Abstract
Context:
Vitiligo is a psychosocial problem which significantly affects quality of life in Indian scenario.
Aims:
The purpose of this study was to compare the changes in quality of life in patients of vitiligo before and after treatment with narrowband ultraviolet B (NBUVB) phototherapy.
Subjects and Methods:
A total of 54 patients had completed the study. The age ranged between 16 and 70 years with a mean age of 26.77±14.2 years. The initial dose of NBUVB was 300 mJ/cm2 in adults and 150 mJ/cm2 in children twice weekly with 20% dose increment on subsequent visits. It was given for a maximum period of 6 months and was followed up for another 6 months to determine stability of repigmentation.
Results:
The average number of exposure given to the patients was 45.63±12.74 while the mean irradiation cumulative dose was 39.8 J/cm2. Mean Dermatology Life Quality Index (DLQI) of the vitiligo patients was 8.64±4.32 while those patients with acrofacial vitiligo had a mean DLQI of 11.78±5.61. After treatment with NBUVB, mean DLQI of all vitiligo patients was significantly reduced to 5.86±2.15 (P<0.01).
Conclusions:
This study showed that phototherapy had a positive therapeutic outcome in vitiligo, especially in younger patients. Even a small, depigmented lesion in a child could be psychosocially devastating.
Keywords: DLQI, NBUVB, phototherapy, vitiligo
Introduction
Vitiligo is caused by selective destruction of epidermal melanocytes resulting in the formation of well-defined depigmented patches. It has a global prevalence of 0.1%–8%.[1]
Parrish and Jaenicke in 1981 first found that a wavelength of 311-nm ultraviolet B (UVB) radiation was most effective for treating psoriasis.[2]
Westerhof and Nieuweboer-Krobotova first compared the effect of narrowband UVB (NBUVB) and psoralen UVA (PUVA) therapy in vitiligo patients in 1997.[3] NBUVB therapy has been reported to be effective and a safe tool in vitiligo during childhood and pregnancy.[4]
Quality of life is an index of different behavioral, social, and cultural factors. Dermatology Life Quality Index (DLQI), developed in 1994, was the first dermatology-specific quality of life instrument.[5]
Vitiligo was referred to as Sweta Kustha which meant “white leprosy” and thus severely impaired the quality of life of patient in Indian scenario.[6]
Many studies have been done to assess the quality of life, but there are few studies that compare the change in quality of life after treatment in vitiligo patients in Indian scenario.[7] The purpose of this study was to determine the impact of NBUVB phototherapy on vitiligo patients on psychological aspect.
Subjects and Methods
This was a prospective study in which patients were selected from the outpatient department over a period of 6 months and were followed for next 6 months. All the patients were included after institutional ethical clearance about the study and after proper informed consent of the patients regarding the protocol.
Sixty-three patients (24 males, 39 females) of vitiligo, with age ranging from 16 to 70 years, were included in the study. Patients with a history of photosensitizing disorders or cancer and suffering from claustrophobia were excluded from the study. Patients with segmental vitiligo or a history of spontaneous repigmentation were also excluded. The selected patients were advised to stop any previous treatment for at least 4 months before NBUVB monotherapy. A complete general, systemic, and dermatological examination was carried out, taking into account the number of depigmented macules, site of involvement and the approximate percentage of body surface area involved. Routine hematological investigations were carried out in all the patients.
DLQI questionnaire designed by Finlay and Khan[8] was used to determine the quality of life impairment in vitiligo patients. The DLQI consisted of 10 different questions, each one with four possible answers scored from 0 to 3, giving a maximum score of 30. The greater the score, the more the patient's quality of life was impaired.
Equipoments used and procedures followed were:
Whole-body, NB unit with 12 tubes (TL-01)-100 W, 6 ft
UV blocking glasses as an eye shield
Black apron to protect other parts while treatment and opening for the affected part to be exposed
Treatment protocols of our center
In Fitzpatrick skin type IV and V, the minimal erythema dose (MED) was not determined, and in those cases the treatment was started with an initial dose of 300 mJ/cm2 in adult cases and 150 mJ/cm2 in children. It was administered 2 days/week on nonconsecutive days. The irradiation dose was increased by 20% on each subsequent visit until the optimal dose was obtained to have a minimal erythema in the lesion
In case of any symptomatic complaints such as erythema, burning, pain, or blistering, treatment was withheld until its resolution and the irradiation dose was decreased by 20% for further treatment. All the patients were asked to use sunscreens during daytime and emollients at night
The maximum period of treatment was 6 months or earlier if 75% or greater repigmentation was achieved. Maintenance therapy once in a week for 4 weeks and once in 2 weeks for another 4 weeks was given. If there was no repigmentation even after 6 months of therapy, NBUVB was discontinued. All the patients were examined by two independent dermatologists at 4-week intervals, and lesional photographs were taken at baseline and thereafter to document the pattern and extent of repigmentation. All the patients were followed up for further 6 months after termination of therapy to observe the stability of repigmentation. Computerized phototherapy data of all the patients were maintained.
DLQI questionnaire was administered before and after therapy to each patient and the scores were recorded.
Response to treatment was assessed by comparing the photographs of before and after therapy. Statistical methods were employed using SPSS software.
Results
A total of 63 patients were selected for the study out of which there were 9 (14%) dropouts (4 [6%] males and 5 [8%] females). Fifty-four patients were left on whom the study parameters were assessed. The demographic profiles of the patients are depicted in Table 1.
Table 1.
Demographic profile of patients
Of the 54 patients, 12 (22.22%) patients showed <25% repigmentation, 27 (50%) patients showed 25%–75% repigmentation, and 11 (20.34%) patients showed >75% repigmentation. Only 4 (7.4%) cases showed complete repigmentation. Average number of treatment sessions given to the patients was 45.63±12.74 while the mean irradiation cumulative dose was 39.8 J/cm2.
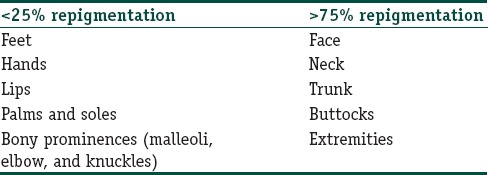
Various anatomic sites responded in different patterns as shown in Table 2. The lesions on the posterior aspect of the trunk responded better as compared to the anterior aspect of the trunk. Spotty repigmentation was seen on palms of 3 (5%) patients.
Table 2.
Anatomic sites and percentage of repigmentation
The disease activity significantly decreased after NBUVB therapy. Before therapy, about 39 (72%) had active disease, whereas after therapy, the disease had stabilized in 48 (89%) of the cases, and the remaining 4 (7%) continued to develop new lesions on previously uninvolved skin during the follow-up period, while 2 (4%) patients developed depigmentation on previously repigmented site.
Mean DLQI of the vitiligo patients was 9.64 ± 4.32 while those patients with acrofacial vitiligo had a mean DLQI of 13.78 ± 5.61 before treatment. After treatment with NBUVB, mean DLQI significantly reduced to 4.86±2.15 (P<0.001).
Adverse effects of NBUVB therapy were minimal, and none of the patients required termination of therapy. Eight (15%) patients reported mild erythema with telangiectasia or burning, 4 (7%) patients complained of xerosis, and 1 (2%) patient complained of blister formation. All these side effects resolved on tapering the irradiation dose or with topical application of an emollient and were mostly self-limiting.
Discussion
Although PUVA therapy is an established first-line therapy for vitiligo, various studies have shown that NBUVB therapy is far more effective, superior, and less dangerous when compared to PUVA therapy.[5,9]
NBUVB may exert its pigmentary effect in vitiligo through a two-step process. Both the steps occur simultaneously; the first step is stabilization of the depigmenting process and second step is the stimulation of residual follicular melanocytes in the outer root sheath of a hair follicle, which are activated to proliferate and produce melanin.[10,11]
Westerhof and Nieuweboer-Krobotova[3] compared twice weekly NBUVB phototherapy to twice weekly topical PUVA and found that after 4 months of therapy, 67% of patients undergoing NBUVB phototherapy showed repigmentation compared with 46% of patients receiving topical PUVA. Even more than 75% repigmentation of lesional skin was found in 32 patients (63%) after 12 months of NBUVB therapy, while in our study, >75% repigmentation was found in 15 (27.74%) patients after 6 months of therapy. Scherschun et al.[12] reported that 5 out of 7 patients attained more than 75% repigmentation with a mean of 19 treatments while mean duration of the disease was 13 months.
In this study, although MED was not calculated, the NBUVB therapy was initiated with a dose of 300 mJ/cm2 and gradually increased by 20% till MED. Nicolaidou et al.[13] suggested that initiation of phototherapy at 300 mJ/cm2 for skin types III-V with increments at 50 mJ/cm2 till MED. Kishan Kumar et al.[14] suggested 20% increment in dose of NBUVB till erythema. We also follwed that in our study.[14] Serish and Srinivas[15] reported that mean MED for NBUVB was 300 mJ/cm2 for the Indian skin.
In this study, mean number of exposures (45.63±12.74) was required to achieve a repigmentation of 38.65%, and the mean cumulative dose was (39.8 J/cm2) whereas Njoo et al.[16] reported similar repigmentation with more number of exposures (76.3±16.7). Similar observations were reported in another study by Yones et al. also.[17]
In this study also, certain anatomical sites such as face, neck, back, and trunk responded faster with better repigmentation to NBUVB therapy, and a poorer response was observed over the acral areas and this was comparable to various previous studies.[14,18]
It had been observed that younger the age better was the response with good repigmentation (>75%), with lesser number of exposures and lesser cumulative dose of NBUVB. Similar observations were reported by Kishan Kumar et al.[14]
Our repigmentation result in skin phototype III and IV patients was similar to Sitek et al.[19] Nearly, 30% of our patients achieved more than 75% repigmentation. Various studies did not find any relation between the skin type and the response.[18]
Hamzavi et al. in a study of left-right comparison of NBUVB exposure reported that after 6 months of therapy, in the treated side the repigmentation was 42.9% whereas in the untreated side, the repigmentation was 3.3% (P<0.001).[20]
The mean DLQI score in this study was 8.64±4.32 that was higher than that obtained by Finlay and Khan (mean 7.3)[8] and Kent and Al-Abadie[21] study (mean 4.82) but lower than Parsad et al.[22] (mean 10.67) and Sangma et al.[23] (mean 9.08±4.46). This difference might be due to literacy level regarding disease as well as due to vitiligo causing greater quality of life impairment in the darker skin due to contrast.
In our study, patients with acrofacial vitiligo had a mean DLQI of 11.78±5.61 which was similar to Sangma et al.[23] whereas those with lesions on exposed parts had a DLQI of 10.25±4.02. This was probably because vitiligo on acrofacial parts or exposed areas were evident to other people and were matter of concern for the patients.
In our study after NBUVB phototherapy, DLQI reduced from 8.64±4.32 to 5.86±2.15. Similarly in a Chinese study by Mou et al.[24] reported that DLQI before and after NBUVB therapy were 6.3±4.8 and 3.1±2.4 respectively and the difference was significant.
Relapse of depigmented macules on repigmented skin is a common sequela after NBUVB therapy. This finding similar to our study had also been reported by Natta et al.[25] who reported a relapse of 25% and 43% at 1 and 1.5 years, respectively, in 9 patients while Nicolaidou et al.[13] found a relapse rate of 44% within 1 year of stopping the treatment.
The adverse effects in this study were of short term and were easily resolved, and none of the patients discontinued the therapy because of the side effects. The adverse effects noticed in our study were similar to that reported in various other studies.[13,20]
Conclusion
Our study showed that vitiligo affected the DLQI of the patients and the suffering became less with repigmentation of lesions with NBUVB therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206–12. doi: 10.1111/j.1365-4632.2011.05377.x. [DOI] [PubMed] [Google Scholar]
- 2.Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359–62. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- 3.Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs. topical psoralen plus UV-A. Arch Dermatol. 1997;133:1525–8. [PubMed] [Google Scholar]
- 4.Dogra S, Kanwar AJ. Narrow band UVB phototherapy in dermatology. Indian J Dermatol Venereol Leprol. 2004;70:205–9. [PubMed] [Google Scholar]
- 5.Mishra N, Rastogi MK, Gahalaut P, Agrawal S. Dermatology specific quality of life in vitiligo patients and its relation with various variables: A hospital based cross-sectional study. J Clin Diagn Res. 2014;8:YC01–3. doi: 10.7860/JCDR/2014/8248.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattoo SK, Handa S, Kaur I, Gupta N, Malhotra R. Psychiatric morbidity in vitiligo: Prevalence and correlates in India. J Eur Acad Dermatol Venereol. 2002;16:573–8. doi: 10.1046/j.1468-3083.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- 7.Pahwa P, Mehta M, Khaitan BK, Sharma VK, Ramam M. The psychosocial impact of vitiligo in Indian patients. Indian J Dermatol Venereol Leprol. 2013;79:679–85. doi: 10.4103/0378-6323.116737. [DOI] [PubMed] [Google Scholar]
- 8.Finlay AY, Khan GK. Dermatology life quality index (DLQI) – A simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 9.Hercogová J, Buggiani G, Prignano F, Lotti T. A rational approach to the treatment of vitiligo and other hypomelanosis. Dermatol Clin. 2007;25:383–92. doi: 10.1016/j.det.2007.04.008. ix. [DOI] [PubMed] [Google Scholar]
- 10.Norris DA, Horikawa T, Morelli JG. Melanocyte destruction and repopulation in vitiligo. Pigment Cell Res. 1994;7:193–203. doi: 10.1111/j.1600-0749.1994.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 11.Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol. 1991;97:410–6. doi: 10.1111/1523-1747.ep12480997. [DOI] [PubMed] [Google Scholar]
- 12.Scherschun L, Kim JJ, Lim HW. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J Am Acad Dermatol. 2001;44:999–1003. doi: 10.1067/mjd.2001.114752. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaidou E, Antoniou C, Stratigos A, Katsambas AD. Narrowband ultraviolet B phototherapy and 308-nm excimer laser in the treatment of vitiligo: A review. J Am Acad Dermatol. 2009;60:470–7. doi: 10.1016/j.jaad.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 14.Kishan Kumar YH, Rao GR, Gopal KV, Shanti G, Rao KV. Evaluation of narrow-band UVB phototherapy in 150 patients with vitiligo. Indian J Dermatol Venereol Leprol. 2009;75:162–6. doi: 10.4103/0378-6323.48662. [DOI] [PubMed] [Google Scholar]
- 15.Serish, Srinivas CR. Minimal erythema dose (Med) to narrow band ultraviolet-B (NB-UVB) broad band ultraviolet-B (BB-UVB) – A pilot study. Indian J Dermatol Venereol Leprol. 2002;68:63–4. [PubMed] [Google Scholar]
- 16.Njoo MD, Bos JD, Westerhof W. Treatment of generalized vitiligo in children with narrow-band (TL-01) UVB radiation therapy. J Am Acad Dermatol. 2000;42:245–53. doi: 10.1016/S0190-9622(00)90133-6. [DOI] [PubMed] [Google Scholar]
- 17.Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized double-blind trial of treatment of vitiligo: Efficacy of psoralen-UV-A therapy vs.narrowband-UV-B therapy. Arch Dermatol. 2007;143:578–84. doi: 10.1001/archderm.143.5.578. [DOI] [PubMed] [Google Scholar]
- 18.Adauwiyah J, Suraiya HH. A retrospective study of narrowband-UVB phototherapy for treatment of vitiligo in Malaysian patients. Med J Malaysia. 2010;65:297–9. [PubMed] [Google Scholar]
- 19.Sitek JC, Loeb M, Ronnevig JR. Narrowband UVB therapy for vitiligo: Does the repigmentation last? J Eur Acad Dermatol Venereol. 2007;21:891–6. doi: 10.1111/j.1468-3083.2007.01980.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H, et al. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: The vitiligo area scoring index. Arch Dermatol. 2004;140:677–83. doi: 10.1001/archderm.140.6.677. [DOI] [PubMed] [Google Scholar]
- 21.Kent G, al-Abadie M. Factors affecting responses on dermatology life quality index items among vitiligo sufferers. Clin Exp Dermatol. 1996;21:330–3. [PubMed] [Google Scholar]
- 22.Parsad D, Pandhi R, Dogra S, Kanwar AJ, Kumar B. Dermatology life quality index score in vitiligo and its impact on the treatment outcome. Br J Dermatol. 2003;148:373–4. doi: 10.1046/j.1365-2133.2003.05097_9.x. [DOI] [PubMed] [Google Scholar]
- 23.Sangma LN, Nath J, Bhagabati D. Quality of life and psychological morbidity in vitiligo patients: A study in a teaching hospital from North-East India. Indian J Dermatol. 2015;60:142–6. doi: 10.4103/0019-5154.152508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mou KH, Han D, Liu WL, Li P. Combination therapy of orally administered glycyrrhizin and UVB improved active-stage generalized vitiligo. Braz J Med Biol Res. 2016;49 doi: 10.1590/1414-431X20165354. pii: S0100-879X2016000800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natta R, Somsak T, Wisuttida T, Laor L. Narrowband ultraviolet B radiation therapy for recalcitrant vitiligo in Asians. J Am Acad Dermatol. 2003;49:473–6. doi: 10.1067/s0190-9622(03)01484-1. [DOI] [PubMed] [Google Scholar]


