Abstract
Cutaneous fungal infections affect more than one-fourth of world's population. The pathogenesis and severity of fungal infection depend on various immunological and nonimmunological factors. The rampant use of antifungal therapy in immunocompromised individuals marked the onset of antifungal drug resistance. Fungal resistance can be microbiological or clinical. Microbiological resistance depends on various fungal factors which have established due to genetic alteration in the fungi. Clinical resistance is due to host- or drug-related factors. All these factors may cause fungal resistance individually or in tandem. In addition to standardized susceptibility testing and appropriate drug dosing, one of the ways to avoid resistance is the use of combinational antifungal therapy. Combination therapy also offers advantages in increased synergistic action with enhanced spectrum activity. Newer insights into mechanisms of drug resistance will help in the development of appropriate antifungal therapy.
Keywords: Antifungal resistance, dermatophytes, mechanisms
Introduction
Fungi have been present for around 1500 million years with more than 1.5 million species out of which only about 300 species are known to cause human infections.[1,2] Fungi were recognized earlier than bacteria as a pathogenic agent of human disease with David Gruby describing etiological agent of favus, ectothrix, and endothrix into three genera, Epidermophyton, Microsporum, and Trichophyton.[3] Despite their early discovery, most widespread infectious diseases in the 19th century were attributed to bacterial, parasitic, and to viral origins.[4] From the mid-20th century, the incidence of severe systemic fungal infections increased significantly, mainly due to increase in the number of patients with compromised immune system such as acquired immunodeficiency syndrome (AIDS) or postorgan transplantation and chemotherapy. The indiscriminate use of antibiotics added to the worsening of this picture, leading to the installation of fungal infections.[5]
Among all fungal infections, superficial mycoses are the most frequent forms of human infections, affecting more than 20%–25% of the world's population.[6] It is also estimated that 30%–70% of adults are asymptomatic carriers of these pathogens.[7] In addition, species of dermatophytes are divided into zoophilic, geophilic, or anthropophilic, depending on their primary habitat (animals, soil, or humans, respectively). Zoophilic species are responsible for about 30% of human dermatophytoses and usually cause acute inflammatory features. Anthropophilic species represent about 70% of infections on these hosts, causing a chronic infection of slow progression, suggesting that the fungus has adapted to the human host.[8]
Pathogenesis of Fungal Infections
The successful initiation of infection is a process closely related to the capability of the infecting dermatophyte to overcome the host resistance mechanisms.[5]
Factors affecting fungal virulence are:
Defect in normal physiological barriers such as physical and chemical structure of skin, normal microflora present, exposure to ultraviolet light, temperature, and humidity[6]
Adherence of dermatophytes and hyphae penetration – adherence of dermatophytes is due to arthroconidia, which are genetically programmed disarticulated septate hyphae. It occurs 3–4 hours after contact.[8,9] Hyphae penetration into the stratum corneum is a result of breakage of disulfide bridges followed by secretion of variety of enzymes such as metalloendoproteases (fungalysins) formerly called keratinases, multiple serine-subtilisins, proteases, lipases, elastases, collagenases, phosphatases, and esterases.[7,8,10] Reduction of disulfide bridges depends on a sulfite efflux pump encoded by the TruSSU1 gene, a proposed target for new antifungal treatment. Sulfite secretion by this transporter allows the cleavage of the cystine present in keratin, thereby making it accessible to the action of endo and exoproteases.[8,10,11]
-
Host response:
- Nonimmunological factors such as inhibitory effect of sebum, unsaturated transferrin, α2-macroglobulin keratin inhibitor, long-chain saturated fatty acids in the scalp, serum inhibitory factors (beta-globulins, ferritin, and other metal chelators) binding to iron essential for growth of dermatophytes inhibit fungal invasion. Macerated and hydrated skin, altered skin barrier, and immunodeficiency disorders can promote entry of dermatophytes[8,10]
- The immunological mechanism is cell-mediated, also more effective and protective, and is mediated by macrophages as effector cells and cytokines secretion from type 1 T-helper lymphocytes.[10]
Fungal response – dermatophytes have developed mechanisms that allow them to avoid the host response such as reduction of inflammation and phagocytosis by fungal mannans. Mannans also inhibit the proliferation of keratinocytes, allowing the establishment of a persistent chronic infection.[5,8]
The common antifungal agents and their mechanisms of action depending on the above are given in Figure 1.
Figure 1.
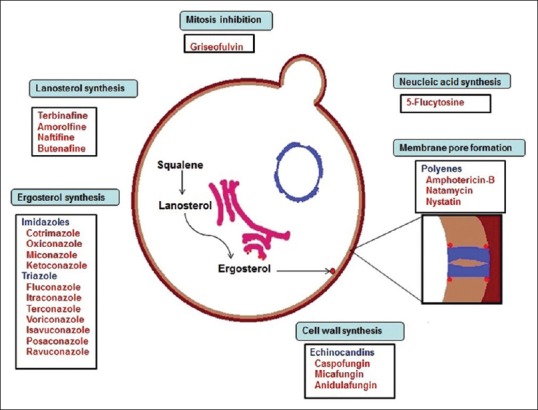
The common antifungals and their mechanism of action
Antifungal Resistance
The evolution of antimicrobial drug resistance is an almost inevitable process that is universal in the microbial world. Although fungal resistance is not at par with bacterial resistance, the economic facets associated with fungal infections remains unacceptably high.[1] Also considering the limited number of antifungal drugs available, one of the main strategies of improving therapy in mycoses is overcoming antifungal resistance.[12,18]
In general, clinical resistance is considered to be the persistence or progression of an infection despite appropriate antimicrobial therapy.[5,13] Clinical resistance to antifungals was rare during the early phases of antifungal therapy.[14] Among the earliest reported case of drug resistance of dermatophytes was to griseofulvin by Michaelides et al. in 1961.[15] Antifungal resistance to azoles was first reported in 1980s to ketoconazole used in patients of chronic mucocutaneous candidiasis.[13,16] Mukherjee et al. in 2003 reported primary resistance of Trichophyton rubrum to terbinafine in patients with onychomycosis.[17] A study of Candida species in patients with AIDS showed 33% of late-stage patients with drug-resistant strains of Candida albicans in their oral cavities.[13,18]
Azole resistance in dermatophytes has been reported to be as high as 19% in certain regions of the world.[19] Occurrence of antifungal resistance has to be considered independently for each antifungal class and for each fungal genus.[14]
Fungal resistance can be:
The factors responsible for fungal resistance are given in Table 1.[5,13,20]
Table 1.
Factors for fungal resistance

Microbiological resistance refers to nonsusceptibility of a fungus to an antifungal agent by in vitro susceptibility testing, in which the minimum inhibitory concentration (MIC) of the drug exceeds the susceptibility breakpoint for that organism. Microbiological resistance can be primary (intrinsic), where the fungi are resistant to a drug before exposure and secondary (acquired), which develops in response to exposure to an antimicrobial agent.[13] Certain fungal species are intrinsically resistant such as Candida krusei to fluconazole and Cryptococcus neoformans to echinocandins and nonalbicans Candida to 5-flucytosine (5FC).[13,20]
Secondary resistance develops among previously susceptible strains after exposure to the antifungal agent and is usually dependent on altered gene expression,[20] for example, terbinafine resistance in T. rubrum, fluconazole resistance among C. albicans.
Clinical resistance is defined as the failure to eradicate a fungal infection despite the administration of an antifungal agent with in vitro activity against the organism. Although clinical resistance cannot always be predicted, it highlights the importance of individualizing treatment strategies on the basis of the clinical situation.[20]
Fungal factors, host factors, and drug pharmacology play a role in fungal resistance in isolation or in association with other.
The fungal molecular mechanism can result from gene amplification, gene transfer, gene deletion, point mutations, loss of cis- and trans-acting regulatory elements, and transcriptional activation. All these effects could be on genes directly involved in the combat against the cytotoxic compounds and/or could be on genes involved in their regulation or processing.[5]
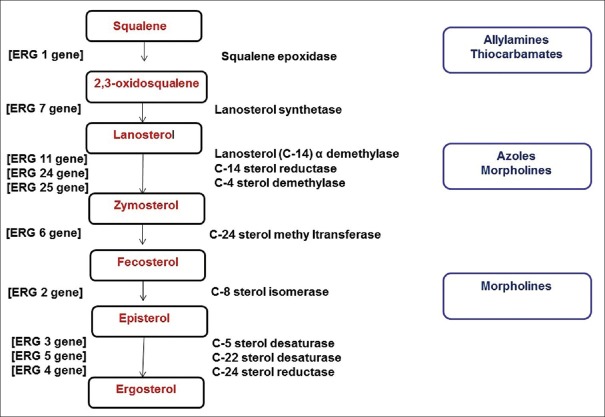
The genes responsible for ergosterol synthesis is given in Figure 2.[13]
Figure 2.
The genes involved in ergosterol synthesis
Fungal Factors
Decreased accumulation of drug within fungi
Reducing the accumulation of the drug within the fungal cell is done by increasing the drug efflux mechanism. Multidrug efflux transporters are membrane proteins found in all living organisms. These proteins bind to a variety of structurally and chemically dissimilar compounds and actively extrude them from the cells.[5] Mutations (upregulation or overexpression) of the genes encoding these efflux pumps result in decreased accumulation of the drug in the cell.[21]
Efflux systems affecting the antifungal drugs vary. Common drug efflux systems that modulate azole resistance are the ATP-binding cassette (ABC) superfamily and the major facilitator superfamily. Overexpression of TruMDR1 and TruMDR2 genes in T. rubrum, which encoded an ABC transporter was seen following azole and terbinafine therapy.[5]
Decreasing the affinity of the drug for its target
A mutation or overexpression of the gene coding for target enzymes is another mechanism developed by fungi.
A point mutation in the ERG11 gene that codes for lanosterol 14α-demethylase leads to the complete inhibition of the binding capacity of the azole drug to its target.[14] Mutation in the squalene epoxide (SE) gene (ERG1) leads to an amino acid substitution in the SE making the fungi about 1000-fold less susceptible to terbinafine.[5]
The overexpression of target enzymes is either by gene amplification or upregulation of the corresponding gene.[5] In chromosomal anomalies like isochromosome formation, an increase in the number of azole-resistant genes can occur.[21]
Modifications of metabolism to counterbalance the drug effect De novo synthesis of pyrimidines
The antifungal drug 5FC competes with the regular pyrimidine intermediate metabolites for incorporation into nucleic acids.[14,22] De Novo increase in pyrimidine synthesis leads to 5FC resistance as seen in Candida glabrata.[14]
Paradoxical effect
Few yeasts and filamentous fungi are able to grow in elevated echinocandin concentrations much higher than the MICs. This phenomenon, called paradoxical effect or “eagle effect,” is strain dependent and is due to the upregulation of the chitin synthesis in the fungal cell wall after drug administration.[14,20]
Modifications of the ergosterol biosynthetic pathway
The antifungal activity of azole drugs depends on the depletion of ergosterol from the fungal membrane and accumulation of the toxic product 14α-methyl-3,6-diol, leading to growth arrest. Alteration in the late steps of the ergosterol biosynthetic pathway through inactivation of the ERG3 gene can lead to the total inactivation of C5 sterol desaturase and also can give rise to cross-resistance to all azole drugs. This mechanism is identified in C. albicans.[14,20]
Plasma membrane composition variation
A decrease or total absence of ergosterol in the plasma membrane through mutations in nonessential genes of the ergosterol is a rare mechanism of resistance among polyene drugs, for example, ERG3 mutation in clinical isolates of C. albicans, ERG6 mutation in C. glabrata.[14]
Biofilms
Biofilms are sessile microbial communities surrounded by extracellular polymeric substances with increased resistance to antimicrobial agents and host defenses. Both T. rubrum and T. mentagrophytes are capable of producing biofilms.[23]
Biofilms by yeast and certain molds are frequently polymicrobial and are resistant to almost all the currently used antifungals, with the exceptions of echinocandins and lipid formulations of amphotericin B. Biofilm resistance is probably the result of multiple factors such as an increased expression of efflux pumps, a modification of plasma membrane composition, and the drug sequestration in biofilm-produced extracellular matrix.[14,21]
Cellular response to stress or stress adaptation
Fungi are remarkably adaptive and have numerous signal-transduction pathways to sense and ensure appropriate physiological mechanisms to adapt to environmental stress following exposure to an antifungal agent.[5,21] Hsp90, Hsp104, ubiquitin, calcineurin, esterases, and oxidative-stress proteins such as glutathione synthatase are key cellular regulators critical for orchestrating cellular responses to drug-induced stress, e.g., in T. rubrum following azoles, griseofulvin and amphotericin B.[5]
Stress adaptation may not induce clinical resistance but stabilizes the cell in the presence of drug and allows it to develop more profound resistance mechanisms over time that are manifested as clinical resistance.[21]
Clinical Resistance
Clinical resistance depends on a multiple host- and drug-related factors which are as follows:[13,20]
Patients with severe degree of immunosuppression with invasive fungal infections may not respond to antifungals
Delay in initiation of adequate dose of antifungal results in increased chances of treatment failure
Fluconazole has better cerebrospinal fluid (CSF) penetration as compared to itraconazole, therefore, making it a better choice in treating fungal meningitis. When the site of infection is necrotic with poor blood supply, a debulking surgery is essential to overcome antifungal treatment resistance
Compliance in patients requiring long-term therapies.
Drug-Related Factors
Various factors such as the fungistatic nature of most drugs, inappropriate antifungal usage (in cases where the etiological agent is known), treatment with low antifungal dosage, long duration of treatment, drug interactions, and the cost of therapy play a role in fungal resistance.[21] Combination of polyenes and azoles with other nephrotoxic drugs can result in treatment failure.[20]
Environmental resistance
In recent years, the role of environment as a driver for resistance has become prominent. Fungicidal use in agriculture differs hugely between different regions, with the United States using about a tenth of as much as Europe. Azole-based fungicides are used in grape and cereal production in European countries. A strong link has been found between countries where azole-based fungicides are used and the incidence of antifungal resistance.[21,24]
Antifungal susceptibility testing
Antifungal susceptibility testing (AFST) has undergone considerable change from a nonstandardized procedure that generated results lacking clinical utility to a standardized procedure. Standardized microdilution-based procedures by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antibiotic Susceptibility Testing are universally accepted for performing AFST[25] The CLSI, formerly the National Committee on Clinical Laboratory Standards has released four standard methods for antifungal susceptibility testing, namely, M27-A3 for macrobroth and microtiter yeast testing, M38-A2 for microtiter mold testing, M44-A for yeast disc diffusion testing, and M51-P for mold disc diffusion testing.[26,27,28,29,30,31]
AFST patterns in dermatophytes show individual and regional variation in their outcome. Studies on AFST showed that T. rubrum and T. mentagrophytes, which were the common species isolated, showed a higher MIC to fluconazole. Although generally itraconazole, terbinafine had lower MICs, few studies demonstrated a higher MIC to itraconazole and terbinafine. Systemic griseofulvin and topical amorolfine had lower MIC than fluconazole. A study done by Koga et al. showed the lowest MIC to be with luliconazole.[32,33,34,35,36] Rex and Pfaller proposed the “90–60 Rule” which states that infections caused by isolates that have MICs considered susceptible respond favorably to appropriate therapy approximately 90% of the time, whereas infections caused by isolates with MICs considered resistant respond favorably approximately 60% of the time.[37]
Treatment
Clinically, antifungal resistance may be suspected in patients with recurrent episodes of infection, unresponsiveness to the first line of therapy, generalized involvement, or atypical form of presentation with usual history of similar lesions in the family.
One of the important treatment measures in recurrent cutaneous fungal infections is prevention of disease among family members. Therefore, nonpharmacological measures like good hygiene play an important adjunct along with medications.[38]
Good skin hygiene measures include handwash; clipping of nail; regular bathing and complete drying of the skin; use of nonocclusive shoes, absorbent socks, and powder; avoidance of sharing of combs, towels, brushes, bedding, and hats; and avoidance of barefoot walk in public bathroom.[39,40]
Pharmacological measures like prudent use of antifungals in proper dosing and duration form the mainstay of therapy. In general, topical agents should be applied once to twice daily over the lesions and up to at least 2 cm beyond the margins of the lesions for 2–4 weeks and continued for 1 week after the rash resolves.[41] Topical allylamines maintain the mycological cure for longer periods compared to azole drugs.[42] With respect to systemic antifungals, in addition to a proper dose, duration, and knowledge of pharmacokinetics, dosage updosing (pulse or boosted oral antifungal) and combination antifungals can be tried in recurrent dermatophytoses. Boosted oral antifungal treatment is designed to target dormant chlamydospores and arthroconidia to produce sensitive hyphae which are less refractory to antifungal treatment.[43,44]
Combination Antifungal Therapy
The availability of new antifungal agents with unique mechanisms of action and improved tolerability has widened the possibilities for the use of combination antifungal therapy for difficult-to-treat opportunistic mycoses.[45] Combining two systemic antifungal agents has been used in invasive mycoses caused by Candida, Aspergillus and Cryptococcus.[46] In dermatophytoses, combination antifungal therapy has been used, wherein topical and systemic antifungal are combined. Gupta et al. used sequential therapy with itraconazole and terbinafine pulse in toenail onychomycosis.[44,47] The advantages of combination therapy are increased rate and extent of fungal killing (synergy), enhanced spectrum of activity, and decreased likelihood of resistance or tolerance.[45] Azoles can act in a synergic way when combined with terbinafine providing good results against Candida, dimorphic molds, dematiaceous fungi and yeasts, such as C. glabrata. Azoles with amphotericin B have mixed response.[46,49]
The disadvantages are increased toxicity, increased drug interactions, increased cost, and poorly standardized methods of testing efficacy of the combination.[45] Itraconazole and voriconazole are potent inhibitors of cytochrome P450 3A4 and associated with a greater risk of broader range of potentially severe drug interactions and hepatotoxicity than fluconazole.[48] At present, checkerboard dilution assay is the most common laboratory method for evaluating antifungal combinations.[45]
Antifungal Drugs Plus Nonantifungal Drugs
Combination of antifungal drugs with nonantimicrobial agents such as calcineurin inhibitors (cyclosporine A and tacrolimus), proton-pump inhibitors, antiarrhythmic agents, cholesterol-lowering agents, immunomodulators, antineoplastic drugs, antiparasitic agents, microbial metabolites, and human recombinant antibodies has shown beneficial effects.[46,50] Cyclosporine singularly is not able to inhibit the fungal growth but increases susceptibility to fluconazole due to efflux pump deletion or alteration of stress response caused by calcineurin during azole therapy.[51]
Studies show that statins are active against Microsporum canis and T. mentagrophytes. Synergistic interactions were noticed when simvastatin was combined with terbinafine against dermatophytes.[52]
Hemopoietic growth factors, such as granulocyte colony stimulating factor or granulocyte-macrophage colony stimulating factor and Th1 cytokines have activity on the antifungal function of phagocytes and the efficacy of antifungal agents.[46,53,54]
Fractional carbon-dioxide laser therapy combined with topical antifungals such as terbinafine, amolorfine, or luliconazole was found to be effective in the treatment of onychomycosis.[55]
Newer Antifungal Drugs
An efficient antifungal should act against a wide range of fungi having no or low toxicity to the host.[5] One of the major challenges in developing antifungal drugs lies in the similarities shared between fungi and their hosts, thereby restricting the target molecules for antifungal drugs. Most newer antifungal drugs are designed considering the following:
Drugs having pharmacological similarity with the older drugs which share the target molecules, with lower MIC level and specific indications (e.g., isavuconazole, micafungin, luliconazole)[56,57]
Repurposing of established medications where an old compound with a known pharmacology is used alone or in combination with another drug for a newer indication, for example, calcineurin inhibitors, target of rapamycin inhibitors, Hsp90 inhibitors, in synergy with azoles[20,50,58,59]
Recent advances in fungal genomics and proteomics have revealed target genes, proteins, or virulence factors required during infection of the host tissues by dermatophytes, for example, efflux pump inhibitors (derived compounds of milbemycin), the transcription factor PacC, a wide-domain regulatory protein involved in pathogenicity events or the sulfite transporters were proposed as interesting targets for antifungal drugs in dermatology because of their role in the proteolytic digestion of hard keratin).[5,20,60,61,62]
Conclusion
While deeper mycoses are still difficult to treat, common fungal infections in dermatology were considered easily treatable. Lately, fungi causing superficial mycoses also have evolved to develop resistance against commonly used drugs. Current solutions to this could be good skin hygiene measures, prudent use of antifungals in proper dosing and duration, appropriate use of susceptibility testing, usage of older molecules such as griseofulvin or topical keratolytics in combination with newer drugs and in appropriate dosages and combination therapy with two systemic antifungals or systemic antifungal with topical antifungals and/or topical keratolytics.
The key to managing the widespread nature of this emerging problem would involve development of drugs with newer targets, decreased drug interactions, at affordable price, which at this stage appears distant in our scenario, knowing the resources required and the economics involved. However, newer insight on drug resistance mechanisms could lead to advanced treatment strategies in managing fungal infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Srinivasan A, Lopez-Ribot JL, Ramasubramanian AK. Overcoming antifungal resistance. Drug Discov Today Technol. 2014;11:65–71. doi: 10.1016/j.ddtec.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawksworth DL. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol Res. 2001;105:1422–32. [Google Scholar]
- 3.Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8:240–59. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapil A, editor. 9th ed. Hyderabad: University Press Private Limited; 2013. Ananthanarayan & Paniker's Textbook of Microbiology; pp. 589–615. [Google Scholar]
- 5.Martinez-Rossi NM, Peres NT, Rossi A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia. 2008;166:369–83. doi: 10.1007/s11046-008-9110-7. [DOI] [PubMed] [Google Scholar]
- 6.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 7.Nigam PK. Antifungal drugs and resistance: Current concepts. Our Dermatol Online. 2015;6:212–21. [Google Scholar]
- 8.Peres NT, Maranhão FC, Rossi A, Martinez-Rossi NM. Dermatophytes: Host-pathogen interaction and antifungal resistance. An Bras Dermatol. 2010;85:657–67. doi: 10.1590/s0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 9.Coelho LM, Aquino-Ferreira R, Maffei CM, Martinez-Rossi NM. In vitro antifungal drug susceptibilities of dermatophytes microconidia and arthroconidia. J Antimicrob Chemother. 2008;62:758–61. doi: 10.1093/jac/dkn245. [DOI] [PubMed] [Google Scholar]
- 10.Tainwala R, Sharma Y. Pathogenesis of dermatophytoses. Indian J Dermatol. 2011;56:259–61. doi: 10.4103/0019-5154.82476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Léchenne B, Reichard U, Zaugg C, Fratti M, Kunert J, Boulat O, et al. Sulphite efflux pumps in Aspergillus fumigatus and dermatophytes. Microbiology. 2007;153:905–13. doi: 10.1099/mic.0.2006/003335-0. [DOI] [PubMed] [Google Scholar]
- 12.Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2:73–85. doi: 10.1016/s1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 13.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol 2012. 2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaelides P, Rosenthal SA, Sulzberger MB, Witten VH. Trichophyton tonsurans infection resistant to griseofulvin. A case demonstrating clinical and in vitro resistance. Arch Dermatol. 1961;83:988–90. doi: 10.1001/archderm.1961.01580120100025. [DOI] [PubMed] [Google Scholar]
- 16.Ryley JF, Wilson RG, Barrett-Bee KJ. Azole resistance in Candida albicans. Sabouraudia. 1984;22:53–63. [PubMed] [Google Scholar]
- 17.Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA, et al. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother. 2003;47:82–6. doi: 10.1128/AAC.47.1.82-86.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law D, Moore CB, Wardle HM, Ganguli LA, Keaney MG, Denning DW. High prevalence of antifungal resistance in Candida spp. from patients with AIDS. J Antimicrob Chemother. 1994;34:659–68. doi: 10.1093/jac/34.5.659. [DOI] [PubMed] [Google Scholar]
- 19.Ghannoum M. Azole resistance in dermatophytes: Prevalence and mechanism of action. J Am Podiatr Med Assoc. 2016;106:79–86. doi: 10.7547/14-109. [DOI] [PubMed] [Google Scholar]
- 20.Kanafani ZA, Perfect JR. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin Infect Dis. 2008;46:120–8. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 21.Perlin DS, Shor E, Zhao Y. Update on antifungal drug resistance. Curr Clin Microbiol Rep. 2015;2:84–95. doi: 10.1007/s40588-015-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polak A, Scholer HJ. Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy. 1975;21:113–30. doi: 10.1159/000221854. [DOI] [PubMed] [Google Scholar]
- 23.Costa-Orlandi CB, Sardi JC, Santos CT, Fusco-Almeida AM, Mendes-Giannini MJ. In vitro characterization of Trichophyton rubrum and T. Mentagrophytes biofilms. Biofouling. 2014;30:719–27. doi: 10.1080/08927014.2014.919282. [DOI] [PubMed] [Google Scholar]
- 24.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57:513–20. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 25.Antifungal Susceptibility Testing: Current role from the clinical laboratory perspective Brunella Posteraro, Riccardo Torelli, Elena De Carolis, Patrizia Posteraro and Maurizio Sanguinetti. Mediterr J Hematol Infect Dis. 2014:6–e2014030. doi: 10.4084/MJHID.2014.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLSI. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard-CLSI Document M27-A3. [Google Scholar]
- 27.CLSI. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard CLSI Document M38-A2. [Google Scholar]
- 28.CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Filamentous fungi. Approved Guideline. CLSI Document M51-P. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 29.NCCLS. Wayne, PA: National Committee for Clinical Laboratory Standards; 2004. Reference Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. Approved Guideline. NCCLS Document M44-A. [Google Scholar]
- 30.Fothergill AW. Antifungal susceptibility testing: Clinical laboratory and standards institute (CLSI) methods. In: Hall GS, editor. Interactions of Yeasts, Moulds, and Antifungal Agents: How to Detect Resistance. New York: Springer; 2012. pp. 65–74. [Google Scholar]
- 31.CLSI/NCCLS. Wayne, PA: National Committee for Clinical Laboratory Standards; 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidial Forming Filamentous Fungi. Approved Standard NCCLS M38-A. [Google Scholar]
- 32.Indira G. In vitro antifungal susceptibility testing of 5 antifungal agents against dermatophytic species by CLSI (M38-A) micro dilution method. Clin Microbiol. 2014;3:145. [Google Scholar]
- 33.Favre B, Hofbauer B, Hildering KS, Ryder NS. Comparison of in vitro activities of 17 antifungal drugs against a panel of 20 dermatophytes by using a microdilution assay. J Clin Microbiol. 2003;41:4817–9. doi: 10.1128/JCM.41.10.4817-4819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jessup CJ, Warner J, Isham N, Hasan I, Ghannoum MA. Antifungal susceptibility testing of dermatophytes: Establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J Clin Microbiol. 2000;38:341–4. doi: 10.1128/jcm.38.1.341-344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adimi P, Hashemi SJ, Mahmoudi M, Mirhendi H, Shidfar MR, Emmami M, et al. In vitro activity of 10 antifungal agents against 320 dermatophyte strains using microdilution method in Tehran. Iran J Pharm Res. 2013;12:537–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Koga H, Nanjoh Y, Makimura K, Tsuboi R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med Mycol. 2009;47:640–7. doi: 10.1080/13693780802541518. [DOI] [PubMed] [Google Scholar]
- 37.Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis. 2002;35:982–9. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 38.Rezabek GH, Friedman AD. Superficial fungal infections of the skin. Diagnosis and current treatment recommendations. Drugs. 1992;43:674–82. doi: 10.2165/00003495-199243050-00004. [DOI] [PubMed] [Google Scholar]
- 39.Clinard VB, Smith JD. Cutaneous fungal infections. US Pharm. 2015;40:35–9. [Google Scholar]
- 40.Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–52. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 41.Kim D, Lockey R. Dermatology for the allergist. World Allergy Organ J. 2010;3:202–15. doi: 10.1097/WOX.0b013e3181e2eb2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotta I, Otuki MF, Sanches AC, Correr CJ. Efficacy of topical antifungal drugs in different dermatomycoses: A systematic review with meta-analysis. Rev Assoc Med Bras (1992) 2012;58:308–18. [PubMed] [Google Scholar]
- 43.Piérard GE, Piérard-Franchimont C, Arrese JE. The boosted oral antifungal treatment for onychomycosis beyond the regular itraconazole pulse dosing regimen. Dermatology. 2000;200:185–7. doi: 10.1159/000018363. [DOI] [PubMed] [Google Scholar]
- 44.Singal A, Khanna D. Onychomycosis: Diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77:659–72. doi: 10.4103/0378-6323.86475. [DOI] [PubMed] [Google Scholar]
- 45.Kontoyiannis DP, Lewis RE. Toward more effective antifungal therapy: The prospects of combination therapy. Br J Haematol. 2004;126:165–75. doi: 10.1111/j.1365-2141.2004.05007.x. [DOI] [PubMed] [Google Scholar]
- 46.Carrillo-Muñoz AJ, Finquelievich J, Tur-Tur C, Eraso E, Jauregizar N, Quindós G, et al. Combination antifungal therapy: A strategy for the management of invasive fungal infections. Rev Esp Quimioter. 2014;27:141–58. [PubMed] [Google Scholar]
- 47.Gupta AK, Lynde CW, Konnikov N. Single-blind, randomized, prospective study of sequential itraconazole and terbinafine pulse compared with terbinafine pulse for the treatment of toenail onychomycosis. J Am Acad Dermatol. 2001;44:485–91. doi: 10.1067/mjd.2001.110644. [DOI] [PubMed] [Google Scholar]
- 48.Groll AH, Piscitelli SC, Walsh TJ. Clinical pharmacology of systemic antifungal agents: A comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 49.Cuenca-Estrella M. Combinations of antifungal agents in therapy – What value are they? J Antimicrob Chemother. 2004;54:854–69. doi: 10.1093/jac/dkh434. [DOI] [PubMed] [Google Scholar]
- 50.Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother. 2000;44:2373–81. doi: 10.1128/aac.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onyewu C, Eads E, Schell WA, Perfect JR, Ullmann Y, Kaufman G, et al. Targeting the calcineurin pathway enhances ergosterol biosynthesis inhibitors against Trichophyton mentagrophytes in vitro and in a human skin infection model. Antimicrob Agents Chemother. 2007;51:3743–6. doi: 10.1128/AAC.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyilasi I, Kocsubé S, Krizsán K, Galgóczy L, Papp T, Pesti M, et al. Susceptibility of clinically important dermatophytes against statins and different statin-antifungal combinations. Med Mycol. 2014;52:140–8. doi: 10.3109/13693786.2013.828160. [DOI] [PubMed] [Google Scholar]
- 53.Farmaki E, Roilides E. Immunotherapy in patients with systemic mycoses: A promising adjunct. BioDrugs. 2001;15:207–14. doi: 10.2165/00063030-200115040-00001. [DOI] [PubMed] [Google Scholar]
- 54.Casadevall A, Pirofski LA. Adjunctive immune therapy for fungal infections. Clin Infect Dis. 2001;33:1048–56. doi: 10.1086/322710. [DOI] [PubMed] [Google Scholar]
- 55.Bhatta AK, Keyal U, Huang X, Zhao JJ. Fractional carbon-dioxide (CO2) laser-assisted topical therapy for the treatment of onychomycosis. J Am Acad Dermatol. 2016;74:916–23. doi: 10.1016/j.jaad.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Cross SA, Scott LJ. Micafungin: A review of its use in adults for the treatment of invasive and oesophageal candidiasis, and as prophylaxis against Candida infections. Drugs. 2008;68:2225–55. doi: 10.2165/00003495-200868150-00010. [DOI] [PubMed] [Google Scholar]
- 57.Miceli MH, Kauffman CA. Isavuconazole: A New broad-spectrum triazole antifungal agent. Clin Infect Dis. 2015;61:1558–65. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 58.Cruz MC, Goldstein AL, Blankenship J, Del Poeta M, Perfect JR, McCusker JH, et al. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob Agents Chemother. 2001;45:3162–70. doi: 10.1128/AAC.45.11.3162-3170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butts A, Krysan DJ. Antifungal drug discovery: Something old and something new. PLoS Pathog. 2012;8:e1002870. doi: 10.1371/journal.ppat.1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma M, Manoharlal R, Shukla S, Puri N, Prasad T, Ambudkar SV, et al. Curcumin modulates efflux mediated by yeast ABC multidrug transporters and is synergistic with antifungals. Antimicrob Agents Chemother. 2009;53:3256–65. doi: 10.1128/AAC.01497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreira-Nozawa MS, Silveira HC, Ono CJ, Fachin AL, Rossi A, Martinez-Rossi NM. The pH signaling transcription factor PacC mediates the growth of Trichophyton rubrum on human nail in vitro . Med Mycol. 2006;44:641–5. doi: 10.1080/13693780600876553. [DOI] [PubMed] [Google Scholar]
- 62.Odds FC. Genomics, molecular targets and the discovery of antifungal drugs. Rev Iberoam Micol. 2005;22:229–37. doi: 10.1016/s1130-1406(05)70048-6. [DOI] [PubMed] [Google Scholar]