Abstract
Background:
Grape exosome-like nanovesicles (GELNs) have the advantage of inherent biocompatibility and biodegradability, the potential to be used as oral delivery vehicles. The objective of this research was to evaluate the efficiency of Syrah GELN purification and their effects on the intestinal stem cells when orally administrated to the rats.
Materials and Methods:
In this experimental study, Syrah GELN isolated by differential centrifugation and sucrose gradient ultracentrifugation method, then the concentration of protein, size, and zeta potential were measured as well as nanoparticles morphology. The stability of nanoparticles was investigated in the solution that mimicked the condition encountered in the stomach and intestine. To demonstrate transfection efficiency of intestinal stem cells, real-time PCR was carried out using rat leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5)-specific primers on cDNA derived from total RNA extracted from the upper part of the small intestine of GELN-treated rats and their controls.
Results:
The mean size, zeta potential, and concentration of nanoparticles were 205.1 nm, −12.5 mV, and 250 μg/ml, respectively. The result of stability test demonstrated that Syrah GELN were resistant to the harsh environment of the stomach. Lgr5 gene expression was increased by tenfold in GELN-treated rats compared with the controls.
Conclusions:
As intestinal stem cells are poorly accessible by common exogenous agents in vivo, oral delivery of GELNs provides a new approach to modulate the stem cell microenvironment for intestinal remodeling. This novel and effective method would help to overcome conditions such as inflammatory bowel disease, colorectal cancer, and applicable in regenerative medicine.
Keywords: Grape exosome-like nanoparticles, intestinal stem cell, leucine-rich repeat-containing G-protein-coupled receptor 5
Introduction
Maintenance and repair of adult tissues depend on the stem cells residing in that tissue. As a definition, stem cells are cells that preserve themselves over long periods of time (self-renewal) and producing all differentiated cell types of that tissue (multipotency).[1,2] Mature stem cells can potentially follow three cell division patterns: symmetric division for the production of 2 stem cells, asymmetric division for the production of 1 stem cell and 1 transit amplifying (TA) cell, or symmetric division for the production of 2 TA cells.[1] At approximately 95% of the time, a stem cell divides asymmetrically, resulting in one stem cell and one TA cell.[3] In the small intestine, the stem cells are believed to reside at position +4, relative to the bottom of the crypt, with positions 1–3 occupied by Paneth cells. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) is a candidate stem cell marker when it was found to be selectively expressed in a tiny population of slender, morphologically immature cells interspersed with the Paneth cells at the very crypt base. Each crypt contains about 10–15 Lgr5+ cells, of which 10% are in the +4 position. The cell cycle of the intestinal stem cell is slow and continuous; producing cells that are rapidly differentiated, and after epithelial damage, they rebuild it and so the differentiated cells migrate along the crypt-villus axis.[4,5] Evidence suggests that Wnt signaling pathway is the only dominant power in controlling the fate of a cell in the crypt-villus axis and nuclear β-catenin is observed as a Wnt signaling characteristic across the crypt. In adult mice, the control of the Wnt receptor complex by Dickkopf-1 causes complete crypt loss.[1] Achieving the intestinal stem cell to deliver the drug or nucleic acid due to obstacles such as the mucosal endothelium of the intestine and intestinal enzymes and inaccessible is difficult, and finding a way to deliver nucleic acids or drug is important and can lead to many studies in the field of gene therapy in these cells. Exosomes are specialized membranous Nano-sized vesicles released from a variety of cells.[6,7,8,9,10,11] Exosomes can carry a range of proteins, lipids, mRNAs, and/or microRNAs and can transfer their cargo to target cells and regulate gene expression of target mRNAs through de novo translation and posttranslational modification[1] However, more specifically, there is no information about the role of Syrah exosome in expression and the amount of expression of Lgr5 and also its effect on liver and kidney markers. In this study, we demonstrate that Syrah grape exosome-like nanoparticles (GELNs) can pass the intestinal mucus barrier, and rat intestinal stem cells can take up GELNs and through the Wnt/β-catenin pathway causes significant induction expression of Lgr5+ intestinal stem cells.
Materials and Methods
This experimental study includes preparation of Syrah GELNs and in vivo study in rats.
Isolation and purification of grape exosome-like nanoparticles
The fresh Syrah grape was purchased from a local market and washed three times with water in a plastic basket. After the final washing, grape skin removed manually and then the grape extract was collected by squeezing and then diluted 1:1 with cold phosphate-buffered saline (PBS). The Syrah grape juice was then differentially centrifuged at 1000 g for 10 min, 3500 g for 30 min, and 10,000 g for 60 min at 4°C. After 10,000 g centrifugation, the supernatant was then centrifuged at 100,000 g for 90 min, the pellet was resuspended in cold PBS and transferred to a sucrose step gradient (8%, 30%, 45%, and 60%) to isolate and purify the exosome-like nanoparticles and centrifuged at 150,000 g for 120 min at 4°C. The bands between the 30% and 45% layers were harvested and noted as GELNs. Immediately after being washed, sucrose-purified pellets of GELNs were weighed and then suspended in cold PBS and saved as mg of GELNs/ml of PBS. The protein concentration of the samples was determined using the Bio-Rad Protein Quantitation Assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard.
Particle size and surface charge analysis
The sizes and zeta potentials of GELNs can be determined by dynamic light scattering.[12] The average diameter of the nanoparticles is considered as a key parameter for the therapeutic efficacy of nanoparticles. Nano-size GELNs also increase the possibility of DNA or drug transfer to the bottom of intestinal crypts and improve access to the intestinal stem cells. For this purpose, GELNs were diluted with cold PBS and then the size and zeta potential were measured using Zetasizer Nano ZS (ZE3600, Malvern Instruments) and Microtrac (Microtrac ZETA-check, USA).
Atomic force microscopy
GELNs were prepared for AFM using a conventional procedure.[13] In brief, the GELNs were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 4 h, at 4°C. After extensively washing with 0.1 M cacodylate buffer (pH 7.4), GELNs were fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.4) for 1 h at 4°C and dehydrated in a graded ethanol (25% for 20 min, 50% for 20 min, 75% for 20 min, 95% for 20 min, 100% for 30 min, and 100% for 30 min). The GELNs were then examined using an AFM scope (NanoWizard II JPK, Germany).
In vitro stability of Syrah grape exosome-like nanoparticles
Conditions of digestion were performed in vitro based on a previous description.[14] In brief, 1 mL of Syrah GELNs in a water solution was incubated at 37°C for 60 min after the addition of 1.34 mL of 18.5% w/v HCl and 24 mL of a pepsin solution (80 mg/mL in 0.1N of HCl, pH 2.0, Sigma) to form a stomach-like solution. Then, 80 mL of a mixture containing 24 mg/mL of bile extract and 4 mg/mL of pancreatin (Sigma) in 0.1M of NaHCO3 was added to the stomach-like solution. The pH of the bulk solution was adjusted to 6.5 with 1M NaHCO3, which was referred to as an intestinal solution. Syrah GELNs were incubated for an additional 60 min in the intestinal solution. The stability of Syrah GELNs was evaluated by measuring particle size and surface charge.
In vivo up taking grape exosome-like nanoparticles
Male Sprague-Dawley rats of 200 g weight were obtained from Pasteur Institute of Iran. All animal procedures were approved by the Isfahan University of Medical Science Institutional Animal Care. Three animals were placed in each cage under the control of room temperature and humidity with a dark light cycle of 12:12 h in the instructions of Isfahan University of Medical Sciences and were monitored as feed by formal rodent chew. Ten rats were divided into two groups of A and B. Group A received gavage administering of 0.5 mg GELN/Rat in 1 ml PBS daily continued for 2 weeks as a case and Group B received 1 ml PBS daily continued for 2 weeks as a control group. Then, 15 days after gavage, the rats were killed so that at the last night, rats were placed under a diet of water to completely drain and wash their stomachs and intestines.
RNA extraction and real-time-polymerase chain reaction
We used real-time-PCR to confirm whether GELNs were able to penetrate the intestinal mucosa barrier and take up by rat intestinal and enter the stem cells and cause signifcant induction of Lgr5+ intestinal stem cells through the Wnt/β-catenin pathway. For this purpose, initial 3 cm of the small intestine was dissected. The tissue was homogenized under liquid nitrogen and the entire RNA was isolated by TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to acid guanidium thiocyanate–phenol–chloroform method.[14] Then, cDNA was synthesized from 5 μg of RNA using kit (cDNA synthesis kit, Thermo scientific, USA) according to the manufacturer's instructions. Lgr5 was amplified from the cDNA by real-time PCR. The PCR conditions consisted of 5 min at 95°C one cycle, 30 s at 95°C, 30 s at 55°C, 30 s at 72°C, 35 cycles and 5 min at 72°C. The primer sequences were F-CTCCCAGGTCTGGTGTGTTG and R-GAGGTCTAGGTAGGAGGTGAAG for Lgr5; and F-TGCCAAGTGGGTGGTATAGAG and R-TGGGATGGTGGGTGTAAGAG for β-actin as a housekeeping gene.
Aspartate transaminase and alanine transaminase measurement
To test for hepatotoxicity, levels of aspartate transaminase (ALT) and alanine transaminase (AST) activity of fasting blood were measured in two groups of test and control group on the first day as the day of intervention and days 8 and 15 after intervention using BT 3000 autoanalyzer (Biotechnica, Italy).
Blood urea nitrogen and creatinine measurement
To test for kidney function and damage after intervention with GELNs, levels of blood urea nitrogen (BUN) and creatinine activity of fasting blood were measured in two groups of test and control group on the 1st day as the day of intervention and days 8 and 15 after intervention using BT 3000 autoanalyzer (Biotechnica, Italy).
Statistical analysis
All tests were performed three times. Statistical analysis was performed using SPSS software (IBM, New York, NY, USA) version 20. For evaluating differences among the groups, Student paired t-test was performed. The data were represented as mean ± standard deviation statistical significance was defined as P < 0.05.
Results
Characterization of grape exosome-like nanoparticless
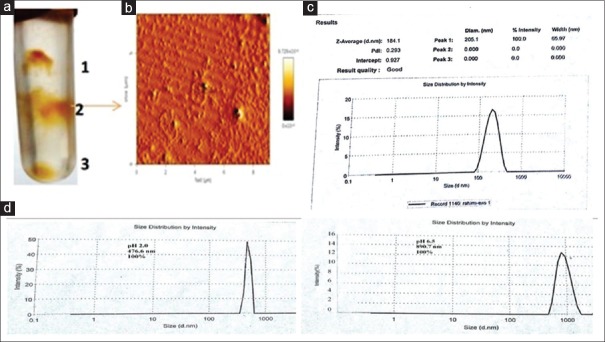
GELNs were isolated from fresh Syrah grape using a sucrose gradient centrifugation method. The majority of the GELNs accumulated at the 30% to 45% interface (band 2) [Figure 1a] of the sucrose gradient. The concentration of protein in band 2 was 250 μg/ml. GELNs integrity and size were evaluated by AFM [Figure 1b] and a nano zeta sizer [Figure 1c]. The results showed that the size distribution of the nanoparticles of isolated GELNs ranged from 91.28 to 712.4 nm in diameter, with an average diameter of 205.1 nm. Zeta potential measurements indicated that grape nanoparticles had a negative zeta potential value ranging from −11.2 mV to −13.8 mV.
Figure 1.
Identification and characterization of Syrah grape-derived nanoparticles (GELNs). (a) Three bands from sucrose-banded Syrah grape samples were formed after gradient ultracentrifugation. (b) GELN particles were visualized by atomic force microscopy. (c) Size distribution of the particles were determined using a ZetasizerNano ZS. (d) Syrah GELN was incubated in a stomach-like solution (left panel) for 60 min at 37°C and subsequently in small intestinal-like solution (right panel) for an additional 60 min at 37°C. Size distribution were measured by Zetasizer Nano ZS results
Stability
To test the stability of Syrah grape nanoparticles under physiological conditions, we mimicked in vivo conditions by suspending Syrah grape nanoparticles in a stomach-like solution (pH 2.0) or a small intestinal-like solution (pH 6.5). The results showed that compared to the size of Syrah grape nanoparticles in PBS [Figure 1d], the diameter of Syrah grape nanoparticles was increased in a stomach-like solution and was further enlarged in a small intestine like solution. Moreover, the Syrah grape nanoparticles surface charge in a stomach-like solution changed from negative to a positive charge (−13.8 mV change to 2.5 mV), whereas in a small intestine-like solution, the Syrah grape nanoparticles shifted back from a positive to a negative charged surface (−12.9 mV).
Intestinal stem cells take up grape exosome-like nanoparticles
The results of real-time-PCR demonstrated that oral administration with Syrah grape nanoparticles enhanced the expression of LGR5 gene that serves as markers of the intestinal stem cells [Figure 2]. Given that the Lgr5+ intestinal stem cells are at the end of the crypt, then we can conclude that Syrah grape nanoparticles can go to the bottom of the intestine crypt and penetrate to the epithelial mucosal and take up by the intestinal stem cells.
Figure 2.
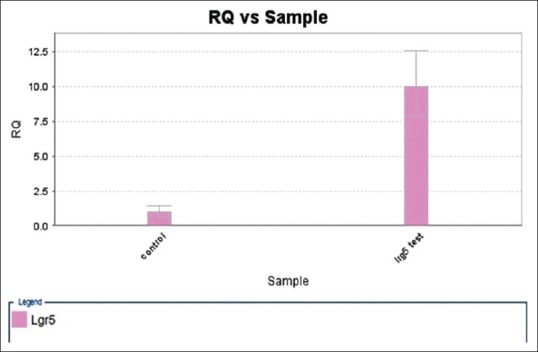
Real-time analysis of mRNA expression of Lgr5 gene in the small intestine from Male Sprague-Dawley rats. Values are mean ± standard deviation. The significant level of comparison between the two groups was P = 0.001
The effect of oral administration of Syrah grape nanoparticles on hepatotoxicity
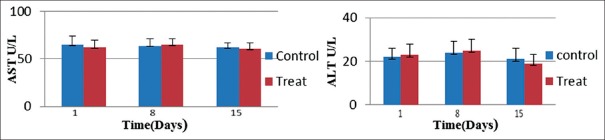
ALT and AST are two of the most useful measures of liver cell injury[15] that performed in this study. Analysis of data by SPSS showed that statistically, there was no significant difference between the two groups receiving GELNs and the PBS group (P > 0.05) [Figure 3]. Furthermore, the AST/ALT ratio that is useful in medical diagnosis to differentiate between causes of liver damage or hepatotoxicity[16] in treated group was 2.80 and in control group was 2.85.
Figure 3.
Levels of serum alanine transaminase (left) and aspartate transaminase (right) in the treat group oral administration of Syrah grape-like exosome nanoparticles compared with control (phosphate-buffered saline treated) group. Values are mean ± standard deviation
The effect of oral administration of Syrah grape nanoparticles on the levels of serum BUN and creatinine.
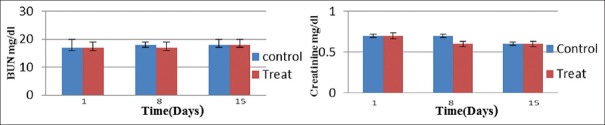
As BUN and Cr are well-recognized biomarkers for estimation of kidney function and damage,[17] that performed in this study. Analysis of data by SPSS showed that statistically, there is no significant difference between the two groups receiving GELNs and the PBS group (P > 0.05) [Figure 4].
Figure 4.
Levels of serum blood urea nitrogen (left) and creatinine (right) in the treat group oral administration of Syrah grape-like exosome nanoparticles compared with control (phosphate-buffered saline treated) group. Values are mean ± standard deviation
Discussion
In this study, we extracted exosome-like nanoparticles of Syrah grape using a sucrose gradient centrifugation method and then confirmed with conventional methods for exosome approval including assessment of concentration of protein, particle size and surface charge analysis, and AFM[18] Then, we tested the stability of Syrah grape nanoparticle with mimicked solution stomach-like solution (pH 2.0) and small intestinal-like solution (pH 6.5). Also in this study, we demonstrated that Syrah grape nanoparticle can travel within the gut, migrate through the intestinal mucus, be taken up by rat intestinal stem cells, and subsequently promote the proliferation of Lgr5+ intestinal stem cells. In this study, the exosomal group that received Syrah grape nanoparticle showed a tenfold increase in proliferation of Lgr5+ intestinal stem cells in comparison with the group that received PBS. Ju et al. reported threefold increase in expression of Lgr5 intestinal stem cell C57BL/6j mice after seven days treatment with 2 mg grape exosome-like nanoparticle in 200 μl PBS compared to PBS-treated mice.[19] The expression level of Lgr5 versus control group in Ju et al. study does not match with our study, which may be attributed to the difference in the type of grape used in two studies.
In this study, we investigated the effect of Syrah grape nanoparticles on liver and kidney markers such as AST, ALT, BUN, and creatinine. No liver and kidney toxicity effect of Syrah grape nanoparticles was observed. In addition, we indicated that edible Syrah grape nanoparticles are highly resistant to digestion by both gastric pepsin solution and intestinal pancreatic and bile extract solution. Significant amounts of Syrah grape nanoparticles are taken up by Lgr5+ intestinal stem cells. Mu and Wang in two separate studies showed that edible plant nanoparticles are highly resistant to digestion by both gastric pepsin solution and intestinal pancreatic and bile extract solution. Significant amounts of edible plant nanoparticles are taken up by intestinal macrophages.[12,20] Oral delivery is the most reasonable and acceptable route of administration, especially when the target cell is in the gastrointestinal tract.[21]
Conclusion
Regarding the role of exosomes in inducing intestinal stem cells and resistant to digestion by both gastric pepsin solution and intestinal pancreatic and bile extract solution and also safety, cheap, and most importantly, inherent biocompatibility and biodegradability could be a good option for nucleic acid and drug delivery in intestinal stem cells. Then can provide new approach for the treatment of colorectal cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–96. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Gil-Sanchis C, Cervelló I, Mas A, Faus A, Pellicer A, Simón C, et al. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) as a putative human endometrial stem cell marker. Mol Hum Reprod. 2013;19:407–14. doi: 10.1093/molehr/gat014. [DOI] [PubMed] [Google Scholar]
- 3.Yatabe Y, Tavaré S, Shibata D. Investigating stem cells in human colon by using methylation patterns. Proc Natl Acad Sci U S A. 2001;98:10839–44. doi: 10.1073/pnas.191225998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt GH, Winton DJ, Ponder BA. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988;103:785–90. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- 5.Roth KA, Hermiston ML, Gordon JI. Use of transgenic mice to infer the biological properties of small intestinal stem cells and to examine the lineage relationships of their descendants. Proc Natl Acad Sci U S A. 1991;88:9407–11. doi: 10.1073/pnas.88.21.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–20. [PubMed] [Google Scholar]
- 7.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 8.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang S, et al. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006;176:7385–93. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–85. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 11.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 12.Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–73. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo P. RNA nanotechnology: Methods for synthesis, conjugation, assembly and application of RNA nanoparticles. Methods. 2011;54:201–3. doi: 10.1016/j.ymeth.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. 2003;79:307–12. doi: 10.1136/pmj.79.932.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyblom H, Björnsson E, Simrén M, Aldenborg F, Almer S, Olsson R, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26:840–5. doi: 10.1111/j.1478-3231.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsue Y, van der Meer P, Damman K, Metra M, O’Connor CM, Ponikowski P, et al. Blood urea nitrogen-to-creatinine ratio in the general population and in patients with acute heart failure. Heart. 2017;103:407–13. doi: 10.1136/heartjnl-2016-310112. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345–57. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522–34. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momenzadeh S, Sadeghi A, Vatandoust N, Salehi R. Evaluation of in vivo transfection efficiency of eudragit coated nanoparticles of chitosan-DNA: A pH-sensitive system prepared for oral DNA delivery. Iran Red Crescent Med J. 2015;17:e16761. doi: 10.5812/ircmj.17(4)2015.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]