ABSTRACT
Bariatric surgery results in long‐term weight loss and improvement or resolution in obesity‐related comorbidities. However, mounting evidence indicates that it adversely affects bone health. This review summarizes clinical research findings about the impact of bariatric surgery on skeletal outcomes. The literature is the largest and strongest for the Roux‐en‐Y gastric bypass (RYGB) procedure, as RYGB was the most commonly performed bariatric procedure worldwide until it was very recently overtaken by the sleeve gastrectomy (SG). Because SG is a newer procedure, its skeletal effects have not yet been well defined. Epidemiologic studies have now demonstrated an increased risk of fracture after RYGB and biliopancreatic diversion with duodenal switch, both of which include a malabsorptive component. As these epidemiologic data have emerged, patient‐oriented studies have elucidated the bone tissue‐level changes that may account for the heightened skeletal fragility. Bariatric surgery induces early and dramatic increases in biochemical markers of bone turnover. A notable feature of recent patient‐oriented clinical studies is the application of advanced skeletal imaging modalities; studies address the limitations of dual‐energy X‐ray absorptiometry (DXA) by using quantitative computed tomography (QCT)‐based modalities to examine volumetric bone mineral density and compartment‐specific density and microstructure. RYGB results in pronounced declines in bone mass at the axial skeleton demonstrated by DXA and QCT, as well as at the appendicular skeleton demonstrated by high‐resolution peripheral quantitative computed tomography (HR‐pQCT). RYGB has detrimental effects on trabecular and cortical microarchitecture and estimated bone strength. Skeletal changes after RYGB appear early and continue even after weight loss plateaus and weight stabilizes. The skeletal effects of bariatric surgery are presumably multifactorial, and mechanisms may involve nutritional factors, mechanical unloading, hormonal factors, and changes in body composition and bone marrow fat. Clinical guidelines address bone health and may mitigate the negative skeletal effects of surgery, although more research is needed to direct and support such guidelines. © 2018 The Authors. JBMR Plus is published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
Keywords: BONE QCT/μCT, DXA, BIOCHEMICAL MARKERS OF BONE TURNOVER, FRACTURE RISK ASSESSMENT, BONE–FAT INTERACTIONS
Introduction
Obesity is a public health concern worldwide. If not rapidly addressed, it is predicted that in 2025, 18% of men and 21% of women will be categorized as obese, and 6% of men and 9% of women will be severely obese with body mass index (BMI) ≥40 kg/m2.1 The United States has already passed that mark, as 38% of US adults were obese in 2014, and 8% had BMI ≥40 kg/m2.2 Reflecting the obesity epidemic, the number of bariatric surgeries performed internationally is rising.3 This gain in popularity for bariatric surgery is explained by several factors, including its established efficacy for long‐term weight loss4 as well as for improved glycemic control and even diabetes remission in people with type 2 diabetes.5 Furthermore, bariatric surgery improves or resolves multiple comorbidities associated with obesity, including dyslipidemia,6 obstructive sleep apnea,7 and cardiovascular disease,8 and also disease‐related mortality.9 However, mounting evidence suggests that bariatric surgery adversely affects bone health.
Since several review articles were written on this topic 4 years ago,10, 11, 12, 13, 14 understanding of the impact of bariatric surgery on bone health has evolved. This review aims to summarize recent evidence about the impact of bariatric surgery on bone outcomes, including fracture risk, bone turnover markers, bone mineral density (BMD), and bone microarchitecture and strength. It will also recapitulate the data on potential mechanisms for the skeletal changes after bariatric procedures, namely nutritional and hormonal factors, body composition and bone marrow changes, and mechanical unloading. Moreover, it will discuss the clinical implications of these postoperative skeletal changes, including the impact of interventions to mitigate fracture risk after bariatric surgery. Finally, a research agenda is proposed to stimulate investigation to address knowledge gaps.
Overview of the Bariatric Procedures
Traditionally, bariatric procedures have been classified into restrictive, malabsorptive, or combined restrictive and malabsorptive surgeries based on the mechanisms by which they promote weight loss. Restrictive surgeries limit food intake by reducing the size of the stomach. Adjustable gastric banding and sleeve gastrectomy belong to this category. Of note, sleeve gastrectomy also induces functional malabsorption by altering nutrient transit time. Combined restrictive and malabsorptive surgeries include Roux‐en‐Y gastric bypass (RYGB) and biliopancreatic diversion with duodenal switch (BPD‐DS). In addition to their restrictive component, these procedures limit the absorption of food and nutrients by bypassing sections of the small intestine.
However, it is now recognized that hormonal changes account for some of the benefits of sleeve gastrectomy, RYGB, and BPD‐DS by reducing appetite and by improving glucose homeostasis.15 Bariatric procedures induce hormonal changes due to weight loss (eg, changes in adipose tissue–derived hormones such as estrogens, adiponectin, and leptin) and due to the anatomical changes induced by surgery (eg, changes in gastrointestinal‐derived hormones such as ghrelin, glucagon‐like peptide 1, and peptide YY).
Laparoscopic adjustable gastric banding (LAGB)
LAGB is a purely restrictive procedure. It was once the most popular restrictive bariatric procedure, but because of its relatively modest weight loss (excess weight loss of 40% to 50%), high rates of weight regain, and late complications, it is now on the decline.3, 16 LAGB involves inserting a small ring in the proximal stomach to create a pouch above the ring of about 15 to 20 mL (Fig. 1 A). Ring diameter can be reduced as needed by injecting sterile saline through a port inserted subcutaneously.
Figure 1.
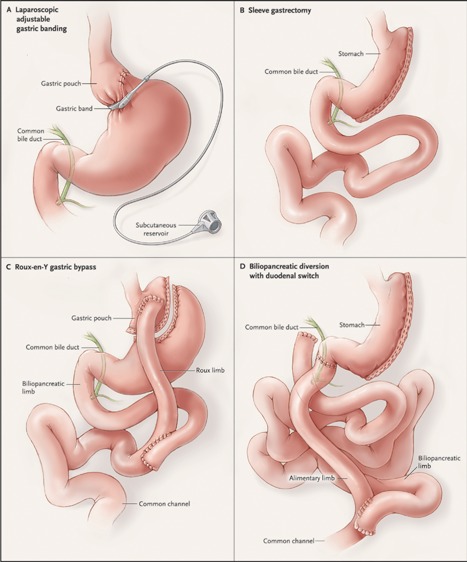
Bariatric procedures. Restrictive procedures include laparoscopic adjustable gastric banding (A) and sleeve gastrectomy (B). Although both Roux‐en‐Y gastric bypass (C) and biliopancreatic diversion with duodenal switch (BPD‐DS) (D) are mixed restrictive and malabsorptive procedures, the malabsorptive component is greater for BPD‐DS. (From DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–83. Copyright © 2007 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society).
Sleeve gastrectomy (SG)
SG is now the most commonly performed bariatric procedure worldwide.3 It involves the longitudinal resection of the lateral part of the stomach from the fundus to the antrum to create a narrow tubular stomach, leaving only about 20% of the stomach in place (Fig. 1 B). While SG restricts the amount of food intake through reduced stomach size, it also promotes satiety by decreasing ghrelin levels and by increasing glucagon‐like peptide 1 (GLP‐1) and peptide YY (PYY) levels.17 SG results in a significant and sustained weight loss of >50% excess weight loss in the long term.18
RYGB
RYGB has long been the favored bariatric procedure worldwide, but SG recently surpassed it.3 RYGB is a restrictive procedure, but it also involves a malabsorptive component that contributes to weight loss. First, a small stomach pouch of about 30 mL is created (Fig. 1 C). Then, the small intestine is transected at a variable distance distal to the ligament of Treitz, typically 30 to 50 cm, and the remaining small intestine is anastomosed to the gastric pouch (alimentary limb). The biliopancreatic limb is connected 75 to 150 cm distal to the gastric pouch, creating a common channel of roughly 400 cm where absorption of food and nutrients occurs. RYGB leads to several hormonal changes that control hunger and glycemia, including increased GLP‐1 and PYY levels.19, 20 It results in excess weight loss of about 70% that persists in the long term.4, 21
BPD‐DS
BPD‐DS represents only 1% of all bariatric procedures worldwide.3 The restrictive part of the procedure is a SG (Fig. 1 D). For the intestinal bypass, the duodenum is first transected 3 cm distal to the pylorus. Then, the small intestine is transected at 250 cm from the ileocecal valve and the distal end is connected to the duodenum to create an alimentary limb. Finally, the biliopancreatic limb is connected approximately 100 cm from the ileocecal valve to create a common channel. BDP‐DS thus results in substantial malabsorption. GLP‐1 and PYY responses after glucose ingestion are enhanced.22, 23 Excess weight loss after BPD‐DS is approximately 70% to 80%.21
Bone Outcomes After Bariatric Surgery
Fracture risk
Since 2012, a number of epidemiologic studies have evaluated the impact of various bariatric procedures on fracture risk.24, 25, 26, 27, 28, 29 (See Table 1 for a summary of the studies.) All except one of these cohort studies used large population‐based databases from the US, the UK, Canada, and Taiwan (n = 2064 to 12,676 patients in the bariatric groups). Study designs varied in several ways. Sources of heterogeneity include length of follow‐up and the type of bariatric procedure studied, with most including a mixture of several bariatric procedures. In addition, studies differed in the covariates used to match the control group with the bariatric groups; most studies did not match controls for BMI, and some studies did not adjust findings for several important confounders. However, some interesting findings have emanated.
Table 1.
Summary of Epidemiologic Studies Reporting Fracture Risk After Bariatric Surgery
| First author (year) | Design, country | Sample size for bariatric and control groups | Selected baseline characteristics of study cohort | Bariatric procedure (%) | Fracture events (n) | Mean follow‐up (years) | Potential confounders taken into ccount | Results on fracture risk | Strengths (+) and limitations (−) |
|---|---|---|---|---|---|---|---|---|---|
| Lalmohamed (2012)27 | Cohort study using data from the UK General Practice Research Database (1987–2010) UK | Bariatric group: 2079 Control group matched for age, sex, BMI, year, and practice: 10,442 | Women: 84% Mean age: 45 years Ethnicity: unknown Mean BMI: 43 kg/m2 History of fracture: 20% | AGB (60%) RYGB (29%) | Total: 245 Bariatric group: 38 | 2.2 | Age, sex, BMI, history of fracture, inflammatory bowel disease, cerebrovascular disease, history of falls in previous 6–12 months, medication use | Adjusted RR (95% CI): Overall: 0.89 (0.60–1.33) Osteoporotic fracture sites (spine, hip, forearm, humerus): 0.67 (0.34–1.32) AGB: 0.82 (0.50–1.36) RYGB: 0.77 (0.27–2.16) Other: 1.28 (0.42–3.92) Trend toward an increase in fracture risk 3–5 years after surgery | Large population‐based sample (+) Controls matched for BMI (+) Adjustment for several potential confounders (+) Short follow‐up (−) Mainly AGB (−) Number of fracture events too small to assess fracture risk by site and by type of bariatric procedure (−) |
| Nakamura (2013)26 | Historical cohort (1985–2004), single institution US | Bariatric group: 258 Fracture incidence rates compared with those in a cohort matched for age, sex, and calendar year | Women: 82% Mean age: 44 years Whites (97%) Mean BMI: 49 kg/m2 History of fracture: 36% | RYGB (94%) VBG (5%) Other (<1%) | Bariatric group: 132 | 8.9 | Sex, physical activity status, clinical diagnosis of calcium/vitamin D deficiency or general malnutrition, smoking, alcohol use, alcoholism, history of fracture | SIR (95% CI): Overall: 2.3 (1.8–2.8) Osteoporotic sites (hip, wrist, spine, humerus): 2.0 (1.3–3.0) Non‐osteoporotic sites: 2.4 (1.8–3.0) Moderate‐trauma fractures: 3.2 (2.4–4.1) 0–5 years after surgery: 1.8 (1.3–2.5) 5−<10 years after surgery: 2.9 (2.0–4.1) >10 years after surgery: 2.9 (1.5–5.0)a Forearm: 2.0 (0.97–3.5) Humerus: 5.0 (2.2–9.9) Clinical spine: 3.1 (1.4–5.9) Pelvic: 1.0 (0.03–5.7) Femur: 5.5 (1.5–14) Leg: 2.4 (1.5–3.7) | Long‐term follow‐up (+) Small sample (−) Single institution, not necessarily representative of population (−) Controls not matched for BMI (−) Mainly RYGB (−) No adjustment for medication use, falls history, comorbidities (−) |
| Lu (2015)28 | Cohort study using the National Health Insurance Program (2001–2009) Taiwan | Bariatric group: 2064 Control group matched by propensity score: 5027 | Women: 64% Mean age: 32 years Taiwanese: 100% Mean BMI: unknown Patients with a history of fracture excluded | Restrictive procedures (86%) Malabsorptive procedures (14%) | Total: 557 Bariatric group: 183 | 4.8 | Age, sex, Charlson comorbidity index, history of diabetes, hypertension, hyperlipidemia, and year obesity was diagnosed | Adjusted HR (95% CI): Overall: 1.21 (1.01–1.44) Malabsorptive procedures: 1.47 (1.01–2.15) Restrictive procedures: 1.17 (0.97–1.41) Analysis by time period: NS Forearm: NS Humerus: NS Spine: NS Pelvis: NS Femur: NS Leg: NS Clavicle/scapula/sternum: 2.16 (1.27–1.68) Feet/toes: 1.53 (1.02–2.30) | Data representative of the Taiwanese population (+) Controls not matched for BMI (−) No adjustment for medication use and falls history (−) Exclusion of patients with a history of fracture (−) Number of fracture events too small to evaluate fracture risk by site and by specific bariatric procedure (−) |
| Douglas (2015)29 | Cohort study using data from the UK Clinical Practice Datalink (1987–2014) UK | Bariatric group: 3882 Control group matched by propensity score: 3882 | Women: 81% Mean age: 45 years Ethnicity: unknown Mean BMI: 45 kg/m2 Patients with a history of fracture excluded | AGB (47%) RYGB (37%) SG (16%) Other (<1%) | Total: 71 Bariatric group: 39 | 3.4 | Propensity‐score matching for age, sex, BMI, clinical practice, calendar year, type 2 diabetes, hypertension, coronary heart disease, cerebrovascular disease, peripheral vascular disease, other atheroma, smoking status, alcohol consumption, use of insulin, oral hypoglycemic agents, and statins | HR (95% CI): Overall: 1.26 (0.79–2.01) Hip: 1.15 (0.42–3.18) Wrist: 1.56 (0.86–2.84) Spine: 1.50 (0.69–3.23) | Large population‐based study (+) BMI‐ and propensity score–matched controls (+) Exclusion of patients with a history of fracture (−) Number of events too small to evaluate fracture outcome (−) |
| Rousseau (2016)24 | Cohort study using healthcare administrative databases (2001–2014) Canada | Bariatric group: 12,676 Obese control group matched for age and sex: 38,028 Non‐obese control group matched for age and sex: 126,760 | Women: 72% Mean age: 43 years Mainly whites Mean BMI: unknown History of fracture: 11% | For the period 2006–2014: AGB (42%) SG (28%) BPD (21%) RYGB (9%) | Total: 4535 Bariatric group: 514 | 4.4 | Duration of follow‐up, material and social deprivation, area of residence, history of fracture, number of comorbidities in previous 5 years | Adjusted RR (95% CI): Bariatric versus non‐obese group: 1.44 (1.29–1.59) Bariatric versus obese group: 1.38 (1.23–1.55) Fracture risk by site (bariatric versus non‐obese group): Distal lower limb: 1.15 (0.98–1.35) Clinical spine: 1.70 (1.06–2.73) Pelvis/hip/femur: 1.88 (1.37–2.58) Upper limb: 1.65 (1.42–1.91) By bariatric procedure (bariatric group versus non‐obese group): AGB: 1.08 (0.83–1.39) SG: 1.27 (0.86–1.88) RYGB: 1.13 (0.67–1.92) BPD: 1.60 (1.25–2.03) | Large population‐based sample (+) Large number of fracture events (+) Controls not matched for BMI (−) No adjustment for medication use, physical activity, falls history (−) Follow‐up too short for SG and not enough RYGB to assess fracture risk (−) |
| Yu (2017)25 | Cohort study using claims data from a large US commercial health plan (2005–2013) US | RYGB group: 7516 AGB control group matched by propensity score: 7516 | Women: 79% Mean age: 44 years Ethnicity: unknown Mean BMI: unknown Osteoporosis: 1.4% | RYGB (50%) AGB (50%) | Bariatric group: 281 (163 RYGB, 118 AGB) | 2.3 | Propensity‐score matching for age, sex, comorbidities, medications, osteoporosis diagnosis, history of falls, BMD testing, health care utilization, geographic location, surgical year | HR (95% CI): Non‐vertebral osteoporotic sites (humerus, wrist, hip, pelvis): 1.43 (1.13–1.81)a Wrist: 1.45 (1.01–2.07) Humerus: 1.23 (0.73–2.07) Hip: 1.54 (1.03–2.30) Pelvis: 1.29 (0.51–3.26) <45 years: 1.37 (0.86–2.16) 45–55 years: 1.90 (1.33–2.71) >55 years: 0.93 (0.59–1.45) Increase in fracture risk after >2 years | Large sample, population‐based (+) Propensity score–matched bariatric group as control (+) Short follow‐up (−) Only RYGB and AGB (−) |
AGB = adjustable gastric banding; BMI = body‐mass index; BPD = biliopancreatic diversion; CI = confidence interval; HR = hazard ratio; RR = relative risk; RYGB = Roux‐en‐Y gastric bypass; SG = sleeve gastrectomy; SIR = standardized incidence ratio; VBG = vertical‐banded gastroplasty.
First, valuable information on the typical patient undergoing bariatric surgery has been provided by these studies. Whereas in the US, the UK, and Canada mostly women in their mid 40s are opting for bariatric surgery,24, 25, 26, 27, 29 mean age at surgery is about 10 years younger in Taiwan.28 Mean BMI at time of surgery varies from 43 to 49 kg/m2 in the studies for which data are available.25, 26, 27, 29 Moreover, the study by Rousseau and colleagues built upon the knowledge already gathered on fracture risk in people with obesity. Although it confirmed that people with obesity and, more so those with severe obesity, are at higher risk of fracture than those without obesity, it revealed that fracture risk is site‐specific in obesity, affecting predominantly the distal lower limb (tibia, ankle, feet).24 In studies that did not exclude those with a history of fracture, between 11% and 36% had experienced at least one fracture episode before surgery.24, 26, 27 In summary, patients with severe obesity who undergo bariatric surgery are mainly women in their mid 30s to 40s, a significant minority of whom have a history of fracture, most likely of the distal lower limb.
Second, available data suggest that fracture risk after bariatric surgery varies depending on the bariatric procedure. On one hand, LAGB does not appear to be associated with an increased risk of fracture, at least in the short term (mean follow‐up of 2.2 years).27 On the other hand, mixed malabsorptive and restrictive procedures including RYGB and BPD‐DS are associated with a relative risk increase of 1.4 to 2.3, depending on the study.24, 25, 26, 28 Although absolute fracture risk in this generally young population is still low, affecting an estimated 10 per 1000 person‐years,25 it is important to consider that as the population ages and women pass through menopause, this may translate into an important fracture burden. There are insufficient data to make conclusions about whether fracture risk is associated with SG.
Third, most studies did not have enough fracture events in the bariatric group to assess fracture risk by site.27, 28, 29 The studies that had been able to evaluate this outcome indicate that bariatric surgery increases fracture risk at osteoporotic sites with an increase in wrist, humerus, clinical spine, hip, or femur fractures.24, 25, 26 However, the study by Lu and colleagues from Taiwan described a predominance of fractures of the clavicle, scapula, sternum, feet, and toes after bariatric surgery.28 It is unclear if the distinct findings of this study are because of ethnicity; however, the small number of fracture events may have contributed to the lack of significant results at osteoporotic sites. Finally, Rousseau and colleagues highlighted that fracture pattern changes after bariatric surgery, from a pattern associated with obesity to a pattern of fracture typically found in osteoporosis (ie, fractures of the upper limb, clinical spine, and hip/femur/pelvis).24
Fourth, available evidence suggests that fracture risk starts to increase between 2 and 5 years after surgery. In the studies by Rousseau and colleagues and Yu and colleagues, fracture risk rose as early as 2 to 3 years after surgery.24, 25 Moreover, Nakamura and colleagues showed that although fracture risk 0 to 5 years after surgery was already higher than in the general population, it was even greater in years 5 to 10 and 10+ after surgery.26 Finally, Lalmohamed and colleagues found a trend toward an increase in fracture risk 3 to 5 years after surgery.27 Of note, Rousseau and colleagues reported that after the first peak at 3 years, fracture risk plateaued, then started to increase again at year 8 to reach a second higher peak at year 11 after surgery.24 For the average bariatric surgery patient—a premenopausal woman—this second peak may correspond to her passage through menopause. This is speculative, however, as menopausal status was not assessed and because attrition may also explain this finding.
In summary, evidence gathered from epidemiologic studies suggest that mixed restrictive and malabsorptive procedures such as RYGB and BPD‐DS are associated with an increased risk of fracture at osteoporotic sites and that fracture risk starts to manifest between 2 and 5 years after surgery. It remains uncertain if menopausal status influences fracture risk in the bariatric population because very few studies had a follow‐up that was long enough to capture a large number of menopausal women. Although LAGB appears not to increase fracture risk at least in the short term, it is not possible at this point to determine whether SG is safe for skeletal health. Large population‐based cohort studies are necessary that compare various bariatric procedures in the long term in groups matched for important confounding factors including BMI.
Bone turnover, mass, and microarchitecture
As epidemiologic studies have emerged demonstrating increased fracture risk after bariatric surgery, mounting evidence from patient‐oriented studies has elucidated the bone tissue‐level changes that may account for the increase in skeletal fragility. In this section, data will be summarized and synthesized from human studies with bone turnover, mass, and microarchitecture outcomes. The most notable feature of the recent studies is the application of advanced skeletal imaging modalities. In the first wave of studies, skeletal effects of bariatric surgery were assessed by dual‐energy X‐ray absorptiometry (DXA).10, 11 However, assessment of areal BMD (aBMD) by DXA may be biased in the setting of marked weight loss because of changes in the composition of the soft tissue surrounding bone.30, 31 As a result, uncertainty lingered about whether and to what extent reported postoperative aBMD declines might be the result of DXA artifact. Furthermore, DXA cannot distinguish cortical from trabecular bone compartments, nor can it evaluate elements of bone microstructure—aspects of “bone quality”—or estimate bone strength. More recent studies of the skeletal effects of bariatric surgery address these limitations of DXA by using QCT to assess volumetric BMD (vBMD) at the axial skeleton and/or high‐resolution peripheral quantitative computed tomography (HR‐pQCT) to assess vBMD, microstructure, and estimated strength at the appendicular skeleton.32, 33, 34, 35, 36, 37 Although obesity and weight loss can also influence QCT assessments,38 QCT technology has strengthened and advanced the knowledge base.
For all of these patient‐oriented skeletal outcomes, the literature is by far the largest and strongest for RYGB, as RYGB was the most commonly performed bariatric procedure worldwide until very recently.3 Fewer data exist for LAGB and BPD‐DS, as the use of those procedures declined over the years that interest in studying bone outcomes grew. Because SG is a newer procedure, its skeletal effects have not yet been well defined.
Bone turnover markers
Bariatric surgery induces early and dramatic increases in biochemical markers of bone turnover.10, 11, 14, 39 After RYGB, serum C‐terminal telopeptide (CTx) elevation has been documented as early as 10 days postoperatively,40 then marker levels peak by 6 to 12 months but remain elevated.33, 41, 42 The bone resorption marker serum CTx typically increases by 200% during the first postoperative year.10, 11 Biochemical markers of bone formation increase but typically to a lesser extent,10, 11, 14 suggesting a potential “uncoupling” of resorption from formation that has also been reported in rat models of RYGB.43, 44, 45 The few studies to compare marker increases after RYGB and SG have observed either similar increases for the two procedures41 or greater increases after RYGB.5, 36 Bone turnover markers increase after BPD‐DS,46 and studies of LAGB have variably shown increases from baseline47, 48 or no change40, 49 in marker levels. It is now clear that after RYGB, bone turnover marker elevations are sustained: In a trial of adults with type 2 diabetes randomized to bariatric surgery or medical diabetes therapy, serum CTx was higher after RYGB than in the medical therapy group at 5 years.5 After BPD‐DS, marker levels were elevated at 4 years.46
Bone mass
After RYGB, BMD declines at the axial skeleton, demonstrated by DXA and also by QCT‐based imaging modalities. Since 2004, many prospective studies have used DXA to examine BMD change after RYGB; a published meta‐analysis summarized the clear decreases in aBMD reported by studies published before 2014,39 and subsequent studies have yielded generally consistent DXA findings.32, 33, 35, 36, 41, 50, 51, 52, 53 At the proximal femur, the magnitude of the aBMD decline by DXA is particularly striking, with 12‐month decreases ranging from 6% to 11%, roughly comparable to the bone mass a woman might lose over the first 3 to 4 years of menopause. Two published studies have now assessed proximal femur vBMD by QCT and have observed declines in vBMD, although declines are smaller in magnitude than aBMD declines by DXA.33, 36 In the first study, no loss of bone mass was detected by QCT during the first year, despite a substantial decline in aBMD by DXA, but then by 2 years, vBMD by QCT was 7% lower in RYGB participants compared with nonsurgical controls.33 In the second study, total hip vBMD by QCT did decrease significantly during the first year, although to a lesser extent than total hip aBMD by DXA.36 Together, these findings suggest that bone mass does decrease at the hip after RYGB, although DXA might overestimate the decline. At the lumbar spine, DXA‐assessed aBMD generally declines, although the magnitude of change is usually smaller than at the hip33, 35, 36, 39, 41, 50, 53 and in some studies has not reached statistical significance.32, 51, 52 Three studies assessing spinal BMD by both DXA and QCT,33, 34, 36 however, have demonstrated decreases in spinal vBMD by QCT that are larger in magnitude than aBMD declines by DXA and even on par with the magnitude of the DXA‐detected declines at the proximal femur.33, 34, 36 For example, at 12 months after RYGB in one cohort, aBMD at the femoral neck had decreased by a mean of 8.0%, and vBMD at the spine had similarly decreased by 8.1%, even though DXA did not detect a statistically significant change in aBMD at the spine.34 In light of the fact that spinal aBMD by DXA may be susceptible to spurious elevation in the presence of degenerative disease and other processes,54 this raises suspicion for artifactual confounding of the DXA results at the spine. Taken together, the studies’ spinal findings suggest that bone mass does decrease at the spine after RYGB, and DXA might underestimate the decline.
After RYGB, BMD declines at the appendicular skeleton as well. Reported changes in aBMD at the forearm by DXA have been variable, often with decreases at the ultradistal and total radius during the first postoperative year but no change at the 1/3 distal radius.32, 34, 55, 56, 57, 58, 59 Four studies have now used HR‐pQCT to examine the skeletal effects of RYGB and have documented declines in vBMD at the radius and tibia.32, 33, 34, 35 Measured declines in vBMD at the radius and tibia by HR‐pQCT have been smaller than at the spine and hip, but experiments with HR‐pQCT phantoms wrapped in simulated fat indicate that HR‐pQCT underestimates vBMD decrease in the setting of decreasing fat mass.34 The observation that detrimental changes occur at both weight‐bearing and non‐weight‐bearing sites (tibia and radius) signals that the skeletal effects of RYGB are at least in part systemic in nature. In examining the individual cortical and trabecular compartments, the studies employing HR‐pQCT identify a consistent pattern: At the radius, the decrease in total vBMD is driven by a decrease in trabecular vBMD,33, 34, 35 whereas at the tibia, the total vBMD decline is due to vBMD change either within the cortical compartment32, 34 or within both compartments.33, 35
Temporal trends have emerged from the studies of BMD changes after RYGB. First, changes appear early, with decreases documented by DXA, QCT, and HR‐pQCT just 6 months postoperatively.34, 41, 42, 52, 59, 60 Second, BMD continues to decrease with time, even after weight loss plateaus and weight stabilizes. In two RYGB studies, weight loss plateaued between 12 and 24 months, but BMD decreased progressively throughout the 24 months.33, 51 In a cohort of 59 women, there was a 10.2% decline in DXA‐assessed femoral neck aBMD in the first year, and then—despite mild weight regain between years 1 and 3—an additional 2.7% aBMD decline during that period.61 An extension of one of the 24‐month studies has now demonstrated continued declines in vBMD at the spine, radius, and tibia between 2 and 5 years after surgery, despite stability in weight.62
The effects of RYGB on bone mass may particularly impact postmenopausal women. Because approximately 80% of bariatric surgery patients are women,63 and the average age at bariatric surgery is the early to mid 40s,24, 25, 26, 27, 29 studies of RYGB and bone health have included very few men and postmenopausal women or have restricted enrollment to premenopausal women to decrease heterogeneity.41, 50 In one study of women with sufficient numbers for comparison of pre‐ and postmenopausal women, postmenopausal women not only had lower mean DXA‐assessed aBMD values 3 years postoperatively but also had greater aBMD declines at the femoral neck and spine.61 In a recent study enrolling premenopausal women, postmenopausal women, and men, preoperative bone mass was lowest among postmenopausal women, as one would expect.34 One year postoperatively, absolute and percentage declines in aBMD at the total hip by DXA, vBMD at the spine by QCT, and vBMD at the tibia by HR‐pQCT were worse for postmenopausal women than for premenopausal women or men. For example, femoral neck aBMD declined 12.2% in postmenopausal women, 7.2% in premenopausal women, and 6.8% in men (p < 0.05 for difference between postmenopausal women and each of the other groups). Heightened vulnerability of the postmenopausal skeleton may assist with hypotheses about mechanisms for the skeletal effects of bariatric surgery, and it may have implications for clinical care, discussed below.
Far fewer data exist about the effects of other bariatric surgery procedures on bone mass than about the effects of RYGB, as the use of LAGB and BPD‐DS waned over the years that interest in studying bone outcomes waxed, and as SG is a newer procedure. After BPD‐DS, aBMD by DXA has been shown to decrease;46 no study has directly compared BMD effects of BPD‐DS with other procedures. After LAGB, aBMD by DXA appears to decrease modestly at the hip but not the spine,47, 49, 64 with declines at the hip smaller than those after RYGB,64 and with decline in whole‐body bone mineral content comparable to that observed in medical weight management nonsurgical controls.65 After SG, BMD appears to decrease, but it is unclear whether it decreases as much as it does after RYGB, and SG studies have been limited by small sample size, short duration, lack of prospective design, and/or the exclusive use of DXA.36, 37, 41, 50, 51, 64, 66, 67, 68, 69 In one study comparing women undergoing SG and RYGB, for example, DXA‐assessed aBMD appeared to decrease more after RYGB than SG, although the difference was not statistically significant.50 Two recent studies showed similar declines in aBMD, including an analysis of a randomized trial of SG, RYGB versus medical therapy for diabetes.41, 51 In that randomized trial, for example, mean 24‐month declines in total hip aBMD after RYGB and SG were 9.5% and 9.2%, respectively.51 Two studies have used QCT to assess axial vBMD after SG.36, 37 In one, in 7 RYGB and 14 SG participants, no changes in spinal vBMD were detected within or between groups after 6 months.37 In the other, in 9 RYGB and 10 SG participants, 12‐month decline in hip aBMD by DXA was greater in the RYGB group than the SG group, but changes in hip vBMD by QCT were similar, as were declines in spinal aBMD and vBMD.36 Now that SG and RYGB are the predominant bariatric procedures performed, it will be important for fully informed decision‐making to understand the relative effects of the two procedures on BMD. Further, as the bariatric surgery landscape continues to evolve, comparisons of procedural effects will remain a priority.
Bone microarchitecture
Because cortical and trabecular bone microarchitecture influences bone quality and strength,70 some studies of the skeletal effects of bariatric surgery have included microarchitectural outcomes. In a study of obese adults undergoing BPD‐DS, iliac crest bone biopsies preoperatively and 4 years postoperatively demonstrated changes including increased osteoid volume and decreased cortical thickness.46 Four studies to date to have used HR‐pQCT to quantitatively characterize compartmental microstructure.32, 33, 34, 35 These studies, which have involved participants undergoing RYGB33, 34, 35 or RYGB, SG, and LAGB,32 have documented deterioration in trabecular and cortical architecture. Within the trabecular compartment, trabecular number decreases and trabecular separation and heterogeneity increase.33, 34, 35 Cortical thickness decreases and trabecular area increases, consistent with endocortical resorption.32, 33, 34, 35 Cortical porosity increases dramatically.33, 34, 35 Bone strength, estimated by micro‐finite element analysis, declines at both the radius and the tibia,33, 34 consistent with the increase in fracture risk increasingly documented in epidemiologic studies, described above.
Potential Mechanisms for Postoperative Bone Changes
The negative skeletal effects of bariatric surgery are presumably multifactorial, and mechanisms may involve nutritional factors, mechanical unloading, hormonal factors, and changes in body composition and bone marrow fat.
Nutritional factors
Before bariatric surgery, micronutrient and macronutrient deficiencies are commonly encountered in patients with severe obesity.71 The intake of nutrient‐poor food may lead to insufficient intake of nutrients that are important for bone health, including vitamin D, calcium, and protein. If not addressed properly, nutrient deficiencies may be aggravated after all bariatric procedures and especially after procedures with a malabsorptive component such as RYGB and BPD‐DS.71
In a recent systematic review of observational studies, mean serum 25OHD concentrations before surgery were <20 ng/mL and from 20 to 30 ng/mL in 42% and 33% of included studies, respectively.72 Obese individuals may be predisposed to vitamin D deficiency because of sequestration or volumetric dilution of the fat‐soluble hormone in fat stores and inadequate sunlight exposure.73 After bariatric surgery, vitamin D is malabsorbed, and in the great majority of studies, mean serum 25OHD concentrations remain <30 ng/mL despite diverse vitamin D supplementation regimens.72 Preoperative calcium intakes are below recommended dietary allowance for almost half of the bariatric surgery population,74 and the combination of vitamin D deficiency and low dietary calcium intake likely explains why secondary hyperparathyroidism is so prevalent in obesity, with prevalence rates ranging from 21% to 66%.75, 76, 77, 78 After RYGB, intestinal calcium absorption declines.52, 79 This occurs even in the setting of optimized vitamin D status: Schafer and colleagues demonstrated that despite maintaining adequate vitamin D status (most participants with 25OHD >30 ng/mL) and calcium intake (total daily intake of 1200 mg from diet and calcium citrate supplements), intestinal fractional calcium absorption (FCA) decreased dramatically—from a mean of 33% to 7%—6 months after RYGB.52 In parallel, parathyroid hormone (PTH) concentrations increased and 24‐hour urinary calcium excretion decreased. There was an inverse correlation between change in FCA and change in bone resorption marker serum CTx, suggesting that the decline in FCA may be detrimental to bone health in the long term. The effects of other bariatric procedures on FCA have not been determined. Overall, in light of malabsorption and often inadequate intakes of vitamin D and calcium after bariatric surgery, it is not surprising that secondary hyperparathyroidism is common postoperatively, reaching about 40%, 57%, 74%, and 70% after LAGB, SG, RYGB, and BPD‐DS, respectively, at 5 years.75, 80
As early as 3 months after RYGB, an increase in most amino acids was observed, which possibly reflects muscle catabolism.81 In the settings of nonsurgical caloric restriction and after bariatric surgery, adequate protein intake has been shown to minimize muscle and bone loss.53, 82 However, meeting dietary recommendations for protein intake may be a challenge after all bariatric procedures because of very restricted caloric intake and/or protein intolerance.83, 84, 85
Mechanical unloading
The skeleton adapts to mechanical strain, and bone mass and architecture are maintained and enhanced in response to loading or diminished in response to disuse.86 Thus, detrimental effects on bone mass and microarchitecture have been documented with bed rest,87 restricted weight‐bearing after orthopedic surgery,88 and space flight.89 After bariatric surgery, dramatic weight loss results in the relative unloading of the skeleton. One study documented postoperative increases in the osteocyte‐secreted, load‐responsive hormone sclerostin, and increases in sclerostin correlated with increases in bone turnover markers and decreases in aBMD.41 In several bariatric surgery studies, greater weight loss has been associated with greater decline in proximal femur aBMD by DXA.32, 34, 51, 58 However, an association between extent of weight loss and BMD decline might not be attributable to the effects of mechanical unloading, and instead could exist if those with greater weight loss have more dramatic changes in nutritional or hormonal factors. Furthermore, mechanical unloading cannot account for the skeletal changes that occur at the non‐weight‐bearing radius after bariatric surgery, nor the continued loss of bone mass even after weight loss plateaus.33, 51 Thus, mechanical unloading may contribute to but is insufficient to explain skeletal effects of bariatric surgery.
Hormonal factors
The potential role of hormonal factors in the bone loss after bariatric surgery has been covered in detail in other reviews10, 11, 12, 13 and will be summarized here. After bariatric surgery, hormonal changes occur as a consequence of weight loss and of anatomical changes induced by surgery. Indeed, fat mass loss increases adiponectin, IGF‐1, and total testosterone, whereas it reduces leptin, estradiol, and insulin. Moreover, most bariatric procedures increase GLP‐1 and peptide YY concentrations, with variable effects on ghrelin.15 It is anticipated, based on preclinical studies, that the increase in adiponectin and peptide YY and the reduction in estradiol, leptin, insulin, and potentially ghrelin will diminish bone mass, while the rise in testosterone, GLP‐1, and IGF‐1 will favor bone mass gain.12 Although the involvement of adipose tissue and gut‐derived hormones in the pathophysiology of bone loss after bariatric surgery is appealing, conflicting results have emerged from the small observational studies where associations between changes in bone turnover markers or BMD and changes in hormonal factors have been sought.35, 50, 51, 68, 90, 91 Moreover, most studies have looked at these associations after RYGB and SG, while to our knowledge, only one concerned LAGB and none addressed BPD‐DS.
Among all participants in the STAMPEDE trial (RYGB, SG, and intensive medical diabetes therapy groups), an association between reduction in leptin and hip BMD loss by DXA at 2 years was no longer significant after adjustment for weight loss, suggesting that change in leptin may be a mediator of the relationship between weight loss and hip bone loss.51 However, the findings of Bruno and colleagues suggested that change in leptin after RYGB might play a direct role in the pathophysiology of bone loss. Indeed, they found that the decrease in leptin was a significant predictor of the increase in the bone resorption marker N‐terminal telopeptide (NTx) at 6 months after RYGB, independent of change in BMI.89 In addition, the increase in adiponectin at 1 year after RYGB correlated with the decrease in total BMD by DXA, and this was unrelated to changes in body composition parameters.60 In a study by Carrasco and colleagues, reduction in ghrelin was also associated with total BMD loss by DXA after RYGB and with lumbar spine BMD loss after RYGB and SG.50 In a small study, Yu and colleagues found that changes in fasting peptide YY displayed a strong correlation with both 10‐day and 1‐year changes in CTx (r = 0.70, p < 0.001) and 1‐year change in P1NP (r = 0.77, p = 0.014) after RYGB.40 No correlation was found between changes in IGF‐1 and changes in BMD by DXA 1 year after RYGB and SG,68, 90 as well as between changes in insulin, bone turnover markers, and BMD by DXA 2 years after RYGB.35 Finally, although bariatric surgery is associated with an increase in testosterone,92 an association between changes in sex hormones and bone outcomes after bariatric surgery was not identified in a very small sample of men undergoing RYGB.34 In summary, larger studies are required to determine whether and which hormonal changes play a role in the bone loss after various bariatric procedures.
Body composition and bone marrow fat
Muscle provides critical anabolic mechanical stimulus for bone tissue,93, 94 and muscle mass and strength are also important for physical function and avoidance of falls. Bariatric surgery results in loss of muscle mass,95, 96, 97, 98, 99, 100 although the relative loss of fat mass is greater than muscle.60, 94 Most muscle mass loss occurs in the first 6 postoperative months,94, 101 and after that, it is highly variable, with some patients experiencing muscle mass maintenance or gain and others continued loss.100 It is possible that absolute or relative decreases in muscle mass exacerbate decreases in bone mass and quality, whereas muscle improvements could mitigate the negative effects of bariatric surgery on bone. Indeed, a number of studies have reported that those with greater decline in lean mass have greater decline in aBMD by DXA35, 41, 51, 56, 61 or deterioration in microstructure by HR‐pQCT.35
The bone marrow is a depot for adipose tissue, but the physiological significance of bone marrow fat remains uncertain. Greater bone marrow fat is associated with lower bone mass,102, 103, 104, 105, 106 more rapid bone loss,107 and vertebral fracture.108 The regulation of marrow fat appears distinct from the regulation of other fat depots, as caloric restriction paradoxically increases marrow fat in mice,109 and women with anorexia nervosa have high marrow fat.110, 111 These findings have led to the proposal that if marrow fat increases with dramatic weight loss after bariatric surgery, that increase might be a mechanism for the postoperative decline in skeletal health. Three published studies have examined marrow fat after bariatric surgery, quantifying marrow fat with proton magnetic resonance spectroscopy (1H‐MRS).36, 37, 112 Ivaska and colleagues found no significant 6‐month change in vertebral marrow fat content in 21 participants (14 SG and 7 RYGB) undergoing bariatric surgery.37 Bredella and colleagues observed 12‐month increases in vertebral and femoral marrow fat in 10 participants undergoing SG but no changes in 11 undergoing RYGB.36 Kim and colleagues found that among women undergoing RYGB, vertebral marrow fat actually decreased over 6 months among those with diabetes (n = 13).111 Among those without diabetes (n = 12), marrow fat did not change on average, but those who lost more total body fat were more likely to have marrow fat increases. Further, marrow fat changes correlated with BMD decline, such that women with increases in marrow fat content had greater decreases in femoral neck aBMD by DXA and spinal vBMD by QCT. This finding suggests that while marrow fat cannot alone explain the decline in bone mass after bariatric surgery, it could contribute to negative skeletal effects.
Clinical Implications
The field of bone health after bariatric surgery is evolving rapidly. However, there are almost no randomized controlled trials on which the clinician can rely to optimize the management of patients before and after various bariatric procedures. There are a number of published guidelines that provide advice on how to manage the bariatric patient.113, 114, 115, 116 In addition to general guidelines, the American Society for Metabolic and Bariatric Surgery (ASMBS) issued a position statement specifically addressing metabolic bone changes after bariatric surgery.117 However, most recommendations are based on low‐quality evidence or expert opinion. The management approach we propose (Table 2) thus rests on personal opinion derived from available knowledge.
Table 2.
Strategies to Promote Bone Health Before and After Bariatric Surgery
| Before surgery | |
| Biochemical assessment | Measure 25OHD level and treat vitamin D deficiency |
| BMD assessment | DXA when indicated based on screening guidelines for general population Consider DXA in select additional patients Consider DXA forearm or QCT spine in select patients |
| After surgery | |
| Nutrition | Calcium (as citrate) to achieve total daily calcium intakes (diet + supplements): LAGB, SG, RYGB: Calcium 1200–1500 mg/d BPD‐DS: Calcium 1800–2400 mg/d Vitamin D3 3000 IU with titration to 25OHD level ≥30 ng/mL Protein 60–75 g/d |
| Biochemical assessment | Calcium, albumin, PTH, 25OHD every 6 months for 2 years, then annually 24‐hour urinary calcium if additional data are needed (eg, elevated PTH despite 25OHD at goal) |
| Exercise | Moderate aerobic physical activity (at least 150 min/week) plus strength training (2–3 times/week) |
| BMD assessment | DXA when indicated based on screening guidelines for general population Consider after 1–2 years in higher‐risk patients Consider DXA forearm or QCT spine in select patients |
LAGB = laparoscopic adjustable gastric banding; BPD‐DS = biliopancreatic diversion with duodenal switch; RYGB = Roux‐en‐Y gastric bypass; SG = sleeve gastrectomy.
Given that patients with obesity are at high risk of vitamin D deficiency, one should measure serum 25OHD and correct vitamin D deficiency preoperatively.114, 116 After all types of bariatric procedures, routine monitoring of serum 25OHD, calcium, albumin, and PTH levels is indicated. The recommended frequency of these measurements varies between guidelines.114, 116 A reasonable approach is to perform routine biochemical screening every 6 months for the first 2 years and then annually. Yet, testing frequency should be adjusted based on clinical grounds. Measurement of 24‐hour urinary calcium and of serum bone turnover markers may be useful at times.
After all bariatric procedures, sufficient calcium, vitamin D, and protein intake and adequate physical activity are recommended to mitigate negative impacts of the procedures on bone and muscle. Evidence that these measures may be effective collectively comes from a recent randomized controlled trial conducted by Muschitz and colleagues.53 The two‐arm trial tested a multimodal approach composed of preoperative vitamin D supplementation (28,000 IU of vitamin D3 per week for 8 weeks), then postoperatively, a combination of 28,000 IU of vitamin D3 per week, calcium citrate 1000 mg daily, BMI‐adjusted protein intake (35 to 60 g of protein daily), and a physical activity program (Nordik walking and strength training). The control group received no preoperative vitamin D, no postoperative supplementation with vitamin D, calcium, or protein, and no requirement for physical activity. Over 2 years, the multipronged intervention reduced—although did not prevent—the negative impact of RYGB and SG on bone turnover markers, aBMD by DXA, and lean mass. Additional trials will be needed to understand the relative importance of the individual components of the multimodal intervention, but the results are encouraging. In another recent study, a supervised weight‐bearing and aerobic program twice a week for 36 weeks also attenuated the decrease in BMD and lean mass seen after RYGB.118
Regarding vitamin D supplementation, the ASMBS guidelines suggest an initial dose of 3000 IU of vitamin D3 daily after LAGB, SG, and RYGB, with titration to serum 25OHD >30 ng/mL. Higher vitamin D doses are often required for BPD. Regarding calcium supplementation, calcium citrate is preferred over calcium carbonate, with 2 to 3 split doses to achieve a total daily calcium intake (from diet plus supplements) of 1200 to 1500 mg daily for LAGB, SG, and RYGB, and 1800 to 2400 mg daily for BPD.115, 116 Choice of calcium citrate is mainly based on a small study that reported higher bioavailability of calcium citrate compared with calcium carbonate after RYGB.119 As highlighted in the study by Schafer and colleagues,52 recommended calcium intake may not be sufficient for a substantial proportion of patients, at least after RYGB and possibly also after BPD, and thus monitoring with PTH and (when appropriate) 24‐hour urinary calcium is imperative.
The utility of DXA before and after bariatric surgery is debated. Indeed, DXA might underestimate fracture risk in obesity, and BMD changes by DXA may be inaccurate in the context of acute weight loss.38, 59, 120 Further, average age at bariatric surgery is the mid 40s, and in the majority of pre‐ and early postoperative patients, BMD is robust.121 As a result, there is a lack of agreement between guidelines about in whom and when BMD should be assessed in the bariatric population. We believe that DXA could be performed preoperatively in higher‐risk patients, including postmenopausal women, men aged >50 years, and those with risk factors for osteoporosis. It could also be considered postoperatively, perhaps after 2 years, in select patients. DXA of the distal one‐third of the forearm or QCT of the spine may be useful if a patient's weight exceeds the DXA table limit, although modern DXA scanners can accommodate increasingly heavy patients. Forearm scans may also be of interest in patients with secondary hyperparathyroidism, as this condition affects predominantly cortical bone. Moreover, spinal QCT may be useful if DXA results are difficult to interpret.
No study has assessed the effect of osteoporosis pharmacotherapy in the context of bone loss after bariatric surgery. In patients with a moderate or a high risk of fracture, antiresorptive agents may be envisaged after secondary causes of osteoporosis are addressed and only after vitamin D and calcium supplementation is deemed sufficient based on measurement of serum 25OHD, corrected calcium, PTH, and potentially 24‐hour urinary calcium. Indeed, this population is particularly at risk of severe hypocalcemia after administration of potent antiresorptive therapy.122 The parenteral route is preferred because of concerns about adequate absorption and potential anastomotic ulceration with oral bisphosphonates.
Research Agenda
Although research addressing bone health after bariatric surgery is growing, there are still many knowledge gaps to be filled. Questions remain unanswered about the nature of bariatric surgery's skeletal effects. These include questions about the impact of individual bariatric procedures on fracture risk; fracture risk among particular populations such as men, postmenopausal women, older adults in general, and adolescents; and long‐term postoperative changes in bone mass and microarchitecture. Now that SG and RYGB are the predominant bariatric procedures performed, it will be important for the fully informed decision‐making of patients and providers alike, as well as for effective postoperative care, to understand the relative effects of the two procedures on skeletal health. Future studies should include measures of bone quality and strive to minimize confounding in the radiographic assessment of BMD during marked weight loss. Questions also remain about the mechanisms underlying bone loss and fracture risk after bariatric surgery, as an understanding of mechanism is important for the development of appropriate preventive and treatment strategies. In particular, the roles of gut‐secreted hormones, marrow fat, and the gut microbiome should be investigated further. Finally, numerous clinical questions must be addressed. These include questions about the doses and types of calcium and vitamin D supplements to use based on type of surgery; recommended protein intake; and type and extent of physical activity to prescribe. Additional research is necessary to determine the best clinical use of DXA (or QCT) before or after bariatric surgery. Moreover, rigorous randomized controlled trials are needed to determine whether current osteoporosis pharmacologic therapies and emerging therapies such as anti‐sclerostin antibodies are effective and safe to treat osteoporosis in this population, and whether use of these agents in high‐risk patients without osteoporosis might mitigate the detrimental skeletal effects of bariatric surgery.
Disclosures
CG has received honoraria for conference and advisory board meetings from Mantra Pharma. ALS has received donations of dietary supplements for research studies from Bariatric Advantage and Tate & Lyle.
Acknowledgments
CG's research is supported by grants from Diabetes Canada, Diabète Québec, and Canadian Institutes of Health Research. ALS's research is supported by the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (R01 DK107629 and R21 DK112126).
Authors’ roles: CG and ALS both reviewed the literature and drafted, revised, and approved the manuscript.
References
- 1. Non‐communicable Disease Risk Factor Collaboration. Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017; 390:2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flegal KM, Kruszon‐Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016; 315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017; 27:2279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long‐term durability of weight loss. JAMA Surg. 2016; 151:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crawford MR, Pham N, Khan L, Bena JF, Schauer PR, Kashyap SR. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery vs. medical therapy at least 5 years. Endocr Pract. Epub 2017. Nov 16. [DOI] [PubMed]
- 6. Ikramuddin S, Korner J, Lee WJ, et al. Roux‐en‐Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013; 309:2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarkhosh K, Switzer NJ, El‐Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013; 23:414–23. [DOI] [PubMed] [Google Scholar]
- 8. Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long‐term cardiovascular events. JAMA. 2012; 307:56–65. [DOI] [PubMed] [Google Scholar]
- 9. Adams TD, Gress RE, Smith SC, et al. Long‐term mortality after gastric bypass surgery. N Engl J Med. 2007; 357:753–61. [DOI] [PubMed] [Google Scholar]
- 10. Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014; 29:1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014; 2:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hage MP, El‐Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux‐en‐Y gastric bypass. Osteoporos Int. 2014; 25:423–39. [DOI] [PubMed] [Google Scholar]
- 13. Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2013; 14:52–67. [DOI] [PubMed] [Google Scholar]
- 14. Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab. 2014; 16:1204–13. [DOI] [PubMed] [Google Scholar]
- 15. Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016; 77:28–37. [DOI] [PubMed] [Google Scholar]
- 16. Arapis K, Tammaro P, Parenti LR, et al. Long‐term results after laparoscopic adjustable gastric banding for morbid obesity: 18‐year follow‐up in a single university unit. Obes Surg. 2017; 27:630–40. [DOI] [PubMed] [Google Scholar]
- 17. Ramon JM, Salvans S, Crous X, et al. Effect of Roux‐en‐Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012; 16:1116–22. [DOI] [PubMed] [Google Scholar]
- 18. Diamantis T, Apostolou KG, Alexandrou A, Griniatsos J, Felekouras E, Tsigris C. Review of long‐term weight loss results after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014; 10:177–83. [DOI] [PubMed] [Google Scholar]
- 19. Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux‐en‐Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005; 90:359–65. [DOI] [PubMed] [Google Scholar]
- 20. Madsbad S, Holst JJ. GLP‐1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes. 2014; 63:3172–4. [DOI] [PubMed] [Google Scholar]
- 21. Nelson DW, Blair KS, Martin MJ. Analysis of obesity‐related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012; 147:847–54. [DOI] [PubMed] [Google Scholar]
- 22. Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F, Alexandrides TK. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis. 2013; 9:667–77. [DOI] [PubMed] [Google Scholar]
- 23. Kotidis EV, Koliakos G, Papavramidis TS, Papavramidis ST. The effect of biliopancreatic diversion with pylorus‐preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight‐reducing effect of this procedure? Obes Surg. 2006; 16:554–9. [DOI] [PubMed] [Google Scholar]
- 24. Rousseau C, Jean S, Gamache P, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case‐control study. BMJ. 2016; 354:i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu EW, Lee MP, Landon JE, Lindeman KG, Kim SC. Fracture risk after bariatric surgery: Roux‐en‐Y gastric bypass versus adjustable gastric banding. J Bone Miner Res. 2017; 32:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population‐based study. Osteoporos Int. 2014; 25:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012; 345:e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu CW, Chang YK, Chang HH, et al. Fracture risk after bariatric surgery: a 12‐year nationwide cohort study. Medicine (Baltimore). 2015; 94:e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med. 2015; 12:e1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tothill P, Hannan WJ, Cowen S, Freeman CP. Anomalies in the measurement of changes in total‐body bone mineral by dual‐energy X‐ray absorptiometry during weight change. J Bone Miner Res. 1997; 12:1908–21. [DOI] [PubMed] [Google Scholar]
- 31. Van Loan MD. Is dual‐energy X‐ray absorptiometry ready for prime time in the clinical evaluation of body composition? Am J Clin Nutr. 1998; 68:1155–6. [DOI] [PubMed] [Google Scholar]
- 32. Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013; 98:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu EW, Bouxsein ML, Putman MS, et al. Two‐year changes in bone density after Roux‐en‐Y gastric bypass surgery. J Clin Endocrinol Metab. 2015; 100:1452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crawford MR, Pham N, Khan L, Bena JF, Schauer PR, Kashyap SR. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery vs. medical therapy at least 5 years. J Bone Miner Res. Epub 2017. Dec 27. [DOI] [PubMed] [Google Scholar]
- 35. Shanbhogue VV, Stoving RK, Frederiksen KH, et al. Bone structural changes after gastric bypass surgery evaluated by HR‐pQCT: a two‐year longitudinal study. Eur J Endocrinol. 2017; 176:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux‐en‐Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017; 95:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017; 95:47–54. [DOI] [PubMed] [Google Scholar]
- 38. Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012; 27:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu C, Wu D, Zhang JF, et al. Changes in bone metabolism in morbidly obese patients after bariatric surgery: a meta‐analysis. Obes Surg. 2016; 26:91–7. [DOI] [PubMed] [Google Scholar]
- 40. Yu EW, Wewalka M, Ding SA, et al. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J Clin Endocrinol Metab. 2016; 101:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muschitz C, Kocijan R, Marterer C, et al. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015; 100:891–901. [DOI] [PubMed] [Google Scholar]
- 42. Frederiksen KD, Hanson S, Hansen S, et al. Bone structural changes and estimated strength after gastric bypass surgery evaluated by HR‐pQCT. Calcif Tissue Int. 2016; 98:253–62. [DOI] [PubMed] [Google Scholar]
- 43. Stemmer K, Bielohuby M, Grayson BE, et al. Roux‐en‐Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology. 2013; 154:2015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abegg K, Gehring N, Wagner CA, et al. Roux‐en‐Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol. 2013; 305:R999–R1009. [DOI] [PubMed] [Google Scholar]
- 45. Canales BK, Schafer AL, Shoback DM, Carpenter TO. Gastric bypass in obese rats causes bone loss, vitamin D deficiency, metabolic acidosis, and elevated peptide YY. Surg Obes Relat Dis. 2014; 10:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marceau P, Biron S, Lebel S, et al. Does bone change after biliopancreatic diversion? J Gastrointest Surg. 2002; 6:690–8. [DOI] [PubMed] [Google Scholar]
- 47. Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burckhardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: potential role of serum C telopeptides for follow‐up. Int J Obes (Lond). 2005; 29:1429–35. [DOI] [PubMed] [Google Scholar]
- 48. Riedl M, Vila G, Maier C, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008; 93:2307–12. [DOI] [PubMed] [Google Scholar]
- 49. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004; 53:918–21. [DOI] [PubMed] [Google Scholar]
- 50. Carrasco F, Basfi‐Fer K, Rojas P, et al. Changes in bone mineral density after sleeve gastrectomy or gastric bypass: relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes Surg. 2014; 24:877–84. [DOI] [PubMed] [Google Scholar]
- 51. Maghrabi AH, Wolski K, Abood B, et al. Two‐year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring). 2015; 23:2344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015; 30:1377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muschitz C, Kocijan R, Haschka J, et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS Study. J Bone Miner Res. 2016; 31:672–82. [DOI] [PubMed] [Google Scholar]
- 54. Jones G, Nguyen T, Sambrook PN, Kelly PJ, Eisman JA. A longitudinal study of the effect of spinal degenerative disease on bone density in the elderly. J Rheumatol. 1995; 22:932–6. [PubMed] [Google Scholar]
- 55. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004; 12:40–7. [DOI] [PubMed] [Google Scholar]
- 56. Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004; 89:1061–5. [DOI] [PubMed] [Google Scholar]
- 57. Johnson JM, Maher JW, DeMaria EJ, Downs RW, Wolfe LG, Kellum JM. The long‐term effects of gastric bypass on vitamin D metabolism. Ann Surg. 2006; 243:701–4; discussion 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008; 93:3735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yu EW, Bouxsein ML, Roy AE, et al. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014; 29:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009; 19:41–6. [DOI] [PubMed] [Google Scholar]
- 61. Vilarrasa N, San Jose P, Garcia I, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3‐year follow‐up. Obes Surg. 2011; 21:465–72. [DOI] [PubMed] [Google Scholar]
- 62. Yu E, Greenblatt LB, Lindeman K, Rourke C, Bouxsein M, Finkelstein J. Longitudinal 5‐year changes in bone density and microarchitecture after Roux‐en‐Y gastric bypass. J Bone Miner Res. 2017; 32(Suppl 1). Available at: http://www.asbmr.org/education/AbstractDetail?aid=6200c148-355f-4ba1-9aff-86a5b6eb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fuchs HF, Broderick RC, Harnsberger CR, et al. Benefits of bariatric surgery do not reach obese men. J Laparoendosc Adv Surg Tech A. 2015; 25:196–201. [DOI] [PubMed] [Google Scholar]
- 64. Hsin MC, Huang CK, Tai CM, Yeh LR, Kuo HC, Garg A. A case‐matched study of the differences in bone mineral density 1 year after 3 different bariatric procedures. Surg Obes Relat Dis. 2015; 11:181–5. [DOI] [PubMed] [Google Scholar]
- 65. Dixon JB, Strauss BJ, Laurie C, O'Brien PE. Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity (Silver Spring). 2007; 15:1187–98. [DOI] [PubMed] [Google Scholar]
- 66. Nogues X, Goday A, Pena MJ, et al. [Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass]. Cir Esp. 2010; 88:103–9. [DOI] [PubMed] [Google Scholar]
- 67. Pluskiewicz W, Buzga M, Holeczy P, Bortlik L, Smajstrla V, Adamczyk P. Bone mineral changes in spine and proximal femur in individual obese women after laparoscopic sleeve gastrectomy: a short‐term study. Obes Surg. 2012; 22:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vilarrasa N, de Gordejuela AG, Gomez‐Vaquero C, et al. Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy. Obes Surg. 2013; 23:2086–91. [DOI] [PubMed] [Google Scholar]
- 69. Ruiz‐Tovar J, Oller I, Priego P, et al. Short‐ and mid‐term changes in bone mineral density after laparoscopic sleeve gastrectomy. Obes Surg. 2013; 23:861–6. [DOI] [PubMed] [Google Scholar]
- 70. Sornay‐Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007; 22:425–33. [DOI] [PubMed] [Google Scholar]
- 71. Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009; 56:1105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chakhtoura MT, Nakhoul NN, Shawwa K, Mantzoros C, El Hajj Fuleihan GA. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016; 65:574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000; 72:690–3. [DOI] [PubMed] [Google Scholar]
- 74. Dagan SS, Zelber‐Sagi S, Webb M, et al. Nutritional status prior to laparoscopic sleeve gastrectomy surgery. Obes Surg. 2016; 26:2119–26. [DOI] [PubMed] [Google Scholar]
- 75. Wei JH, Lee WJ, Chong K, et al. High incidence of secondary hyperparathyroidism in bariatric patients: comparing different procedures. Obes Surg. 2018; 28(3):798–804. [DOI] [PubMed] [Google Scholar]
- 76. Wolf E, Utech M, Stehle P, Busing M, Stoffel‐Wagner B, Ellinger S. Preoperative micronutrient status in morbidly obese patients before undergoing bariatric surgery: results of a cross‐sectional study. Surg Obes Relat Dis. 2015; 11:1157–63. [DOI] [PubMed] [Google Scholar]
- 77. Sanchez A, Rojas P, Basfi‐Fer K, et al. Micronutrient deficiencies in morbidly obese women prior to bariatric surgery. Obes Surg. 2016; 26:361–8. [DOI] [PubMed] [Google Scholar]
- 78. de Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Cabezas G. Micronutrient status in morbidly obese women before bariatric surgery. Surg Obes Relat Dis. 2013; 9:323–7. [DOI] [PubMed] [Google Scholar]
- 79. Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux‐en‐Y gastric bypass surgery. Obesity (Silver Spring). 2006; 14:1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tardio V, Blais JP, Julien AS, et al. Serum parathyroid hormone and 25‐hydroxyvitamin D concentrations before and after biliopancreatic diversion. Obes Surg. 2018; 10. doi: 10.1007/s11695‐017‐3101‐z. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 81. Nicoletti CF, Morandi Junqueira‐Franco MV, dos Santos JE, Marchini JS, Salgado W Jr, Nonino CB. Protein and amino acid status before and after bariatric surgery: a 12‐month follow‐up study. Surg Obes Relat Dis. 2013; 9:1008–12. [DOI] [PubMed] [Google Scholar]
- 82. Sukumar D, Ambia‐Sobhan H, Zurfluh R, et al. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res. 2011; 26:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bavaresco M, Paganini S, Lima TP, et al. Nutritional course of patients submitted to bariatric surgery. Obes Surg. 2010; 20:716–21. [DOI] [PubMed] [Google Scholar]
- 84. Moize V, Geliebter A, Gluck ME, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux‐en‐Y gastric bypass. Obes Surg. 2003; 13:23–8. [DOI] [PubMed] [Google Scholar]
- 85. Chou JJ, Lee WJ, Almalki O, Chen JC, Tsai PL, Yang SH. Dietary intake and weight changes 5 years after laparoscopic sleeve gastrectomy. Obes Surg. 2017; 27:3240–6. [DOI] [PubMed] [Google Scholar]
- 86. Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987; 219:1–9. [DOI] [PubMed] [Google Scholar]
- 87. Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990; 5:843–50. [DOI] [PubMed] [Google Scholar]
- 88. Kazakia GJ, Tjong W, Nirody JA, et al. The influence of disuse on bone microstructure and mechanics assessed by HR‐pQCT. Bone. 2014; 63:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long‐duration spaceflight. J Bone Miner Res. 2004; 19:1006–12. [DOI] [PubMed] [Google Scholar]
- 90. Bruno C, Fulford AD, Potts JR, et al. Serum markers of bone turnover are increased at six and 18 months after Roux‐en‐Y bariatric surgery: correlation with the reduction in leptin. J Clin Endocrinol Metab. 2010; 95:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vilarrasa N, Gomez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009; 19:860–6. [DOI] [PubMed] [Google Scholar]
- 92. Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity‐associated hypogonadotropic hypogonadism: a systematic review and meta‐analysis. Eur J Endocrinol. 2013; 168:829–43. [DOI] [PubMed] [Google Scholar]
- 93. Bonewald LF, Kiel DP, Clemens TL, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2013; 28:1857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Frost HM. Muscle, bone, and the Utah paradigm: a 1999 overview. Med Sci Sports Exerc. 2000;32:911–7. [DOI] [PubMed] [Google Scholar]
- 95. Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one‐year follow‐up. Obes Surg. 2006; 16:1602–8. [DOI] [PubMed] [Google Scholar]
- 96. Hue O, Berrigan F, Simoneau M, et al. Muscle force and force control after weight loss in obese and morbidly obese men. Obes Surg. 2008; 18:1112–8. [DOI] [PubMed] [Google Scholar]
- 97. Miller GD, Nicklas BJ, You T, Fernandez A. Physical function improvements after laparoscopic Roux‐en‐Y gastric bypass surgery. Surg Obes Relat Dis. 2009; 5:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zalesin KC, Franklin BA, Lillystone MA, et al. Differential loss of fat and lean mass in the morbidly obese after bariatric surgery. Metab Syndr Relat Disord. 2010; 8:15–20. [DOI] [PubMed] [Google Scholar]
- 99. Stegen S, Derave W, Calders P, Van Laethem C, Pattyn P. Physical fitness in morbidly obese patients: effect of gastric bypass surgery and exercise training. Obes Surg. 2011; 21:61–70. [DOI] [PubMed] [Google Scholar]
- 100. Lyytinen T, Liikavainio T, Paakkonen M, Gylling H, Arokoski JP. Physical function and properties of quadriceps femoris muscle after bariatric surgery and subsequent weight loss. J Musculoskelet Neuronal Interact. 2013; 13:291–300. [PubMed] [Google Scholar]
- 101. Tamboli RA, Hossain HA, Marks PA, et al. Body composition and energy metabolism following Roux‐en‐Y gastric bypass surgery. Obesity (Silver Spring). 2010; 18:1718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteoporos Rep. 2011; 9:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cohen A, Dempster DW, Stein EM, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2012; 97:2782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971; 80:147–54. [DOI] [PubMed] [Google Scholar]
- 105. Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001; 22:1620–7. [PMC free article] [PubMed] [Google Scholar]
- 106. Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI‐measured bone marrow adipose tissue is inversely related to DXA‐measured bone mineral in Caucasian women. Osteoporos Int. 2007; 18:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Griffith JF, Yeung DK, Leung JC, Kwok TC, Leung PC. Prediction of bone loss in elderly female subjects by MR perfusion imaging and spectroscopy. Eur Radiol. 2011; 21:1160–9. [DOI] [PubMed] [Google Scholar]
- 108. Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013; 98:2294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Devlin MJ, Cloutier AM, Thomas NA, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010; 25:2078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009; 94:2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fazeli PK, Bredella MA, Freedman L, et al. Marrow fat and preadipocyte factor‐1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012; 27:1864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim TY, Schwartz AV, Li X, et al. Bone marrow fat changes after gastric bypass surgery are associated with loss of bone mass. J Bone Miner Res. 2017; 32:2239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mechanick JI, Youdim A, Jones DB, et al Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient––2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013; 9:159–91. [DOI] [PubMed] [Google Scholar]
- 114. Isom KA, Andromalos L, Ariagno M, et al. Nutrition and metabolic support recommendations for the bariatric patient. Nutr Clin Pract. 2014; 29:718–39. [DOI] [PubMed] [Google Scholar]
- 115. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post‐bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010; 95:4823–43. [DOI] [PubMed] [Google Scholar]
- 116. Parrott J, Frank L, Rabena R, Craggs‐Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: micronutrients. Surg Obes Relat Dis. 2017; 13:727–41. [DOI] [PubMed] [Google Scholar]
- 117. Kim J, Brethauer S, American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. Metabolic bone changes after bariatric surgery. Surg Obes Relat Dis. 2015; 11:406–11. [DOI] [PubMed] [Google Scholar]
- 118. Campanha‐Versiani L, Pereira DAG, Ribeiro‐Samora GA, et al. The effect of a muscle weight‐bearing and aerobic exercise program on the body composition, muscular strength, biochemical markers, and bone mass of obese patients who have undergone gastric bypass surgery. Obes Surg. 2017; 27:2129–37. [DOI] [PubMed] [Google Scholar]
- 119. Tondapu P, Provost D, Adams‐Huet B, Sims T, Chang C, Sakhaee K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux‐en‐Y gastric bypass. Obes Surg. 2009; 19:1256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010; 25:292–7. [DOI] [PubMed] [Google Scholar]
- 121. Scibora LM, Ikramuddin S, Buchwald H, Petit MA. Examining the link between bariatric surgery, bone loss, and osteoporosis: a review of bone density studies. Obes Surg. 2012; 22:654–67. [DOI] [PubMed] [Google Scholar]
- 122. Rosen CJ, Brown S. Severe hypocalcemia after intravenous bisphosphonate therapy in occult vitamin D deficiency. N Engl J Med. 2003; 348:1503–4. [DOI] [PubMed] [Google Scholar]


