = 22) were positioned on the side-alternating OVP (Novaplate; Fitness Evolution, São Paulo, Brazil), barefoot. In the first session, the individuals were seated on a chair in front of the OVP with the hands kept on their knees and the feet positioned on the base of the OVP corresponding to the PPD of 2.5, 5.0, and 7.5 mm with the gravitational force of 0.12, 0.25, and 0.35 g, respectively. The frequency of 5 Hz was used in the first session.21,22 The work time was 1 minute with 1 minute rest in each PPD. This procedure was performed 3 times with total time of WBV exercise of 18 minutes. From the second to the last session, patients were subjected to the same protocol, however standing on the base of the OVP with a squat static position (knees flexed at 130°).39 The frequency used in the second session was 6 Hz and was increased 1 Hz in each session. Fourteen Hz was used in the last session. The total number of sessions was 10.
The individuals of the CG were in all the same positions (squat static position and the feet positioned on the base of the OVP in the PPD of 2.5, 5.0, and 7.5 mm) for the same time of the individuals of the WBVeG; however, the OVP was turned off. This protocol was firstly used in studies involving WBVe and MetS.21,22
Statistical Analysis
Forty-four individuals were recruited. Four did not complete the protocol of intervention and 1 was unable to perform the SPPB, thus being excluded from the study in the initial evaluation. Consequently, 39 individuals participated in this study: CG (n = 17) and WBVeG (n = 22).
Descriptive data are given as mean and standard deviation (SD). Shapiro-Wilk normality test was used to assess the distribution of the variable. The total score of the SPPB and of the 3 tests was presented through median (minimum-maximum) values and the time of execution of the gait speed test and of the 5CS test was presented through mean and SD. Wilcoxon rank test and unpaired t test were used. The results were analyzed with the GraphPad Prism 6 program and P ≤ .05 was considered statistically significant.
Results
Table 1 shows the main anthropometric data of the 2 subgroups (CG and WBVeG), and no significant differences were found between them (P < .05). This is justified due to the slight difference in some anthropometric parameters, demonstrating the homogeneity of the 2 groups. Considering the total number of individuals in each group, similar percentages for smoking habit, T2DM, hypertension, and physical activity were found.
Table 1.
Anthropometrics Data of the Individuals of Study.a
| Variable | CG (n = 17) | WBVeG (n = 22) | P Value |
|---|---|---|---|
| Height (m), mean (SD) | 1.61 (0.08) | 1.63 (0.07) | .32 |
| Body mass (kg), mean (SD) | 87.43 (18.02) | 83.65 (16.27) | .42 |
| BMI (kg/m2), mean (SD) | 32.79 (6.94) | 31.16 (5.35) | .15 |
| Age (years), mean (SD) | 58.20 (9.11) | 61.18 (8.39) | .31 |
| WC (cm), mean (SD) | 108.33 (15.55) | 103.17 (11.09) | .22 |
| Smoker (%) | 2 (13.33) | 1 (5.26) | --- |
| T2DM (%) | 7 (46.66) | 7 (36.84) | --- |
| Hypertension (%) | 13 (86.66) | 17 (89.47) | --- |
| Physical activity (%) | 5 (33.33) | 4 (21.05) | --- |
Abbreviations: BMI, body mass index; CG, control group; SD, standard deviation; T2DM, type 2 diabetes mellitus; WBVeG, whole-body vibration exercise group; WC, waist circumference.
a P ≤ .05.
Table 2 shows the median (min and max) values of baseline of the SPPB total score. Comparing the total score, before and after the protocol, no significant difference was found in the CG. However in the WBVeG, a significant difference was found. In general, this may suggest that the performance of individuals with MetS who underwent WBVe improved with the proposed protocol.
Table 2.
Baseline SPPB Total Score.a
| SPPB Total Score | Before Protocol, Median (Min-Max) | After Protocol, Median (Min-Max) | P Value | P Value#, Before/After Protocol |
|---|---|---|---|---|
| CG (n = 17) | 9 (7-10) | 8 (7-11) | .56 | |
| WBVeG (n = 22) | 9 (6-11) | 10 (6-12) | .005 | .82/.16 |
Abbreviations: CG, control group; SPPB, Short Physical Performance Battery; WBVeG, whole-body vibration exercise group.
a P Value #- intergroup, P ≤ .05.
Tables 3, 4, 5, 6, and 7 show the score of each test of the SPPB, before and after the protocol WBVe of the 2 subgroups, in addition to the time of execution of the gait speed and 5CS test.
Table 3.
Balance Test Score.a
| Balance Test Score | Before Protocol, Median (min-max) | After Protocol, Median (min-max) | P Value | P Value#, Before/After Protocol |
|---|---|---|---|---|
| CG (n = 17) | 4 (3-4) | 4 (3-4) | .07 | |
| WBVeG (n = 22) | 3.5 (3-4) | 4 (4) | .01 | .88/.0017 |
Abbreviations: CG, control group; WBVeG, whole-body vibration exercise group.
a P Value #: intergroup, P ≤ .05.
Table 4.
Gait Speed Test Score.a
| Gait Speed Test Score | Before Protocol, Median (Min-Max) | After Protocol, Median (Min-Max) | P Value | P Value #, Before/After Protocol |
|---|---|---|---|---|
| CG (n = 17) | 4 (2-4) | 4 (2-4) | .57 | |
| WBVeG (n = 22) | 3 (2-4) | 4 (3-4) | .006 | .96/.08 |
Abbreviations: CG, control group; WBVeG, whole-body vibration exercise group.
a P Value #: intergroup, P ≤ .05.
Table 5.
Gait Speed Test Time.a
| Gait Speed Test Time (seconds) | Before Protocol, Mean (SD) | After Protocol, Mean (SD) | P Value | P Value#, Before/After Protocol |
|---|---|---|---|---|
| CG (n = 17) | 3.97 (0.34) | 3.90 (0.33) | .93 | |
| WBVeG (n = 22) | 5.05 (0.50) | 4.62 (0.37) | .43 | .10/.17 |
Abbreviations: CG, control group; SD, standard deviation; WBVeG, whole-body vibration exercise group.
a P Value #: intergroup, P ≤ .05.
Table 6.
5CS Test Score.a
| 5CS Test Score | Before Protocol, Median (Min-Max) | After Protocol, Median (Min-Max) | P Value | P Value#, Before/After Protocol |
|---|---|---|---|---|
| CG (n = 17) | 2 (0-2) | 2 (0-3) | .45 | |
| WBVeG (n = 22) | 1 (0-3) | 2 (1-4) | .03 | .75/.15 |
Abbreviations: 5CS, five-chair stand; CG, control group; WBVeG, whole-body vibration exercise group.
a P Value #: intergroup, P ≤ .05.
Table 7.
The 5CS Test Time in Control and WBVe Groups.a
| 5CS Test Time (seconds) | Before Protocol, Median (SD) | After Protocol, Median (SD) | P Value | P Value#, Before/After Protocol |
|---|---|---|---|---|
| CG (n = 17) | 15.27 (1.51) | 14.50 (1.43) | .10 | |
| WBVeG (n = 22) | 17.61 (0.87) | 17.34 (1.14) | .82 | .16/.12 |
Abbreviations: 5CS, five-chair stand; CG, control group; SD, standard deviation; WBVeG, whole-body vibration exercise group.
a P Value #: intergroup, P ≤ .05.
Table 3 shows the score of the balance test, which significantly increased for individuals of WBVeG (P = .01), and a significant difference was found between the 2 subgroups (P = .0017).
Table 4 shows the score of the gait speed test, which increased in WBVeG (P = .006). Table 5 shows the time of execution of the gait speed test (in seconds). Although there was reduction in time in both groups, no significant difference was found.
Table 6 shows significant difference in the score of the 5CS test in WBVeG (P = .03), indicating improvement due to the WBVe. Table 7 shows the results of the 5CS test time. No significant effects were found in both subgroups.
Discussion
Effects of the WBVe on the functionality of individuals with MetS have not yet been precisely established. However, this study aimed to point out some functional considerations in this population, based on previous findings described in individuals with MetS.21,22
Vieira et al32 reported that the evaluation of functionality may help to determine the degree of physical decline in people with the MetS, thus encouraging exercise prescription to counteract this impairment. It was also demonstrated that individuals with MetS had greater metabolic risk factors and less functional capacity, related to their reduced limb power, muscle strength, and relative leg lean mass. The decreased muscle strength and power in elderly women with the MetS20,21 reinforces the importance of preventive programs, including exercise, in this population. Sievänen et al40 demonstrated that WBVe performed in the side-alternating OVP and low frequency (12 Hz) was feasible among elderly people for increased physical function. Zhang et al,41 using an amplitude of 1 to 3 mm and 6 to 26 Hz as frequency, suggested beneficial effects of WBVe in improving the mobility function of the lower extremity and general health status in the frail elderly people. Cheung et al,42 using low-magnitude high-frequency vibration, demonstrated its ability to reduce fall incidences throughout an 18-month intervention; other than improving balancing ability and muscle strength, this type of exercise being of great potential to produce sustainable effects on muscle among elderly people.
Cross-sectional observational study has shown that muscular strength is inversely associated with the prevalence of MetS.43 Ilanne-Parikka et al44 and Phelan et al45 reported that weight reduction alone or combined with lifestyle intervention is associated with a significant reduction in the prevalence of MetS.
Effects of the WBVe as a Component of the Interventional Strategy in MetS
Srikanth and Deedwania46 reported that lifestyle modifications (weight loss and regular exercise) are essential components in the management of patients with MetS. Whole-body vibration exercise is a neuromuscular intervention modality used for strength training.47-50 This intervention exhibits the greatest impact on muscle strength in elderly individuals with limited muscle function.51 However, in the current study, all individuals submitted to WBVe were able to perform it correctly and negative effects were not reported. Additionally, adherence to WBVe was found and only about 10% gave up.
The SPPB
This is the first pseudorandomized controlled trial study evaluating the functionality of individuals with MetS exposed to WBVe using the SPPB. Simple objective measures of physical performance (standing balance, gait time and sitting to rising) and muscle strength have been used to predict the onset of disability in older community-dwelling populations,52 which present comorbidities that characterize MetS. For Hanson et al,53 the 5CS test (1 of the 3 test that composed the SPPB) is one of the best predictors of functional performance.
Total Score of the SPPB
The total score of the SPPB of this current study was significantly changed after the intervention in the WBVeG, which shows that, in general, the functionality of these individuals increased due to the WBVe.
Score of the SPPB to Evaluate Effects of the WBVe on the Balance
The score of the balance test significantly increased in individuals exposed to WBVe (WBVeG), with a significant difference being found between these patients and the controls. This indicates an improvement in the balance of these individuals with MetS.
This finding is in line with previous studies, reporting positive effects of WBVe intervention in improving the balance in individuals with anterior cruciate ligament injury,54 chronic stroke,55,56 MS,57 elderly patients with diabetic neuropathy,49 and elderly individuals.41,58 As in all of these clinical conditions mentioned, there is a loss of mobility and functional independence, and to promote an improvement in the balance becomes a good strategy to rescue this independence and maintain the quality of life of these individuals.
Score of the SPPB to Evaluate Effects of the WBVe on the Gait
Whole-body vibration exercise is reported to improve the gait time in different clinical conditions: Parkinson disease;59 postmenopausal women;60 osteogenesis imperfecta;61 patients with stroke,55,56 and children with disabling conditions.62
In the current study, the WBVeG presented a significant increase in the score (P = .006) and a decrease in the time of execution of the gait speed test (P < .05). It suggests that the WBVe would be relevant to the management of individuals with MetS, particularly those with some limitation in mobility. Whole-body vibration exercise is safe, feasible, and without negative effects on health.
Score of the SPPB to Evaluate Effects of the WBVe on the Lower Limb Muscle Strength
The evaluation of the 5CS test using the SPPB permits to have information about the lower limb muscle strength.25
Bernabeu-Mora et al25 reported that the 5CS test had the highest value for assessing mobility limitations, because standing up from a sitting position is a very common, essential activity that reflects the potential for other daily life activities, such as walking. Brochu et al63 also reported that lower limb muscle strength is one of the factors correlated with postural control in aging population.
The 5CS test has been cited in other studies with different denominations: sit-to-stand test (Hilgers et al64; Spielmanns et al65), “chair stand” (Coqueiro et al66), and 5-repetition sit-to-stand test (Patel et al26). All these denominations described an identical way of performing this test.
Authors reported significant results of this test in protocols involving WBVe. Kawanabe et al67 found that the lower limb muscular strength and balance performance improved significantly in elderly people receiving 12 to 20 Hz vibration training. Hilgers et al64 found significant results to 3-week WBV training in addition to a standard rehabilitation program that improves walking ability in patients with MS.
Spielmanns et al65 evaluated the exercise capacity in patients with chronic obstructive pulmonary disease (COPD) through this same test and no significant effects were found with the training protocol used. Similarly, in this current study, no significant values were found in the 5CS test score, also in the time of this test. However, the increase in the time of the execution of this test does not present impairment of the functionality of the lower limbs of this population.
For the individuals with MetS evaluated in this study, the group that performed this exercise modality presented significant results in the 5CS test (P = .03), representing an improvement in the strength of lower limbs.
Although no statistical difference was found, the execution time of this test was reduced in both groups, after the execution of the protocol. This may suggest that SPPB is an easy execution tool for this population, which notably has poor functionality.
Some limitations can be declared in this study. The modality, the frequency and the intensity of physical activity, and the presence of CVD risk factors (smoking, blood pressure, diabetes, and age) may influence the performance of the individuals during the evaluation of functionality. In this study, the vibration wasn’t controlled by weight with no tailoring of intervention based on patients comorbidities or tolerance.
In conclusion, the protocol proposed in this study showed the WBVe is a feasible physical activity for individuals with MetS. Through the adhesion to this type of aerobic activity, some functional parameters such as balance, gait, and lower limb strength are improved. Consequently, a better quality of life can be offered to these individuals, contributing to what is advocated as intervention strategy in this syndrome: a change to a better lifestyle. However, larger and higher quality trials are needed to determine the influence of other factors on functional effects.
Footnotes
Author’s Note: Sá-Caputo Danúbia da Cunha is also affiliated with Centro Universitário Serra dos Órgãos, Teresópolis, RJ, Brazil.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank the Brazilian Government agencies (CNPq and FAPERJ) and UERJ for the support.
ORCID iD: Laisa Liane Paineiras-Domingos  http://orcid.org/0000-0003-3451-5056
http://orcid.org/0000-0003-3451-5056
References
- 1. Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165(22):2644–2650. [DOI] [PubMed] [Google Scholar]
- 2. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Hajian-Tilaki K, Heidari B, Hajian-Tilaki A, Firouzjahi A, Bagherzadeh M. The discriminatory performance of body mass index, waist circumference, waist-to-hip ratio and waist-to-height ratio for detection of metabolic syndrome and their optimal cutoffs among Iranian adults. J Res Health Sci. 2014;14(4):276–281. [PubMed] [Google Scholar]
- 4. Beavers KM, Hsu FC, Houston DK, et al. The role of metabolic syndrome, adiposity and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2013;68(5):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Zhang H, Kan L, Zhang C, Wang P. The effect of whole body vibration therapy on the physical function of people with type II diabetes mellitus: a systematic review. J Phys Ther Sci. 2016;28(9):2675–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chanou K, Gerodimos V, Karatrantou K, Jamurtas A. Whole-body vibration and rehabilitation of chronic diseases: a review of the literature. J Sports Sci Med. 2012;11(2):187–200. [PMC free article] [PubMed] [Google Scholar]
- 8. Rauch F. Vibration therapy. Dev Med Child Neurol. 2009;51(suppl 4):166–168. [DOI] [PubMed] [Google Scholar]
- 9. Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52(6):901–908. [DOI] [PubMed] [Google Scholar]
- 10. Giunta M, Rigamonti AE, Agosti F, et al. Combination of external load and whole body vibration potentiates the GH-releasing effect of squatting in healthy females. Horm Metab Res. 2013;45(8):611–616. [DOI] [PubMed] [Google Scholar]
- 11. Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. [DOI] [PubMed] [Google Scholar]
- 12. Da Silva-Grigoletto ME, De Hoyo M, Sañudo B, et al. Determining the optimal whole-body vibration dose-response relationship for muscle performance. J Strength Cond Res. 2011;25(12):3326–3333. [DOI] [PubMed] [Google Scholar]
- 13. Arias P, Chouza M, Vivas J, et al. Effect of whole body vibration in Parkinson’s disease: a controlled study. Mov Disord. 2009;24(6):891–898. [DOI] [PubMed] [Google Scholar]
- 14. Trans T, Aaboe J, Henriksen M, Christensen R, Bliddal H, Lund H. Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee. 2009;16(4):256–261. [DOI] [PubMed] [Google Scholar]
- 15. Wunderer K, Schabrun SM, Chipchase LS. Effects of whole body vibration on strength and functional mobility in multiple sclerosis. Physiother Theory Pract. 2010;26(6):374–384. [DOI] [PubMed] [Google Scholar]
- 16. Paineiras-Domingos LL, Sá-Caputo DC, Moreira-Marconi E, et al. Can whole body vibration exercises affect growth hormone concentration? A systematic review. Growth Factors. 2017;35(4-5):189–200. [DOI] [PubMed] [Google Scholar]
- 17. Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abercromby AF, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski WH. Vibration exposure and biodynamic responses during whole-body vibration training. Med Sci Sports Exerc. 2007;39(10):1794–1800. [DOI] [PubMed] [Google Scholar]
- 19. Bruyere O, Wuidart MA, Di Palma E, et al. Controlled whole body vibration to decrease fall risk and improve health-related quality of life of nursing home residents. Arch Phys Med Rehabil. 2005;86(2):303–307. [DOI] [PubMed] [Google Scholar]
- 20. Carriere I, Peres K, Ancelin ML, et al. Metabolic syndrome and disability: findings from the prospective three-city study. J Gerontol A Biol Sci Med Sci. 2014;69(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carvalho-Lima RP, Sá-Caputo DC, Moreira-Marconi E, et al. Quality of life of patients with metabolic syndrome is improved after whole body vibration exercises. Afr J Tradit Complement Altern Med. 2017;14(4 suppl):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sá-Caputo Dda C, Ronikeili-Costa P, Carvalho-Lima RP, et al. Whole body vibration exercises and the improvement of the flexibility in patient with metabolic syndrome. Rehabil Res Pract. 2014;2014:628518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37(4):430–435. [DOI] [PubMed] [Google Scholar]
- 25. Bernabeu-Mora R, Medina-Mirapeix F, Llamazares-Herrán E, et al. The Short Physical Performance Battery is a discriminative tool for identifying patients with COPD at risk of disability. Int J Chron Obstruct Pulmon Dis. 2015;10:2619–2626. Erratum in: Int J Chron Obstruct Pulmon Dis. 2016;11:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel MS, Mohan D, Andersson YM, et al. Phenotypic characteristics associated with reduced short physical performance battery score in COPD. Chest. 2014;145(5):1016–1024. [DOI] [PubMed] [Google Scholar]
- 27. Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121(9):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puthoff ML. Outcome measures in cardiopulmonary physical therapy: short physical performance battery. Cardiopulm Phys Ther J. 2008;19(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- 29. Wolinsky FD, Miller TR, Malmstrom TK, et al. Four-year lower extremity disability trajectories among African American men and women. J Gerontol A Biol Sci Med Sci. 2007;62(5):525–530. [DOI] [PubMed] [Google Scholar]
- 30. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):221–223. [DOI] [PubMed] [Google Scholar]
- 31. Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vieira DC, Tibana RA, Tajra V, et al. Decreased functional capacity and muscle strength in elderly women with metabolic syndrome. Clin Interv Aging. 2013;8:1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alberti K, Zimmet P, Shaw J. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366(9491):1059–1062. [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization (WHO). Diabetes Programme. 2016. http://www.who.int/diabetes/en/. Accessed December 02, 2017.
- 35. National Health and Nutrition Examination Survey. 2013. https://www.cdc.gov/nchs/data/nhanes/databriefs/adultweight.pdf. Accessed November 14, 2017.
- 36. Akdeniz S, Hepguler S, Öztürk C, Atamaz FC. The relation between vitamin D and postural balance according to clinical tests and Tetrax posturography. J Phys Ther Sci. 2016;28(4):1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balachandran A, Signorile JF. How to improve reporting of the Short Physical Performance Battery scores. J Gerontol A Biol Sci Med Sci. 2015;70(12):1595–1596. [DOI] [PubMed] [Google Scholar]
- 38. Lee TT. Management of functional decline in older adults. Physicians Singapore. 2011;37:8–17. [Google Scholar]
- 39. Subashi GHMJ, Matsumoto Y, Griffin MJ. Modelling resonances of the standing body exposed to vertical whole-body vibration: effects of posture. J Sound Vib. 2008;317:400–418. [Google Scholar]
- 40. Sievänen H, Karinkanta S, Moisio-Vilenius P, Ripsaluoma J. Feasibility of whole-body vibration training in nursing home residents with low physical function: a pilot study. Aging Clin Exp Res. 2014;26(5):511–517. [DOI] [PubMed] [Google Scholar]
- 41. Zhang L, Weng C, Liu M, Wang Q, Liu L, He Y. Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: a pilot randomized controlled trial. Clin Rehab. 2014;28(1):59–68. [DOI] [PubMed] [Google Scholar]
- 42. Cheung WH, Mok HW, Qin L, Sze PC, Lee KM, Leung KS. High-frequency whole-body vibration improves balancing ability in elderly women. Arch Phys Med Rehabil. 2007;88(7):852–857. [DOI] [PubMed] [Google Scholar]
- 43. Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113(22):2642–2650. [DOI] [PubMed] [Google Scholar]
- 44. Ilanne-Parikka P, Eriksson JG, Lindström J, et al. ; Finnish Diabetes Prevention Study Group. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care. 2008;31(4):805–807. [DOI] [PubMed] [Google Scholar]
- 45. Phelan S, Wadden TA, Berkowitz RI, et al. Impact of weight loss on the metabolic syndrome. Int J Obes. 2007;31(9):1442–1448. [DOI] [PubMed] [Google Scholar]
- 46. Srikanth S, Deedwania P. Management of dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Curr Hypertens Rep. 2016;18(10):76. [DOI] [PubMed] [Google Scholar]
- 47. Delecluse C, Roelants M, Verschueen S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003;35(6):1033–1041. [DOI] [PubMed] [Google Scholar]
- 48. Torvinen S, Kannu P, Sievänen H, et al. Effect of a vibration exposure on muscular performance and body balance. Randomized cross-over study. Clin Physiol Funct Imaging. 2002;22(2):145–152. [DOI] [PubMed] [Google Scholar]
- 49. Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med. 2013;231(4):305–314. [DOI] [PubMed] [Google Scholar]
- 50. Santin-Medeiros F, Santos-Lozano A, Cristi-Montero C, Garatachea Vallejo N. Effect of 8 months of whole-body vibration training on quality of life in elderly women. Res Sports Med. 2017;25(1):101–107. [DOI] [PubMed] [Google Scholar]
- 51. Figueroa A, Jaime SJ, Alvarez-Alvarado S. Whole-body vibration as a potential countermeasure for dynapenia and arterial stiffness. Integr Med Res. 2016;5(3):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hardy R, Cooper R, Aihie Sayer A, et al. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: the HALCyon programme. PLoS One. 2013;8(2):e56483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hanson ED, Srivatsan SR, Agrawal S, et al. Effects of strength training on physical function: influence of power, strength, and body composition. J Strength Cond Res. 2009;23(9):2627–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roberts D, Andersoon G, Friden T. Knee joint proprioception in ACL-deficient knees is related to cartilage injury, laxity and age: a retrospective study of 54 patients. Acta Orthop Scand. 2004;75(1):78–83. [DOI] [PubMed] [Google Scholar]
- 55. van Nes IJ, Geurts AC, Hendricks HT, Duysens J. Short-term effects of whole-body vibration on postural control in unilateral chronic stroke patients: preliminary evidence. Am J Phys Med Rehabil. 2004;83(11):867–873. [DOI] [PubMed] [Google Scholar]
- 56. Choi ET, Kim YN, Cho WS, Lee DK. The effects of visual control whole body vibration exercise on balance and gait function of stroke patients. J Phys Ther Sci. 2016;28(11):3149–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schuhfried O, Mittermaier C, Jovanovic T, Pieber K, Paternostro-Sluga T. Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clin Rehabil. 2005;19(8):834–842. [DOI] [PubMed] [Google Scholar]
- 58. Ko MC, Wu LS, Lee S, et al. Whole-body vibration training improves balance control and sit-to-stand performance among middle-aged and older adults: a pilot randomized controlled trial. Eur Rev Aging Phys Act. 2017;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chouza M, Arias P, Vinas S, Cudeiro J. Acute effects of whole-body vibration at 3, 6, and 9 Hz on balance and gait in patients with Parkinson’s disease. Mov Disord. 2011;26(5):920–921. [DOI] [PubMed] [Google Scholar]
- 60. Iwamoto J, Sato Y, Takeda T, Matsumoto H. Whole body vibration exercise improves body balance and walking velocity in postmenopausal osteoporotic women treated with alendronate: Galileo and Alendronate Intervention Trail (GAIT). J Musculoskelet Neuronal Interact. 2012;12(3):136–143. [PubMed] [Google Scholar]
- 61. Sá-Caputo DC, Dionello CDF, Frederico ÉHFF, et al. Whole-body vibration exercise improves functional parameters in patients with osteogenesis imperfecta: a systematic review with a suitable approach. Afr J Tradit Complement Altern Med. 2017;14(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moreira-Marconi E, Sá-Caputo DC, Dionello CF, et al. Whole-body vibration exercise is well tolerated in patients with Duchenne muscular dystrophy: a systematic review. Afr J Tradit Complement Altern Med. 2017;14(4 suppl):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brochu M, Savage P, Lee M, et al. Effects of resistance training on physical function in older disabled women with coronary heart disease. J Appl Physiol. 2002;92(2):672–678. [DOI] [PubMed] [Google Scholar]
- 64. Hilgers C, Mündermann A, Riehle H, Dettmers C. Effects of whole-body vibration training on physical function in patients with multiple sclerosis. NeuroRehabilitation. 2013;32(3):655–663. [DOI] [PubMed] [Google Scholar]
- 65. Spielmanns MA, Gloeckl R, Groppa JM, et al. Whole-body vibration training during a low frequency outpatient exercise training program in chronic obstructive pulmonary disease patients: a randomized, controlled trial. J Clin Med Res. 2017;9(5):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Coqueiro R, Santos K, Schettino L, Barbosa A, Pereira R, Fernandes M., “Chair stand” test as predictor of Brazilian elderly men fallers. Macedonian J Med Sci. 2014;7:316–318. [Google Scholar]
- 67. Kawanabe K, Kawashima A, Sashimoto I, Takeda T, Sato Y, Iwamoto J. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J Med. 2007;56(1):28–33. [DOI] [PubMed] [Google Scholar]