Short abstract
Objective
This study was performed to establish a novel model of citric acid-induced chronic cough in guinea pigs and to investigate the pathogenesis of cough hypersensitivity.
Methods
Healthy conscious guinea pigs inhaled citric acid (0.4 M) for 3 minutes twice daily for 25 days. Cough reactivity was evaluated, substance P (SP) and calcitonin gene-related peptide (CGRP) in bronchoalveolar lavage fluid were detected, and transient receptor potential cation channel subfamily V member 1 (TRPV1) protein expression in the trachea and bronchus was determined. Tracheal and bronchial tissues were examined for TRPV1.
Results
Inhalation of 0.4 M citric acid increased coughing in a time-dependent manner: coughing peaked at 15 days and reached the lowest level at 25 days. This was accompanied by similar changes in SP, CGRP, and TRPV1 protein expression. TRPV1 was mainly observed in the mucosal and submucosal layer of the trachea and bronchi. The areas of TRPV1 positivity in the trachea and bronchi of citric acid-treated animals were significantly larger than in the control group.
Conclusions
Repeated inhalation of citric acid can be employed to establish a chronic cough model in guinea pigs. Cough hypersensitivity in this model is related to tracheal TRPV1 activation and neurogenic inflammation.
Keywords: Chronic cough, cough hypersensitivity, animal model, citric acid, concentration, transient receptor potential cation channel subfamily V member 1
Introduction
Chronic cough refers to coughing that lasts more than 8 weeks in the absence of abnormalities on chest radiographs.1 A frequent and continuous chronic cough may confer adverse effects on patients’ physiology, psychology, and social function, significantly impairing their quality of life.2 Although the causes of chronic cough vary among individuals, cough hypersensitivity is a shared characteristic. The concept of cough hypersensitivity syndrome has been paid much attention to date,3 and several causes of chronic cough have been regarded as the clinical phenotypes and/or triggers of cough hypersensitivity.4,5
Animal models are effective tools with which to study human diseases. However, only a few animal models are currently available for the investigation of chronic cough. Thus, some studies have used a simple animal model of acute cough to investigate the pathogenesis of cough and the effects of antitussive drugs,6–8 and other studies have used models of cigarette smoke-induced chronic cough.9,10 Notably, airway inflammation and remodeling is the pathophysiologic mechanism common to all types of chronic cough.11 One component of airway remodeling is inflammation-induced altered plasticity of afferent vagus nerve C-fibers and their receptor [transient receptor potential cation channel subfamily V member 1 (TRPV1)], which is a key factor in the formation of cough hypersensitivity.12 However, no reports have described airway remodeling, including plasticity alteration of TRPV1, in acute cough models. Therefore, acute cough models fail to reflect the actual pathophysiology of chronic cough; additionally, cigarette smoke-induced chronic bronchial inflammation is not a major cause of chronic cough. Thus, results from these animal models are not representative of or applicable to human diseases.
Studies have shown that cough itself may act as a repeated mechanical stimulation that causes damage to the airway mucosal epithelium and induces the production of proinflammatory cytokines (such as macrophage inflammatory protein 2, interleukin-1β, tumor necrosis factor-α, and nerve growth factor) in the airways, deteriorating airway inflammation7 and forming a vicious circle. This may be a major cause of uncontrollable cough. Chronic airway inflammation may cause repeated damage to and repair of the airway, leading to airway remodeling characterized by thickening of the basement membrane, increased vascular density, and hyperplasia of goblet cells. This has been found to be the main cause of chronic cough.11,13
In the present study, repeated aerosol inhalation of citric acid was employed to establish a novel guinea pig model of chronic cough without eosinophilic infiltration to further investigate the pathogenesis of cough hypersensitivity. Our study may provide a new tool for investigations of chronic cough treatment and the pharmacology of antitussive drugs.
Materials and methods
Animals
Male Hartley guinea pigs (n = 130) weighing 300 to 350 g were purchased from Songjiang Songlian Experimental Animal Center of Shanghai (License: SCXK[Hu] 2007-0011). Experiments were conducted according to the Guide for Animal Care and Use of Tongji Hospital of Tongji University. This study was approved by the Ethics Committee of Tongji Hospital. The animals in all experiments were used with observation of the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Testing, and Education by the New York Academy of Sciences, Ad Hoc Animal Research Committee.
Determination of optimal citric acid inhalation
Sixty guinea pigs were allowed to acclimate to the research environment for 1 week before the study. The animals were placed in a noninvasive whole-body plethysmograph, in which they could move freely, for the detection of lung function. When the animals had become calm, 0.9% normal saline, 0.4 M citric acid, and 0.8 M citric acid were used to induce cough (n = 20 per group) for 3 minutes twice daily. The automatic atomization program of EMKA (Paris, France) was employed for aerosol inhalation (particle diameter, 0.5 to 2.0 µm; flow rate, 0.2 mL/min), and the lung function detection system of EMKA was used to determine the number of coughs. Cough stimulation continued for 25 days. Three guinea pigs died on days 3, 11, and 17, respectively, after inhalation of 0.8 M citric acid.
Investigation of pathogenesis of the novel chronic cough model
Another 70 guinea pigs were used to investigate the pathogenesis of the novel chronic cough model. Fifty healthy conscious guinea pigs underwent 0.4 M citric acid inhalation as described above, and the number of coughs, the cough pressure waveform, and the sound waves were recorded with the EMKA system. Aerosol inhalation continued for 25 days. Bronchoalveolar lavage fluid (BALF), tracheal tissue, and bronchial tissue were collected at 5, 10, 15, 20, and 25 days (n = 10 per time point). In addition, 10 healthy guinea pigs underwent inhalation of 0.9% normal saline as described above. After 25 days of treatment, their BALF, tracheal tissue, and bronchial tissue were collected as controls. Another 10 healthy conscious guinea pigs underwent 0.4 M citric acid inhalation as described above for only 15 days; they then underwent inhalation of 0.9% normal saline for the next 10 days.
Determination of number of coughs
Conscious animals were placed in the transparent chamber of the whole-body plethysmograph and allowed to move freely. The number of coughs was determined with the cough module of the EMKA system. Automatic atomization (citric acid at 0.4 M for 3 minutes daily for 25 days) and monitoring programs were initiated. Coughing was determined by observation of the animals’ cough action. The cough sound and the change in the chamber pressure were monitored to define the cough. The number of coughs was recorded during and 1 minute after the inhalation of citric acid.
Sample collection
Fifty guinea pigs underwent continuous citric acid (0.4 M) inhalation. Ten guinea pigs were killed on day 5, 10, 15, 20, and 25, respectively. The BALF, tracheal tissue, and bronchial tissue were collected at each time point. These specimens were also collected after 25 days of treatment in the 10 healthy guinea pigs that underwent inhalation of 0.9% saline.
The animals were killed by intraperitoneal injection of 3% pentobarbital at 80 mg/kg. Thoracotomy was performed, and the right main bronchus was ligated. The left lung was perfused, and BALF was harvested into a sterilized Eppendorf tube (70% recovery rate). The BALF was filtered through a 48-µm nylon mesh, and 10 µL of solution was then subjected to counting of viable cells after trypan blue staining. The BALF was centrifuged at 4°C for 10 minutes at 1500 rpm/min, and the supernatant was harvested and stored at −80°C. The cells at the bottom were resuspended in phosphate-buffered solution (PBS) at a density of 1 × 106/mL. Next, 25 µL of BALF was processed for hematoxylin and eosin (HE) staining. Cells were differentiated according to standard morphological criteria, and 400 nucleated cells were counted. The trachea (length, 0.8 to 1.0 cm) and bronchi were also placed in liquid nitrogen and stored at −80°C.
Detection of substance P and calcitonin gene-related peptide in BALF
BALF was harvested as described above and then centrifuged. The supernatant was collected, and the contents of substance P (SP) and calcitonin gene-related peptide (CGRP) were detected with an SP ELISA Kit (R&D Systems, Minneapolis, MN, USA) and CGRP RIA Kit (R&D Systems) according to the manufacturer’s instructions.
Detection of TRPV1 expression in the trachea and bronchus by western blotting
The tracheal and bronchial tissues were washed in ice-cold PBS. Total proteins were extracted from tissues with M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific, Waltham, MA, USA). Protein concentrations were determined with the bicinchoninic acid method. The proteins (30 mg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using Tris-HCl Precast Gels (Bio-Rad Laboratories, Hercules, CA, USA) and then transferred onto a polyvinylidene difluoride membrane. The resulting membrane was blocked with PBS containing 0.05% Tween-20 (PBS-T) and 3% nonfat milk at room temperature for 2 hours, followed by washing in PBS-T. The membrane was incubated with specific primary antibody (mouse anti-guinea pig TRPV1; 1:1000; ab203103; Abcam, Cambridge, UK) at 4°C overnight. The membrane was then washed with PBS-T followed by incubation with secondary antibody (goat anti-mouse; 1:5000; Jackson ImmunoResearch, West Grove, PA, USA) conjugated with horseradish peroxidase (HRP) at room temperature for 1 hour. The membrane was washed with PBS-T and then treated with the Electrochemiluminescence Plus Western Blotting Detection System (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Normalization was made against β-actin expression.
HE staining and immunohistochemistry
Tracheal and bronchial tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. After deparaffinization, sections were subjected to routine HE staining and pathological examination. The paraffin-embedded sections were subjected to immunohistochemistry for TRPV1 to evaluate vascularization. Briefly, after deparaffinization in xylene and rehydration in a series of alcohol concentrations, the sections were incubated in hydrogen peroxide to block endogenous peroxidase activity. They were then incubated with primary monoclonal antibody (mouse anti-guinea pig TRPV1) at 4°C overnight. After washing with PBS three times for 5 minutes each, the sections were incubated with goat anti-mouse secondary antibody conjugated with HRP (1:5000). After washing in PBS three times for 5 minutes each, these sections were incubated with streptavidin conjugated with HRP at 37°C for 30 minutes, followed by washing in PBS three times for 5 minutes each. Visualization was performed using DAB or NBT/BCIP for 3 minutes. Five fields were randomly selected from each section and analyzed with ImageJ (National Institutes of Health, Bethesda, MD, USA). The staining intensity was quantified according to the optical density within a 25-µm2 area including the four corners and the central area. The optical density (mean intensity of positive granules in cells) and the proportion of the positive area (positive area/total area × 100%) were determined.
Statistical analysis
Statistical analysis was performed with SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Data are expressed as mean ± standard deviation. The number of different cell types and content of inflammatory cytokines in BALF were compared with analysis of variance, followed by post hoc analysis with Tukey’s test. A P value of <0.05 was considered statistically significant. Animals that died during model establishment were not included in the final analysis.
Results
Number of coughs at different time points
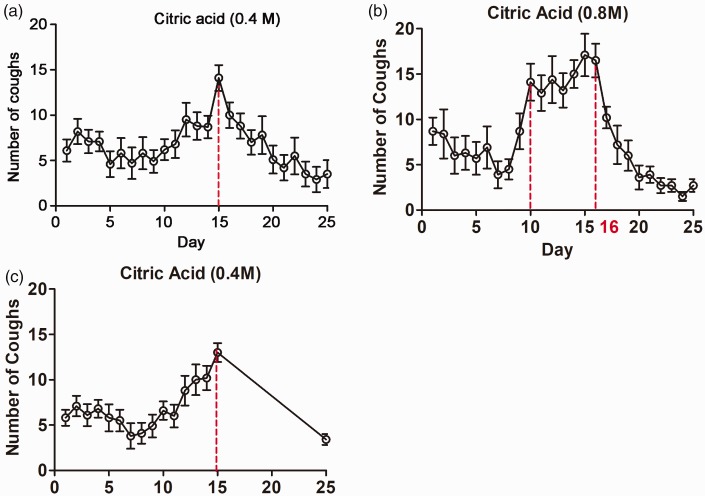
After inhalation of 0.4 M citric acid, the number of coughs changed over time: it increased soon after citric acid inhalation, peaked at 15 days, thereafter gradually decreased, and reached the lowest level at 24 to 25 days. The mean number of coughs was 6.20 ± 2.28 at baseline, 13.35 ± 4.69 at 15 days, and 2.61 ± 1.02 at 25 days, indicating that the cough hypersensitivity peaked after 15 days of citric acid inhalation. In the guinea pigs that stopped inhalation of citric acid after 15 days, the mean number of coughs was 5.80 ± 1.87 at baseline, 13.00 ± 3.30 at 15 days, and 3.40 ± 0.89 at 25 days (Figure 1).
Figure 1.
(a) Change in number of coughs after inhalation of 0.4 M citric acid (n = 20). (b) Change in number of coughs after inhalation of 0.8 M citric acid (n = 17). (c) Change in number of coughs after inhalation of 0.4 M citric acid for 15 days and 0.9% normal saline for 10 days (n = 10).
Differential cell counts in BALF at different time points
The proportion of neutrophils was significantly higher after citric acid inhalation for 15, 20, and 25 days when compared with the control group and 5-day group (P < 0.05), but it tended to be lower at 25 days than at 15 and 20 days (Table 1).
Table 1.
Differential cell count in bronchoalveolar lavage fluid from guinea pigs with chronic cough
| Group | Total cells ×105/mL | Mac % | Neu % | Lym % | Eos % |
|---|---|---|---|---|---|
| Normal control | 3.78 ± 0.86 | 72.5 ± 2.6 | 17.6 ± 5.2 | 5.9 ± 2.0 | 5.2 ± 2.3 |
| Inhalation of citric acid for 5 days | 3.99 ± 0.91 | 67.1 ± 3.6 | 20.3 ± 5.6 | 6.1 ± 1.9 | 6.6 ± 1.8 |
| Inhalation of citric acid for 10 days | 4.28 ± 1.19 | 45.8 ± 2.2 | 39.6 ± 6.3#,* | 7.5 ± 2.8 | 8.0 ± 2.9 |
| Inhalation of citric acid for 15 days | 5.63 ± 1.65#,* | 29.3 ± 4.8 | 53.8 ± 9.3#,* | 8.1 ± 2.2 | 8.6 ± 3.3 |
| Inhalation of citric acid for 20 days | 5.52 ± 1.36#,* | 31.1 ± 5.3 | 53.3 ± 8.7#,* | 8.9 ± 2.1 | 7.9 ± 2.2 |
| Inhalation of citric acid for 25 days | 5.03 ± 1.28# | 39.2 ± 6.1 | 45.1 ± 7.9#,* | 7.7 ± 2.3 | 8.1 ± 2.5 |
Mac, macrophages; Neu, neutrophils; Lym, lymphocytes; Eos, eosinophils.
*P < 0.05 vs. 5 days; #P < 0.05 vs. normal control.
Contents of SP and CGRP in BALF at different time points
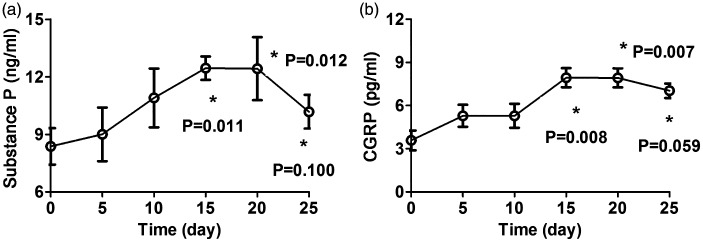
After 15 days of citric acid inhalation, the contents of SP and CGRP in BALF peaked. The contents of SP and CGRP in BALF were significantly higher after 15 and 25 days of citric acid inhalation when compared with the control group (P < 0.05), but they tended to be lower at 25 days than at 15 and 20 days (Table 2 and Figure 2).
Table 2.
Contents of SP and CGRP in bronchoalveolar lavage fluid of guinea pigs with chronic cough
| Group | SP (ng/mL) | CGRP (pg/mL) |
|---|---|---|
| Normal control | 8.02 ± 1.45 | 5.13 ± 0.81 |
| Inhalation of citric acid for 5 days | 8.86 ± 1.97 | 5.96 ± 1.26 |
| Inhalation of citric acid for 10 days | 10.52 ± 2.28 | 5.66 ± 1.55 |
| Inhalation of citric acid for 15 days | 12.03 ± 0.65#,* | 8.52 ± 1.37#,* |
| Inhalation of citric acid for 20 days | 11.91 ± 2.56#,* | 8.61 ± 1.82#,* |
| Inhalation of citric acid for 25 days | 10.22 ± 1.89#,* | 7.03 ± 1.31#,* |
SP, substance P; CGRP, calcitonin gene-related peptide.
*P < 0.05 vs. 5 days; #P < 0.05 vs. normal control.
Figure 2.
(a) Content of substance P in bronchoalveolar lavage fluid of guinea pigs with chronic cough at different time points. (b) Content of calcitonin gene-related peptide in bronchoalveolar lavage fluid of guinea pigs with chronic cough at different time points. *P < 0.05 vs. baseline. CGRP, calcitonin gene-related peptide.
HE staining
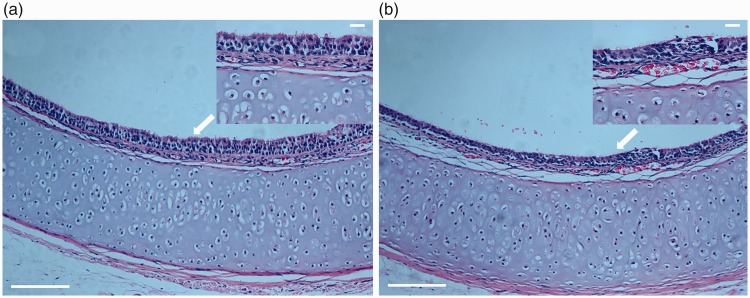
HE staining showed shedding of epithelial cells in the airway mucosa, submucosal edema, infiltration of inflammatory cells in the mucosa, and increased secretion of mucus in the cough group (Figure 3(a)). In the control group, the tracheal and bronchial epithelial cells were intact, and no mucus on the mucosa or infiltration of inflammatory cells in the mucosa was evident (Figure 3(b)).
Figure 3.
(a) Trachea in normal control group (hematoxylin–eosin staining; ×100; upper panel: ×400). (b) Trachea in chronic cough group (hematoxylin–eosin staining; ×100; upper panel: ×400). Arrow: bronchial ciliary lodging and shedding.
Protein expression of TRPV1 in the trachea and bronchi
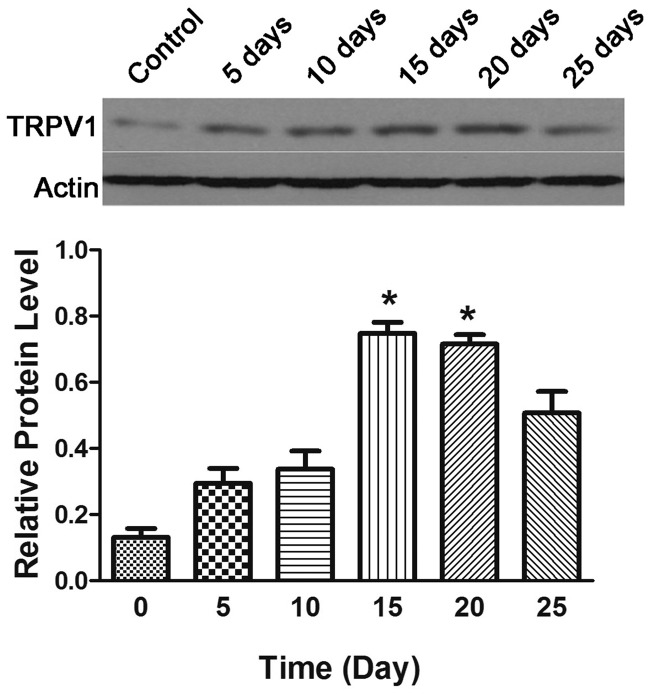
After inhalation of 0.4 M citric acid, western blotting showed that the TRPV1 protein expression in the trachea and bronchi changed over time: it gradually increased soon after citric acid inhalation and peaked at 15 days. TRPV1 protein expression in the trachea and bronchus at 15 and 20 days was significantly higher than that in the normal controls (P < 0.05), but it decreased at 25 days (Figure 4 and Table 3).
Figure 4.
TRPV1 protein expression in the bronchi and trachea of guinea pigs with chronic cough. *P < 0.05 vs. normal control.
Table 3.
TRPV1 protein expression in the bronchus and trachea of guinea pigs with chronic cough
| Group | Relative expression |
|---|---|
| Normal control | 0.15 ± 0.06 |
| Inhalation of citric acid for 5 days | 0.26 ± 0.11 |
| Inhalation of citric acid for 10 days | 0.32 ± 0.13 |
| Inhalation of citric acid for 15 days | 0.73 ± 0.29* |
| Inhalation of citric acid for 20 days | 0.70 ± 0.25* |
| Inhalation of citric acid for 25 days | 0.51 ± 0.20 |
*P < 0.05 vs. normal control.
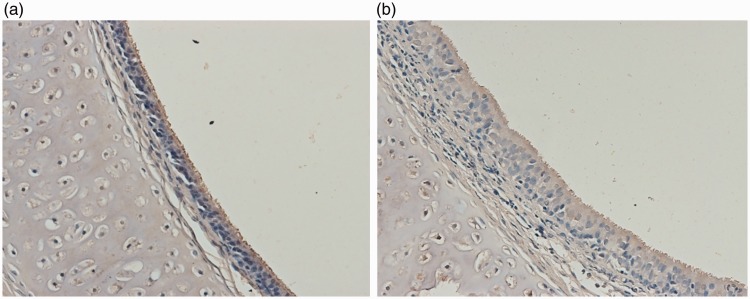
On immunohistochemical examination, TRPV1 was mainly observed in the mucosa and submucosal layer of the trachea and bronchi. The positive area of TRPV1 in the trachea and bronchi was significantly higher in citric acid-treated animals than in the control group (13.02% ± 3.56% vs. 3.09% ± 0.52%, respectively; P < 0.05) (Figure 5(a) and (b)).
Figure 5.
TRPV1 protein expression in the trachea and bronchi of the (a) control group and (b) chronic cough group (immunohistochemistry; ×200).
Discussion
The two main findings of our study were as follows. First, repeated inhalation of 0.4 M citric acid increased the guinea pigs’ cough sensitivity, which peaked at 15 days and thereafter decreased. Second, the citric acid-induced cough hypersensitivity was accompanied by an increase in BALF neutrophils, elevation of BALF SP and CGRP, increase in TRPV1 protein expression in the trachea and bronchi, and increase in airway mucosal injury. Three of 20 (15%) guinea pigs died after inhalation of 0.8 M citric acid, indicating that 0.8 M might not be an appropriate concentration for model establishment. Thus, 0.8 M was not used to create the chronic cough model in this study.
The use of mice or rats to establish cough models is controverisal;14,15 instead, most investigators accept that guinea pigs are the ideal animals for the establishment of cough models. The coughing sound of guinea pigs is similar to that of humans, the cough reflex is easy to induce, and the system used to monitor coughing is commercially available. In this study, repeated inhalation of citric acid was employed to establish a repeatable animal model of chronic cough. In addition, airway inflammation is a shared characteristic of chronic cough. Cough-variant asthma and eosinophilic bronchitis are common causes of chronic cough involving chronic inflammation characterized by eosinophil infiltration. In a fraction of patients with cough-variant asthma, there is also evidence of neutrophil infiltration in the submucosal layer of the airway.16 In patients with non-asthmatic chronic cough (such as upper airway cough syndrome or gastroesophageal reflux-induced chronic cough), the airway inflammation is characterized by infiltration of neutrophils, mast cells, and lymphocytes with a concomitant increase in IL-8 expression.17 Therefore, airway inflammation may be a basic event in the development of chronic cough of any cause.18–20 Conversely, a long-lasting cough may act as a mechanical stimulation that induces airway inflammation, leading to the deterioration of cough symptoms. As shown in our study, the inhalation of citric acid not only increased the cough hypersensitivity but also elevated the proportion of neutrophils in the airway and the secretion of mediators in neurogenic inflammation (SP and CGRP); this was also accompanied by loss of the airway epithelium, thickening of the basement membrane, ciliary lodging and shedding, infiltration of inflammatory cells in the airway, and increased mucus in the airway lumen. These findings imply that repeated inhalation of citric acid can be used to establish a model of airway inflammation-associated chronic cough that mimics the pathology of chronic cough in humans.21 Thus, the model used in the present study was a successfully established animal model of chronic cough.
Cough receptors are distributed between epithelial cells and within the epithelial basal layer of the bronchi and trachea. These receptors can be divided into afferent vagus nerve Aδ-fibers that sense mechanical and acid stimulation and afferent vagus nerve C-fibers that sense chemical stimulation. The C-fibers are highly sensitive to chemical stimuli in the airway (such as capsaicin, citric acid, and different inflammatory mediators).22,23 Citric acid can also stimulate retinoic acid receptors and acid-sensitive ion channels besides C-fibers, leading to the induction of cough.24,25 This avoids the stimulation of a single receptor and is thus more consistent with the pathogenesis of chronic cough in the real world.
TRPV1 is a ligand-gated nonselective cation channel protein that is localized in the endings of C-fibers. TRPV1 is the main cough receptor and a central participant in the transmission of cough-stimulating signals into the central nervous system. Capsaicin, high temperature, H+, and inflammatory mediators may activate TRPV1.22,23 The activated TRPV1 may induce the influx of calcium and sodium, leading to rapid depolarization of neurons and generation of nerve impulses. The subsequently activated C-fibers then release neuropeptides such as SP and CGRP to induce neurogenic inflammation in the airway, increasing the cough sensitivity.26,27 Neuropeptides such as SP and CGRP may not only cause C-fiber–mediated cough but also induce tissue edema and airway smooth muscle contraction, indirectly activating retinoic acid receptors and thus causing cough.23 Sekizawa et al.22 found that aerosol inhalation of SP could not cause cough in healthy subjects but that it could induce cough in patients with respiratory infection or idiopathic cough. This may be explained by the fact that the neutral endopeptidase in the respiratory mucosal epithelium can degrade SP. In the presence of airway inflammation, the degradation of SP in the airway is significantly compromised due to the epithelial injury, and SP then accumulates in the epithelium and subepithelium to stimulate cough sensors, resulting in cough. Alternatively, patients with respiratory infection or idiopathic cough might have cough hypersensitivity, and the threshold of SP-induced cough is reduced. In the present study, the cough reactivity increased over time in the early phase of citric acid inhalation; it then peaked at 15 days, which was accompanied by increased SP and CGRP in the BALF and elevated TRPV1 protein expression in the bronchi and trachea. This indicates that the chronic cough secondary to repeated inhalation of citric acid is ascribed to the release of SP and CGRP by C-fibers after TRPV1 activation and the subsequent neurogenic inflammation in the airway. The neuropeptides may in turn act on the C-fibers in the airway, leading to the increase in TRPV1 expression and alteration of airway plasticity, forming a positive feedback loop in the development of cough hypersensitivity.
In the present study, after 15 days of inhalation of citric acid, the cough hypersensitivity gradually decreased and reached the lowest level at 25 days. This indicates that guinea pigs develop resistance to citric acid-induced cough. After 15 days of exposure to citric acid, the cough hypersensitivity did not recover within 10 days, indicating that the tolerance to citric acid was continuously present in the guinea pigs. A similar phenomenon is also found in guinea pigs that have acclimated to high altitudes.28 The specific mechanism underlying this phenomenon is still poorly understood. The phenomenon has been proposed to be associated with downregulated expression of TRPV1 in vagus nerve C-fibers and the exhaustion or reduced release of neuropeptides in these nerve fibers, which may lead to attenuation of C-fiber excitability, tolerance to citric acid-induced cough, and subsequent relief of cough hypersensitivity. However, the absence of reductions in airway TRPV1 protein expression and BALF SP and CGRP does not support this hypothesis. Considering that the neuropeptides released by C-fibers after TRPV1 activation should bind to their corresponding receptors, which in turn activate C-fibers or Aδ-fibers by inducing neurogenic inflammation in the airway, we speculate that the reduced sensitivity of receptors of neuropeptides such as SP and CGRP may be a dominant cause of attenuation of cough hypersensitivity. However, the specific mechanism requires further study. Notably, after 15 days of exposure to citric acid, the cough hypersensitivity did not recover within 10 days, suggesting that the injured neuropeptide receptors at the nerve endings did not recover. The recovery might therefore take a relatively long time. Further studies are needed to determine the time required for recovery of cough hypersensitivity after stopping inhalation of citric acid.
Taken together, the findings in this study indicate that repeated inhalation of citric acid in guinea pigs can be used to establish a chronic cough model. Such an animal model will serve as a tool with which to investigate the pathogenesis of chronic cough as well as the pharmacodynamics and pharmacology of antitussive drugs. The cough hypersensitivity in guinea pigs may be related to airway TRPV1 activation and neurogenic inflammation. The passive cough secondary to inhalation of citric acid and the single stimulation differ from the active cough of humans in complex environments, which is one of the limitations of our animal model.
Acknowledgments
The authors thank Siwan Wen from the Department of Pulmonary and Critical Care Medicine, Tongji Hospital, Tongji University School of Medicine for providing assistance with English usage and paper revision.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81770097, 81670092, and 81470276) and the Project of Science and Technology Commission of Shanghai Municipality (Nos. 17411970800, 15411965500, and 14411971700).
References
- 1.Xu X, Chen Q, Liang S, et al. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to antireflux therapy. Chest 2014; 145: 1264–1270. [DOI] [PubMed] [Google Scholar]
- 2.Ma W, Yu L, Wang Y, et al. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough 2009; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014; 44: 1132–1148. [DOI] [PubMed] [Google Scholar]
- 4.Morice AH, McGarvey LP, Dicpinigaitis PV. Cough hypersensitivity syndrome is an important clinical concept: a pro/con debate. Lung 2012; 190: 3–9. [DOI] [PubMed] [Google Scholar]
- 5.Morice AH, Millqvist E, Belvisi MG, et al. Cough hypersensitivity syndrome: clinical measurement is the key to progress. Eur Respir J 2015; 45: 1509–1510. [DOI] [PubMed] [Google Scholar]
- 6.Dicpinigaitis PV, Canning BJ, Garner R, et al. Effect of memantine on cough reflex sensitivity: translational studies in guinea pigs and humans. J Pharmacol Exp Ther 2015; 352: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao HY, Su WW, Li PB, et al. Therapeutic effects of naringin in a guinea pig model of ovalbumin-induced cough-variant asthma. Pulm Pharmacol Ther 2015; 33: 59–65. [DOI] [PubMed] [Google Scholar]
- 8.Wex E, Bouyssou T. Olodaterol attenuates citric acid-induced cough in naive and ovalbumin-sensitized and challenged guinea pigs. PLoS One 2015; 10: e0119953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo YL, Li PB, Zhang CC, et al. Effects of four antitussives on airway neurogenic inflammation in a guinea pig model of chronic cough induced by cigarette smoke exposure. Inflamm Res 2013; 62: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 10.Zhong S, Nie YC, Gan ZY, et al. Effects of Schisandra chinensis extracts on cough and pulmonary inflammation in a cough hypersensitivity guinea pig model induced by cigarette smoke exposure. J Ethnopharmacol 2015; 165: 73–82. [DOI] [PubMed] [Google Scholar]
- 11.Niimi A, Torrego A, Nicholson AG, et al. Nature of airway inflammation and remodeling in chronic cough. J Allergy Clin Immunol 2005; 116: 565–570. [DOI] [PubMed] [Google Scholar]
- 12.Lieu T, Undem BJ. Neuroplasticity in vagal afferent neurons involved in cough. Pulm Pharmacol Ther 2011; 24: 276–279. [DOI] [PubMed] [Google Scholar]
- 13.Niimi A. Structural changes in the airways: cause or effect of chronic cough? Pulm Pharmacol Ther 2011; 24: 328–333. [DOI] [PubMed] [Google Scholar]
- 14.Qiu Z, Yu L, Xu S, et al. Cough reflex sensitivity and airway inflammation in patients with chronic cough due to non-acid gastro-oesophageal reflux. Respirology 2011; 16: 645–652. [DOI] [PubMed] [Google Scholar]
- 15.Shi CQ, Yu L, Wei WL, et al. Effect of Airway Inflammation on Pathogenesis of Upper Airway Cough Syndrome. Chin J Resp Critical Care Med 2009; 8: 256–258. [Google Scholar]
- 16.Yu L, Xu X, Lv H, Qiu Z. Advances in upper airway cough syndrome. Kaohsiung J Med Sci 2015; 31: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couto M, de Diego A, Perpini M, et al. Cough reflex testing with inhaled capsaicin and TRPV1 activation in asthma and comorbid conditions. J Investig Allergol Clin Immunol 2013; 23: 289–301. [PubMed] [Google Scholar]
- 18.Khalid S, Murdoch R, Newlands A, et al. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol 2014; 134: 56–62. [DOI] [PubMed] [Google Scholar]
- 19.Patberg KW. The female preponderance to cough hypersensitivity syndrome: another clue pointing to the role of TRPV1 in cough. Lung 2011; 189: 257–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadofsky LR, Ramachandran R, Crow C, et al. Inflammatory stimuli up-regulate transient receptor potential vanilloid-1 expression in human bronchial fibroblasts. Exp Lung Res 2012; 38: 75–81. [DOI] [PubMed] [Google Scholar]
- 21.Hara J, Fujimura M, Ueda A, et al. Effect of pressure stress applied to the airway on cough-reflex sensitivity in Guinea pigs. Am J Respir Crit Care Med 2008; 177: 585–592. [DOI] [PubMed] [Google Scholar]
- 22.Sekizawa K, Jia YX, Ebihara T, et al. Role of substance P in cough. Pulm Pharmacol 1996; 9: 323–328. [DOI] [PubMed] [Google Scholar]
- 23.Undem BJ, Carr MJ. Targeting primary afferent nerves for novel antitussive therapy. Chest 2010; 137: 177–184. [DOI] [PubMed] [Google Scholar]
- 24.Gibson PG, Simpson JL, Ryan NM, et al. Mechanisms of cough. Curr Opin Allergy Clin Immunol 2014; 14: 55–61. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Xu XH, Chen Q, et al. Gastro-esophageal reflux induced cough with airway hyperresponsiveness. Int J Clin Exp Med 2014; 7: 728–735. [PMC free article] [PubMed] [Google Scholar]
- 26.Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J 2013; 19: 18954. [PubMed] [Google Scholar]
- 27.Targum SD. Identification and treatment of antidepressant tachyphylaxis. Innov Clin Neurosci 2014; 11: 24–28. [PMC free article] [PubMed] [Google Scholar]
- 28.Hsia CC, Carbayo JJ, Yan X, et al. Enhanced alveolar growth and remodeling in Guinea pigs raised at high altitude. Respir Physiol Neurobiol 2005; 147: 105–115. [DOI] [PubMed] [Google Scholar]